Abstract
Objectives
To investigate the prognostic value of neutrophil-to-albumin ratio (NAR) in critically ill patients with cardiogenic shock (CS).
Design
A retrospective cohort study.
Setting
A single centre in Boston, USA.
Participants
475 patients with CS were included, among which 272 (57.3%) were men and 328 (69.1%) were white.
Primary and secondary outcome measures
The primary outcome was 90-day mortality and the secondary outcomes were 30-day and 365-day mortality.
Results
A significant positive correlation between NAR levels and 90-day, 30-day or 365-day mortality was observed. For 90-day mortality, the adjusted HR (95% CI) values given NAR levels 23.54–27.86 and >27.86 were 1.71 (1.14 to 2.55) and 1.93 (1.27 to 2.93) compared with the reference (NAR<23.47). Receiver operator characteristic curve analysis showed that NAR had a certain prognostic value in predicting 90-day mortality of CS, which was more sensitive than the neutrophil percentage or the serum albumin level alone (0.651 vs 0.509, 0.584). For the secondary outcomes, the upward trend remained statistically significant.
Conclusions
NAR level was associated with the mortality of CS patients. The prognostic value of NAR was more sensitive than the neutrophil percentage or the serum albumin level alone, but not as good as Sequential Organ Failure Assessment or Simplified Acute Physiology Score.
Keywords: cardiology, adult cardiology, intensive & critical care
Strengths and limitations of this study.
This was the first study to explore the prognostic effect of neutrophil-to-albumin ratio (NAR) in patients with cardiogenic shock.
Multivariate Cox proportional hazards model was applied in the study.
This was a retrospective observational study in a single centre.
The sample size of patients selected was small.
NAR was measured only when patients first admitted to the intensive care unit.
Introduction
Cardiogenic shock (CS), a lethal complication of cardiac emergencies, is traditionally thought to begin with depression of myocardial contractility, followed by intractable hypotension, coronary insufficiency and further loss of cardiac output, causing multiple organ failure and eventually death.1 2 For decades, the prevalence of CS has risen from 4.1% to 7.7% of all admissions to the intensive care unit (ICU),3 of which approximately 6.4%–40% mortality were reported despite intensive care.4 5 Hence, considering the high mortality of CS in ICU, finding effective and convenient prognostic biomarker may be beneficial to assist physicians to make medical decisions and identify patients at high risk.6–8
Inflammation plays an important role in the pathogenesis of CS.9 Among the inflammatory mediators, the neutrophil, well known as a marker of inflammation,10 has been widely studied regarding the development of various diseases, including CS. Serum albumin level was shown to be associated with cardiovascular mortality.11 12 Also, neutrophil or albumin has already been used in several clinical scoring systems. However, it is unclear whether the combination of neutrophils and albumin has a higher prognostic value. Neutrophil-to-albumin ratio (NAR), an integrated biomarker of neutrophils and albumin, is a cost-efficient and readily available biomarker which can be easily obtained from routine blood test. Recently, NAR has been used to evaluate the prognosis of cancer,13 14 but to the best of our knowledge, no study has explored the prognostic significance of NAR in patients with CS. Therefore, we performed a retrospective cohort study to identify the associations between NAR and mortality in patients with CS.
Material and methods
Data source
All data in our study were extracted from the Medical Information Mart for Intensive Care Database III V.1.4 (MIMIC-III V.1.4), a large, open, free and single-centred database including information from more than 50 000 adult patients admitted to various critical care units at Beth Israel Deaconess Medical Center (Boston, MA, USA) from 2001 to 2012.15 The setting and use of this database were approved by the institutional review boards of the Massachusetts Institute of Technology (Boston, MA) and Beth Israel Deaconess Medical Centre (Cambridge, MA). All personal information included in the database have been de-identified to safeguard privacy.
Population selection criteria
More than 50 000 ICU admissions to the MIMIC-III database were recorded, and only patients diagnosed with CS were extracted. Among these patients, we selected those who attained more than 16 years of age at first admission while remained in the hospital for more than 48 hours. Exclusion criteria were as follows: (1) patients diagnosed with haematologic neoplasms, including leukaemia, lymphoma, myelodysplastic syndrome, multiple myeloma and others; (2) more than 10% individual data were missing; (3) individual data values exceeded the mean±3 times the SD.
CS was determined according to the Ninth Revision of International Classification of Diseases, coded as R57.001. CS was defined that the systolic blood pressure (SBP) dropped below 90 mm Hg for more than 30 min or a need for catecholamine to maintain SBP above 90 mm Hg and also signs of end-organ hypoperfusion occurred (urine volume <30 mL/h, lactic acid >2.0 mmol/L, cold extremities or altered mental status).
Date extraction
Data were extracted through Structured Query Language (SQL)16 with MySQL tools from MIMIC-III. We extracted the baseline data within 24 hours at patients’ first admission, containing demographic parameters, basic vital signs, laboratory indicators and scoring systems.
Demographic parameters contained age, gender and ethnicity, while basic vital signs included SBP, diastolic blood pressure (DBP), mean blood pressure (MBP), heart rate, respiratory rate, temperature and percutaneous oxygen saturation (SPO2). The following laboratory indicators were extracted: neutrophils, albumin, white blood counts (WBC), haematocrit, haemoglobin, platelet count, serum bicarbonate, serum sodium, serum potassium, serum chloride, serum glucose, serum bilirubin, blood urea nitrogen (BUN), serum creatinine (SCr), partial thromboplastin time (PTT), prothrombin time (PT) and international normalised ratio (INR). We additionally extracted relevant comorbidities, like congestive heart failure (CHF), coronary heart disease (CHD), atrial fibrillation (AF), stroke, chronic obstructive pulmonary disease (COPD), pneumonia, acute respiratory distress syndrome and other diseases.
Severity-of-illness scores, including the Sequential Organ Failure Assessment (SOFA)17 score and the Simplified Acute Physiology Score II (SAPS II)18 were also calculated for every individual. These scores were assessed and calculated on the basis of published recommendations and accepted formulas.
The primary outcome was 90-day mortality and the secondary outcomes were 30-day mortality and 1-year mortality. Follow-up began when the patients first admitted to the ICU. The date of mortality was got from Social Security Death Index records.
Assessment of NAR
NAR was defined as the ratio of neutrophil percentage to serum albumin level. The indicators both came from the first measured data within 24 hours of ICU admission. Neutrophil percentage was analysed by the automatic flow cytometer, while albumin level was generated by biochemical analyser.
Statistical analysis
Categorical data were shown as frequency (percent), while continuous ones as mean (SD) or median (IQR). We did comparisons between groups by the χ2 test19 or Fisher’s exact test20 for categorical variables and the variance analysis or the Kruskal-Wallis test21 for continuous ones.
Cox proportional hazards models22 were used to examine the associations between NAR and outcomes. The outcomes were respectively analysed according to the tertiles of the NAR level. The first tertile group was regarded as the reference group. The results were presented as HRs with 95% CIs. Multivariate analyses were performed using two adjusted models. The confounders selected in our models were based on their associations with outcome or a change in the effect estimate exceeding 10%.23 In model I, we adjusted covariates for age, gender and ethnicity. In model II, covariates were adjusted further for SBP, DBP, heart rate, respiratory rate, SPO2, anion gap, serum bicarbonate, serum potassium, SCr, BUN, haematocrit, platelet count, WBC count, PTT, PT, INR, stroke, pneumonia, COPD, chronic liver disease, chronic renal disease, malignancy, vasoactive agent, renal replacement therapy (RRT), SOFA score and SAPSII score. The trend tests were performed to examine the differences between groups.
In addition, we performed stratification analysis to confirm whether the effect of NAR differs in each of the subgroups that were classified by vital signs (eg, SBP, DBP, heart rate, respiratory rate, temperature, SPO2), laboratory parameters (eg, anion gap, serum bicarbonate, serum sodium, serum potassium, serum chloride, serum bilirubin, serum glucose, SCr, BUN, haematocrit, haemoglobin, WBC count, platelet count, PTT, PT, INR), comorbidities (CHD, CHF, AF, stroke, pneumonia, respiratory failure, chronic liver disease, chronic renal disease, RRT, malignancy), vasoactive drug use and scoring systems (SOFA and SAPSII scores).
To further assess the predictive value of NAR, we did receiver operator characteristic (ROC) curve analysis for the 90-day mortality according to the neutrophil percentage, the serum albumin level, NAR, SOFA score and SPASII score.
A two-tailed p<0.05 was deemed statistically significant. We applied EmpowerStats V.2.17.8 (http://www.empowerstats.com/cn/) and R software V.3.42 for all statistical analysis.
Results
Patient characteristics
After excluding the patients who did not meet the inclusion criteria, a total of 475 patients were included. Among the patients included, 272 (57.3%) were men and 328 (69.1%) were white.
We divided the patients into three groups according to the tertiles of NAR. Baseline characteristics classified by NAR tertiles were presented in table 1. Patients in the higher NAR group tended to be white and had higher serum chloride, BUN, WBC count, PTT, PT, INR and lower serum bicarbonate, haematocrit, haemoglobin. Patients with higher NAR also had a higher SOFA and SAPSII scores than those with lower NAR (<23.47). These patients, however, had no apparent differences in age, gender, vital signs, vasoactive drug use or comorbidities.
Table 1.
Baseline characteristics of the study population
| NAR | P value | |||
| <23.47 | 23.54–27.86 | >27.86 | ||
| n | 158 | 158 | 159 | |
| NAR | 19.8±3.8 | 25.6±1.2 | 34.1±8.0 | <0.001 |
| Neutrophil | 72.6±15.4 | 83.2±9.6 | 85.8±7.8 | <0.001 |
| Albumin | 3.7±0.5 | 3.3±0.4 | 2.6±0.5 | <0.001 |
| Death, n (%) | ||||
| 30 day | 42 (26.6) | 67 (42.4) | 71 (44.7) | 0.001 |
| 90 day | 54 (34.2) | 76 (48.1) | 88 (55.3) | <0.001 |
| 365 day | 63 (39.9) | 93 (58.9) | 108 (67.9) | <0.001 |
| Age, years | 69.2±14.9 | 70.1±13.3 | 70.9±13.8 | 0.661 |
| Gender, n(%) | 0.065 | |||
| Female | 57 (36.1) | 68 (43.0) | 78 (49.1) | |
| Male | 101 (63.9) | 90 (57.0) | 81 (50.9) | |
| Ethnicity, n (%) | 0.039 | |||
| White | 112 (70.9) | 107 (67.7) | 109 (68.6) | |
| Black | 13 (8.2) | 3 (1.9) | 13 (8.2) | |
| Other | 33 (20.9) | 48 (30.4) | 37 (23.3) | |
| Vital signs | ||||
| Heart rate, beats/min | 86.6±17.5 | 90.1±17.3 | 90.4±18.0 | 0.077 |
| SBP, mm Hg | 108.3±15.2 | 106.0±13.3 | 104.7±13.9 | 0.058 |
| DBP, mm Hg | 58.7±9.9 | 57.5±8.9 | 57.5±11.5 | 0.303 |
| MBP, mm Hg | 75.2±9.5 | 74.4±9.3 | 73.3±10.0 | 0.114 |
| Respiratory rate, beats/minute | 19.9±4.2 | 20.1±3.9 | 20.2±4.1 | 0.554 |
| Temperature, °C | 36.8±0.8 | 36.8±0.9 | 36.7±0.9 | 0.442 |
| SPO2, % | 96.3±4.6 | 96.5±4.5 | 96.4±5.2 | 0.089 |
| Laboratory parameters | ||||
| Anion gap, mmol/L | 14.6±4.0 | 14.7±4.1 | 14.7±3.9 | 0.942 |
| Serum bicarbonate, mmol/L | 20.2±5.4 | 19.6±5.3 | 18.6±5.3 | 0.042 |
| Serum sodium, mmol/L | 134.7±5.4 | 135.0±6.6 | 135.3±5.3 | 0.632 |
| Serum potassium, mmol/L | 3.8±0.6 | 3.8±0.6 | 3.8±0.6 | 0.616 |
| Serum chloride, mmol/L | 99.5±7.0 | 101.2±7.6 | 101.9±6.2 | 0.010 |
| Serum glucose, mg/dL | 119.7±43.6 | 123.6±46.3 | 121.4±52.4 | 0.433 |
| Serum bilirubin, μmol/L | 1.0±1.7 | 0.9±1.0 | 1.3±2.9 | 0.625 |
| BUN, mg/dL | 33.2±23.0 | 35.2±24.9 | 38.5±23.9 | 0.031 |
| SCr, mg/dL | 1.7±1.5 | 1.7±1.3 | 1.8±1.4 | 0.629 |
| Haematocrit, % | 32.4±7.6 | 30.4±6.1 | 28.0±6.3 | <0.001 |
| Haemoglobin, g/dL | 11.0±2.6 | 10.2±2.1 | 9.3±2.0 | <0.001 |
| Platelet count, 109/l | 195.3±91.3 | 207.6±118.3 | 209.3±113.4 | 0.712 |
| WBC count, 109/l | 9.9±5.5 | 12.3±5.9 | 12.6±5.7 | <0.001 |
| PTT, s | 35.3±18.6 | 36.8±18.0 | 41.7±24.3 | 0.005 |
| PT, s | 15.5±5.8 | 16.3±5.7 | 16.5±5.1 | <0.001 |
| INR | 1.6±1.8 | 1.6±1.1 | 1.6±0.7 | <0.001 |
| Comorbidities, n (%) | ||||
| CHD | 89 (56.3) | 91 (57.6) | 79 (49.7) | 0.315 |
| CHF | 58 (36.7) | 58 (36.7) | 61 (38.4) | 0.940 |
| AF | 65 (41.1) | 68 (43.0) | 67 (42.1) | 0.943 |
| Stroke | 6 (3.8) | 6 (3.8) | 9 (5.7) | 0.752 |
| COPD | 1 (0.6) | 0 (0.0) | 4 (2.5) | 0.133 |
| Pneumonia | 50 (31.6) | 60 (38.0) | 53 (33.3) | 0.471 |
| ARDS | 2 (1.3) | 3 (1.9) | 0 (0.0) | 0.214 |
| Respiratory failure | 69 (43.7) | 82 (51.9) | 84 (52.8) | 0.200 |
| Chronic liver disease | 5 (3.2) | 11 (7.0) | 6 (3.8) | 0.250 |
| Chronic renal disease | 30 (19.0) | 28 (17.7) | 42 (26.4) | 0.122 |
| RRT | 16 (10.1) | 27 (17.1) | 30 (18.9) | 0.074 |
| Malignancy | 21 (13.3) | 15 (9.5) | 18 (11.3) | 0.568 |
| Vasoactive drug, n (%) | 113 (71.5) | 126 (79.7) | 126 (79.2) | 0.151 |
| Scoring systems | ||||
| SOFA | 6.7±3.6 | 6.8±4.0 | 7.9±3.7 | 0.005 |
| SAPSII | 45.5±15.3 | 46.5±15.1 | 52.8±16.1 | <0.001 |
Mean±SD and categorical variables are presented as n (%).
AF, atrial fibrillation; ARDS, acute respiratory distress syndrome; BUN, blood urea nitrogen; CHD, coronary heart disease; CHF, congestive heart failure; COPD, chronic obstructive pulmonary disease; DBP, diastolic blood pressure; INR, international normalised ratio; MBP, mean blood pressure; N, number; NAR, neutrophil-to-albumin ratio; PT, prothrombin time; PTT, partial thromboplastin time; RRT, renal replacement therapy; SAPSII, Simplified Acute Physiology Score; SBP, systolic blood pressure; SCr, serum creatinine; SOFA, Sequential Organ Failure Assessment; SPO2, percutaneous oxygen saturation; WBC, white blood count.
NAR levels and mortality
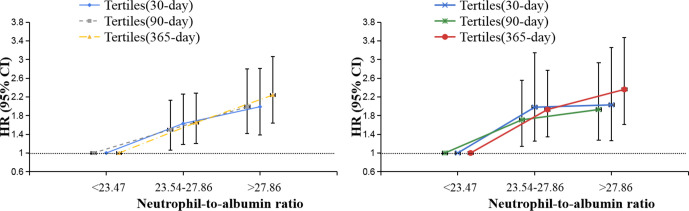
A total of 180, 218 and 264 deaths were recorded in the 30-day, 90-day and 365-day follow-up periods, respectively. Results of the relationship between NAR and mortality in CS patients were shown in table 2 and figure 1.
Table 2.
Association between NAR and mortality in CS patients
| Non-adjusted | Model I | Model II | ||||
| HR (95% CI) | P value | HR (95% CI) | P value | HR (95% CI) | P value | |
| 90-day mortality | ||||||
| NAR (per 0.1 change) | 1.03 (1.01 to 1.04) | <0.0001 | 1.03 (1.01 to 1.04) | <0.0001 | 1.02 (1.00 to 1.04) | 0.0276 |
| Fitted groups | ||||||
| <23.47 | 1.0 | 1.0 | 1.0 | |||
| 23.54–27.86 | 1.56 (1.10 to 2.22) | 0.0122 | 1.50 (1.06 to 2.13) | 0.0229 | 1.71 (1.14 to 2.55) | 0.0092 |
| >27.86 | 1.95 (1.39 to 2.73) | 0.0001 | 1.99 (1.42 to 2.80) | <0.0001 | 1.93 (1.27 to 2.93) | 0.0022 |
| P for trend | 0.0001 | <0.0001 | 0.0037 | |||
| 30-day mortality | ||||||
| NAR (per 0.1 change) | 1.02 (1.01 to 1.04) | 0.0020 | 1.02 (1.01 to 1.04) | 0.0031 | 1.02 (1.00 to 1.04) | 0.1371 |
| Fitted groups | ||||||
| <23.47 | 1.0 | 1.0 | 1.0 | |||
| 23.54–27.86 | 1.72 (1.17 to 2.53) | 0.0060 | 1.63 (1.10 to 2.40) | 0.0139 | 1.98 (1.25 to 3.15) | 0.0036 |
| >27.86 | 1.96 (1.34 to 2.87) | 0.0006 | 1.99 (1.36 to 2.92) | 0.0004 | 2.03 (1.26 to 3.26) | 0.0036 |
| P for trend | 0.0008 | 0.0005 | 0.0096 | |||
| 365-day mortality | ||||||
| NAR (per 0.1 change) | 1.03 (1.02 to 1.04) | <0.0001 | 1.03 (1.02 to 1.04) | <0.0001 | 1.03 (1.01 to 1.04) | 0.0024 |
| Fitted groups | ||||||
| <23.47 | 1.0 | 1.0 | 1.0 | |||
| 23.54–27.86 | 1.69 (1.23 to 2.33) | 0.0013 | 1.65 (1.20 to 2.28) | 0.0022 | 1.93 (1.34 to 2.77) | 0.0004 |
| >27.86 | 2.17 (1.59 to 2.97) | <0.0001 | 2.24 (1.64 to 3.06) | <0.0001 | 2.36 (1.61 to 3.47) | <0.0001 |
| P for trend | <0.0001 | <0.0001 | <0.0001 | |||
Models I and II were derived from Cox proportional hazards regression models: model I covariates were adjusted for age; gender; ethnicity; model II covariates were adjusted for age; gender; ethnicity; heart rate; SBP; DBP; respiratory rate; SPO2; anion gap; serum bicarbonate; serum potassium; SCr; BUN; haematocrit; platelet count; WBC count; PTT; PT; INR; stroke; pneumonia; COPD; chronic liver disease; chronic renal disease; RRT; malignancy; vasoactive agent; SOFA; SAPSII.
BUN, blod urea nitrogen; COPD, chronic obstructive pulmonary disease; CS, cardiogenic shock; DBP, diastolic blood pressure; INR, international normalised ratio; NAR, neutrophil-to-albumin ratio; PT, prothrombin time; PTT, partial thromboplastin time; RRT, renal replacement therapy; SAPSII, Simplified Acute Physiology Score; SBP, systolic blood pressure; SCr, serum creatinine; SOFA, Sequential Organ Failure Assessment; SPO2, percutaneous oxygen saturation.
Figure 1.
HRs (95% CIs) for mortality across tertile groups of NARs. (Tertiles: model I and model II). NAR, neutrophil-to-albumin ratio.
For the primary outcome of 90-day mortality, we found that higher NAR was related to increased risk of mortality. The HR (95% CI) values of the mid-tertile (NAR=23.54–27.86) and the upper tertile (NAR>27.86) were 1.56 (1.10 to 2.22) and 1.95 (1.39 to 2.73), respectively, when compared with the reference (NAR<23.47). After adjusted for age, gender and ethnicity in model I, an increasing trend was also observed and the adjusted HR (95% CI) values for 90-day mortality given NAR of 23.54–27.86 and >27.86 were 1.50 (1.06 to 2.13) and 1.99 (1.42 to 2.80). After further adjusted for potential confounders in model II, the upward trend remained statistically significant (mid-tertile: 1.71 (1.14 to 2.55); upper tertile: 1.93 (1.27 to 2.93)).
The similar trends were also observed for the secondary outcomes of 30-day and 365-day mortality.
Subgroup analysis
Subgroup analysis was conducted to determine the consistency of association between NAR and 90-day mortality in patients with CS. Partial results were shown in table 3 and the full results were in the online supplemental table. Most subgroup factors showed low significance with 90-day mortality, except for the serum sodium (p=0.0270), the serum bilirubin (p=0.0343), respiratory failure (p=0.0102) and RRT (p=0.0044). NAR particularly showed significant interactions in patients without RRT. Patients without the therapy of RRT had a significant higher 90-day mortality risk for NAR>27.86 (HR (95% CI): 2.29 (1.58 to 3.32)). In addition, patients without respiratory failure also had higher mortality risks.
Table 3.
(Partial). Subgroup analysis of the association between NAR and 90-day mortality
| N | NAR stratification | P value | |||
| <23.47 | 23.54–27.86 | >27.86 | |||
| Laboratory parameters | |||||
| Serum sodium, mmol/L | 0.0270 | ||||
| ≤134 | 192 | 1.0 | 1.11 (0.66 to 1.88) | 1.23 (0.74 to 2.03) | |
| >134 | 283 | 1.0 | 2.01 (1.24 to 3.27)** | 2.90 (1.81 to 4.67)*** | |
| Serum potassium, mmol/L | 0.3218 | ||||
| ≤3.6 | 211 | 1.0 | 1.71 (0.94 to 3.11) | 1.94 (1.08 to 3.46)* | |
| >3.6 | 264 | 1.0 | 1.44 (0.93 to 2.24) | 2.24 (1.45 to 3.45)c | |
| Serum chloride, mmol/L | 0.0702 | ||||
| ≤100 | 206 | 1.0 | 1.59 (0.97 to 2.61) | 1.44 (0.86 to 2.39) | |
| >100 | 269 | 1.0 | 1.50 (0.91 to 2.48) | 2.56 (1.59 to 4.12)*** | |
| Serum bilirubin, μmol/L | 0.0343 | ||||
| ≤0.5 | 192 | 1.0 | 1.91 (1.07 to 3.42)* | 2.87 (1.63 to 5.05)*** | |
| >0.5 | 239 | 1.0 | 1.46 (0.89 to 2.37) | 1.75 (1.08 to 2.84)* | |
| Comorbidities | |||||
| Respiratory failure | 0.0102 | ||||
| No | 240 | 1.0 | 1.73 (1.02 to 2.95)* | 3.14 (1.89 to 5.20)*** | |
| Yes | 235 | 1.0 | 1.22 (0.76 to 1.94) | 1.19 (0.75 to 1.90) | |
| RRT | 0.0044 | ||||
| No | 402 | 1.0 | 1.42 (0.96 to 2.11) | 2.29 (1.58 to 3.32)*** | |
| Yes | 73 | 1.0 | 0.87 (0.36 to 2.09) | 0.45 (0.18 to 1.11) | |
| Stroke | 0.0742 | ||||
| No | 454 | 1.0 | 1.44 (1.01 to 2.05)* | 1.85 (1.31 to 2.61)*** | |
| Yes | 21 | 1.0 | NA | NA | |
| Malignancy | 0.3513 | ||||
| No | 421 | 1.0 | 1.53 (1.05 to 2.25)* | 2.15 (1.48 to 3.12)*** | |
| Yes | 54 | 1.0 | 1.58 (0.59 to 4.26) | 1.08 (0.41 to 2.85) | |
P value: *p< 0.05, **p< 0.01, ***p< 0.001.
N/A, not applicable; RRT, renal replacement therapy;
NAR, neutrophil-albumin ratio.;
bmjopen-2020-039860supp001.pdf (122.2KB, pdf)
ROC curve analysis
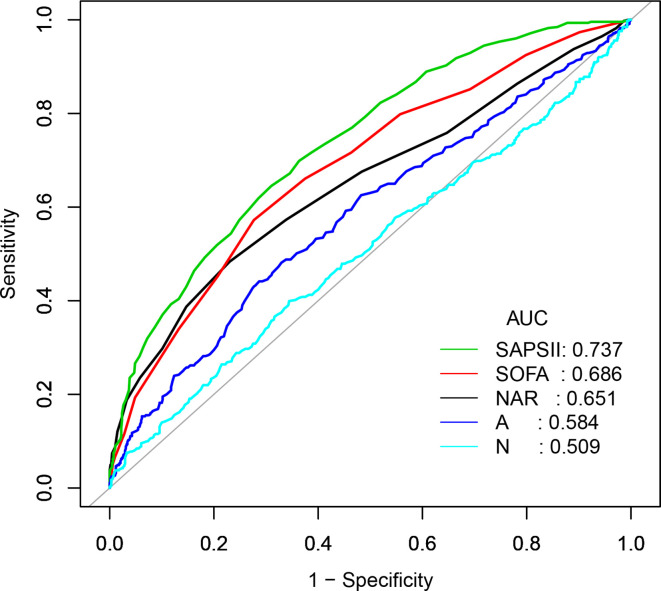
ROC curve analysis (figure 2) was performed to further test the potential prognostic value of NAR in predicting the survival of CS patients. Compared with the neutrophil percentage or the serum albumin level alone, NAR was more sensitive in predicting 90-day mortality of CS (AUC: 0.651 vs 0.509, 0.584). The C statistic for NAR, however, was lower than that of SOFA or SAPSII scores (0.651 vs 0.686, 0.737). However, when ROC curve analysis was performed combining NAR and SOFA score or SAPS II, NAR contributed very little to these already known and well-consolidated prognostic indices (figures 3 and 4).
Figure 2.
ROC curve for 90-day mortality of CS patients. (N: Neutrophil percentage, A: Albumin). AUC, area under the curve; CS, cardiogenic shock; NAR, neutrophil-to-albumin; SOFA, Sequential Organ Failure Assessment; SAPSII, Simplified Acute Physiology Score; ROC, receiver operator characteristic curve.
Figure 3.
ROC curve for combining SAPSII and NAR. (Model a: SAPSII+NAR; model b: SAPSII). AUC, area under the curve; NAR, neutrophil-to-albumin; SAPSII, Simplified Acute Physiology Score; ROC, receiver operator characteristic curve.
Figure 4.
ROC curve for combining SOFA and NAR. (Model a: SOFA+NAR; model b: SOFA). AUC, area under the curve; NAR, neutrophil-to-albumin; SOFA, Sequential Organ Failure Assessment; ROC, receiver operator characteristic curve.
Discussion
In our study, we found a significant positive association between NAR levels and mortality in patients with CS. In particular, a high level of NAR was associated with growing risk of mortality. In addition, NAR was more sensitive in predicting mortality of CS than the neutrophil percentage or the serum albumin level alone. The predictive value of NAR, however, was not as good as SOFA or SAPSII score.
CS is a lethal complication of cardiovascular diseases with an extremely high mortality. Inflammation has been shown to play a vital role in the pathogenesis of CS. Studies in recent decades have suggested the prognostic value of inflammatory mediators in CS, including blood cells,24 cytokines,25 26 complement27 and enzymes.28 29 Furthermore, the use of albumin, the main serum protein, to predict the mortality of cardiovascular disease as well as all-cause mortality has already been described.11 30 31 Recent studies have combined these inflammatory mediators to predict the outcome of diseases. The NAR, a combination of the neutrophil percentage and the serum albumin level, is a novel and readily available biomarker. Prior to our work, the prognostic value of NAR has recently been shown. Samuel et al14 demonstrated that NAR was a significant prognostic marker in patients with palliative pancreatic cancer. Tawfik et al13 investigated the association between NAR and pathological complete response in rectal cancer patients after neoadjuvant chemoradiation. Based on these evidences, an inference may be put forward that NAR could predict the mortality in patients with CS.
It remains unclear why NAR, the combined biomarker, could have such a significant prognostic value in patients with CS. On the one hand, neutrophil, which is a vital type of leucocytes, has been well studied regarding the development of various diseases, including CS. Sionis et al32 recently found that distinct microparticles released by neutrophils (CD15+) significantly increased in patients with CS. This result suggested high activation of neutrophils in CS and further indicated the significance of inflammation in that condition. Given the severe systemic inflammatory response in CS, it has been demonstrated that patients with higher leucocyte count had a higher mortality in CS.33 However, whether the increase of inflammatory mediators in CS results from the heart itself, from intestinal bacterial translocation, or from ischemia-reperfusion injury remains unknown.9 On the other hand, serum albumin, synthesised in the liver, is the major plasma protein in human blood. Albumin has already been used to predict mortality especially in critically ill patients in ICUs.34 35 It has already become a part of major risk scores, such as the Acute Physiology and Chronic Health Evaluation III Prognostic system. Low albumin levels were demonstrated to be related to some inflammatory mediators;36 37 therefore, the association between serum albumin and mortality may result from subclinical inflammation, as Mutsert et al38 found in their study. However, whether the prognostic value of albumin only reflects inflammation or whether there is an independent role of albumin itself remains to be clear. As albumin plays an important role in maintaining the plasma colloid osmotic pressure, low albumin levels may disorganise the fluid distribution in the internal environment so as to destroy the balance of the haemodynamics, resulting in poor outcomes.39 Another interpretation may involve the state of nutrition. Studies have shown that low albumin may be related to malnutrition, emaciation or cachexia.40 However, other studies have indicated that albumin is a lousy nutrition marker. The relationship between albumin and nutritional status remains controversial. Furthermore, as the most abundant carrier protein in plasma, albumin can change the existing form of some toxins by binding to them, leading to changes in their biological effects. The recent study of Watanabe et al41 indicated that, when combined with lower albumin levels, levels of indoxyl sulfate, a protein-bound uremic toxin, might be a prognostic marker for cardiovascular diseases, because lower albumin levels might increase free indoxyl sulfate levels, possibly activating a signal transduction pathway and subsequently exerting toxic effects. Further studies are needed to confirm these hypotheses.
CS in critically ill patients has an extremely high mortality. This poor outcome may be affected by a number of factors, including basic vital signs (ie, DBP,42 MBP),43 some laboratory parameters (ie, serum bicarbonate levels,44 cardiac power index,43 vasopressor support),45 severity-of-illness scores (ie, SAPSII),43 as well as other comorbidities. In subgroup analysis, patients were stratified according to potential confounders and statistically significant interactions were observed for some factors, such as respiratory failure and RRT. Patients without a history of respiratory failure or without the therapy of RRT might have a higher risk of 90-day mortality. In patients with the therapy of RRT, prognosis might be meliorated through metabolites clearance. While in patients with a history of respiratory failure, the improved survival might contribute to the systemic antimicrobial therapy and advanced assisted ventilation strategies. The real mechanism, however, remained unclear.
Our study was the first study to explore the prognostic effect of NAR in patients with CS. The period of follow-up in our study was quite long. The limitations of this study, however, cannot be ignored. First and foremost, it was a retrospective observational study in a single centre. The biases inherent in this type of study and selection bias in this design should be highlighted. Therefore, we should further perform studies based on multiple centres. Second, owing to the relatively low incidence of CS, the sample size of patients selected in our study was small, suggesting that larger prospective studies are needed. Third, NAR was measured only when patients first admitted to the ICU, possibly causing biases to a certain extent. Therefore, the dynamic evaluation of NAR during the ICU stay can make a difference. Furthermore, merely measuring NAR does not adequately reflect genuine levels of inflammation. Therefore, simultaneous measurement of other inflammatory factors would make a better demonstration of our conclusions. Last but not the least, to set up NAR as a prognostic biomarker, its clinical significance must further be verified.
Conclusions
NAR level was associated with the mortality of CS patients. NAR was an potential prognostic biomarker of mortality in CS patients. Its predictive value was more sensitive than the neutrophil percentage or the serum albumin level alone, but not as good as SOFA or SAPSII score. However, further prospective studies with larger sample size are needed to confirm our findings.
Supplementary Material
Footnotes
Contributors: JW and CL conceived and designed the research. YP and YX participated in statistical analysis and drafted the manuscript. JW, HX and KJ participated in data collection, data processing.
Funding: This work was supported by the National Natural Science Foundation of China (No. 81573185).
Competing interests: None declared.
Patient consent for publication: Not required.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data availability statement: Data are available in a public, open access repository. All data in our study were extracted from a freely accessible database, the Medical Information Mart for Intensive Care Database III version 1.4 (MIMIC-III v1.4). The setting and use of this database were approved by the institutional review boards of the Massachusetts Institute of Technology (Boston, MA) and Beth Israel Deaconess Medical Center (Cambridge, MA). Anyone who want to get access to the database must complete the online course of the National Institutes of Health and pass the Examination for the Protection of Human Research Participants. We have finished it and acquired the certificate (No. 8043591).
Supplemental material This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
References
- 1.Tewelde SZ, Liu SS, Winters ME. Cardiogenic shock. Cardiol Clin 2018;36:53–61. 10.1016/j.ccl.2017.08.009 [DOI] [PubMed] [Google Scholar]
- 2.van Diepen S, Katz JN, Albert NM, et al. . Contemporary management of cardiogenic shock: a scientific statement from the American heart association. Circulation 2017;136:e232–68. 10.1161/CIR.0000000000000525 [DOI] [PubMed] [Google Scholar]
- 3.Puymirat E, Fagon JY, Aegerter P, et al. . Cardiogenic shock in intensive care units: evolution of prevalence, patient profile, management and outcomes, 1997-2012. Eur J Heart Fail 2017;19:192–200. 10.1002/ejhf.646 [DOI] [PubMed] [Google Scholar]
- 4.Azoulay E, Adrie C, De Lassence A, et al. . Determinants of postintensive care unit mortality: a prospective multicenter study. Crit Care Med 2003;31:428–32. 10.1097/01.CCM.0000048622.01013.88 [DOI] [PubMed] [Google Scholar]
- 5.Knaus WA, Wagner DP, Zimmerman JE, et al. . Variations in mortality and length of stay in intensive care units. Ann Intern Med 1993;118:753–61. 10.7326/0003-4819-118-10-199305150-00001 [DOI] [PubMed] [Google Scholar]
- 6.Nguyen HL, Yarzebski J, Lessard D, et al. . Ten‐Year (2001–2011) trends in the incidence rates and Short‐Term outcomes of early versus late onset cardiogenic shock after hospitalization for acute myocardial infarction. J Am Heart Assoc 2017;6 10.1161/JAHA.117.005566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Aushev A, Ripoll VR, Vellido A, et al. . Feature selection for the accurate prediction of septic and cardiogenic shock ICU mortality in the acute phase. PLoS One 2018;13:e0199089. 10.1371/journal.pone.0199089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Goldberg RJ, Spencer FA, Gore JM, et al. . Thirty-year trends (1975 to 2005) in the magnitude of, management of, and hospital death rates associated with cardiogenic shock in patients with acute myocardial infarction: a population-based perspective. Circulation 2009;119:1211–9. 10.1161/CIRCULATIONAHA.108.814947 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Geppert A, Huber K. Inflammation and cardiovascular diseases: lessons that can be learned for the patient with cardiogenic shock in the intensive care unit. Curr Opin Crit Care 2004;10:347–53. 10.1097/01.ccx.0000139364.53198.fd [DOI] [PubMed] [Google Scholar]
- 10.Gibson PH, Croal BL, Cuthbertson BH, et al. . Preoperative neutrophil-lymphocyte ratio and outcome from coronary artery bypass grafting. Am Heart J 2007;154:995–1002. 10.1016/j.ahj.2007.06.043 [DOI] [PubMed] [Google Scholar]
- 11.Grimm G, Haslacher H, Kampitsch T, et al. . Sex differences in the association between albumin and all-cause and vascular mortality. Eur J Clin Invest 2009;39:860–5. 10.1111/j.1365-2362.2009.02189.x [DOI] [PubMed] [Google Scholar]
- 12.Takata Y, Ansai T, Soh I, et al. . Serum albumin levels as an independent predictor of 4-year mortality in a community-dwelling 80-year-old population. Aging Clin Exp Res 2010;22:31–5. 10.1007/BF03324812 [DOI] [PubMed] [Google Scholar]
- 13.Tawfik B, Mokdad AA, Patel PM, et al. . The neutrophil to albumin ratio as a predictor of pathological complete response in rectal cancer patients following neoadjuvant chemoradiation. Anticancer Drugs 2016;27:879–83. 10.1097/CAD.0000000000000411 [DOI] [PubMed] [Google Scholar]
- 14.Tingle SJ, Severs GR, Goodfellow M, et al. . NARCA: a novel prognostic scoring system using neutrophil-albumin ratio and CA19-9 to predict overall survival in palliative pancreatic cancer. J Surg Oncol 2018;118:680–6. 10.1002/jso.25209 [DOI] [PubMed] [Google Scholar]
- 15.Johnson AEW, Pollard TJ, Shen L, et al. . MIMIC-III, a freely accessible critical care database. Sci Data 2016;3:160035. 10.1038/sdata.2016.35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jamison DC. Structured query language (SQL) fundamentals. Curr Protoc Bioinformatics 2003;Chapter 9:9.2.1–9.2.29. 10.1002/0471250953.bi0902s00 [DOI] [PubMed] [Google Scholar]
- 17.Vincent JL, Moreno R, Takala J, et al. . The SOFA (sepsis-related organ failure assessment) score to describe organ dysfunction/failure. on behalf of the Working group on sepsis-related problems of the European Society of intensive care medicine. Intensive Care Med 1996;22:707–10. 10.1007/BF01709751 [DOI] [PubMed] [Google Scholar]
- 18.Le Gall JR, Lemeshow S, Saulnier F. A new simplified acute physiology score (SAPs II) based on a European/North American multicenter study. JAMA 1993;270:2957–63. 10.1001/jama.1993.03510240069035 [DOI] [PubMed] [Google Scholar]
- 19.Tallarida RJ, Murray RB. Chi-Square Test : Tallarida RJ, Murray RB, Manual of pharmacologic calculations: with computer programs. New York, NY: Springer New York, 1987: 140–2. [Google Scholar]
- 20.Rédei G. Fisher’s Exact Test : Rédei G, Encyclopedia of genetics, genomics, proteomics and informatics. Springer Netherlands: Dordrecht, 2008: 690. [Google Scholar]
- 21.Dalgaard P. Analysis of variance and the Kruskal-Wallis test, in introductory statistics with R. Springer New York: New York, NY, 2002: 111–27. [Google Scholar]
- 22.Kirch W. Cox proportional hazards model, in encyclopedia of public health. Dordrecht: Springer Netherlands, 2008: 176. [Google Scholar]
- 23.Jaddoe VWV, de Jonge LL, Hofman A, et al. . First trimester fetal growth restriction and cardiovascular risk factors in school age children: population based cohort study. BMJ 2014;348:g14. 10.1136/bmj.g14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gagnon DR, Zhang TJ, Brand FN, et al. . Hematocrit and the risk of cardiovascular disease--the Framingham study: a 34-year follow-up. Am Heart J 1994;127:674–82. 10.1016/0002-8703(94)90679-3 [DOI] [PubMed] [Google Scholar]
- 25.Prondzinsky R, Unverzagt S, Lemm H, et al. . Interleukin-6, -7, -8 and -10 predict outcome in acute myocardial infarction complicated by cardiogenic shock. Clin Res Cardiol 2012;101:375–84. 10.1007/s00392-011-0403-3 [DOI] [PubMed] [Google Scholar]
- 26.Prondzinsky R, Unverzagt S, Lemm H, et al. . Acute myocardial infarction and cardiogenic shock: prognostic impact of cytokines: INF-γ, TNF-α, MIP-1β, G-CSF, and MCP-1β. Med Klin Intensivmed Notfmed 2012;107:476–84. 10.1007/s00063-012-0117-y [DOI] [PubMed] [Google Scholar]
- 27.Granger CB, Mahaffey KW, Weaver WD, et al. . Pexelizumab, an anti-C5 complement antibody, as adjunctive therapy to primary percutaneous coronary intervention in acute myocardial infarction: the complement inhibition in myocardial infarction treated with angioplasty (CoMMA) trial. Circulation 2003;108:1184–90. 10.1161/01.CIR.0000087447.12918.85 [DOI] [PubMed] [Google Scholar]
- 28.Dominguez-Rodriguez A, Samimi-Fard S, Abreu-Gonzalez P, et al. . Prognostic value of admission myeloperoxidase levels in patients with ST-segment elevation myocardial infarction and cardiogenic shock. Am J Cardiol 2008;101:1537–40. 10.1016/j.amjcard.2008.02.032 [DOI] [PubMed] [Google Scholar]
- 29.Cotter G, Kaluski E, Milo O, et al. . LINCS: L-NAME (a NO synthase inhibitor) in the treatment of refractory cardiogenic shock: a prospective randomized study. Eur Heart J 2003;24:1287–95. 10.1016/S0195-668X(03)00193-3 [DOI] [PubMed] [Google Scholar]
- 30.Goldwasser P, Feldman J. Association of serum albumin and mortality risk. J Clin Epidemiol 1997;50:693–703. 10.1016/S0895-4356(97)00015-2 [DOI] [PubMed] [Google Scholar]
- 31.Okamura T, Hayakawa T, Kadowaki T, et al. . A combination of serum low albumin and above-average cholesterol level was associated with excess mortality. J Clin Epidemiol 2004;57:1188–95. 10.1016/j.jclinepi.2004.02.019 [DOI] [PubMed] [Google Scholar]
- 32.Sionis A, Suades R, Sans-Roselló J, et al. . Circulating microparticles are associated with clinical severity of persistent ST-segment elevation myocardial infarction complicated with cardiogenic shock. Int J Cardiol 2018;258:249–56. 10.1016/j.ijcard.2017.10.044 [DOI] [PubMed] [Google Scholar]
- 33.Barron HV, Cannon CP, Murphy SA, et al. . Association between white blood cell count, epicardial blood flow, myocardial perfusion, and clinical outcomes in the setting of acute myocardial infarction: a thrombolysis in myocardial infarction 10 substudy. Circulation 2000;102:2329–34. 10.1161/01.CIR.102.19.2329 [DOI] [PubMed] [Google Scholar]
- 34.Gillum RF, Makuc DM. Serum albumin, coronary heart disease, and death. Am Heart J 1992;123:507–13. 10.1016/0002-8703(92)90667-K [DOI] [PubMed] [Google Scholar]
- 35.Weijenberg MP, Feskens EJ, Souverijn JH, et al. . Serum albumin, coronary heart disease risk, and mortality in an elderly cohort. Epidemiology 1997;8:87–92. 10.1097/00001648-199701000-00014 [DOI] [PubMed] [Google Scholar]
- 36.Sullivan DH, Roberson PK, Johnson LE, et al. . Association between inflammation-associated cytokines, serum albumins, and mortality in the elderly. J Am Med Dir Assoc 2007;8:458–63. 10.1016/j.jamda.2007.04.004 [DOI] [PubMed] [Google Scholar]
- 37.Nelson JJ, Liao D, Sharrett AR, et al. . Serum albumin level as a predictor of incident coronary heart disease: the Atherosclerosis risk in communities (ARIC) study. Am J Epidemiol 2000;151:468–77. 10.1093/oxfordjournals.aje.a010232 [DOI] [PubMed] [Google Scholar]
- 38.de Mutsert R, Grootendorst DC, Indemans F, et al. . Association between serum albumin and mortality in dialysis patients is partly explained by inflammation, and not by malnutrition. J Ren Nutr 2009;19:127–35. 10.1053/j.jrn.2008.08.003 [DOI] [PubMed] [Google Scholar]
- 39.Rackow EC, Fein IA, Leppo J. Colloid osmotic pressure as a prognostic indicator of pulmonary edema and mortality in the critically ill. Chest 1977;72:709–13. 10.1378/chest.72.6.709 [DOI] [PubMed] [Google Scholar]
- 40.Evans WJ, Morley JE, Argilés J, et al. . Cachexia: a new definition. Clin Nutr 2008;27:793–9. 10.1016/j.clnu.2008.06.013 [DOI] [PubMed] [Google Scholar]
- 41.Watanabe I, Tatebe J, Fujii T, et al. . Prognostic significance of serum indoxyl sulfate and albumin for patients with cardiovascular disease. Int Heart J 2019;60:129–35. 10.1536/ihj.18-116 [DOI] [PubMed] [Google Scholar]
- 42.Axler O. Low diastolic blood pressure as best predictor of mortality in cardiogenic shock*. Crit Care Med 2013;41:2644–7. 10.1097/CCM.0b013e31829cb36e [DOI] [PubMed] [Google Scholar]
- 43.Popovic B, Fay R, Cravoisy-Popovic A, et al. . Cardiac power index, mean arterial pressure, and simplified acute physiology score II are strong predictors of survival and response to revascularization in cardiogenic shock. Shock 2014;42:22–6. 10.1097/SHK.0000000000000170 [DOI] [PubMed] [Google Scholar]
- 44.Wigger O, Bloechlinger S, Berger D, et al. . Baseline serum bicarbonate levels independently predict short-term mortality in critically ill patients with ischaemic cardiogenic shock. Eur Heart J Acute Cardiovasc Care 2018;7:45–52. 10.1177/2048872616683526 [DOI] [PubMed] [Google Scholar]
- 45.Geppert A, Dorninger A, Delle-Karth G, et al. . Plasma concentrations of interleukin-6, organ failure, vasopressor support, and successful coronary revascularization in predicting 30-day mortality of patients with cardiogenic shock complicating acute myocardial infarction. Crit Care Med 2006;34:2035–42. 10.1097/01.CCM.0000228919.33620.D9 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2020-039860supp001.pdf (122.2KB, pdf)