Abstract
Background:
Cervical cancer is the fourth most common cancer among women worldwide, with over 300,000 deaths each year. In addition to screening and prevention, effective cancer treatment is needed to reduce cervical cancer mortality. We discuss the role of imaging in cervical cancer management and estimate the potential survival impact of scaling up imaging in different contexts.
Methods:
Using a previously developed microsimulation model of global cancer survival we estimated stage-specific cervical cancer 5-year net survival in 200 countries/territories. We evaluated the potential survival impact of scaling up treatment (chemotherapy, surgery, radiotherapy, targeted therapy) and imaging modalities (ultrasound, X-ray, computerized tomography [CT], magnetic resonance imaging [MRI], positron emission tomography [PET], single photon emission computed tomography [SPECT]) to the mean level of high-income countries, both individually, and as packages.
Findings:
Estimated global cervical cancer five-year net survival is 42·1% (95% UI 33·8–48·5). Among individual imaging modalities, expanding MRI would yield the largest survival gains globally, while scaling up ultrasound would yield the largest gains in low-income countries, followed by expanding CT, which would have the most impact in Latin America and Oceania, with PET yielding the largest gains in high-income countries. Scaling up all treatment modalities could improve global 5-year net survival to 52·4% (95% UI 44·6–62·0). In addition to expanding treatment, improving quality of care could raise survival to 57·5% (95% UI 51·2–63·5), and the cumulative impact of scaling up all imaging modalities together with expanded treatment and quality of care improves survival to 62·5% (95% UI 57·7–67·8).
Interpretation:
Comprehensive scale-up of treatment, imaging, and quality of care could improve global cervical cancer 5-year net survival by 20 percentage points, with quality of care and imaging improvements each contributing about 25% of these total potential gains. These findings suggest that a narrow focus on the availability of treatment modalities may forgo substantial survival gains. Investments in imaging equipment and personnel, as well as quality of care efforts will also be needed to successfully scale-up cervical cancer treatment worldwide.
INTRODUCTION
Cervical cancer is the fourth most common cancer among women worldwide, with nearly 600,000 women diagnosed and over 300,000 women dying from cervical cancer each year.1 Nearly 90% of cervical cancer deaths occur in low-income and middle-income countries,1 with large variations in incidence and survival rates affected by socio-demographic and individual-level factors which influence the risk of developing cervical neoplasia, availability of effective screening and treatment of pre-cancer, and access to early diagnosis and effective treatment for invasive cancer.
In high-income countries, population-based and opportunistic screening programs have contributed to substantial decreases in cervical cancer incidence and a downward shift in stage at diagnosis.2–4 However, effective coverage rates for cervical cancer screening are very low outside of developed countries, with women with the highest risk of developing cervical cancer the least likely to be screened.5 Since 2006, vaccines to prevent infection with human papillomavirus (HPV), the causative agent for nearly all cervical cancers, have become available.6 However, uptake of the HPV vaccine has varied considerably by income group, with less than 3% of girls and women aged 10–20 years having received the full course of the HPV vaccine in low-income and middle-income countries, compared to 33% in high-income countries.7 Worldwide, across all country income groups, women with lower socioeconomic status, especially those residing in rural areas, are less likely to have access to cervical cancer prevention, screening, and treatment.8–10
The World Health Organization (WHO) is developing a strategy to eliminate global cervical cancer which includes triple-intervention coverage targets by 2030: 90% HPV vaccination coverage, 70% coverage of twice-lifetime cervical screening, and 90% treatment availability for pre-invasive lesions and invasive cancer.11 A recent modelling analysis finds that while vaccination alone would have minimal impact on cervical cancer mortality in the near future, additionally scaling up screening and treatment would reduce mortality by 34·2% (95% UI 23·3–37·8) in low-income and middle-income countries by 2030, with a 99% reduction in cervical cancer mortality possible over the next century by successful implementation of the WHO elimination strategy.12
In addition to expanding access to screening and prevention, improving the availability of effective cancer treatment will thus be needed to reduce the burden of cervical cancer mortality. Resource-stratified clinical practice guidelines for women with cervical cancer have been published by the American Society of Clinical Oncology13 and the National Comprehensive Cancer Network.14 Both provide guidance for the use of imaging, which plays several roles in the management of invasive cervical cancer. First, imaging complements physical examination in the determination of cancer staging and primary treatment. Second, imaging guides the selection of fields for radiotherapy, one of the critical modalities for cervical cancer treatment. Third, imaging is used to assess treatment response and to evaluate possible recurrence or progression of disease. The presence or absence of good imaging capability is thus a major determinant of the quality of cervical cancer treatment and care. In this analysis we discuss the role of imaging in cervical cancer management and estimate the potential survival impact of scaling up the availability of different imaging modalities while expanding treatment and quality of care.
METHODS
Overview
To estimate cervical cancer survival we used a previously developed microsimulation (individual-level) model of stage-specific cancer survival in 200 countries/territories which takes into account the availability of specific treatment modalities (chemotherapy, radiotherapy [including brachytherapy], surgery, targeted therapy), and imaging modalities (ultrasound, X-ray, computed tomography [CT], magnetic resonance imaging [MRI], positron-emission tomography [PET], single-photon emission computed tomography [SPECT]).15 The model also accounts for quality of care, capturing health-system and facility-level factors that account for residual differences in survival not explained by cancer stage or treatment and imaging availability (e.g. interpreting radiologist expertise, technologist acquiring images, nursing standards, infection control, etc).15 We simulated cervical cancer survival in each country, and evaluated the potential impact of scaling up treatment and imaging modalities, while improving quality of care.
Survival impact of treatment/imaging modalities
To estimate prior probability distributions for the impact of specific treatment and imaging modalities on cervical cancer 5-year net survival, we performed a two-stage survey to elicit expert opinions, described elsewhere.15 A sample of actively practicing physicians was selected from collaborating institutions based on demonstrable expertise in their field (imaging or treatment of cancer patients), both in high-income and low-resource settings. Respondents were asked to indicate the impact of each treatment/imaging modality on stage-specific five-year net cervical cancer survival using a four-point scale. We received between 17–35 responses for each modality. To provide consensus results, responses with at least 75% agreement were accepted as final responses, while responses with lower levels of agreement were discussed by a panel of experts to forge final consensus. As a simplifying assumption, expert opinion responses for treatment impacts were based on recommended treatment for patients at first presentation. Similarly, survey responses regarding imaging are limited to initial staging. These expert consensus results thus reflect best contemporary care for patients initially diagnosed at different stages of cancer.
Stage at diagnosis
Initial staging and treatment recommendations for women with invasive cervical cancer are primarily based upon physical examination and imaging. The International Federation of Gynecologists and Obstetricians (FIGO) provides guidelines for cervical cancer staging.16 These were recently updated to include data from imaging or surgical assessment, which in some cases can provide critical information regarding lymph node metastatic disease. Cervical cancer survival varies substantially by stage. For example, estimates of stage-specific survival from 2010–2016 in the US from the Surveillance, Epidemiology, and End Results (SEER) Program reveal a large gradient in 5-year (net) survival by stage: Stage I = 91%; Stage II = 67%; Stage III = 49%; Stage IV = 17%.17
While it is commonly assumed that cancers in low-income and middle-income countries are diagnosed at later stages, actual data are scarce on the global stage distribution of cervical cancer. To fill this gap, as part of the model development we performed a literature review of reported stage distribution (I-IV) by country, described elsewhere.15 Estimates of cervical cancer stage at diagnosis were available from 83 studies in 55 countries. We used a hierarchical modeling approach to regularize the reported estimates and estimate stage distribution for countries for which no data were aviailable.15
Survival estimates
Using a previously developed microsimulation model of global cancer survival, we estimated five-year net survival in each country for cervical cancer patients diagnosed in 2018 (based on GLOBOCAN 2018 estimates). Model inputs regarding the availability of treatment modalities were based on previously published estimates, and the availability of imaging modalities was estimated based on data obtained from the International Atomic Energy Agency (IAEA) IMAGINE database.18 The model was calibrated to empirical data on five-year net cancer survival in 2010–14 from CONCORD-3.19 Full details on the model development are described elsewhere.15
We simulated stage-specific cervical cancer survival in each country, and evaluated the potential impact of individual policy interventions which expand the availability of specific treatment and imaging modalities to the mean level of high-income countries. We also simulated more comprehensive packages of scale-up which simultaneously expand the availability of multiple treatment and imaging modalities. Specifically, we evaluated the incremental survival benefits of sequentially adding imaging modalities to packages of expanded treatment availability and improved quality of care. We estimated the cumulative impact of sequentially expanding access along a continuum: 1) treatment availability (all modalities); 2) quality of care; 3) ultrasound; 4) CT (including x-ray); 5) MRI; 6) SPECT; 7) PET. We ran 1,000 simulations of the model for all policy scenarios, sampling from the 100 best-fitting parameter sets identified by calibration.15 We report the mean and 95% uncertainty intervals (UI), calculated as the 2·5 and 97·5 percentiles of the simulation results. The simulation model was developed in Java (version 1.8.0).
Role of the funding source
The funders of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report. The corresponding author had full access to all of the data and the final responsibility to submit for publication.
RESULTS
Expert opinion consensus results for the impact of each treatment and imaging modality on five-year net cervical cancer survival are presented in Figure 1. The findings indicate that chemoradiotherapy is critical for managing invasive cervical cancer, while surgery is only beneficial for early-stage cancers. For imaging, expert opinion suggests that MRI is necessary for all stages of cervical cancer, while PET and CT are recommended for more advanced stages.
Figure 1:
Expert opinion consensus of impact of treatment and imaging modalities on 5-year cervical cancer net survival given initial stage at diagnosis
Assessing the model fit, we found that the model prediction intervals (95% UI) for the training set of cervical cancer calibration targets overlapped with the CONCORD 95% CIs 93·0% of the time, with coverage probabilities of 82·5% and a mean absolute error of 5·35 percentage points.15 Although the test set of CONCORD estimates (not used for calibration) only comprised three estimates for cervical cancer, the model performed well compared to this small test set: the prediction intervals contained these estimates with 100% coverage and a mean absolute error of 1·96 percentage points.15 These predictive accuracy checks on data not used to fit the model help to build confidence in the robustness of the model estimates.
Posterior means and 95% UIs of cervical cancer stage distribution and stage-specific survival are reported from the calibrated simulation model by country income group and geographic region in Table 1. We estimate that 49·0% (95% UI 15·3–78·5) (35,200/71,800) of cases are diagnosed at advanced stage (III-IV) in low-income countries, compared to 39·8% (95% UI 29·7–51·4) (28,900/72,500) in high-income countries. We also find that stage-specific survival varies widely by country income group and geographic region, and estimate that overall global five-year net survival is 42·1% (95% UI 33·8–48·5), with large variation by country (see Figure 2).
Table 1:
Cervical cancer stage at diagnosis and survival by income group and region, means (95% UI)
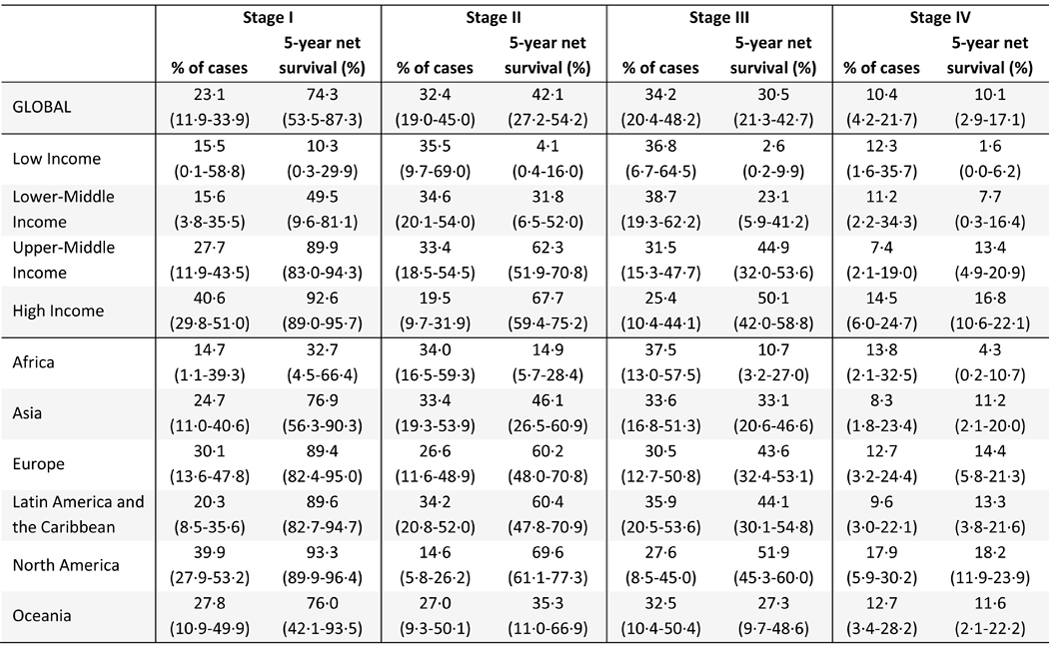 |
Figure 2.
Estimated 5-year cervical cancer net survival by country
We find that the scale-up of specific treatment modalities that would yield the largest survival gains varies by income group and region (see Table 2). Specifically, we find that scaling up access to radiotherapy would yield the largest survival gains in low-income countries, and in Africa and Oceania in general, while expanding surgery availability would yield the largest gains in lower-middle income countries and upper-middle income countries, and Asia as a whole. In contrast, we find that expanding access to chemotherapy would yield the largest survival gains in Latin America, while improving the quality of care would bring the largest benefits in high-income countries and in Europe and North America, as the availability of treatment/imaging modalities is already high.
Table 2:
Five-year net survival by income group and region under various policies that expand availability of single treatment modalities to the mean level of high-income countries, means (95% UI)
 |
For imaging, we also find that the scale-up of individual modalities that would yield the largest survival gains varies by context (see Table 3). Expanding the availability of MRI would yield the largest survival gains globally, and in lower-middle income and upper-middle countries in particular. Expanding ultrasound would yield the largest survival gains in low-income countries, followed by CT, which is estimated to yield the largest survival benefits in Latin America and Oceania. In contrast, expanding PET is estimated to yield the largest benefits in high-income countries. However, the gains from expanding any single treatment or imaging modality individually are small.
Table 3:
Five-year net survival by income group and region under various policies that expand availability of single imaging modalities to the mean level of high-income countries, means (95% UI)
| Baseline | Ultrasound | CT (+X-ray) | MRI | PET | SPECT | ||
|---|---|---|---|---|---|---|---|
| Global | Survival (%) | 42·1 (33·8–48·5) |
42·3 (34·0–48·7) |
42·5 (34·0–48·8) |
42·7 (34·0–49·9) |
42·4 (34·0–48·8) |
42·2 (33·8–48·6) |
| Gain (%) | --- |
0·2 (0·0–1·1) |
0·4 (0·0–1·2) |
0·6 (0·1–2·1) |
0·3 (0·0–0·8) |
0·1 (0·0–0·2) |
|
| Low income | Survival (%) | 4·4 (0·4–16·4) |
4·9 (0·4–19·7) |
4·5 (0·4–17·0) |
4·5 (0·4–17·0) |
4·4 (0·4–16·4) |
4·4 (0·4–16·4) |
| Gain (%) | --- |
0·5 (0·0–3·7) |
0·2 (0·0–0·9) |
0·1 (0·0–0·6) |
0·0 (0·0–0·1) |
0·0 (0·0–0·0) |
|
| Lower middle income | Survival (%) | 28·5 (7·5–45·5) |
28·9 (7·6–45·5) |
29·0 (7·5–45·7) |
29·3 (7·7–47·3) |
28·8 (7·5–45·6) |
28·6 (7·5–45·5) |
| Gain (%) | --- |
0·3 (0·0–2·4) |
0·5 (0·0–2·1) |
0·8 (0·0–3·1) |
0·3 (0·0–1·2) |
0·0 (0·0–0·1) |
|
| Upper middle income | Survival (%) | 61·1 (56·2–66·8) |
61·1 (56·2–66·8) |
61·5 (56·2–67·0) |
61·9 (56·7–67·3) |
61·6 (56·6–67·5) |
61·2 (56·5–66·8) |
| Gain (%) | --- |
0·0 (0·0–0·1) |
0·4 (0·0–1·5) |
0·8 (0·0–3·3) |
0·5 (0·0–1·9) |
0·1 (0·0–0·4) |
|
| High income | Survival (%) | 66·0 (64·2–68·2) |
66·0 (64·2–68·2) |
66·1 (64·3–68·2) |
66·1 (64·3–68·4) |
66·2 (64·4–68·3) |
66·0 (64·3–68·2) |
| Gain (%) | --- |
0·0 (0·0–0·0) |
0·1 (0·0–0·6) |
0·1 (0·0–0·6) |
0·2 (0·0–0·8) |
0·0 (0·0–0·3) |
|
| Africa | Survival (%) | 13·5 (7·4–20·9) |
13·9 (7·5–21·8) |
13·7 (7·4–21·6) |
13·8 (7·4–21·5) |
13·6 (7·5–21·1) |
13·5 (7·4–21·0) |
| Gain (%) | --- |
0·5 (0·0–2·2) |
0·3 (0·0–0·8) |
0·4 (0·0–1·6) |
0·1 (0·0–0·6) |
0·0 (0·0–0·1) |
|
| Asia | Survival (%) | 46·4 (33·1–56·9) |
46·6 (33·1–56·9) |
46·7 (33·1–57·2) |
47·2 (33·2–58·9) |
46·7 (33·1–57·2) |
46·5 (33·1–56·9) |
| Gain (%) | --- |
0·2 (0·0–1·5) |
0·3 (0·0–1·4) |
0·8 (0·0–2·8) |
0·3 (0·0–1·0) |
0·1 (0·0–0·3) |
|
| Europe | Survival (%) | 58·1 (53·8–62·2) |
58·1 (53·8–62·2) |
58·4 (54·0–62·5) |
58·7 (54·1–63·9) |
58·6 (54·0–62·7) |
58·1 (53·9–62·4) |
| Gain (%) | --- |
0·0 (0·0–0·2) |
0·3 (0·0–2·2) |
0·6 (0·0–3·1) |
0·5 (0·0–1·8) |
0·0 (0·0–0·3) |
|
| Latin America and the Caribbean | Survival (%) | 56·2 (49·9–61·8) |
56·2 (49·9–61·8) |
57·0 (50·2–62·8) |
56·5 (50·0–64·0) |
56·8 (50·6–62·5) |
56·3 (49·9–61·9) |
| Gain (%) | --- |
0·0 (0·0–0·3) |
0·8 (0·0–3·4) |
0·3 (0·0–3·2) |
0·6 (0·0–2·5) |
0·1 (0·0–0·5) |
|
| Northern America | Survival (%) | 64·9 (60·1–70·6) |
64·9 (60·1–70·6) |
64·9 (60·1–70·6) |
64·9 (60·1–70·6) |
64·9 (60·1–70·6) |
65·0 (60·3–70·7) |
| Gain (%) | --- |
0·0 (0·0–0·0) |
0·0 (0·0–0·1) |
0·0 (0·0–0·0) |
0·0 (0·0–0·0) |
0·1 (0·0–0·7) |
|
| Oceania | Survival (%) | 40·7 (27·3–61·9) |
40·9 (27·3–61·9) |
41·1 (27·3–62·4) |
40·8 (27·3–62·6) |
40·9 (27·3–62·1) |
40·7 (27·4–61·9) |
| Gain (%) | --- |
0·3 (0·0–2·9) |
0·4 (0·0–3·2) |
0·2 (0·0–1·0) |
0·2 (0·0–1·6) |
0·1 (0·0–0·5) |
|
We find that scaling up the availability of all treatment modalities to the mean level of high-income countries could improve global 5-year net survival to 52·4% (95% UI 44·6–62·0) (see Table 4). Increasing treatment availability to the mean level of high-income countries while also improving quality of care could raise survival to 57·5% (95% UI 51·2–63·5), and including investments in imaging further improves survival to 62·5% (95% UI 57·7–67·8). We therefore find that comprehensive scale-up of treatment, imaging, and quality of care could potentially improve global 5-year net survival of cervical cancer by 20 percentage points, with quality of care and imaging improvements each contributing 25% of the survival gains (i.e. half of the total increase). Survival would improve most in low-income and lower-middle income countries (Figure 3a) and Africa and Oceania (Figure 3b).
Table 4:
Policy scenarios to scale-up availability of treatment/imaging modalities to the mean level of high-income countries
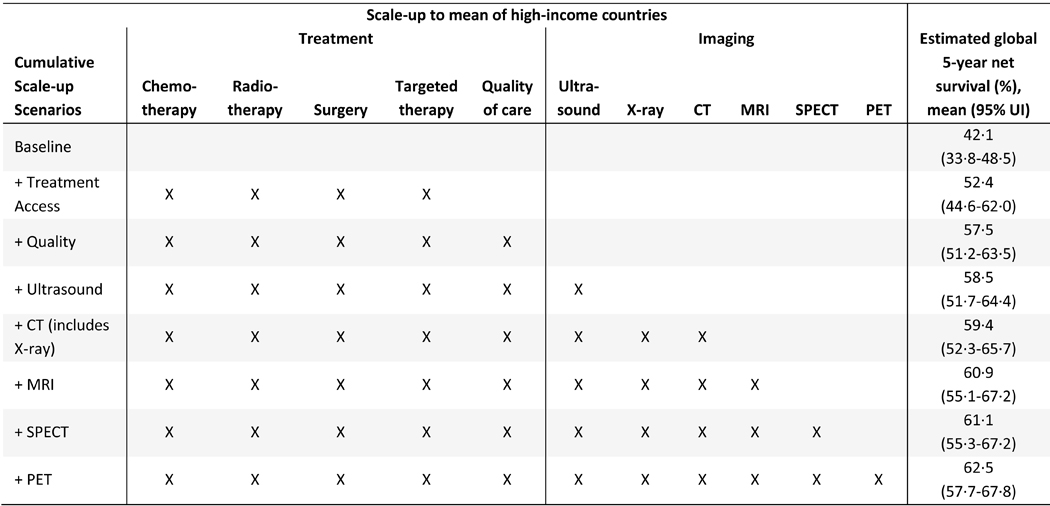 |
Figure 3.
Estimated 5-year cervical cancer net survival with cumulative scale-up of treatment and imaging (A) By income group. (B) By geographical region. Shaded regions indicate 95% UI. Dashed vertical line indicates the point at which imaging scale-up begins to be added to the cumulative scale-up policices. UI=uncertainty interval. SPECT=single photon emission CT.
DISCUSSION
We find that imaging is a critical component of cervical cancer management. Globally we find that comprehensive scale-up of treatment, imaging, and quality of care to mean levels in high-income countries could improve five-year net cervical cancer survival by 20 percentage points, with quality of care and imaging together contributing to half of the increase (about 25% each). We find that the stage distribution of cervical cancer varies by income group and region, with worse stage at diagnosis in low-income countries. Stage-specific and overall 5-year net survival also varies widely due to lack of availability of treatment and imaging modalities, and quality of care. We find that among single treatment modality interventions, expanding radiotherapy availability would yield the largest survival gains in low-income countries, while increasing the availability of surgery would yield the largest gains in lower middle-income and upper middle-income countries, with improvements in quality of care producing the most benefit in high-income countries as treatment is already generally available. We also find that the relative priority of single imaging modality policies differs by context. Specifically, we find that expanding access to MRI would yield the largest survival gains globally, and in lower-middle income and upper-middle income countries in particular. In low-income countries, scaling up ultrasound could yield the largest gains, especially in Africa, followed by CT, which would produce the largest benefits in Latin America and Oceania. In contrast, in high-income countries we find that increasing the availability of PET would yield the largest survival gains, although these gains are small as survival is already relatively high in high-income countries.
However, overall we find that the survival impact of expanding the availability of any single imaging/treatment modality is modest even in lower-income countries; more comprehensive packages of scale-up will be needed to substantially improve cervical cancer survival. We estimate that simultaneously expanding the availability of treatment and imaging modalities and improving the quality of care to high-income levels could improve global 5-year net survival by 20 percentage points. Importantly, we find that improvements in quality of care and imaging availability each contribute 25% of the survival gains (i.e. half of the total increase). This highlights the importance of simultaneous investments in quality of care and medical imaging, which helps to improve the management of cervical cancer in a number of ways, such as determination of cancer staging and primary treatment, selection of fields in radiotherapy planning, and assessment of treatment response.
Clinical staging may be inaccurate in 17–32% of patients with early disease (IB) and up to 65% of patients with advanced disease (IIB-IV), thus adversely affecting patient prognosis.20 Recent studies have found that imaging impacts initial stage determination for around 40% of cervical cancer patients.21–23 Improvements in imaging availability and quality may thus improve patient prognosis and also provide more accurate estimates of stage distribution at diagnosis. Our estimates of the impact of improving the availability and quality of imaging are thus likely to be conservative as we assume that the ‘observed’ stage distribution remains unchanged, whereas with imaging one would expect improved care with more precise staging of cervical cancer.
A recent meta-analysis on the diagnostic performance of imaging modalities for determining local disease extent and nodal metastasis in patients with newly-diagnosed cervical cancer finds that MRI is the method of choice for assessing local extent, but where not available ultrasound can be of value, especially for assessing parametrial invasion.24 The study also finds that CT, MRI, and PET have high specificity but poor sensitivity for detection of lymph node metastases.24
In settings with only basic resources, chest X-ray may be used to rule out gross metastatic disease in the lungs or bones of the thorax. In settings with greater imaging capability, women should undergo CT of the chest, abdomen, and pelvis. Where available, MRI or whole-body PET combined with CT can provide greater accuracy than CT alone. Ideally, CT of the abdomen is used to evaluate the kidneys and presence/potential causes of hydronephrosis, but in resource-constrained settings, ultrasound can also be used.
Once the extent of disease has been evaluated, appropriate treatment can be planned. Early-invasive disease (stage IA1) may be treated with conization for women who wish to preserve fertility. Slightly more advanced disease (stage IA2) may be treated with radical trachelectomy, or neoadjuvant chemotherapy, followed by conization. Pelvic MRI is commonly used to evaluate depth of invasion of the uterine cervix and thus guide decision making. If MRI is contraindicated, then pelvic transvaginal ultrasound may be used to evaluate the depth of invasion in the uterine cervix. Women with more advanced disease (stage IB1-IIA) may be considered for primary surgery, generally modified radical hysterectomy.
Primary chemoradiation, including both external beam and intracavitary radiation, is also a treatment option for women with stage IB1, IB2 and IIA disease, and is the only curative treatment option for women with IB3, IIB, IIIB, IIIC and IVA disease. Alternatively, if chemotherapy is not feasible, regional hyperthermia is an option to enhance radiotherapy effect.25 Imaging of the chest, abdomen, and pelvis is therefore of critical importance to identify patients with locally advanced disease but without evidence of para-aortic metastasis, who should be treated with definitive chemoradiation, and those with para-aortic metastasis, who should be treated with extended field radiation with chemotherapy. Similar consideration should be given to radiation of the groin for cervix tumours with distal vaginal involvement.
Accurate imaging of the chest, abdomen, and pelvis can thus be critical for cervical cancer management. In addition to improving survival, determining appropriate primary treatment by evaluating para-aortic (PA) nodes can reduce the number of women referred to surgery, directing them to primary chemoradiotherapy instead, saving on costs of treatment and quality of life decrements due to unnecessary surgery.
CT scans of the abdomen and pelvis are also used for planning fields for radiotherapy. Compared to fluoroscopic simulation, 3D planning allows increased dosing to the tumour and decreased dosing to normal tissues, thus increasing the chance of local cure and decreasing toxicity to the organs at risk. Imaging is also used for intracavitary brachytherapy planning, and can help to position applicators for optimal dose distribution. While MRI or CT would ideally be used, ultrasound may also be used to rule out perforation of the uterus and X-ray used to verify applicator placement. Interstitial brachytherapy may be done with the use of MRI (or CT at a minimum) to determine needle placement and for treatment planning. Newer brachytherapy approaches, especially those using MRI for guidance, result in reduced toxicity and improved outcomes in patients with locally advanced cervical cancer.26,27
PET/CT done at 3 months post-treatment has been found to correlate well with treatment response, risk of recurrence, and disease prognosis, especially after radiation.28 While PET/CT may not be needed for all cases, it can be helpful to assess treatment response for patients with initial high burden of disease, or for whom assessing response by clinical examination is difficult. Women who develop signs suggestive of recurrent cervical cancer should undergo physical examination and imaging of the chest, abdomen, and pelvis. There is however no conclusive data supporting a role for routine imaging as part of surveillance after definitive therapy.
Although we synthesized data from multiple sources, data limitations mean that we had to make assumptions when developing the model. For example, estimates of cervical cancer stage at diagnosis were only available for selected countries, and then only for general stages I-IV. Due to the scarcity of stage data we were not able to estimate trends in stage at diagnosis over time. More information on the distribution (and survival) of incident cervical cancers by detailed FIGO staging (e.g., IIa vs IIb) would allow us to refine our model assumptions and improve the precision of our estimates. The collection and reporting of accurate stage data in population-based cancer registries (both across and within countries) is critical for global cancer research aimed at informing policy and practice to improve cancer outcomes. Additional funding is needed for population-based cancer registries to systematically incorporate and report stage data. We used the best available data that currently exist and can refine our estimates as more data become available in the future.
Our model results suggest that substantial gains in cervical cancer survival could be achieved by scaling up treatment and imaging modalities and quality of care. However, to achieve such scale-up, investments must be made in equipment, training of engineering and health professionals, and infrastructure such as informatics services. For technologies such as MRI and PET/CT, having adequate human resources is an important consideration in addition to the costs of equipment and installation. For example, technicians are needed to operate the equipment, in addition to the radiologists and nuclear medicine physicians trained to read the results and implement the appropriate protocols for diagnosis. Another barrier to effective treatment of cervical cancer is the global shortage of healthcare professionals with expertise in the development of radiation plans for individual patients.29
In addition, efforts aimed at health system strengthening will be needed to improve cancer prevention efforts, early detection of disease, and efficient referral of cancer cases. Indeed, even after scaling up treatment and imaging availability and improving quality of care for invasive cervical cancers, we find that a survival gap still exists between high-income and lower-income countries due to higher stage at diagnosis (see Figure 3A). This highlights the importance of primary and secondary prevention (ie, HPV vaccination and screening) as critical components of comprehensive cancer control efforts. Once detected, women with suspected invasive cancer should be diagnosed and placed on definitive treatment as quickly as possible, with scans completed within 30 days of referral and imaging reports made available promptly (i.e., within 4–7 days of the scan).13,14,30 Effective communication between imaging experts and other members of the medical decision-making team will also be needed as a critical component of comprehensive scale-up of treatment and imaging modalities and quality of care that could yield major benefits for cervical cancer survival worldwide.
Research in context.
Evidence before this study
Recent data on cervical cancer five-year net survival is provided by the CONCORD-3 study. GLOBOCAN 2018, produced by the International Agency for Research on Cancer, also provides modeled mortality estimates for cervical cancer. The World Health Organization is developing a strategy for eliminating cervical cancer globally, which includes targets for vaccination coverage, screening, and treatment. A recent modelling analysis finds that while vaccination alone would have minimal impact on cervical cancer mortality in the near future, additionally scaling up screening and treatment could reduce mortality by 34% in low-income and middle-income countries by 2030. We searched PubMed using the search terms “cervical cancer”, “survival”, “global” and “imaging” on May 11, 2020, without language or publication date restrictions, and find no estimates of the impact of imaging modalities on global cervical cancer survival.
Added value of this study
Using a microsimulation model of global cancer survival this study provides estimates of cervical cancer stage distribution and five-year net survival (stage-specific and overall) for 200 countries and territories. We provide expert opinion consensus on the impact of treatment (chemotherapy, surgery, radiotherapy and targeted therapy) and imaging modalities (ultrasound, X-ray, computerized tomography [CT], magnetic resonance imaging [MRI], positron emission tomography [PET], and single photon emission computed tomography [SPECT]) and discuss the role of imaging in cervical cancer management. We also estimate the potential survival impact of scaling up specific treatment and imaging modalities in different contexts. Among single imaging modalities, we find that expanding MRI would yield the largest survival gains globally, while scaling up ultrasound could yield the largest gains in low-income countries, followed by expanding CT, which would have the most impact in Latin America and Oceania. In contrast, increasing the availability of PET would yield the largest gains in high-income countries.
Implications of all the available evidence
Cervical cancer survival varies substantially by country, largely due to differences in the availability of treatment and imaging modalities, and quality of care. Comprehensive scale-up of treatment and imaging modalities and quality of care could potentially improve global cervical cancer 5-year net survival by 20 percentage points. Quality of care and imaging improvements each contribute about 25% of these gains in 5-year net survival (i.e. half of the total increase). In addition to expanding treatment availability, investments will therefore also be needed in imaging equipment, human resources, and quality of care efforts to successfully scale up treatment of invasive cervical cancer worldwide.
Acknowledgements
This study was funded by the Harvard TH Chan School of Public Health and the National Cancer Institute P30 Cancer Center Support Grant (P30 CA008748) to Memorial Sloan Kettering Cancer Center. ECJ and ELT are employed by NIH.
Funding:
Harvard T.H. Chan School of Public Health, National Cancer Institute P30 Cancer Center Support Grant (P30 CA008748) to Memorial Sloan Kettering Cancer Center
Footnotes
Declaration of interests
We declare no competing interests. HH receives annual compensation for serving on the Board of Directors of Ion Beam Applications (IBA).
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Zachary J. Ward, Center for Health Decision Science, Harvard TH Chan School of Public Health, Harvard University, Boston, MA, USA.
Surbhi Grover, Department of Radiation Oncology, University of Pennsylvania, Philadelphia, PA, USA.
Andrew M. Scott, Olivia Newton-John Cancer Research Institute, Melbourne, Australia Department of Molecular Imaging and Therapy, Austin Health, Melbourne, Australia; School of Cancer Medicine, La Trobe University, Melbourne, Australia; Department of Medicine, University of Melbourne, Melbourne, Australia.
Sungmin Woo, Department of Radiology, Memorial Sloan Kettering Cancer Center, New York, NY, USA.
Dina H. Salama, National Center for Radiation Research and Technology, Egyptian Atomic Energy Authority, Cairo, Egypt
Elizabeth C. Jones, Clinical Center, National Institutes of Health, Bethesda, MD, USA
Tarek El-Diasty, Department of Radiology, Urology and Nephrology Center, University of Mansoura, Mansoura, Egypt.
Bradley R. Pieters, Department of Radiation Oncology, Amsterdam University Medical Centers, University of Amsterdam, Amsterdam, The Netherlands
Edward L. Trimble, National Cancer Institute, National Institutes of Health, Washington DC, USA
H. Alberto Vargas, Department of Radiology, Memorial Sloan Kettering Cancer Center, New York, NY, USA.
Hedvig Hricak, Department of Radiology, Memorial Sloan Kettering Cancer Center, New York, NY, USA.
Rifat Atun, Department of Global Health and Population, Harvard TH Chan School of Public Health, Harvard University, Boston, MA, USA; Department of Global Health and Social Medicine, Harvard Medical School, Harvard University, Boston, MA, USA.
References
- 1.Arbyn M, Weiderpass E, Bruni L, et al. Estimates of incidence and mortality of cervical cancer in 2018: a worldwide analysis. Lancet Glob Health 2019; S2214–109X(19)30482–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yang DX, Soulos PR, Davis B, Gross CP, Yu JB. Impact of widespread cervical cancer screening: number of cancers prevented and changes in race-specific incidence. Am J Clin Oncol 2018; 41(3): 289–294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pesola F, Sasieni P. Impact of screening on cervical cancer incidence in England: a time trend analysis. BMJ Open 2019; 9(1): e026292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dickinson JA, Stankiewicz A, Popadiuk C, Pogany L, Onysko J, Miller AB. Reduced cervical cancer incidence and mortality in Canada: national data from 1932 to 2006. BMC Public Health 2012; 12: 992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gakidou E, Nordhagen S, Obermeyer Z. Coverage of cervical cancer screening in 57 countries: low average levels and large inequalities. PLoS Med 2008; 5(6): e132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tota JE, Chevarie-Davis M, Richardson LA, Devries M, Franco EL. Epidemiology and burden of HPV infection and related diseases: implications for prevention strategies. Prev Med 2011; 53: S12–21. [DOI] [PubMed] [Google Scholar]
- 7.Bruni L, Diaz M, Barrionuevo-Rosas L, et al. Global estimates of human papillomavirus vaccination coverage by region and income level: a pooled analysis. Lancet Glob Health 2016; 4: e453–63. [DOI] [PubMed] [Google Scholar]
- 8.Ng’ang’a A, Nyangasi M, Nkonge NG, et al. Predictors of cervical cancer screening among Kenyan women: results of a nested case-control study in a nationally representative survey. BMC Public Health 2018; 18: 1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chidyaonga-Maseko F, Chirwa ML, Muula AS. Underutilization of cervical cancer prevention services in low and middle income countries: a review of contributing factors. Pan Afr Med J 2015; 21: 231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Akinyemiju T, Ogunsina K, Sakhuja S, Ogbhodo V, Braithwaite D. Life-course socioeconomic status and breast and cervical cancer screening: analysis of the WHO’s Study on Global Ageing and Adult Health (SAGE). BMJ Open 2016; 6(11): e012753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.WHO. Draft global strategy towards the elimination of cervical cancer as a public health problem. December 16, 2019. https://www.who.int/docs/default-source/cervical-cancer/cerv-cancerelimn-strategy-16dec-12pm.pdf (accessed Feb 17, 2020). [Google Scholar]
- 12.Canfell K, Kim JJ, Brisson M, et al. Mortality impact of achieving WHO cervical cancer elimination targets: a comparative modelling analysis in 78 low-income and lower-middle-income countries. Lancet 2020; S0140-6736(20)30157–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chuang LT, Temin S, Camacho R, et al. Management and Care of Women with Invasive Cervical Cancer: American Society of Clinical Oncology Resource-Stratified Clinical Practice Guideline. J Glob Oncol 2016; 2(5): 311–340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.National Comprehensive Cancer Network. NCCN Framework for Resource Stratification of NCCN Guidelines . Cervical Cancer: core resources. Version 2020. https://www.nccn.org/professionals/physician_gls/pdf/cervical.pdf (accessed March 9, 2020). [Google Scholar]
- 15.Ward ZJ, Scott AM, Hricak H, et al. Estimating the impact of treatment and imaging modalities on 5-year net survival of 11 cancers – global, regional, and country-level simulation results. Under review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bhatla N, Berek JS, Cuello Fredes M, et al. Revised FIGO staging for carcinoma of the cervix uteri. Int J Gynaecol Obstet 2019; 145(1): 129–135. [DOI] [PubMed] [Google Scholar]
- 17.Surveillance Epidemiology, and Results End (SEER) Program (www.seer.cancer.gov) SEER*Stat Database: Incidence – SEER 18 Reg Research Data + Hurricane Katrina Impacted Louisiana Cases, Nov 2018 Sub (2000–2016) – Linked To County Attributes – Total U.S., 1969–2017 Counties, National Cancer Institute, DCCPS, Surveillance Research Program, release April 2019, based on the November 2018 submission. [Google Scholar]
- 18.International Atomic Energy Agency. IMAGINE – IAEA Medical imAGIng and Nuclear mEdicine global resources database. https://humanhealth.iaea.org/HHW/DBStatistics/IMAGINE.html [Accessed Feb 13, 2020]. [Google Scholar]
- 19.Allemani C, Matsuda T, Di Carlo V, et al. Global surveillance of trends in cancer survival 2000–14 (CONCORD-3): analysis of individual records 37 513 025 patients diagnosed with one of 18 cancers from 322 population-based registries in 71 countries. Lancet 2018; 391: 1023–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bourgioti C, Chatoupis K, Rodolakis A, et al. Incremental prognostic value of MRI in the staging of early cervical cancer: a prospective study and review of the literature. Clin Imaging 2016; 40(1): 72–8. [DOI] [PubMed] [Google Scholar]
- 21.Cegla P, Urbanski B, Burchardt E, Roszak A, Cholewinski W. Influence of 18F-FDG-PET/CT on staging of cervical cancer. Nuklearmedizin 2019; 58(1): 17–22. [DOI] [PubMed] [Google Scholar]
- 22.Morkel M, Ellmann A, Warwick J, Simonds H. Evaluating the Role of F-18 Fluorodeoxyglucose Positron Emission Tomography/Computed Tomography Scanning in the Staging of Patients With Stage IIIB Cervical Carcinoma and the Impact on Treatment Decisions. Int J Gynecol Cancer 2018; 28(2): 379–384. [DOI] [PubMed] [Google Scholar]
- 23.Zhang W, Chen C, Liu P, et al. Impact of pelvic MRI in routine clinical practice on staging of IB1-IIA2 cervical cancer. Cancer Manag Res 2019; 11: 3603–3609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Woo S, Atun R, Ward ZJ, Scott AM, Hricak H, Vargas HA. Diagnostic performance of conventional and advanced imaging modalities for assessing newly diagnosed cervical cancer: a systematic review and meta-analysis. Eur Radiol In press. 10.1007/s00330-020-06909-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Van der Zee J, González González D, van Rhoon GC, van Dijk JD, van Putten WL, Hart AA. Comparison of radiotherapy alone with radiotherapy plus hyperthermia in locally advanced pelvic tumours: a prospective, randomised, multicentre trial. Dutch Deep Hyperthermia Group. Lancet 2000; 355(9210): 1119–25. [DOI] [PubMed] [Google Scholar]
- 26.Holschneider CH, Petereit DG, Chu C, et al. Brachytherapy: A critical component of primary radiation therapy for cervical cancer: From the Society of Gynecologic Oncology (SGO) and the American Brachytherapy Society (ABS). Brachytherapy 2019; 18(2): 123–132. [DOI] [PubMed] [Google Scholar]
- 27.Sturdza A, Pötter R, Fokdal LU, et al. Image guided brachytherapy in locally advanced cervical cancer: Improved pelvic control and survival in RetroEMBRACE, a multicenter cohort study. Radiother Oncol 2016; 120(3): 428–433. [DOI] [PubMed] [Google Scholar]
- 28.Kim YJ, Han S, Kim YS, Nam JH. Prognostic value of post-treatment 18F-fluorodeoxyglucose positron emission tomography in uterine cervical cancer patients treated with radiotherapy: a systematic review and meta-analysis. J Gynecol Oncol 2019; 30(5): e66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Atun R, Jaffray DA, Barton MB, et al. Expanding global access to radiotherapy. Lancet Oncol 2015; 16: 1153–86. [DOI] [PubMed] [Google Scholar]
- 30.Miller JW, Hanson V, Johnson GD, Royalty JE, Richardson LC. From cancer screening to treatment: service delivery and referral in the National Breast and Cervical Cancer Early Detection Program. Cancer 2014; 120 Suppl 16: 2549–56. [DOI] [PMC free article] [PubMed] [Google Scholar]





