Purpose of review
This is a review of the most up-to-date research on the effectiveness of probiotic supplementation for outcomes related to athletes and physical activity. The focus is on clinical research incorporating exercise and/or physically active participants on the nutritional effectiveness of single and multistrain preparations.
Recent findings
Findings of the included clinical studies support the notion that certain probiotics could play important roles in maintaining normal physiology and energy production during exercise which may lead to performance-improvement and antifatigue effects, improve exercise-induced gastrointestinal symptoms and permeability, stimulate/modulate of the immune system, and improve the ability to digest, absorb, and metabolize macro and micronutrients important to exercise performance and recovery/health status of those physically active.
Summary
The current body of literature highlights the specificity of probiotic strain/dose and potential mechanisms of action for application in sport. These novel findings open new areas research, potential use for human health, and reinforce the potential role for probiotic's in exercise performance. While encouraging, more well designed studies of probiotic supplementation in various sport applications are warranted.
Keywords: exercise, gut microbiome, physical activity, probiotic, sport
INTRODUCTION
In humans, the effects of probiotics in relation to exercise has been less described in comparison with clinical conditions and sedentary populations, and even less so when considering athletic populations. However, the body of probiotic research in physically active individuals and competitive athletes is expanding, including investigations in gastrointestinal health, exercise performance, recovery, physical fatigue, immunity, and body composition [1▪▪]. Probiotic preparations comprise live microorganisms that, when administered in adequate amounts, confer a health benefit on the host [2]. The beneficial effect of probiotic supplementation profoundly relies on strain, dose, duration, form, and host physiology as well as the target population and the outcome of interest [3]. As such, recommendations for probiotics should consider all of these factors and benefits from specific studies should not lead to general conclusions for all probiotic products.
Probiotics are available commercially in capsule or tablet forms, as powder sachets, in the form of liquids, and in specific foods such as yogurt and nutrition bars. Commonly used probiotic strains for the application of exercise include Lactobacillus, Bifidobacterium, and Bacillus genera, however, new microbiome research and technological advances are identifying potential next-generation probiotic candidates [4]. In the context of exercise, and especially athletes, the present body of literature suggests their microbiota has several key differences in comparison with other populations, likely driven, in part, by exercise and diet [5▪]. These characteristics may influence the effects of probiotics on the resident microbiome and host physiology, as well as consideration for probiotic application.
The current article reviews the most up-to-date clinical research on probiotic supplementation in relation to exercise, published in 2019–2020 (Table 1 ). Key concepts explored include the effectiveness of probiotic supplementation on performance and exercise adaptation, gastrointestinal and immune health associated with exercise, and nutrient utilization (Fig. 1). Understanding whether probiotic supplementation plays a role in exercise performance and recovery is of interest to those who work to improve their training, competition performance, and health. Moreover, this knowledge may be of general benefit to human health and is important for clinicians, consumers, industry, and regulating bodies alike.
Table 1.
The effect of oral probiotic supplementation on health outcomes related to sport, exercise, or physical activity in healthy adults: characteristics of included studies (by publication date)
FIGURE 1.
Supplementation with certain probiotic strains has been shown to increase gastrointestinal and immune health, improve nutrient absorption, speed up recovery, and improve athletic performance (illustration by Stephen Somers, Milwaukee, Wisconsin, USA).
Table 1 (Continued).
The effect of oral probiotic supplementation on health outcomes related to sport, exercise, or physical activity in healthy adults: characteristics of included studies (by publication date)
Box 1.

no caption available
Effectiveness of probiotic supplementation on performance and exercise adaptation
Although considered for their role in gastrointestinal health, probiotics have more recently been studied for their potential ergogenic effects on exercise performance. In a 6-week double-blind placebo-controlled clinical study, young healthy amateur runners supplemented with either a low [3 × 1010 colony forming units (CFU)/day] or high (9 × 1010 CFU/day) daily dose Lactobacillus plantarum TWK10 and underwent an exhaustive treadmill exercise test [85% maximal volume of oxygen utilization (VO2MAX)] with performance measurements and related biochemical indexes [6▪]. Both probiotic groups had significant improvements in endurance performance, compared placebo, with greater effects in the high-dose group. There was also an increase in serum glucose concentrations during the maximal treadmill running test in the high-dose group, indicating that L. plantarum TWK10 supplementation may be beneficial for energy harvest during exercise.
While research examining exercise performance has been addressed in previous works, little has been done in terms of physiological stress and adaptation of exercise. To explore these features, Huang et al.[7▪▪] investigated the effects of L. plantarum PS128 supplementation (3 × 1010 CFU/day), in triathletes. L. plantarum PS128 supplementation, combined with training, significantly alleviated circulating markers of oxidative stress (including thioredoxin and myeloperoxidase indices) and positively modulated the inflammatory response (6–13% decrease: TNF-α, IL-6, and IL-8; 55% increase in IL-10). In addition, L. plantarum PS128 substantially increased plasma branched-chain amino acids (BCAA; 24–69%; P ≤ 0.05) and elevated exercise performance (i.e., 30 s Wingate test and VO2MAX endurance test; P ≤ 0.05), as compared with placebo. BCAA's have been reported to play a role in fatigue reduction in endurance exercise and are important for homeostasis of muscle energy metabolism and energy index for adaptation to exercise training [8]. Together, these studies suggest a role in which certain probiotic strains may enhance energy harvesting, and have health-promotion, performance-improvement, and antifatigue effects. Such benefits may be mediated by metabolic products from the microbiota as a result of supplementation with certain probiotic strains, although further research is warranted.
Effectiveness of probiotic supplementation on gastrointestinal health associated with exercise
The terms ‘gastrointestinal health’ or ‘gut health’ have been used increasingly in both the scientific literature and food industry. However, given the varied and far reaching functions of the gastrointestinal tract, it is difficult to discuss or study gastrointestinal health completely. Rather, probiotic strains have been used to assess their efficacy in a variety of gastrointestinal health-related outcomes such as symptoms of gastrointestinal distress or acute illness, microbiome changes, and indirect markers of barrier function. One such issue that is often faced by those who participate in endurance training is exercise-induced gastrointestinal distress. Although it is generally accepted that a reduction in splanchnic blood flow is one of the main factors precipitating exercise-induced gastrointestinal symptoms, there are many other elements that contribute to different symptoms (e.g., nausea, vomiting, bloating, and diarrhea). To evaluate the effects of probiotic supplementation on gastrointestinal symptoms, circulatory markers of gastrointestinal permeability, damage, and markers of immune response during a marathon race, 24 recreational runners were randomly assigned to either supplement with a multistrain probiotic consisting of Lactobacillus acidophilus (CUL60 and CUL21), Bifidobacterium bifidum CUL20, and Bifidobacterium animalis ssp. Lactis (totaling 25 × 109 CFU) or placebo for 28 days prior to a marathon race [9]. Although there were no differences in race finish times between probiotic and placebo groups, those in the probiotic supplement group were better able to maintain their running velocity in the final stages of the race, compared with placebo. Of note, for all participants, a significant correlation was found between reductions in running velocity during the final third of the race and the severity of subjective gastrointestinal symptoms. Supplementation of probiotics, though, resulted in fewer and less severe gastrointestinal symptoms, both in training and during a marathon race using standardized carbohydrate and hydration strategies. In contrast, probiotic supplementation had no effect on sCD14, IL-6, IL-8, IL-10, cortisol, or I-FABP concentrations or gastrointestinal permeability, which all increased postmarathon race. As such, the exact mechanism by which gastrointestinal symptoms were attenuated could not be identified, further highlighting the multifaceted nature of such distress. However, while gastrointestinal permeability and barrier function are not always indicated with functional exercise-associated gastrointestinal symptoms, this does not preclude the potential importance of limiting gastrointestinal barrier disruption during exercise and the therapeutic role probiotics may play.
To assess the efficacy of Lactobacillus salivarius UCC118 (2 × 108 CFU/day) on exercise-induced gastrointestinal permeability and the gut microbiome in healthy adults, Axelrod et al.[10▪▪], performed a randomized, double-blind, placebo-controlled crossover study on seven healthy, endurance trained athletes with 4-week treatment periods. Athletes undertook strenuous treadmill running performed before and after each supplementation period and urine recovery of lactulose, rhamnose, and sucrose was used to assess gastrointestinal permeability. L. salivarius UCC118 significantly reduced sucrose (Δ = 38 ± 13% vs. 169 ± 79%; P < 0.05) recovery, with no substantial change in lactulose or rhamnose. Shotgun metagenomic sequencing of fecal samples revealed that L. salivarius UCC118 supplementation appeared to remodel the gut microbiome with 99 differentially regulated microorganisms, including a significant reduction in the phylum Verrucomicrobia. This apparent remodeling of the gut microbiome, not often observed in clinical probiotic studies [11], may be suggestive of protective effects of L. salivarius UCC118 by orchestrating changes in the gut's resident microorganisms.
Effectiveness of probiotic supplementation on immune health associated with exercise
Of particular relevance to those that exercise (particularly athletes) is the reduction in incidence and/or severity and duration of symptoms from illnesses like upper respiratory tract infections (URTIs). Certain probiotics may offer a proactive approach for preventing these types of illnesses which can minimize training days lost, and in turn enhance exercise performance. This is especially true for those that travel, who may experience higher levels of stress, increased risk of transmission of illness, and circadian disfunction. In a 27-week, double blind randomized controlled trial, a small group of rugby athletes were assigned a high-dose multistrain probiotic (120 × 109 CFU) or placebo group during control, domestic competition, and international competition periods [12▪]. During the final period, there was a significant increase in salivary alpha-amylase (marker of mucosal immune response) in those consuming the probiotic [13]. The mechanism on how the probiotic may have stimulated this increase was not explored in the current study, however, orally administered probiotics may interact with the approximately 200 m2 of gastrointestinal mucosa and gut associated lymphoid tissue where more than 70% of immune cells are localized [14]. It is therefore not surprising that many of the recognized health benefits of probiotics are conferred mainly through stimulation/modulation of the immune system [15].
The mucosal lining of the gastrointestinal tract represents the first line-of-defense against invading pathogens and is an important interface with the host immune system. Exhaustive physical exercise negatively impacts immunity, reducing of the count and function of immune cells, as well as altering the inflammation-responsive, including pro/anti-inflammatory cytokines. Assessing these immune and inflammatory responses, Vaisberg and colleagues had 42 male marathon runners with previous history of postrace URTI ingest a fermented milk containing Lactobacillus casei Shirota (4 × 1010 CFU/day) or placebo 30 days prior to a marathon [16▪]. Supplementation resulted in improved systemic and airways immune responses, including reduced neutrophil infiltration in the nasal mucosa and modulation of pro and anti-inflammatory cytokine response in the upper airway (i.e., decreased IL-6, IL-13, TNF-α, IL-5, & IL-1β; increased IL-10, as compared with placebo). These results demonstrated that the daily intake of the probiotic was able to induce an anti-inflammatory response that can mitigate the deleterious effects of marathon on the mucosal inflammation.
Although the current body of literature is weighted in favor of probiotics ability to reduce the incidence of URTIs and related symptoms, there is a large number of differing strains used with likely narrowly distributed mechanisms [1▪▪]. Moreover, the immune response is complex and not easily measured, exemplified by the large array of measurable immune cells, cytokines, and chemokines. The two studies reviewed above have taken important and necessary steps to investigate both URTI incidence and symptomology, as well as multiple markers of the immune response. Future work should continue this trend to better understand how different probiotic strains may affect immunity.
Effectiveness of probiotic supplementation on nutrient absorption and utilization associated with exercise
More recent work has aimed to assess various probiotic strains for their ability to digest, absorb, and metabolize nutrients important to exercise performance and recovery/health status of those physically active. For example, maintaining adequate carbohydrate availability for skeletal muscle and the central nervous system during longer duration exercise is well noted to improve performance and delay fatigue [17]. However, absorption of ingested large amounts of carbohydrate during exercise is limited by transport systems within the gut. To test whether a probiotic could increase the absorption and oxidation of maltodextrin, Pugh and colleagues had a small group of trained male cyclists perform a 2-h cycling challenge before and after 4 weeks of supplementation with a multistrain probiotic (25 × 109 CFU) or placebo using a double blind, randomized controlled, crossover design [18]. Probiotic supplementation led to a small but significant increase in total carbohydrate oxidation in the 60–120-min exercise periods and the oxidation of the consumed maltodextrin drink, as well as significant increases in the plasma glucose and insulin concentration. However, there were minimal increases in the absorption of the ingested maltodextrin and no effect on subsequent time-trial performance. Although this initial data highlights the potential of probiotics to increase absorption and oxidation of consumed carbohydrates and subsequent exercise metabolism, further studies are needed to replicate these findings.
Probiotics have also been linked to improved protein utilization [19], potentially through optimizing gut microbiota composition and increased proteolytic activity. Different types and quality of dietary protein can affect amino acid absorption following protein supplementation. Compared with animal protein sources, plant protein sources are generally incomplete proteins, contain less BCAAs, and differ in the absorption kinetics and the amount of amino acids absorbed by the host, factors known to affect adaption effects important to athletes such as skeletal muscle protein synthesis. Therefore, there is an interest in nutritional strategies to raise the blood amino acid concentrations after ingesting a plant protein source to overcome compositional shortcomings. To assess the amino acid concentration in the blood after the administration of a plant protein with or without coadministration of a probiotic supplement (5 × 109 CFU Lactobacillus paracasei LP-DG and 5 × 109L. paracasei LPC-S01) a randomized, double-blind, crossover pilot study was performed in physically active males [20▪▪]. Probiotic administration significantly increased methionine (+16.3%), histidine (+49.2%), valine (+24.7%), leucine (+25.2%), isoleucine (+26.1%), tyrosine (+11.6%), total BCAA (+26.8%), and total essential amino acid (+15.6%) maximum concentrations and area under the curve (Fig. 2). To corroborate these findings, in-vitro data showed increased proteolysis and a synergistic effect of the two strains compared with either strain alone for pea protein. The increased amino acids in this study are of key importance to athletes and exercise recovery. This research is also notable because this is some of the first evidence to show that probiotics helps to overcome the compositional shortcomings of plant proteins and could help to elevate BCAA levels in the blood to comparable levels of animal proteins. This would remove the need to increase the dose of plant proteins to achieve similar benefits of protein supplementation on muscle health.
FIGURE 2.
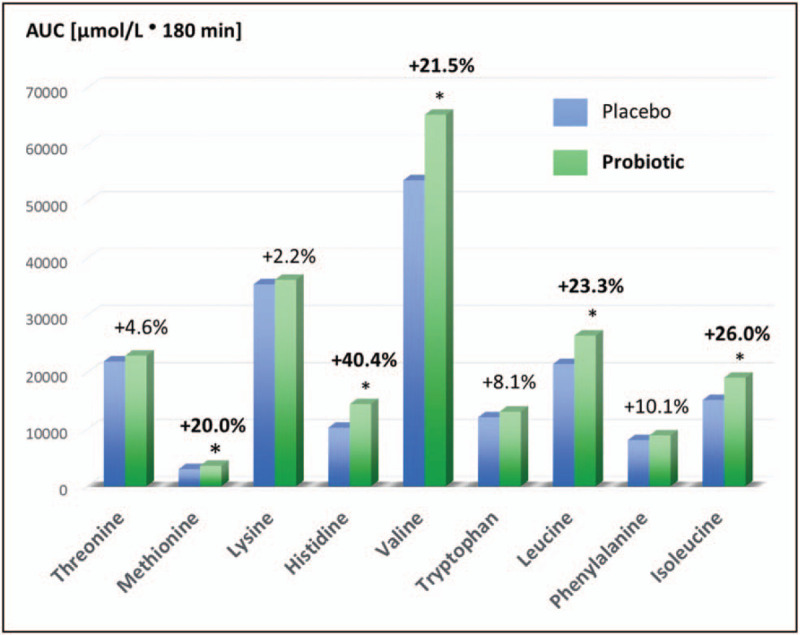
Coadministration of pea protein with Lactobacillus paracasei LP-DG and L. paracasei LPC-S01 significantly improves postprandial changes in blood amino acids: total essential amino acids, total branched-chain amino acids, methionine, histidine, valine, leucine, and isoleucine (∗P < 0.05).
A further area of recent interest in regards to probiotics and nutrient absorption is within the field of inorganic iron supplementation. Iron is crucial for oxygen transport, mitochondrial energy production, and cellular immune responses and has been shown to negatively affect physical performance and adaptation to training when low [21]. Increasing the absorption of iron could be a strategy for improving iron status and avoid the use of traditional high-dose iron supplements and thereby adverse side effects. In a recent double blind, 12 week randomized control trial in nonanemic female athletes with low-iron stores receiving a daily supplement of 20 mg of ferrous fumarate, L. plantarum 299v increased plasma ferritin (iron status), in those with ferritin levels above 20 μg/l at baseline compared with control [22▪]. Significantly, those with ferritin levels below 20 μg/l did not differ from each other in terms of the ferritin response (increase). The results indicated that probiotic intake with 20 mg iron (as ferrous fumarate) could result in a more substantial and rapid improvement in iron status compared with iron alone. Further, the beneficial effects of the probiotic on iron utilization (e.g., serum ferritin) from an inorganic source of iron without any change in inflammatory markers are notable. In a recent meta-analysis this strain has been identified as moderately effective (effect size = 0.55, 95% confidence interval 0.22–0.88, P = 0.001) in iron absorption in humans using a variety of methods [e.g., stable iron kinetics, 59Fe whole-body retention and isotope activities in blood samples, double-isotope (55Fe and 59Fe)] [23].
Currently, research investing the potential of probiotics to aid nutrient metabolism in relation to exercise remains limited. However, the recent positive findings noted above are promising and investigators are encouraged to continue this line of research for both macro and micronutrients. It may be that the exercise associated gut microbiota may be more receptive to improved nutrient utilization as it appears to possess a functional capacity that is primed for tissue repair and a greater ability to harness energy from the diet with increased capacity for carbohydrate, cell structure, and nucleotide biosynthesis [5▪].
CONCLUSION
Although there has been continual research on probiotic supplementation in the application of sport and exercise, many questions remain concerning mechanisms of action and strain/dose specificity. Results from the included studies in this review are encouraging and open up potential new lines of inquiry.
Acknowledgements
None.
Financial support and sponsorship
None.
Conflicts of interest
There are no conflicts of interest.
REFERENCES AND RECOMMENDED READING
Papers of particular interest, published within the annual period of review, have been highlighted as:
▪ of special interest
▪▪ of outstanding interest
REFERENCES
- 1▪▪.Jäger R, Mohr AE, Carpenter KC, et al. International Society of Sports Nutrition position stand: probiotics. J Int Soc Sports Nutr 2019; 16:62. [DOI] [PMC free article] [PubMed] [Google Scholar]; A comprehensive review of potential benefits of probiotic supplementation in athletes, naming the clinically validated strains, and doses.
- 2.Hill C, Guarner F, Reid G, et al. Expert consensus document: the international scientific association for probiotics and prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat Rev Gastroenterol Hepatol 2014; 11:506–514. [DOI] [PubMed] [Google Scholar]
- 3.McFarland LV, Evans CT, Goldstein EJ. Strain-specificity and disease-specificity of probiotic efficacy: a systematic review and meta-analysis. Front Med (Lausanne) 2018; 5:124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Scheiman J, Luber JM, Chavkin TA, et al. Meta-omics analysis of elite athletes identifies a performance-enhancing microbe that functions via lactate metabolism. Nat Med 2019; 25:1104–1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5▪.Mohr AE, Jäger R, Carpenter KC, et al. The athletic gut microbiota. J Int Soc Sports Nutr 2019; 17:24. [DOI] [PMC free article] [PubMed] [Google Scholar]; The review describes the differences in gut microbiota of athletes in comparison with sedentary people, and delineates the exercise and diet influences diversity and the kind of bacteria in an athlete's gut.
- 6▪.Huang WC, Lee MC, Lee CC, et al. Effect of Lactobacillus plantarum TWK10 on exercise of physiological adaptation, performance, and body composition in healthy humans. Nutrients 2019; 11:2836. [DOI] [PMC free article] [PubMed] [Google Scholar]; An important report of the potential of Lactobacillus plantarum TWK10 as a promoter of energy harvesting in the gut microbiota, maintaining normal physiology, and energy production during exercise.
- 7▪▪.Huang WC, Wei CC, Huang CC, et al. The beneficial effects of Lactobacillus plantarum PS128 on high-intensity, exercise-induced oxidative stress, inflammation, and performance in triathletes. Nutrients 2019; 11:353. [DOI] [PMC free article] [PubMed] [Google Scholar]; A primary report of the benefit of probiotic treatment in relation to allieviting physiological stress and promoting adaptation of exercise, mediated, in part, by metabolic products from the gut microbiota.
- 8.Xu M, Kitaura Y, Ishikawa T, et al. Endurance performance and energy metabolism during exercise in mice with a muscle-specific defect in the control of branched-chain amino acid catabolism. PLoS One 2017; 12:e0180989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pugh JN, Sparks AS, Doran DA, et al. Four weeks of probiotic supplementation reduces GI symptoms during a marathon race. Eur J Appl Physiol 2019; 119:1491–1501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10▪▪.Axelrod CL, Brennan CJ, Cresci G, et al. UCC118 supplementation reduces exercise-induced gastrointestinal permeability and remodels the gut microbiome in healthy humans. Physiol Rep 2019; 7:e14276. [DOI] [PMC free article] [PubMed] [Google Scholar]; A unique study reporting the ability of a probiotic, Lactobacillus salivarius UCC118, to orchestrate changes within the gut's resident microorganisms, which may confer benefits to the integrity of the gastrointestinal tract.
- 11.Kristensen NB, Bryrup T, Allin KH, et al. Alterations in fecal microbiota composition by probiotic supplementation in healthy adults: a systematic review of randomized controlled trials. Genome Med 2016; 8:52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12▪.Pumpa KL, McKune AJ, Harnett J. A novel role of probiotics in improving host defence of elite rugby union athlete: a double blind randomised controlled trial. J Sci Med Sport 2019; 22:876–881. [DOI] [PubMed] [Google Scholar]; In this novel and longer duration study, Pumpa et al. assess the ability of a high-dose, multistrain probiotic to support immunity during a control period and then domestic and international travel for competition.
- 13.Fábián TK, Hermann P, Beck A, et al. Salivary defense proteins: their network and role in innate and acquired oral immunity. Int J Mol Sci 2012; 13:4295–4320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lebeer S, Vanderleyden J, De Keersmaecker SCJ. Host interactions of probiotic bacterial surface molecules: comparison with commensals and pathogens. Nature Rev Microbiol 2010; 8:171–184. [DOI] [PubMed] [Google Scholar]
- 15.Mohr AE, Basile AJ, Crawford MS, et al. Probiotic supplementation has a limited effect on circulating immune and inflammatory markers in healthy adults: a systematic review of randomized controlled trials. J Acad Nutr Diet 2020; 120:548–564. [DOI] [PubMed] [Google Scholar]
- 16▪.Vaisberg M, Paixão V, Almeida EB, et al. Daily intake of fermented milk containing Lactobacillus casei shirota (Lcs) modulates systemic and upper airways immune/inflammatory responses in marathon runners. Nutrients 2019; 11:1678. [DOI] [PMC free article] [PubMed] [Google Scholar]; A high-dose probiotic (Lactobacillus casei Shirota) prior to a marathon can atteunate muscal immune and inflammation response immediately after the event.
- 17.Hawley JA, Burke LM. Carbohydrate availability and training adaptation: effects on cell metabolism. Exerc Sport Sci Rev 2010; 38:152–160. [DOI] [PubMed] [Google Scholar]
- 18.Pugh JN, Wagenmakers AJM, Doran DA, et al. Probiotic supplementation increases carbohydrate metabolism in trained male cyclists: a randomized, double-blind, placebo-controlled crossover trial. Am J Physiol Endocrinol Metab 2020; 318:E504–E513. [DOI] [PubMed] [Google Scholar]
- 19.Jäger R, Shields KA, Lowery RP, et al. Probiotic Bacillus coagulans GBI-30, 6086 reduces exercise-induced muscle damage and increases recovery. PeerJ 2016; 2016:e2276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20▪▪.Jäger R, Zaragoza J, Purpura M, et al. Probiotic administration increases amino acid absorption from plant protein: a placebo-controlled, randomized, double-blind, multicenter, crossover study. Probiotics Antimicrob Proteins 2020; doi: 10.1007/s12602-020-09656-5. [DOI] [PMC free article] [PubMed] [Google Scholar]; First evidence of probiotic's use in conjunction with a plant protein to overcome it's compositional shortcomings and elevate BCAA levels in the blood to comparable levels of animal proteins. These findings have important relevance for the use of plant protein supplementation on muscle recovery from exercise.
- 21.Sim M, Garvican-Lewis LA, Cox GR, et al. Iron considerations for the athlete: a narrative review. Eur J Appl Physiol 2019; 119:1463–1478. [DOI] [PubMed] [Google Scholar]
- 22▪.Axling U, Önning G, Combs MA, et al. The effect of Lactobacillus plantarum 299v on iron status and physical performance in female iron-deficient athletes: a randomized controlled trial. Nutrients 2020; 12:E1279. [DOI] [PMC free article] [PubMed] [Google Scholar]; Important for endurance and female athletes, Axling et al. report the ability of a probiotic to increase iron absorption while potentially avoiding the use of traditional high-dose iron supplements and thereby adverse side effects.
- 23.Vonderheid SC, Tussing-Humphreys L, Park C, et al. A systematic review and meta-analysis on the effects of probiotic species on iron absorption and iron status. Nutrients 2019; 11:2938. [DOI] [PMC free article] [PubMed] [Google Scholar]


