Abstract
BACKGROUND
Tumour necrosis factor receptor 1 (TNFR1) signalling mediates the cell death and inflammatory effects of TNF-α.
OBJECTIVE
The current clinical trial investigated the effects of a nebulised TNFR1 antagonist (GSK2862277) on signs of lung injury in patients undergoing oesophagectomy.
DESIGN
Randomised double-blind (sponsor unblind), placebo-controlled, parallel group study.
SETTING
Eight secondary care centres, the United Kingdom between April 2015 and June 2017.
PATIENTS
Thirty-three patients undergoing elective transthoracic oesophagectomy.
INTERVENTIONS
Patients randomly received a single nebulised dose (26 mg) of GSK2862277 (n = 17) or placebo (n = 16), given 1 to 5 h before surgery; 14 and 16, respectively competed the study.
MAIN OUTCOME MEASUREMENTS
Physiological and biochemical markers of lung injury, pharmacokinetic and safety endpoints were measured. The primary endpoint was the change from baseline in pulmonary vascular permeability index (PVPI) at completion of surgery, measured using single-indicator transpulmonary thermodilution. Adjusted point estimates and 95% credible intervals (analogous to conventional confidence intervals) were constructed for each treatment using Bayesian statistical models.
RESULTS
The mean change (with 95% credible intervals) from baseline in PVPI on completion of surgery was 0.00 (−0.23, 0.39) in the placebo and 0.00 (−0.24, 0.37) in the GSK2862277 treatment groups. There were no significant treatment-related differences in PaO2/FiO2 or Sequential Organ Failure Assessment score. Levels of free soluble TNFR1, Macrophage Inflammatory Protein-1 alpha and total protein were significantly reduced in the bronchoalveolar lavage fluid of patients treated with GSK2862277 (posterior probability of decrease with GSK2862277 vs. placebo:≥0.977; equivalent to P < 0.05). The frequency of adverse events and serious adverse events were distributed evenly across the two treatment arms.
CONCLUSION
Pre-operative treatment with a single 26 mg inhaled dose of GSK2862277 did not result in significantly lower postoperative alveolar capillary leak or extra vascular lung water. Unexpectedly small increases in transpulmonary thermodilution-measured PVPI and extra vascular lung water index at completion of surgery suggest less postoperative lung injury than historically reported, which may have also compromised a clear assessment of efficacy in this trial. GSK2862277 was well tolerated, resulted in expected lung exposure and reduced biomarkers of lung permeability and inflammation.
TRIAL REGISTRATION
clinicaltrials.gov: NCT02221037.
Background
Acute respiratory distress syndrome (ARDS) is broadly characterised by rapid-onset, diffuse lung inflammation resulting in hypoxaemia.1 Postoperative pulmonary complications (PPCs), including ARDS, are common in patients undergoing transthoracic oesophagectomy2 in which signs of inflammation and lung injury over the postoperative period have also been reported.3 Most patients having oesophagectomy receive one-lung ventilation (OLV) for a significant part of the operative procedure, while the contralateral lung is collapsed to allow surgical access. This technique is associated with increased risk of developing postoperative lung injury and PPCs, possibly due to underlying increases in pulmonary vascular permeability, inflammation and neutrophil infiltration.3,4 The high rate of pulmonary complications in patients undergoing oesophagectomy, in addition to the more homogeneous cause and controlled timing of injury, indicates oesophagectomy as a useful ‘model’ population for the early evaluation of new treatments for the prevention of PPCs, and ARDS, prior to initiating larger trials in more heterogenous cohorts of patients.5
Evidence from clinical trials with anti-TNF-α antibody therapy in critical illness is contradictory, perhaps because of the diverse range of different therapeutic modalities tested (e.g. short-acting and long-acting, partially selective and pan-TNF-α signalling inhibitors), and variability in trial patient populations (e.g. biologically enriched vs. clinically defined).6 Although patient enrichment strategies are likely to be important when investigating the efficacy of targeted therapies within heterogeneous cohorts of critically ill patients, in the case of anti-TNF-α therapies, differences in mechanism of action may also have contributed to differences in trial outcomes. The pleiotropic effects of TNF-α diverge at the level of its two cellular receptors, TNF receptor 1 (TNFR1) and TNF receptor 2 (TNFR2), with studies confirming the role of TNFR1 in promoting cell death7,8 and inflammation,9 together with an opposing role for TNFR2 in regulating inflammation10 and promoting resolution of injury.11 Correspondingly, TNFR1-deficient mice are protected from lung injury, sepsis and other acute organ injuries, whereas TNFR2-deficient mice are consistently more susceptible to injury in these models.12–16 Such data offer a potential mechanism for why some long-acting pan-TNF inhibitors could be harmful in acute illness, and suggest that selectively antagonising TNFR1, while sparing TNFR2 signalling, could be therapeutically advantageous.
GSK2862277 is a fully human domain antibody (dAb) fragment that is a potent and selective inhibitor of TNFR1 signalling.17 Short-acting selective TNFR1 dAb inhibitors reduce pulmonary inflammation in human and nonhuman primate pulmonary endotoxin challenge models, and modulate neutrophil/endothelial interactions.9 This pilot study investigated the effects of GSK2862277 on measures of lung inflammation and capillary leak in patients undergoing oesophagectomy.
Methods
Study design and data collection
This was a multicentre, randomised placebo-controlled, double-blind (sponsor unblind), parallel group study (Study number TFR116341; NCT02221037).
Following screening for eligibility 7 to 28 days before elective surgery, oesophagectomy was performed (Day 1) under general anaesthesia with invasive positive-pressure ventilation according to local practice. As per protocol, ventilation during OLV procedure utilised tidal volumes of 4 to 5 ml kg−1, positive end-expiratory pressure was up to 5 cmH2O, and SpO2 was maintained at at least 90%. Otherwise, usual peri-operative management was followed with fluid management or two lung ventilation managed at the discretion of the anaesthetist. Physiological and biochemical markers of lung injury in blood were measured pre and postsurgery and on the following 2 to 4 days (Fig. 1), and in bronchoalveolar lavage fluid (BALF) immediately postsurgery. Thereafter, patients were only assessed for safety endpoints up to day 28 (as inpatients or as outpatients if discharged before day 28).
Fig. 1.
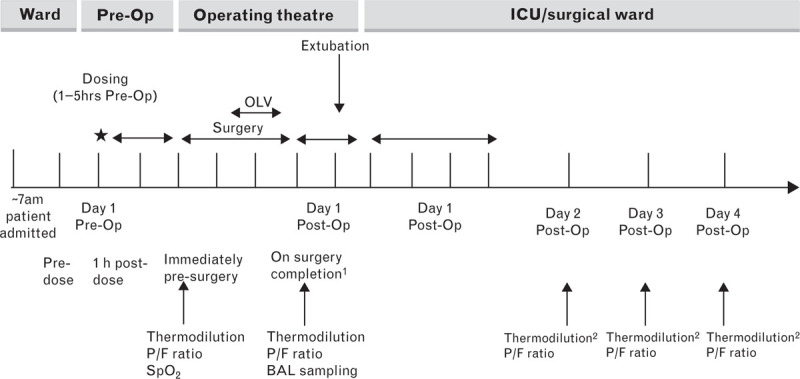
Study schematic. Dosing with nebulised GSK2862277 26 mg or placebo. BAL, bronchoalveolar lavage; OLV, one-lung ventilation; OP, operation. 1On completion of surgery, transpulmonary thermodilution measurement first then bronchoalveolar lavage were performed before tracheal extubation. 2Thermodilution performed if patient remained in ICU with patent indwelling PiCCO catheter.
Patients
Eligible patients were aged 18 to 80 years old undergoing elective oesophagectomy. Full eligibility criteria are available in the online Supplement.
Written informed consent was obtained from all patients before enrolment, and the study was approved by the local ethics committee at each centre (protocol TFR116341; NCT02221037).
Randomisation and treatment allocation
Patients were randomised to GSK2862277 or matching placebo by means of a secure, allocation concealed, central system. In addition patients were randomised to one of four combinations (1 : 1 : 1 : 1) of BALF sampling from the collapsed or ventilated lung using a randomisation schedule generated by GlaxoSmithKline (GSK) Clinical Statistics. A pharmacist/staff nominee at each site was unblinded to the treatment: this was necessary for the preparation of the randomised treatment, and this person was responsible for contacting the central allocation system to obtain the patient randomisation number. On the morning of surgery, active or placebo preparations were reconstituted, placed in an unmarked nebuliser cup and handed to a member of the research team for administration as a single nebulised dose, 1 to 5 h before oesophagectomy and before the initiation of pre-operative procedures. The investigators, who enrolled the participants and performed the surgery, and the patients themselves were blinded to study treatment but not to the BALF sampling site. The sponsor was unblinded to allow for instream analysis of safety data only (see further details below under the Role of the study sponsor).
Outcome measures
Primary endpoint
The primary endpoint was the difference in the pulmonary vascular permeability index (PVPI) between baseline (immediately before surgery) and at the completion of surgery. PVPI was calculated as the ratio of extra vascular lung water (EVLW) to pulmonary blood volume as described previously.18 The EVLW data were obtained from triplicate thermodilution measurements using the pulse contour cardiac output haemodynamic analyser (Pulsion Medical Systems, Feldkirchen, Germany).
Secondary endpoints
Markers of lung injury
Secondary endpoint markers of lung injury were: the difference in postoperative PVPI (days 2 to 4) from baseline; EVLWI (EVLW indexed to the predicted body weight); arterial partial pressure of oxygen (PaO2)/fraction of inspired oxygen (FiO2) ratio at completion of surgery and on postoperative days 2 to 4; Sequential Organ Failure Assessment (SOFA) score on days 2 to 4.19
Safety parameters
Vital signs, ECG, routine haematology and blood chemistry analyses were recorded regularly during the study, pre and postdosing and pre and postsurgery. Antibody responses to GSK2862277 were measured using a validated electrochemiluminescence (ECLIA) bridging assay in serum samples collected predose and on Days 8 and 28 following dosing, as previously described.17 Adverse events and serious adverse events (SAEs) were recorded throughout the study.
Pharmacodynamic and pharmacokinetic measures
Bronchoalveolar lavage (BAL) was performed after completion of surgery, but prior to tracheal extubation. For the BAL, three successive 60 ml aliquots of 0.9% saline were instilled into a subsegment of the middle or lingual lobe (randomised to the collapsed or ventilated lung) and then immediately recovered using gentle suction. The BALF aspirated was centrifuged at 500 × g for 5 min at 4 °C placed in aliquots, and frozen at −70 °C prior to analysis.
Blood samples were processed into plasma for pharmacokinetic, urea and total protein analysis or serum for pharmacodynamic [free and total soluble tumour necrosis factor receptor 1 (sTNFR1)] and biomarker analysis.
Pharmacokinetic, free and total sTNFR1 were measured by ECLIA (MesoScale Discovery platform, 1601 Research Boulevard Rockville, Maryland 20850-3173), and urea and total protein were measured using a Clinical Chemistry analyser (Bayer Diagnostic and Pierce Bicinchoninic acid assays, respectively), conducted by GSK. All other biomarkers were analysed under contract by LGC Ltd (Cambridgeshire, UK). All assessments were made using validated analytical methods and staff undertaking analyses were blinded to treatment allocation.
Full details of the timings for assessments are available in the online Supplement.
Statistical analysis
In this pilot study, a sample size of 80 was based on variability and computer simulation of operating characteristics derived using data from the BALTI-prevention (The Beta Agonist Lung Injury Trial-Prevention) trial3 [with respect to PVPI and PaO2/FiO2 endpoints as described in the protocol (see online supplement for further details)], and on study feasibility.
Two interim analyses using sponsor-unblinded data were planned for this study. The first was a safety review after approximately 10 patients had completed Day 7 of the study protocol, and the second was a further safety review and a futility analysis after approximately 40 patients had completed Day 7 of the protocol. The futility analysis was based on joint modelling of the (baseline adjusted) changes in PVPI and PaO2/FiO2 upon completion of surgery, estimates of treatment effect and variability derived from the interim data to predict the end of study outcome and action taken according to predefined decision rules. The first interim analysis resulted in no change to the planned study conduct; the second resulted in the study being stopped due to futility.
For the efficacy analyses, a per protocol population, comprising patients who received randomised treatment, met all the inclusion/exclusion criteria and were not classed as ‘inoperable’ once in theatre, was used. For primary and secondary endpoints, a Bayesian statistical framework was employed, which allows quantitative statements to be constructed from posterior distributions (since noninformative priors were used, the results can also be expressed as P value equivalents). Data for PVPI and biomarkers in BALF were log transformed before analysis. For endpoints derived from BALF sampling, the BAL sampling locations were pooled (via combinations of statistical model parameters) to obtain posterior distributions of the study medication groups. Adjusted point estimates and 95% credible intervals (Cr I) were constructed for each treatment, and for the comparison of GSK2862277 with placebo using a Bayesian statistical model that adjusts for baseline conditions and other parameters to estimate the true treatment differences (see online supplement for further details). Specific posterior probabilities that the true treatment difference is greater than specified quantities were produced. A posterior probability more than 0.975 is deemed equivalent to a statistically significant difference between treatments at the 5% level (P < 0.05, two-sided test). A posterior probability more than 0.9 indicates a strong trend toward a true treatment difference. All analyses were conducted using SAS version 9.4., Wittington House Henley Road, Medmenham Marlow, Buckinghamshire SL7 2EB.
Plasma GSK2862277 concentration-time data were analysed by noncompartmental methods with WinNonlin Phoenix 6.3 (Quanticate, Hertfordshire, UK).
All safety data were summarised descriptively.
Results
Study population
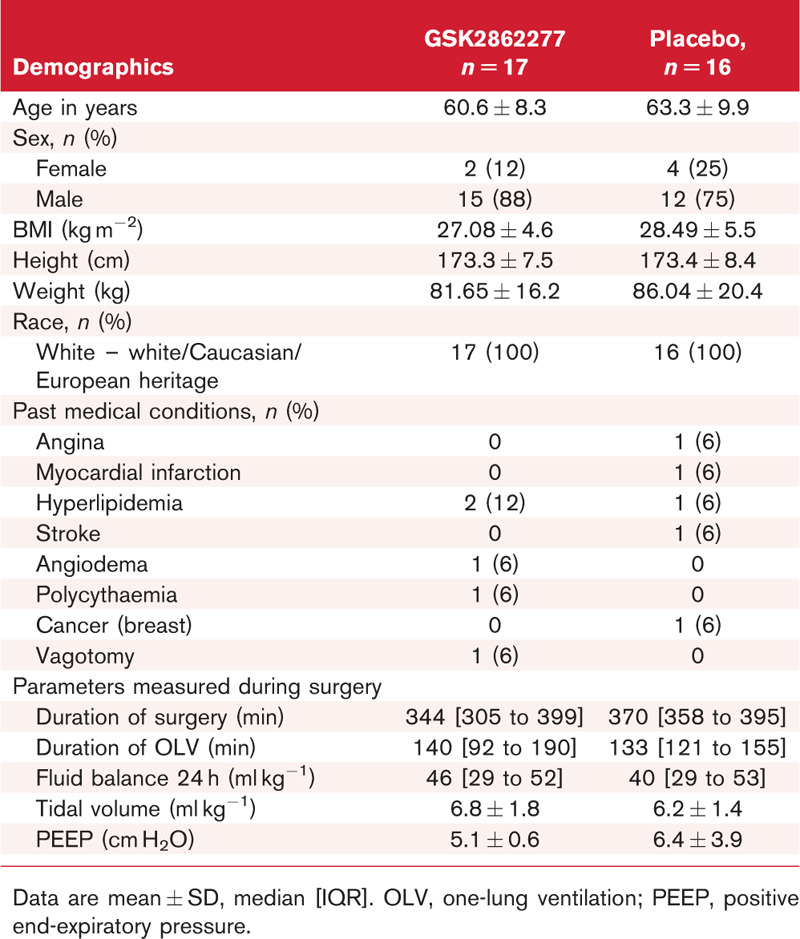
The study was initiated in eight secondary care centres in the United Kingdom between April 2015 and June 2017. Six centres screened patients and five centres enrolled patients. After meeting the futility criteria for the second planned interim analysis, the study was stopped after 33 patients had been enrolled and randomised to treatment. Of those randomised, 16 received placebo and 17 GSK2862277, and 16 and 14, respectively, underwent surgery and had available transpulmonary thermodilution measurements (Fig. 2). For three patients randomised/dosed with GSK2862277, curative oesophagectomy was abandoned. Baseline characteristics and demographics were similar for the two treatment groups (Table 1).
Fig. 2.
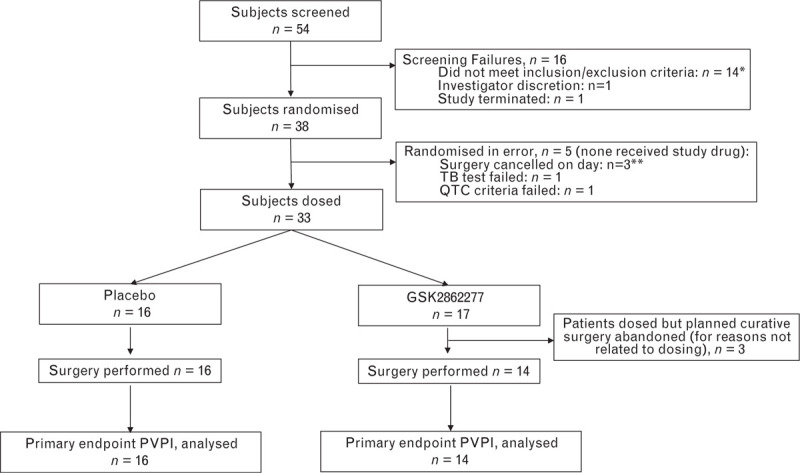
Consort flow diagram. ∗1 subject subsequently rescreened and randomised. ∗∗1 Subject subsequently randomised. PVPI, pulmonary vascular permeability index.
Table 1.
Patient characteristics (safety population)
Primary endpoint
In contrast to previous trials in this population, there was no change in PVPI [mean (95% Cr I)] between baseline and completion of surgery in either group, and no difference between treatment groups: placebo 0.00 (−0.23 to 0.39), GSK2862277 0.00 (−0.24 to 0.37) (Fig. 3, Table 2).
Fig. 3.
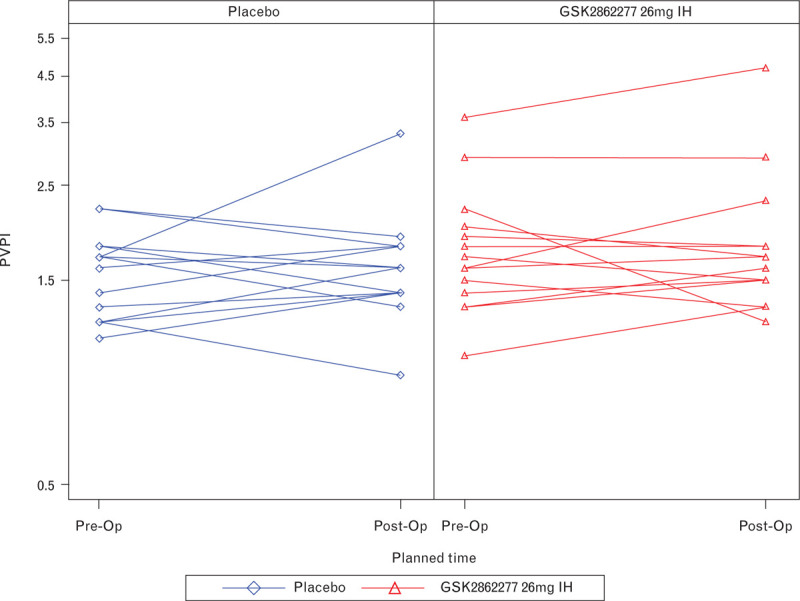
Individual changes from baseline in pulmonary vascular permeability index immediately postsurgery. PVPI, pulmonary vascular permeability index.
Table 2.
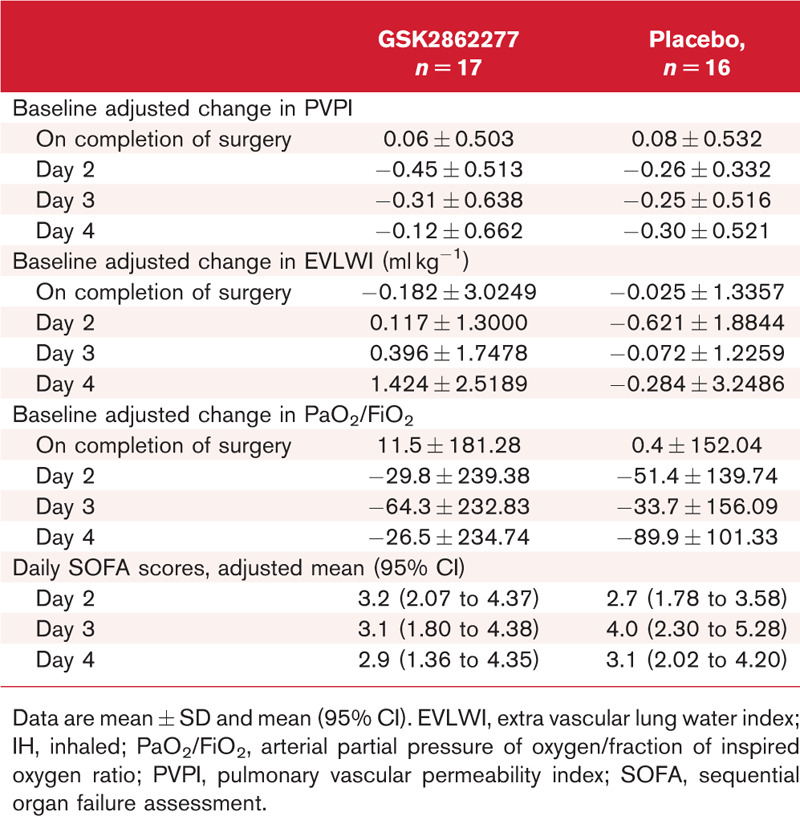
Summary of physiological endpoints
Secondary endpoints
Physiological endpoints
Data for EVLWI were consistent with PVPI data in showing minimal increase (mean change (95% Cr I) from baseline immediately postsurgery: placebo, 0.22 ml kg−1 (−0.80 to 0.75); GSK2862277, 0.30 ml kg−1 (−2.01 to 1.65). There were also no significant changes from baseline observed in both PVPI and EVLWI on days 2 to 4 postsurgery.
In contrast to the thermodilution assessments of lung injury, the expected postoperative decrease in PaO2/FiO2 was apparent in placebo treated patients. However, although the baseline adjusted mean (95% Cr I) PaO2/FiO2 was improved in GSK2862277-treated-patients relative to placebo, 60.4 (−93.2 to 116.1) and −25.4 (−67.5 to 98.1) respectively, this difference was not statistically significant and intersubject variability in PaO2/FiO2 measurements was high. Finally, there were no significant differences in organ failure SOFA scores on days 2 to 4 following surgery [Day 2 adjusted mean (95% Cr I): placebo, 2.67 (1.78 to 3.58); GSK2862277, 3.22 (2.07 to 4.37)]. A summary of secondary physiological endpoint results is shown in Table 2.
Safety
The frequency of adverse events was similar between treatment groups [placebo 15 (94%), GSK2862277 15 (88%) (Table S1, online Supplement)]. Of the more commonly reported adverse events, most were consistent with a patient's underlying illness or associated with major surgery (Table S1, online Supplement). Adverse events reported by investigators as lower respiratory tract infections were more frequent in the GSK2862277 treatment group 5 (29%) compared with the placebo group (0). The incidence of investigator-reported pneumonia was similar between the placebo group 4 (25%) compared with the GSK2862277 treatment group 3 (18%). There were relatively few reports of drug-related adverse events, but the most common was throat irritation, reported by 4 (25%) patients in the placebo group and none in the GSK2862277 treatment group (Table S2, online Supplement). There were no subject withdrawals due to an adverse event.
A post hoc review of respiratory infection events was conducted using a standardised Medical Dictionary for Regulatory Activities query (SMQ) to further understand potential mechanism-related effects on respiratory tract infections.20 SMQs are used to support signal detection and include narrow and/or broad terms. Narrow terms are those that are highly likely to represent the condition of interest.20 The frequency of patients identified using the broad terms within infective pneumonia SMQ (as defined in the online Supplement) was six (38%) in placebo and nine (53%) in GSK2862277 treated participants. Using narrow terms, the frequency was 6 (38%) and three (18%) for placebo and GSK2862277, respectively, with the difference between the broad and narrow search term results being due to the broader inclusion of chest infections (bacterial and other causes).
The overall frequency of SAEs was eight (50%) in the placebo group and nine (53%) in the GSK2862277 group; the most commonly reported SAE was pneumonia [Placebo 2 (13%), GSK2862277 3 (18%)] (Table S3, online Supplement). There were no subject withdrawals due to a SAE.
With respect to adverse events of specific interest as defined in the protocol, only one patient developed ARDS (in the GSK2862277 treatment group). There were no deaths during the study or notable differences between treatment groups in ECGs, vital signs or laboratory measures. No patient tested positive for the development of de novo antibody response to GSK2862277 over the 28-day follow-up period.
Biomarkers in bronchoalveolar lavage fluid
BALF levels of free sTNFR1 were significantly reduced [mean (95% Cr I)] in patients treated with GSK2862277: 37.66 (19.34 to 71.44) pg ml−1 compared with placebo-treated patients 231.04 (125.81 to 435.10) pg ml−1, confirming expected binding of GSK2862277 with its target (Fig. 4a). The Bayesian posterior probability of a reduction on GSK2862277 vs. placebo treatment was 1.00, exceeding the predefined significance level of 0.975 (equivalent to P < 0.05).
Fig. 4.
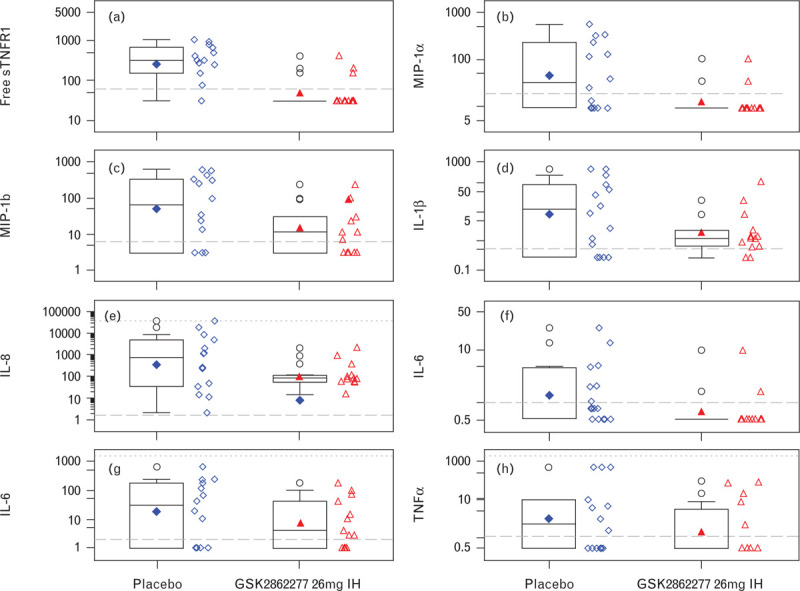
Effect of GSK2862277 on inflammatory biomarkers (pg ml−1): (a) free soluble tumour necrosis factor receptor 1; (b) macrophage inflammatory protein-1 alpha; (c) macrophage inflammatory protein-1 beta; (d) IL-1β; (e) IL-8; (f) IL-10; (g) IL-6; (h) TNF-α. IL, interleukin; MIP1-α, macrophage inflammatory protein-1 alpha; MIP-1β, macrophage inflammatory protein-1 beta; sTNFR1, soluble tumour necrosis factor receptor 1. Boxplots display the median (solid horizontal line), the interquartile range (the box), geometric mean (solid symbol inside the box) and highest and lowest values (whiskers). Open symbols lying outside the whiskers denote outliers. Horizontal long dashed lines are the lower limit of quantification and horizontal short dash lines are the upper limit of quantification.
Compared with placebo, treatment with GSK2862277 resulted in a significant reduction [mean (95% Cr I)] in BALF levels of macrophage inflammatory protein-1 alpha (MIP-1α) immediately postsurgery: placebo 41.86 (15.05 to 116.00) pg ml−1, GSK2862277 10.88 (6.35 to 19.24) pg ml−1, posterior probability 0.990, equivalent to P < 0.05 (Fig. 4b). Similar but less pronounced reductions were observed for other inflammatory biomarkers; MIP-1-beta (β), IL-1β, IL-8, IL-10, IL-6 and TNF-α (Fig. 4c to h; Table S4, online Supplement).
Total protein in BALF [mean (95% Cr I)], a marker of alveolar capillary barrier permeability, was also significantly reduced in the lungs of patients treated with GSK2862277: 100.24 (44.28 to 230.86) mg ml−1 compared with placebo 284.19 (140.38, 581.69) mg ml−1, posterior probability 0.977, equivalent to P < 0.05) (Fig. 5a). Similar results were observed for total protein ratio (Fig. 5b). Nonstatistically significant reductions in markers of alveolar epithelial injury were observed after treatment with GSK2862277 vs. placebo, including soluble Receptor for Advanced Glycation End Products, Club Cell protein 16 and Surfactant Protein-D (Table S5, online Supplement). Markers of endothelial injury in BALF could not be measured due to technical difficulties with the assay.
Fig. 5.
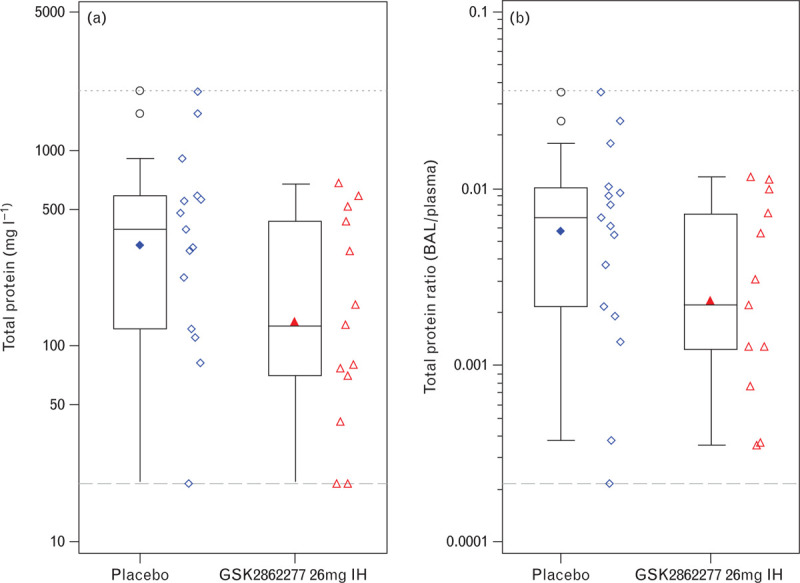
Effect of GSK2862277 on: (a) total protein; (b) total protein ratio. Boxplots display the median (solid horizontal line), the interquartile range (the box), geometric mean (solid symbol inside box) and highest and lowest values (whiskers). Open symbols lying outside the whiskers denote outliers. Horizontal long dashed lines are the lower limit of quantification and horizontal short dash lines are the upper limit of quantification.
GSK2862277 pharmacokinetics
From 17 patients dosed with GSK2862277, 14 had quantifiable drug plasma concentrations. Following single dose administration of GSK2862277, the geometric mean of plasma GSK2862277 concentrations was defined in terms of maximum plasma concentration Cmax (95% CI), 24.19 (15.12 to 38.71) ng ml−1 and cumulative exposure over the study sampling time AUC(0-t) (95% CI), 279.42 (187.38 to 416.68) ng h ml−1. Absorption rate from the lungs was relatively slow with a median time to maximum plasma concentration (Tmax) of ≈7.5 h. Local concentrations in the lung were higher than those observed in plasma (Fig. 6); however, data for lung concentrations were highly variable (coefficient of variation between patients: 387%).
Fig. 6.
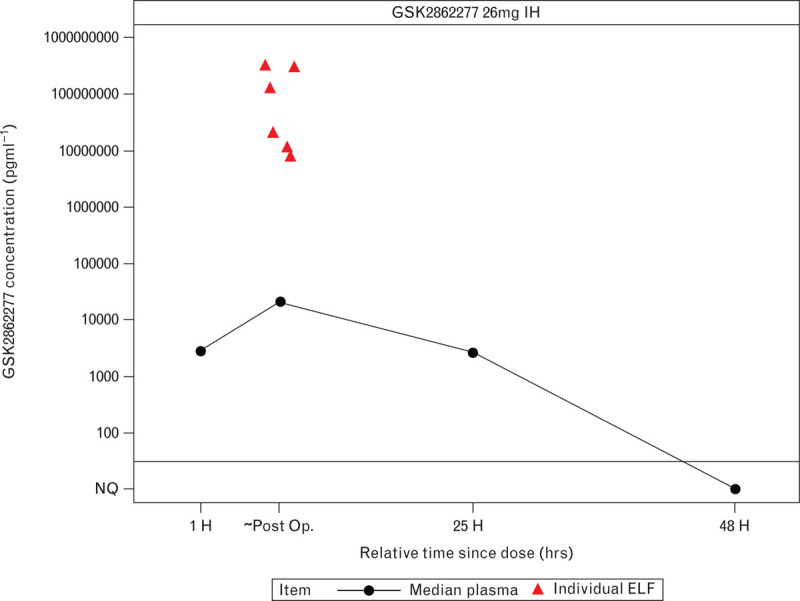
Median plasma concentrations of GSK2862277 and individual patient bronchoalveolar lavage fluid-derived lung epithelial lining fluid concentrations.
Discussion
The current pilot study was the first to assess the effects of inhaled GSK2862277 in patients undergoing oesophagectomy. Adequate exposure of the target tissue (the lungs) to GSK2862277 was achieved at pharmacologically relevant concentrations (90% inhibitory concentration (IC90) in BALF-derived lung epithelial lining fluid), which was confirmed by significant target binding (reduction in levels of free sTNFR1 due to GSK2862277 binding) both in the lungs and in plasma. Oesophagectomy patients were recruited into this trial as they represent a single cause with a reproducible and timed lung injury response that, historically, has been associated with increases in transpulmonary thermodilution-measured postoperative PVPI and EVLWI, inflammation and a high-rate of PPCs, including ARDS.5,21 However, in contrast to previous trials in this population,3,22 minimal postoperative increases in PVPI and EVLWI were observed in the placebo-treated patients, suggesting less lung injury and alveolar capillary leak in patients recruited to this trial.
Furthermore, no treatment-related differences in PVPI and EVLWI could be discerned, either because inhibiting TNFR1 signalling does not attenuate lung capillary leak in these patients, or because the minimal increases in PVPI and EVLWI in addition to the higher than expected intersubject variability observed in this trial precludes a proper assessment of efficacy against these endpoints. Although exploratory in nature, treatment with GSK2862277 did not result in any significant improvements in postoperative oxygenation (PaO2/FiO2) or SOFA score endpoints.
The discrepant postoperative PVPI and EVLWI increases between this and previous trials, could be attributed to changes in anaesthetic practice, including increased adoption of lower tidal volume ventilation strategies and evolving fluid management over the last 7 years.23 This is partially supported by an exploratory comparison of anaesthetic practice across BALTI-prevention, VINDALOO (Vitamin D to Prevent Acute Lung Injury Following Oesophagectomy) trials, and data from this trial (Table S6, online Supplement). Patient related factors, such as cancer type, smoking and pre-operative nutrition and changes in surgical practice may also be contributory.
Due to slower than anticipated recruitment, the second planned interim analysis was completed after 33 patients were enrolled instead of the planned 40 patients. After review of data from these 33 subjects, the study was stopped based on futility of the primary endpoint.
Nebulised GSK2862277 significantly attenuated levels of BALF protein, a biochemical marker of vascular leak and markers of pulmonary inflammation. To ensure the pooling of the BALF samples from collapsed and ventilated lung for each intervention group did not influence these results, checks were made to determine the importance of treatment by BALF sampling location interaction (checking size of estimate relative to the main effect sizes) but, due to the small sample sizes, no formal statistics methodology was employed. However, we are confident that the BALF pooling did not bias the data or its interpretation. The effects of GSK2862277 on biochemical markers of lung injury observed in this patient population are consistent with observations in nonhuman primate and human pulmonary endotoxin lipopolysaccharide challenge studies9 and confirm translation of the effects of GSK2862277 on inflammation and tissue injury to patients. Although the clinical relevance of changes in BALF biomarkers of inflammation and tissue injury is unclear, increased BALF protein levels and inflammatory markers are associated with the development of PPCs and ARDS in surgical cohorts.24,25
Treatment with GSK2862277 was generally well tolerated. Although a greater number of lower respiratory tract infections were observed in the active treatment group compared with the placebo group, when collectively assessed post hoc by a standardised group term (infective pneumonia SMQ), respiratory tract infections were similar between treatment groups. The incidence of postoperative infective pneumonia in oesophagectomy patients is high, and in general surgical cohorts infection in the postoperative period is strongly associated with longer hospital stay and increased in-hospital mortality risk.26 Significantly, serial measurements of postoperative cytokine levels have demonstrated a link between the magnitude of the initial postoperative inflammatory response with the extent of subsequent immunosuppression and susceptibility to postoperative infections including pneumonia.27 These observations raise the possibility that attenuating the immediate peri-operative and postoperative inflammatory response might limit the extent of subsequent immunosuppression, and possibly also reduce the risk of developing postoperative infections. Conversely, further attenuation of the immune system during the postoperative period might also potentially worsen postoperative immunosuppression. Given these dynamic and variable immune responses to surgical trauma, it is important to carefully investigate the potential impact of immunotherapies such as GSK2862277 in these patients. Given the small sample size, the effects of the short-acting, selective TNFR1 inhibitor GSK2862277 on the incidence of postoperative infectious pneumonia cannot be acertained. However, considering the established risks associated with long-acting, nonselective anti-TNF-α therapies,28 it is encouraging that GSK2862277 did not result in significant increases in the incidence of postoperative pneumonia in the oesophagectomy patients. The results for immunogenicity testing were also encouraging as no patients tested positive for the formation of de novo antibodies to GSK2862277.
The current study has some limitations. Both slow recruitment to the study and the early termination of the study on grounds of futility resulted in smaller than planned patient numbers. The small number of patients also hampered the ability to compare differences in drug disposition and biology between hyperinflated and collapsed lungs in treated patients. Finally, this study was designed to investigate the effect of GSK2862277 on inflammation and injury in the lungs, which was presumed to occur because of peri-operative OLV and/or partial lung collapse. Although some systemic exposure to GSK2862277 was anticipated and confirmed by the reduction in free serum sTNFR1 levels on Day 1, as expected, systemic exposure of GSK2862277 was transient and limited. Given the postoperative increase in inflammatory biomarkers observed in the serum of patients, it is plausible that systemic inflammation also probably contributes to the development of both pulmonary and nonpulmonary complications in oesophagectomy patients. Therefore, dosing GSK2862277 systemically (e.g. intravenously), or perhaps both intravenously and via the inhaled route, over a longer duration may improve the likelihood of an effect on postoperative complications in these patients.
Conclusion
In this trial of patients undergoing oesophagectomy, pre-operative treatment with a single 26 mg inhaled dose of GSK2862277 did not result in significantly lower postoperative alveolar capillary leak or lower EVLW. Unexpectedly small increases in transpulmonary thermodilution-measured PVPI and EVLWI at completion of surgery suggest less postoperative lung injury than historically reported in these patients, which may have also compromised a clear assessment of efficacy in this trial. A single nebulised dose of GSK2862277 was well tolerated, resulted in the expected pharmacokinetics and lung exposure, and reduced other biomarkers of lung permeability and inflammation. The potential of GSK2862277 for future clinical use requires further exploration
Supplementary Material
Acknowledgements relating to this article
The authors would like to thank all members of the study site teams (at The James Cook University Hospital, Middlesbrough; Birmingham Heartlands Hospital; University of Birmingham; Castle Hill Hospital, Hull; Belfast City Hospital; Addenbrooke's Hospital, Cambridge) for their help in running the study and caring for participating patients, as well as the staff of the Northern Ireland Clinical Research Network and the National Institute for Health Research (NIHR) Clinical Research Network for help with patient recruitment and data acquisition.
Editorial support in the form of copyediting, collating author comments and fact checking was provided by Kate Hollingworth of Continuous Improvement Ltd, Devon, UK, funded by GSK.
The study (NCT02221037) was funded by GSK.
DFM has received personal funds for consultancy from GSK, and funds to his institution from GSK for the conduct of this trial. All outside the submitted work, DFM has also received personal fees for consultancy for Bayer, DFM's institution has received grants from the NIHR and others for ARDS research and DFM has a patent issued to his institution for a treatment for ARDS. DFM is a Director of Research for the Intensive Care Foundation. JY is supported by a NIHR postdoctoral fellowship and has no competing interests to declare.
PAH and CO’D were funded by GSK research fellows to conduct this study. AMV declares that she has received a research grant from GSK towards attainment of a PhD. CS reports her institution receives research grant funding from GSK for research conducted in her laboratory. GDP has received consultancy fees and reimbursement of expenses from GSK. GDP is a Director of Research for the Intensive Care Foundation. AB, TJW, EM, KH, WP, RW, ALL and AF are employees of, and hold shares in, GSK. There are no other competing interests.
Assistance with the study: none.
Financial support and sponsorship: Role of the sponsor: as the study sponsor, GSK was involved in the study concept and design, study conduct, study analysis and interpretation and the writing up of the results. Although the study was formally defined as sponsor unblind, internal GSK best practices for restricting access to unblinded outputs was followed [randomisation codes were only sought for participants contributing data to any upcoming analyses; all outputs were stored in a restricted access reporting effort (controlled by membership of the ‘tnfrint’ unix group with members limited to study statistics and programming personnel), and dissemination of outputs was kept to the core study team (or Safety Review Team) members].
Presentation: Preliminary data presentation: none.
Availability of data and materials: Anonymised individual participant data and study documents can be requested for further research from www.clinicalstudydatarequest.com.
Authors’ contributions: contributed to study concept and design: AB, DFM, DRT, TJW, EM, KH, WP, RW, ALL, GDP. Contributed to the conduct of the study: JR, DFM JY, DRT, PAH, CO’D, AMV, TJW, EM, KH, CS, MOS, WP, RW, ALL, AF; Performed data analysis: WP; Data interpretation, contributed to the writing of the article, approved the submitted article: All.
Footnotes
Published online 27 May 2020
References
- 1.Ranieri VM, Rubenfeld GD, Thompson BT, et al. ARDS Definition Task Force. Acute respiratory distress syndrome: the Berlin definition. JAMA 2012; 307:2526–2533. [DOI] [PubMed] [Google Scholar]
- 2.Abbott TEF, Fowler AJ, Pelosi P, et al. StEP-COMPAC Group. A systematic review and consensus definitions for standardised end-points in perioperative medicine: pulmonary complications. Br J Anaesth 2018; 120:1066–1079. [DOI] [PubMed] [Google Scholar]
- 3.Perkins GD, Gates S, Park D, et al. BALTI-Prevention Collaborators. The beta agonist lung injury trial prevention. A randomized controlled trial. Am J Respir Crit Care Med 2014; 189:674–683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Proudfoot AG, McAuley DF, Griffiths MJD, et al. Human models of acute lung injury. Dis Model Mech 2011; 4:145–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shyamsundar M, McAuley DF, Shields MO, et al. Effect of simvastatin on physiological and biological outcomes in patients undergoing esophagectomy: a randomized placebo-controlled trial. Ann Surg 2014; 259:26–31. [DOI] [PubMed] [Google Scholar]
- 6.Qiu P, Cui X, Sun J, et al. Antitumor necrosis factor therapy is associated with improved survival in clinical sepsis trials: a meta-analysis. Crit Care Med 2013; 41:2419–2429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tartaglia LA, Rothe M, Hu YF, et al. Tumor necrosis factor's cytotoxic activity is signaled by the p55 TNF receptor. Cell 1993; 7:213–216. [DOI] [PubMed] [Google Scholar]
- 8.Patel BV, Wilson MR, O’Dea KP, et al. TNF-induced death signaling triggers alveolar epithelial dysfunction in acute lung injury. J Immunol 2013; 190:4274–4282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Proudfoot A, Bayliffe A, O’Kane CM, et al. Novel antitumour necrosis factor receptor-1 (TNFR1) domain antibody prevents pulmonary inflammation in experimental acute lung injury. Thorax 2018; 73:723–730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Marchetti L, Klein M, Schlett K, et al. Tumor necrosis factor (TNF)-mediated neuroprotection against glutamate-induced excitotoxicity is enhanced by N-methyl-d-aspartate receptor activation. Essential role of a TNF receptor 2-mediated phosphatidylinositol 3-kinase-dependent NF-kappa B pathway. J Biol Chem 2004; 279:32869–32881. [DOI] [PubMed] [Google Scholar]
- 11.Bluml S, Binder N, Niederreiter B, et al. Analysis of TNFR2-mediated functions on osteoclast precursor cells. Ann Rheum Dis 2010; 69:A35–A36. [Google Scholar]
- 12.Al-Lamki RS, Wang J, Vandenabeele P, et al. TNFR1- and TNFR2-mediated signalling pathways in human kidney are cell type-specific and differentially contribute to renal injury. FASEB J 2005; 19:1637–1645. [DOI] [PubMed] [Google Scholar]
- 13.Luo D, Luo Y, He Y, et al. Differential functions of tumor necrosis factor receptor 1 and 2 signaling in ischemia-mediated arteriogenesis and angiogenesis. Am J Pathol 2006; 169:1886–1898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wilson MR, Goddard ME, O’Dea KP, et al. Differential roles of p55 and p75 tumor necrosis factor receptors on stretch-induced pulmonary edema in mice. Am J Physiol Lung Cell Mol Physiol 2007; 293:L60–L68. [DOI] [PubMed] [Google Scholar]
- 15.Ebach DR, Riehl TE, Stenson WF. Opposing effects of tumor necrosis factor receptor 1 and 2 in sepsis due to cecal ligation and puncture. Shock 2005; 23:311–318. [DOI] [PubMed] [Google Scholar]
- 16.Longhi L, Perego C, Ortolano F, et al. Tumor necrosis factor in traumatic brain injury: effects of genetic deletion of p55 or p75 receptor. J Cereb Blood Flow Metab 2013; 33:1182–1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cordy JC, Morley PJ, Wright TJ, et al. Specificity of human antivariable heavy (VH) chain autoantibodies and impact on the design and clinical testing of a VH domain antibody antagonist of tumour necrosis factor-α receptor 1. Clin Exp Immunol 2015; 182:139–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jozwiak M, Teboul JL, Monnet X. Extravascular lung water in critical care: recent advances and clinical applications. Ann Intens Care 2015; 5:38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vincent JL, Moreno R, Takala J, et al. The SOFA (Sepsis-related Organ Failure Assessment) score to describe organ dysfunction/failure. Intensive Care Med 1996; 22:707–710. [DOI] [PubMed] [Google Scholar]
- 20.MedDRA The Medical Dictionary for Regulatory Activities. MedDRA SMQs. URL: https://www.meddra.org/meddra-smqs [Accessed 30 Novemebr 2018]. [Google Scholar]
- 21.Howells P, Thickett D, Knox C, et al. The impact of the acute respiratory distress syndrome on outcome after oesophagectomy. Br J Anaesth 2016; 117:375–381. [DOI] [PubMed] [Google Scholar]
- 22.Parekh D, Dancer RCA, Scott A, et al. Vitamin D to prevent lung injury following esophagectomy – a randomized, placebo-controlled trial. Crit Care Med 2018; 46:e1128–e1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Howells PA, Aldridge KA, Parekh D, et al. ARDS following oesophagectomy: a comparison of two trials. BMJ Open Respir Res 2017; 4:e000207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Serpa NA, Campos PP, Hemmes SN, et al. PROVE Network Investigators. Kinetics of plasma biomarkers of inflammation and lung injury in surgical patients with or without postoperative pulmonary complications. Eur J Anaesthesiol 2017; 34:229–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Agrawal A, Zhuo H, Brady S, et al. Pathogenetic and predictive value of biomarkers in patients with ALI and lower severity of illness: results from two clinical trials. Am J Physiol Lung Cell Mol Physiol 2012; 303:L634–L639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Arozullah AM, Khuri SF, Henderson WG, et al. Participants in the National Veterans Affairs Surgical Quality Improvement Program. Development and validation of a multifactorial risk index for predicting postoperative pneumonia after major noncardiac surgery. Ann Intern Med 2001; 135:847–857. [DOI] [PubMed] [Google Scholar]
- 27.Torrance HD, Pearse RM, O’Dwyer MJ. Does major surgery induce immune suppression and increase the risk of postoperative infection? Curr Opin Anaesthesiol 2016; 29:376–383. [DOI] [PubMed] [Google Scholar]
- 28.Furst DE. The risk of infections with biologic therapies for rheumatoid arthritis. Semin Arthritis Rheum 2010; 39:327–346. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


