Preliminary testing in a low-resourced setting suggests that a mini-sponge tamponade device may be feasible for use in treatment of postpartum hemorrhage.
Abstract
BACKGROUND:
Postpartum hemorrhage is a leading cause of maternal mortality globally. A tamponade agent that can be quickly and easily placed in a range of settings could advance the treatment of atonic hemorrhage.
METHOD:
We adapted a highly effective trauma dressing for use in postpartum hemorrhage. This mini-sponge tamponade device is comprised of two components: compressed mini-sponges contained within a strong mesh pouch and a tubular applicator. Compressed mini-sponges rapidly absorb blood, expand within seconds, and exert sustained pressure uniformly to bleeding sites. The sponges are deployed within a mesh pouch to facilitate simple vaginal removal.
EXPERIENCE:
We successfully placed the mini-sponge device in nine patients experiencing postpartum hemorrhage after vaginal birth, with resolution of bleeding within 1 minute. The mean time to place the device was 62 seconds. Uterine fill was documented in all cases by ultrasound scan, and device placement was rated as “easy” to “very easy.” Mini-sponges were left in place on average for 1 hour (0.5 hours–14 hours). Bleeding did not recur. There were no adverse events; all patients remained afebrile and did not require subsequent surgical intervention.
CONCLUSION:
This study supports further evaluation of the mini-sponge device for the management of postpartum hemorrhage.
FUNDING:
This study was funded by OBSTETRX, Inc.
Postpartum hemorrhage, defined by the World Health Organization as blood loss of 500 mL or more within 24 hours of birth, is responsible for a quarter of all maternal deaths globally.1 Uterine atony, or the ineffective contraction of the uterus after delivery, is responsible for the bulk of postpartum hemorrhage cases.2,3 Nearly all of these deaths could be prevented by timely and appropriate management.
Uterine tamponade to treat atony has been attempted using sterile gauze, inflated Foley catheters, condom catheters, and silicone obstetric balloons. None of these are ideal, they may be difficult to use without patient anesthesia, and they may be difficult to stock in low-resourced settings.4–7 Despite the range of devices available, none is ideal.
An innovative mini-sponge tamponade device (XSTAT) has proven successful in the acute cessation of traumatic, noncompressible bleeding. The mini-sponge device is capable of stopping high-flow arterial bleeding (ie, greater than 1.5 L/min) within seconds, without external compression.8–10 Because the compressed sponges do not have a fixed shape, they conform to the unique shape of the wound or uterine cavity after insertion.11 In a bleeding wound, the mini-sponges rapidly absorb blood, expand, and then exert outward pressure for up to 24 hours.12 The mini-sponges are distinct from routine laparotomy sponges, which, when saturated, become smaller in size. The effect is not due to a hemostatic agent, but simply holding and maintaining pressure.8,12 The types of injuries treated in trauma settings include penetrating injuries from shrapnel, gunshots, or motor vehicle accidents. Deployment in these settings is rapid and reliable and provides a high degree of hemorrhage control.8–10,13,14 Based on these results, we hypothesized that the mini-sponges could effectively manage postpartum hemorrhage due to atony.
To adapt the mini-sponge device for use in the treatment of postpartum hemorrhage, we developed an obstetric applicator for transcervical placement using a digital vaginal route. The objective of this early feasibility study was to assess the placement, removal, and preliminary efficacy of the mini-sponge tamponade device in patients experiencing postpartum hemorrhage.
ROLE OF THE FUNDING SOURCE
The authors had access to relevant aggregated study data and other information (such as study protocol, analytic plan and report, validated data table, and clinical study report) required to understand and report research findings. The authors take responsibility for the presentation and publication of the research findings, have been fully involved at all stages of publication and presentation development, and are willing to take public responsibility for all aspects of the work. All individuals included as authors and contributors who made substantial intellectual contributions to the research, data analysis, and publication or presentation development are listed appropriately. The role of the sponsor in the design, execution, analysis, reporting, and funding is fully disclosed. The authors' personal interests, financial or nonfinancial, relating to this research and its publication have been disclosed.
METHOD
We obtained ethical review and approval from the institutional review boards at the University Teaching Hospital in Lusaka, Zambia (University of Biomedical Research Ethics Committee), and the Oregon Health & Science University in Portland, Oregon. The Zambia Medicine Regulatory Authority also reviewed and approved the study protocol. The study was conducted from May 20 to June 12, 2019, at a single site, the University Teaching Hospital in Lusaka, Zambia. All pregnant patients presenting to the labor and delivery unit were screened for study eligibility. Patients aged 16 years or older with no serious medical conditions and uncomplicated pregnancies who were able to provide written informed consent were eligible for enrollment. A baseline demographic and health questionnaire was completed at the time of study enrollment. Eligibility for device placement was an estimated blood loss of 500 mL or greater due to atony after vaginal delivery. Blood loss was assessed by visual inspection; quantitative blood loss assessment was not performed at the study site. Patients needing cesarean delivery and those with evidence of infection or experiencing hemodynamic instability necessitating immediate surgical intervention were ineligible for device placement. A study investigator reaffirmed consent and eligibility immediately before device placement, as well as approval from the primary medical team at the bedside. Three physicians placed all of the devices, which was done transcervically after confirming persistent cervical dilation. No speculum was used, and ultrasonography was not used for placement but was used with physical examination to confirm correct location of the sponge pouches. Complete fill of the uterus was defined as the inability to place further sponges within the uterus (physical examination) and ultrasound documentation. At the discretion of the investigator, an additional device could be deployed. The sponge pouches were removed when the patient was deemed to be clinically stable or within 24 hours after placement. Patients were treated with a single dose of intravenous cephazolin. We monitored participants receiving the device from delivery until hospital discharge. For the purpose of the study, we asked participants to return for follow-up approximately 6 days postdischarge. If participants did not return, an attempt was made to contact them by telephone. Ease of placement was assessed by asking the physician whether the placement was easy, moderately difficult, or hard. Our study outcomes included successful placement of the device (uterine fill documented on ultrasound scan), ease of placement, time to place the device, time to hemostasis, estimation of ongoing blood loss, re-bleeding, blood transfusion, and need for further treatment for hemorrhage control (surgical or medical). Enrolled study participants who did not experience postpartum hemorrhage had study participation end at time of delivery.
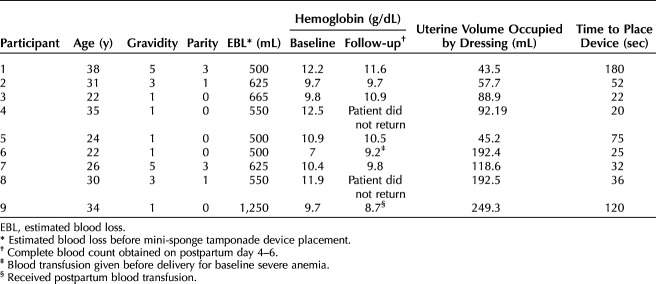
The mini-sponge tamponade device (Fig. 1) was adapted from the trauma device in two key ways: 1) the applicator was enlarged to facilitate placement as well as to accommodate a larger mini-sponge dose for filling a postpartum uterus, and 2) the mini-sponges were secured within a strong, porous pouch to facilitate manual vaginal removal (Fig. 2). The mini-sponges are composed of the same materials used in standard surgical sponges currently approved for use inside the uterus and vagina. Each mini-sponge is compressed to a height of 4–5 mm; on contact with blood, the mini-sponges absorb blood and, if unencumbered, are capable of expanding to their precompressed height of 40–50 mm within approximately 20 seconds. Expansion of the mini-sponges exerts pressure within the uterus for a period of hours. The pouch is highly distensible and durable, with a tensile strength of greater than 200 lbs. Approximately 400 mini-sponges are contained within each pouch. A removal strand is attached to the pouch to facilitate posttreatment removal by means of gentle traction without additional procedures. The pouch was inspected on removal to confirm that it was intact and that all sponges had been removed. In the unlikely event that a pouch broke, the sponges would be visible on ultrasound scan and removed by suction or forceps.
Fig. 1. The study device is composed of a mini-sponge pouch that is delivered with a curved applicator and plunger. Image courtesy of OBSTETRX. Used with permission.
Rodriguez. Mini Sponge Device for Uterine Tamponade. Obstet Gynecol 2020.
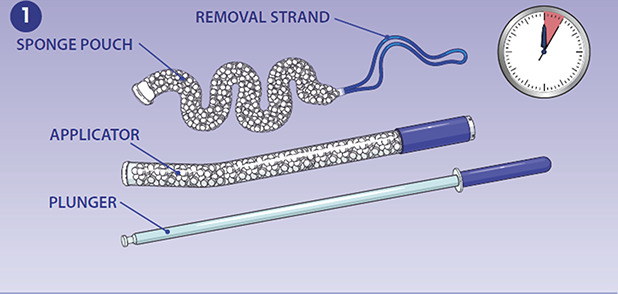
Fig. 2. Placement and removal of study device. The applicator is inserted through the vagina into the lower uterine segment. A. The plunger is depressed, ejecting the mini-sponge pouch into the uterus. B. The applicator is gently removed from the uterus, and the removal strand is left in the vaginal canal. Once inside the uterus, the mini-sponges absorb blood and pack the uterus. C. When the patient is clinically stable and it is deemed safe to remove the dressing, the pouch is removed by applying gentle, steady traction to the removal strand. Images courtesy of OBSTETRX. Used with permission.
Rodriguez. Mini Sponge Device for Uterine Tamponade. Obstet Gynecol 2020.
All data from the investigation were entered into a database and maintained by the independent study team. The sponsor did not collect or analyze the information. We used descriptive statistics to report on study outcomes.
EXPERIENCE
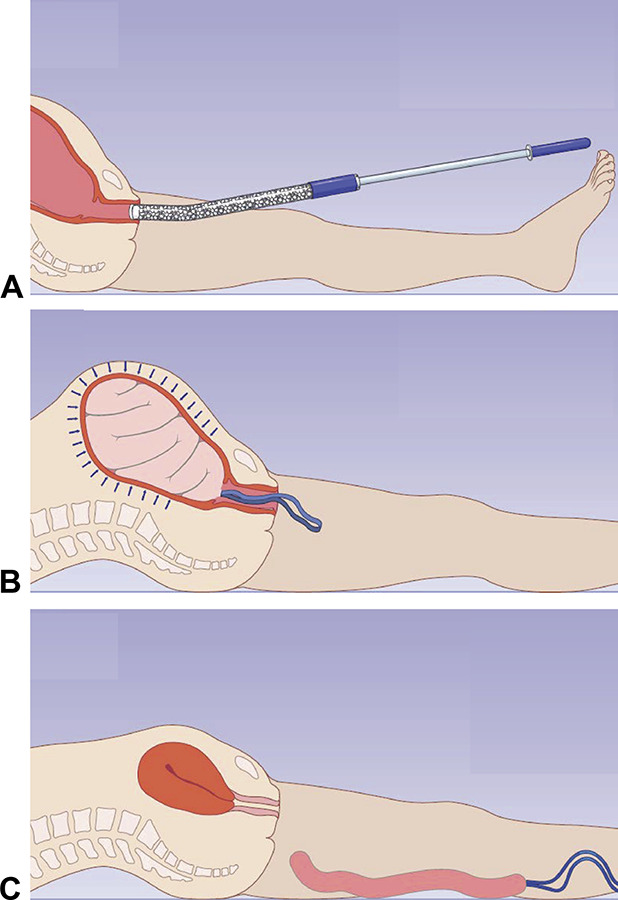
During the study period, 827 patients were admitted for delivery at the study site; of these, 100 consented and were enrolled (Fig. 3). Nine patients were treated with the mini-sponge tamponade device. Participants receiving the study device had a mean age of 29 years (range 22–38) and had a mean of two prior pregnancies (range 1–5) and one birth before the current delivery (range 0–3). One third of the patients reported a history of anemia. One participant had a prior history of blood transfusion, and another had a past history of postpartum hemorrhage. Almost half of the patients ([44% [4/9]) were living with human immunodeficiency virus (HIV). No participant reported a history of malarial infection or sickle cell disease. One enrolled participant received a blood transfusion during labor; however, this was given before her postpartum hemorrhage given her significant baseline anemia (antenatal hemoglobin of 7 g/dL).
Fig. 3. Study enrollment chart. Image courtesy of OBSTETRX. Modified with permission.
Rodriguez. Mini Sponge Device for Uterine Tamponade. Obstet Gynecol 2020.
Before device placement, mean estimated blood loss was 600 mL (range 500–1,250). Cervical dilation at the time of device placement was a mean of 4.5 cm (range 3–5 cm). All participants (N=9) received fundal massage and intramuscular oxytocin before receiving the study device. One participant also received misoprostol. The device was successfully deployed into the uterus with complete fill in all cases. The mean time to place the device was 62 seconds (range 25–120 seconds). Investigators placing their first device took between 75 and 180 seconds; placement of subsequent devices took between 20 and 52 seconds.
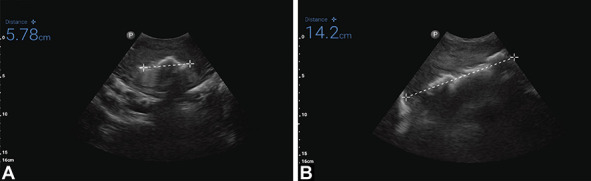
All participants were treated with one mini-sponge tamponade device (ie, one sponge pouch dressing). On ultrasound scan, the uterine cavity appeared to be completely filled by the dressing, and there was no evidence of ongoing bleeding around the dressing, which would have been evidenced by fluid accumulating in the uterine cavity on ultrasound scan. There was no visible bleeding in the perineum after device placement. For all participants, bleeding stopped in less than 1 minute, did not recur, and required no further treatment. In all cases, the dose was more than was needed for uterine fill, with a portion of the pouch containing sponges remaining unused in the vagina and external to the participant. The average volume of dressing in the uterus was 120 mL (range 43.5–249.3 mL). This was assessed by ultrasound scan (Fig. 4). Placement of the device was rated as moderately difficult to easy by obstetrician–gynecologists. Participant 9 received a blood transfusion postpartum given her baseline anemia and estimated blood loss of 1,200 mL.
Fig. 4. Ultrasound images of device within uterus. Sagittal view (A) and longitudinal view (B). Images courtesy of OBSTETRX. Used with permission.
Rodriguez. Mini Sponge Device for Uterine Tamponade. Obstet Gynecol 2020.
Mini-sponge pouches were left in place on average for 1 hour, with a range of 0.5 hours–14 hours. Patients were monitored with visual, physical (fundal height and massage), and ultrasound assessment to confirm that the bleeding had stopped and did not recur over the first 24 hours postpartum. The ongoing use of uterotonics was at the discretion of the clinical team. Only participant 9 received additional oxytocin. No sponges were expelled prematurely. All dressings were successfully removed. Removal of the dressing was rated as easy to moderately easy by study investigators, and removal time ranged from 10 to 30 seconds. All dressings were visually inspected, and the sponge pouch was intact in all cases.
No adverse events related to the device occurred. All participants remained afebrile throughout device retention and until discharge, with scant ongoing bleeding. Participant demographic data and case detail summaries are listed in Table 1. Among those patients who returned at 6 days postpartum or who were reached by telephone (5/9), none reported pain, infection, or bleeding greater than normal lochia.
Table 1.
Patient Demographics and Procedure Outcomes
DISCUSSION
This early feasibility study demonstrated that the mini-sponge tamponade device can be readily placed and removed in patients experiencing postpartum hemorrhage. In all nine cases, the device was quickly placed in the correct intrauterine position without the need for ultrasound guidance, with immediate cessation of bleeding.
In all nine cases, removal of the device was also easy, fast, and complete. We did note a “learning curve” with device placement. This rapid placement time, even with first use, reflects an important comparative advantage of the mini-sponge device, because reducing time from diagnosis of hemorrhage to placement of uterine tamponade balloons reduces morbidity.1 A previous multicountry study demonstrated that more than half (52.1%) of all condom balloon placements were complicated, most commonly owing to balloon displacement, or they required a second attempt at placement.7
In Zambia, we use the World Health Organization's definition of postpartum hemorrhage—blood loss of 500 mL or greater.1 Early diagnosis and swift management of postpartum hemorrhage are essential to reducing maternal morbidity and mortality, particularly in low-resourced settings where the risks to maternal health are high.1 The American College of Obstetricians and Gynecologists updated their postpartum hemorrhage guidance to reflect a new definition of postpartum hemorrhage as blood loss of 1,000 mL or greater or any bleeding that causes hemodynamic stability.14 Our use of the lower threshold for diagnosing postpartum hemorrhage affects the generalizability of our results. We also relied on estimated blood loss, the standard of care at our study site. This is less precise than using quantitative blood loss assessment.
This preliminary investigation supports further evaluation of the mini-sponge tamponade device as an alternative to other devices used to treat atonic postpartum hemorrhage. This device is being developed to offer a low-cost, easy-to-use product that is of similar or greater efficacy than the condom uterine balloon tamponade. It does not require electricity or additional equipment to place, which may facilitate its use in low- and high-resourced settings.
Investigators involved in the testing of this initial prototype suggested to the manufacturer several modifications of the design to improve use characteristics, including reducing the dose of sponge delivered and reducing the pressure needed to deploy the sponges. To evaluate the efficacy of the mini-sponge tamponade device, a larger study is planned, comparing it with the available condom uterine balloon tamponade, with a primary outcome of time from diagnosis of postpartum hemorrhage to cessation of bleeding. Future studies will include a larger number of participants, with quantitative blood loss assessment to determine the device's effect in managing more patients with severe postpartum hemorrhage.
Footnotes
Financial Disclosure Mary Bullard, Kenton Gregory, and Andrew Barofsky are OBSTETRX employees. They participated in device design, development and desktop testing. They were not involved with direct data collection or analysis. Maria Rodriguez has received payments from OBSTETRX; this conflict of interest is reviewed and managed by OHSU's Office of Integrity. Maria Isabel Rodriguez reports that money was paid to her institution from NIH and Merck. She received funds from Bayer. Jeffrey T. Jensen has received payments for consulting from AbbVie, Cooper Surgical, Bayer Healthcare, Merck, Sebela, and TherapeuticsMD. OHSU has received research support from AbbVie, Bayer Healthcare, Daré, Estetra SPRL, Medicines360, Merck, and Sebela. These companies and organizations may have a commercial or financial interest in the results of this research and technology. These potential conflicts of interest have been reviewed and managed by OHSU. Andrew D. Barofsky reports that he is the CEO and a board director of OBSTETRX, Inc. He owns stock in OBSTETRX, Inc. He is an inventor of a patent assigned to OBSTETRX, Inc. that claims technology described in the manuscript. Alison Edelman receives royalties from UpToDate. She is a Nexplanon Trainer with Merck but has declined payments in past 36 months. Her institution receives research monies from Merck, HRA Pharma, and NIH on projects where she is principal investigator. The other authors did not report any potential conflicts of interest.
Each author has confirmed compliance with the journal's requirements for authorship.
Peer reviews and author correspondence are available at http://links.lww.com/AOG/C78.
Figure.

No available caption
REFERENCES
- 1.World Health Organization. WHO guidelines for the prevention and management of postpartum haemorrhage. World Health Organization: Geneva (Switzerland); 2012. [Google Scholar]
- 2.Bakri YN, Amri A, Abdul Jabbar F. Tamponade-balloon for obstetrical bleeding. Int J Gynaecol Obstet 2001;74:139–42. [DOI] [PubMed] [Google Scholar]
- 3.Bakri YN. Uterine tamponade-drain for hemorrhage secondary to placenta previa-accreta. Int J Gynaecol Obstet 1992;37:302–3. [DOI] [PubMed] [Google Scholar]
- 4.Dabelea V, Schultze PM, McDuffie RS., Jr Intrauterine balloon tamponade in the management of postpartum hemorrhage. Am J Perinatol 2007;24:359–64. [DOI] [PubMed] [Google Scholar]
- 5.Vitthala S, Tsoumpou I, Anjum ZK, Aziz NA. Use of Bakri balloon in post-partum haemorrhage: a series of 15 cases. Aust N Z J Obstet Gynaecol 2009;49:191–4. [DOI] [PubMed] [Google Scholar]
- 6.Maier RC. Control of postpartum hemorrhage with uterine packing. Am J Obstet Gynecol 1993;169:317–21. [DOI] [PubMed] [Google Scholar]
- 7.Anger HA, Dabash R, Durocher J, Hassanein N, Ononge S, Frye LJ, et al. The effectiveness and safety of introducing condom-catheter uterine balloon tamponade for postpartum hemorrhage at secondary level hospitals in Uganda, Egypt and Senegal: a stepped wedge, cluster-randomized trial. BJOG 2019;126:1612–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mueller GR, Pineda TJ, Xie HX, Teach JS, Barofsky AD, Schmid JR, et al. A novel sponge-based wound stasis dressing to treat lethal noncompressible hemorrhage. J Trauma Acute Care Surg 2012;73:S134–9. [DOI] [PubMed] [Google Scholar]
- 9.Stuart SM, Zarow G, Walchak A, McLean J, Roszko P. Pilot study of a novel swine model for controlling junctional hemorrhage using the iTClamp in conjunction with hemostatic agents. Mil Med 2019;184:367–73. [DOI] [PubMed] [Google Scholar]
- 10.Cox JM, Rall JM. Evaluation of XSTAT(R) and QuickClot(R) combat gauze(R) in a swine model of lethal junctional hemorrhage in coagulopathic swine. J Spec Oper Med 17:64–7. [DOI] [PubMed] [Google Scholar]
- 11.Suarez S, Conde-Agudelo A, Borovac-Pinheiro A, Suarez-Rebling D, Eckardt M, Theron G, et al. Uterine balloon tamponade for the treatment of postpartum hemorrhage: a systematic review and meta-analysis. Am J Obstet Gynecol 2020;222:293.e1–52. [DOI] [PubMed] [Google Scholar]
- 12.Rodriguez MI, Jensen JT, Gregory K, Bullard M, Longo P, Heidel J, et al. A novel tamponade agent for management of post partum hemorrhage: adaptation of the Xstat mini-sponge applicator for obstetric use. BMC Pregnancy Childbirth 2017;17:187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Warriner Z, Lam L, Matsushima K, Benjamin E, Strumwasser A, Demetriades D, et al. Initial evaluation of the efficacy and safety of in-hospital expandable hemostatic minisponge use in penetrating trauma. J Trauma Acute Care Surg 2019;86:424–30. [DOI] [PubMed] [Google Scholar]
- 14.Sims K, Montgomery HR, Dituro P, Kheirabadi BS, Butler FK. Management of external hemorrhage in tactical combat casualty care: the adjunctive use of XStat compressed hemostatic sponges: TCCC guidelines change 15-03. J Spec Oper Med 2016;16:19–28. [PubMed] [Google Scholar]