Robot-assisted vitreoretinal surgery increases precision and limits tissue damage compared with manual surgery especially for the novice surgeon. The Eyesi Simulator is a feasible platform for investigating robot-assisted vitreoretinal surgery.
Key words: eyesi, randomized, robot-assisted, robotics, simulation, surgery, virtual-reality, vitreoretinal
Abstract
Purpose:
To compare manual and robot-assisted vitreoretinal surgery using a virtual-reality surgical simulator.
Methods:
Randomized controlled crossover study. Ten experienced vitreoretinal surgeons and 10 novice ophthalmic surgeons were included. The participants were randomized to start with either manual or robot-assisted surgery. Participants completed a test session consisting of three vitreoretinal modules on the Eyesi virtual-reality simulator. The automated metrics of performance supplied by the Eyesi simulator were used as outcome measures. Primary outcome measures were time with instruments inserted (seconds), instrument movement (mm), and tissue treatment (mm2).
Results:
Robot-assisted surgery was slower than manual surgery for both novices and vitreoretinal surgeons, 0.24 SD units (P = 0.024) and 0.73 SD units (P < 0.001), respectively. Robot-assisted surgery allowed for greater precision in novices and vitreoretinal surgeons, −0.96 SD units (P < 0.001) and −0.47 SD units (P < 0.001), respectively. Finally, novices using robot-assisted surgery inflicted less tissue damage when compared with that using manual surgery, −0.59 SD units (P = 0.009).
Conclusion:
At the cost of time, robot-assisted vitreoretinal surgery seems to improve precision and limit tissue damage compared with that of manual surgery. In particular, the performance of novice surgeons is enhanced with robot-assisted vitreoretinal surgery.
Vitreoretinal surgery is one of the most demanding surgical fields within ophthalmology, because it requires great surgical precision and dexterity. The surgeons face tasks that would benefit from reduced tremor, higher precision, and better stability unachievable by the human hand.1,2 Thus, there is a need for technological advances that enhance human precision and dexterity.3
Surgical robotics has long held promise of delivering these technological advances, and in other surgical fields such as general surgery, robotic systems such as the da Vinci Surgical System, have achieved success enabling instrument movements not possible during conventional manual surgery.4 Several studies have investigated adaptation of the da Vinci Surgical System to perform ophthalmic surgery, and found that in simulated environments, it can perform corneal suturing, cataract, and pterygium surgery.5–7 However, attempts at using the system in standard vitreoretinal surgery are so far unsuccessful.8 Therefore, attention has shifted to developing a robotic surgical system specifically designed for vitreoretinal surgery.
In vitreoretinal surgery, there has been an increase in the number of described robotic systems.9 Experimental robotic surgical systems designed to perform intraocular surgery use one of several strategies. The main approaches use tele-operation (remote controlling the robot, necessitating the use of a computer interface) or co-manipulation. One of these systems is the Preceyes Surgical System (Preceyes BV, Eindhoven, the Netherlands), with a remote center of motion, designed to enhance surgical precision during vitreoretinal surgery. Its precision and stability allow for prolonged injections in the retinal and subretinal space with micrometer positioning precision, including cannulation of retinal veins and subretinal injections, although it is anticipated that it will also provide benefits for conventional surgical tasks.10–12 A recent study indicates that the system is feasible to use in human subjects, both using general and local anesthesia.12 However, a comparison of the Preceyes Surgical System to conventional surgical methods requires a more rigorous approach where objective quantifiable data are generated and evaluated.
Surgical performance using robotic systems claims to improve the dexterity and surgical outcomes in novice surgeons, but such claims are often based on purely subjective assessment. A safe, standardized test environment that supplies quantifiable data on surgical performance is required. Such an environment can be provided by the Eyesi simulator.
The Eyesi simulator (VRmagic, Mannheim, Germany) is a virtual-reality surgical simulator for both cataract and vitreoretinal surgery. The Eyesi simulator is widely used and thoroughly investigated for training and assessment purposes within ophthalmic surgery.13 Its metrics are shown to be sufficiently robust to allow for the evaluation of surgical performance in vitreoretinal surgery.14 However, it has not previously been used to investigate the possible benefits of robot-assisted vitreoretinal surgery.
In this study, we aim to investigate the use of robot-assisted vitreoretinal surgery compared with that of manual surgery for both novice and experienced vitreoretinal surgeons in a virtual-reality surgical simulator environment.
Methods
Study Design
This study was designed as a randomized controlled, crossover study (allocation ratio 1:1, balanced randomization). The study was conducted at the Copenhagen Academy for Medical Education and Simulation (CAMES) at Rigshospitalet.15 The ethics committee of the Capital Region of Denmark ruled that approval was not required for this study (protocol number H-18015799). The study adheres to the tenets of the Declaration of Helsinki and is reported in accordance with the Consolidated Standards of Reporting Trials (CONSORT) statement for randomized trials using the simulation-based research extensions.16
Participants
We aimed to include 20 participants in the study based on previous sample size calculation.14 Participants consisted of experienced vitreoretinal surgeons (>200 vitreoretinal surgeries performed) and novices. All vitreoretinal surgeons from the Eastern and Northern part of Denmark were invited to participate in the study. Year 3 and 4 ophthalmic residents with no previous intraocular surgical experience were invited to participate in the study as novices. Surgeons and novices who had trained more than 2 hours on the Eyesi simulator in the past 6 months were not eligible to participate in the study. All participants gave written and oral consent before being included in the study.
Intervention
Participants started with either robot-assisted or manual surgery according to their assigned randomization. All participants underwent a warm-up session consisting of 10 minutes of basic warm up and one familiarization session consisting of one repetition of the test procedure on both robot-assisted and manual surgery before starting data collection. The warm-up period was included to allow for acquaintance with the equipment and procedure. An instructor explained the performance and procedural goals of each test. The data collection consisted of two repetitions of the test. After completing the data collection on the first surgical modality, they shifted to the other surgical modality. Figure 1 shows an overview of the study design. Finally, participants also filled out a questionnaire regarding their stereopsis (measured by TNO test), dexterity, and past surgical experience. This was done to document and secure group comparability.
Fig. 1.
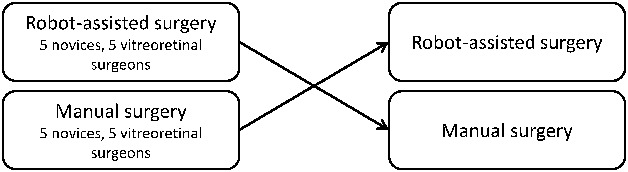
Overview of the crossover study design.
The robot-assisted modality consisted of the Eyesi simulator's vitreoretinal interface (hardware version 2.8; software version 3.2.27 PE VRmagic, Mannheim, Germany) modified for use with a Preceyes Surgical System (hardware version R0-S, software version BL REL R0s 001; Preceyes BV, Eindhoven, the Netherlands) mounted to the side of the table. Figure 2 illustrates the study setup for the robot-assisted surgery. The robot consists of a motion controller that the surgeon holds and an instrument arm that can be supplied with a wide variety of surgical instruments.11 The surgical system enables dynamic motion scaling, tremor filtering, virtual z-boundary, and a freeze position for the instrument inside the eye. The system uses an optical coherence tomography-based distance sensor, integrated inside surgical instruments, to detect the distance between the instrument tip and the retina. This measured distance enables a parking-sensor-like auditory feedback, and a z-boundary function. The z-boundary is a virtual limit to the z-axis that prevents instrument movements past a predefined distance from the retina. Because this optical coherence tomography measurement is not possible in the Eyesi phantom eye, this virtual distance was calculated by the Eyesi, and communicated to the Preceyes Robotic System. The z-boundary distance was set to −10 µm to −20 µm. A negative distance means that the boundary is placed inside the retina, e.g., to allow small indentations required to initiate the surgical tasks. The Preceyes Surgical system and the Eyesi simulator do not use a force control or force feedback functionality. The use of force feedback when designing robot and simulator systems is an ongoing debate.17
Fig. 2.
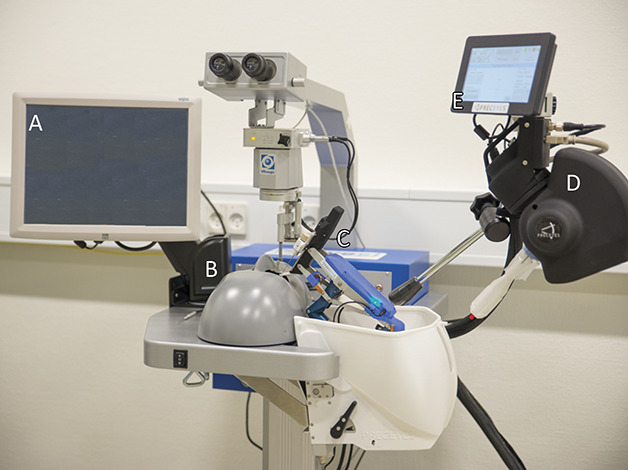
The robot assisted surgery setup. A. EyeSi simulator controls display. B. EyeSi simulator phantom head. C. Robot instrument manipulator. D. Robot motion controller. E. Robot controls display.
To operate the surgical forceps, a foot pedal was used. Depressing the foot pedal resulted in closing of the forceps, whereas releasing the foot pedal resulted in the opening of the forceps.
The manual surgery setup did not differ from the standardized Eyesi simulator.
Outcomes
The automated metrics provided by the vitreoretinal interface of the Eyesi simulator were used as outcome measures to test surgical performance. Table 1 shows an overview of the Eyesi simulator settings used in these experiments.
Table 1.
Overview of Included Eyesi Simulator Modules
| Module No. | Module Name | Type | Task Difficulty Level* | Score |
| 1 | Bimanual | Abstract task | Level 5 out of 5 | 0–100 |
| 2 | ILM peeling | Procedural task | Level 5 out of 6 | 0–100 |
| 3 | ERM peeling | Procedural task | Level 4 out of 6 | 0–100 |
Level 1 being easiest and difficulty increasing with higher levels.
The automated metrics on the EyeSi Simulator are categorized in five different domains, these domains are: efficiency, target achievement, tissue treatment, instrument handling, and microscope handling. Together, these five domains make up the total score between 0 and 100 points on each of the Eyesi simulator modules. From these five domains, three primary outcomes were selected for this study: time taken following instrument insertion to task completion (seconds), instrument movement (distance travelled by the instrument tip measured by an odometer), and tissue treatment measured as injured macular area (mm2). The primary outcomes were chosen because of their relevance to robot-assisted surgery. The remaining domains, such as target achievement and microscope handling, are not directly affected by the nature of the surgical modality and therefore were not investigated in the current study.
As part of this study, we analyzed selected simulator modules for evidence of validity using the manual surgery setup. In particular, we analyzed instrument handling, tissue treatment, and time required to complete tasks by novice and experienced vitreoretinal surgeons.
Two of the authors completed the data collection (M.F.J. and A.S.S.T.). Because of the nature of the study design, it was not possible to mask data collectors/investigators or participants to the intervention.
Randomization and Allocation Sequencing
A computer-generated list of random numbers was used to randomize participant assignment following a balanced permutation (computer-generated random numbers) to the two surgical modalities (manual and robot-assisted surgery). Vitreoretinal surgeons and novices were randomized in each group one at a time. This was done to minimize bias that could result from enabling prediction of the order in which participants were exposed to the different surgical modalities. Two authors performed randomization and allocation (M.F.J. and A.S.S.T.).
Statistics
Our three primary outcome measures, time taken with instruments inserted to complete task (seconds), instrument movement (distance travelled by the instrument tip measured by an odometer), and tissue treatment measured as injured macula area (mm2), were standardized by converting them to z-scores. This was done to allow comparison of robot-assisted surgery and manual surgery between the two groups of participants across the three primary outcomes. Consequentially, a z-score of −1 was equivalent to one SD less than the mean. This translated to less time, fewer instrument movements, and less tissue damage than the mean. Cross-classified mixed-effects regression models were used to examine differences between groups taking into account the outcome measures, module type, and the nested effects of the participants.18 Model fit and regression coefficients were used to examine the effect of the intervention.19 Effect sizes below 0.2 were considered small, 0.50 medium, and 0.80 large. Data compilation and analyses were conducted using Stata 14 (College Station, TX).
Results
From the 4th of May 2018 to the 18th of July 2018, 10 experienced vitreoretinal surgeons and 10 novices were included in the study. All 20 participants completed the study setup and were included in the data analysis. Table 2 shows mean values for descriptive data on study participants.
Table 2.
Descriptive Data on Study Participants (Mean Values)
| Age (Range) | Right Handed | Stereopsis TNO Test (Range) | Vitrectomies Performed | Retinal Detachment Surgeries Performed | Macular Surgeries Performed | |
| Novices | 36 years (31–42) | 60% | 60 seconds arc (60–60) | 0 | 0 | 0 |
| Vitreoretinal surgeons | 49 years (36–66) | 60% | 60 seconds arc (60–60) | 2,339 (100–8,000) | 995 (50–5000) | 733 (30–2000) |
The three included modules had discriminative ability; the novices mean score was lower than the vitreoretinal surgeons concerning the performance measured by instrument handling, tissue treatment, and time on the manual surgery modality, corresponding to 0.39 SD units (P = 0.010).
Overall, we found a significant improvement when using robot-assisted surgery, −0.18 SD units (P = 0.003), when combining all three primary outcomes and analyzing all performances. When considering each of the primary outcomes individually, we found that the robot-assisted surgery required more time, 0.49 SD units (P < 0.001), required less movement of instruments, −0.71 SD units (P < 0.001), and resulted in less tissue damage, −0.32 SD units (P = 0.007). Table 3 shows robot-assisted surgery compared with manual, overall and for each primary outcome for all participants as one group.
Table 3.
Impact of Robot-Assisted Surgery Compared With That of Manual Surgery, Overall and for Each Primary Outcome for all Participants
| Score | Standardized Effect Size (Coef.) | SE | P | 95% Confidence Interval | ||
| Novices and vitreoretinal surgeons | Overall | −0.18 | 0.06 | 0.003 | −0.299 | −0.063 |
| Time | 0.49 | 0.08 | <0.001 | 0.335 | 0.638 | |
| Movement | −0.71 | 0.09 | <0.001 | −0.896 | −0.532 | |
| Tissue treatment | −0.32 | 0.12 | 0.007 | −0.545 | −0.085 | |
Both novices and especially vitreoretinal surgeons were slower when using the robot, 0.24 (P = 0.024) and 0.73 (P < 0.001) SD units, respectively.
Furthermore, we found less movement of instruments when performing tasks robotically, −0.96 SD units (P < 0.001), and −0.47 SD units (P < 0.001) for novices and vitreoretinal surgeons, respectively. Finally, novices caused statistically significant less tissue damage when working robotically, −0.588 SD units (P = 0.009). Whereas, robot-assisted surgery did not have a statistically significant impact on the vitreoretinal surgeons' tissue treatment. Table 4 illustrates the robot-assisted surgery compared with manual surgery for novices and vitreoretinal surgeons separately.
Table 4.
Impact of Robot-Assisted Surgery Compared With That of Manual for Each Primary Outcome for the Two Groups of Participants
| Score | Standardized Effect Size (Coef.) | SE | P | 95% Confidence Interval | ||
| Novices (n = 10) | Time | 0.24 | 0.11 | 0.024 | 0.032 | 0.456 |
| Movement | −0.96 | 0.17 | <0.001 | −1.294 | −0.628 | |
| Tissue treatment | −0.59 | 0.23 | 0.009 | −1.032 | −0.144 | |
| Vitreoretinal surgeons (n = 10) | Time | 0.73 | 0.10 | <0.001 | 0.525 | 0.932 |
| Movement | −0.47 | 0.06 | <0.001 | −0.595 | −0.340 | |
| Tissue treatment | −0.042 | 0.04 | 0.333 | −0.126 | 0.043 | |
Bold entries mark statistical significance on a 0.05 level.
The participants also completed a questionnaire regarding their impression of robot-assisted surgery compared with manual surgery. Figure 3 shows the participants' impression of the ease of use of robot-assisted surgery compared with that of manual surgery. Figure 4 shows the participants' impression of the safety of robot-assisted surgery compared with that of manual surgery. From these questionnaires, it is apparent that overall the novices have a more positive subjective impression of robot-assisted surgery than the vitreoretinal surgeons concerning ease of use and safety.
Fig. 3.
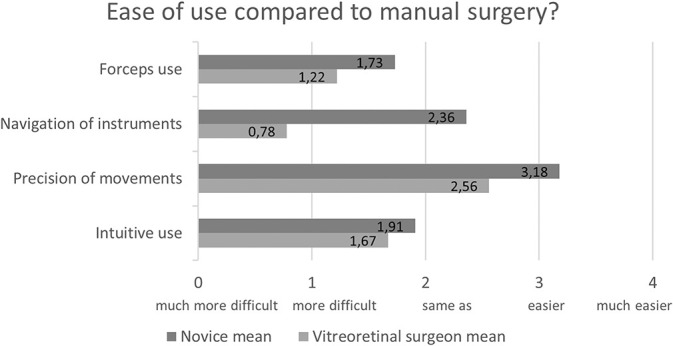
Participant's impression of the ease of use of robot-assisted compared with that of manual surgery.
Fig. 4.
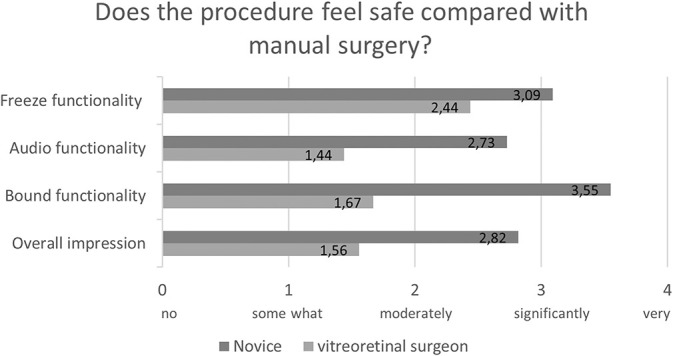
Participant's impression of the safety of robot-assisted compared with that of manual surgery.
Discussion
Our results show that robot-assisted surgery is significantly slower compared with manual surgery for both novices and vitreoretinal surgeons. However, compared with manual surgery, robot-assisted surgery improves precision (i.e., reduces the instrument movements) and it improves tissue treatment of novices, but not vitreoretinal surgeons.
Increased time to complete tasks for robot-assisted surgery compared with manual surgery is similar to a previous Preceyes Surgical System study.13 Particularly, trained vitreoretinal surgeons lost speed when performing robot-assisted surgery. They were not slower than the novices while using the robot, but were significantly faster during manual surgery. Such findings are not surprising, because it is commonly reported during surgical procedures carried out in other surgical disciplines.20,21 The increased surgical time using robot-assisted surgery is attributable to many factors. Precision tasks may likely just require more time. However, the compartmentalized movement pattern when using the robotic motion controller is also a contributing factor, because intraocular movements with the instrument tip cannot be performed in one fluent process. To move the instrument tip across the retina, the surgeon needs to disengage the clutch and readjust the position of the motion controller repeatedly to continue the movement. The regrasping and readjustment continues throughout the surgery and could add to the increased surgical time. For the vitreoretinal surgeons, the time to complete tasks decreased with repetitions, and they were significantly faster on the second repetition of the test session. This is most likely due to a familiarization effect that gradually increases the surgical speed as surgeons become more experienced in the use of the robot. Studies of learning curves are necessary to explore whether repeated practice could make robot-assisted surgery almost as fast as manual-surgery, and finding means of rendering the grasp re-grasp clutching more efficient.
It is noteworthy that instrument movements of both novice and experienced vitreoretinal surgeons can be reduced significantly with robot-assisted surgery. This finding indicates that the robot-assisted surgery is more precise than manual surgery for both novice and vitreoretinal surgeons. This is further supported by previous study findings for experienced vitreoretinal surgeons using the Preceyes robotic-system.12 Furthermore, it is in accordance with previous findings within robot-assisted general surgery.21 The increased surgical precision for both groups of participants could be attributable to core functionalities of the robotic-system. First, the tremor filtering reduces physiological tremor from the order of 100 µm to 10 µm.20,22,23 However, we defined tremor in only three degrees of freedom (XYZ). This enables a degree of precision not achievable by the human hand.24 Furthermore, the dynamic motion scaling feature of the robotic-system changes the motion scaling ratio in relation to the instrument tip's distance to the retina. Lower motion scaling ratios (1:5) are used in the center of the eye and higher motion scaling ratios (1:25) are used when the instrument tip is near the retinal surface. This further enables a higher degree of precision compared with manual surgery, because it increases the economy of movement of the surgical instruments. Finally, the freeze functionality, freezes the position of the instrument when the clutch on the motion controller is disengaged, allowing the surgeon to re-adjust the grip and the position of the motion controller without intraocular movement of the instrument. This greatly reduces the amount of redundant instrument movements and in turn increases precision. However, it is important to acknowledge that the effect of robot-assisted surgery on precision is greatest for novices and less pronounced for experienced vitreoretinal surgeons. Years of experience allow expert surgeons to make very precise movements and reduce the effect of robotic assistance.2
Novices in our study had significantly less tissue damage when using robot-assisted surgery compared with manual. Previously, the possible benefit of robot-assisted surgery for novices has remained largely undocumented, because trials including unsupervised novice performance on patients are not ethically feasible. The reduction in tissue damage primarily occurred in the Eyesi epiretinal membrane module, where novices had the most tissue damage. Meanwhile, vitreoretinal surgeons did not have a significant change in tissue treatment when using the robot-assisted surgery, which is because vitreoretinal surgeons did not have significant tissue damage when operating manually. This is similar to the findings of Edwards et al12 showing that robot-assisted surgery using the Preceyes Surgical System did not have a statistically significant impact on iatrogenic retinal micro-trauma. The functionalities of the robotic-system mentioned previously, including the integrated z-boundary function, plays a significant part in limiting tissue damage. The operator can impose and adjust the distance of this virtual boundary in relation to the retina throughout the operation, and hereby greatly reduce the risk of iatrogenic retinal micro-trauma.
Virtual reality simulators supply a standardized environment without clinical variation and generate quantifiable data that can be used to study surgical performance in different contexts.25,26 However, the simulated setup poses some limitations for the clinical application of our study findings. First, the phantom eye of the Eyesi simulator is not capable of spontaneously moving during simulated surgery. It remains completely static except for the movements induced by the manipulation of the surgeon's instruments. As such, it mimics a patient relaxed under general anesthesia, which is not always the case for patients undergoing vitreoretinal surgery. It would increase the generalizability of the study findings, if the Eyesi simulator was able to mimic the eye movements of a patient under local anesthesia. However, this is currently not possible. Furthermore, the surgeons included in the study all used foot pedal controlled surgical forceps. This is different from standard practice, because most vitreoretinal surgeons worldwide use hand actuated forceps. The motion of actuating the surgical forceps via foot action requires ankle, knee, and hip rotation and may have an effect on hand and tool motion.
There is some evidence that skills demonstrated on the Eyesi Simulator are correlated to real-life surgical performance.26 However, we cannot rule out that robot-assisted surgery may be applied differently in a simulated setting compared with real-life, which is a limitation to the study.
Finally, it is important to underline the fact that the procedures performed on the Eyesi simulator modules differ in some ways from the approach used in the operating theatre by the vitreoretinal surgeons participating in this study. This is apparent in the Eyesi internal limiting membrane peeling module. Clinically, there are multiple approaches to this task. In particular, concerns have been raised about damage to the nerve fiber layer caused by excessive peeling, with a recommendation of keeping the peeled area small.27 The simulator, however, encourages an approach which maximized peeled area. This may affect the generalizability of our study findings and their transferability to a clinical setting. To minimize the effects of this aspect, an instructor explained the performance and procedural goals for each task in the performance test to the study participants to ensure that all participants had an equal and full understanding of the task at hand. However, despite the abovementioned limitation, one clear advantage of the study design is that the participants serve as their own control, because of the nature of the crossover study design. Furthermore, in addition to the abovementioned, the crossover study design and the balanced randomization of participants minimized the bias caused by any transfer of skill between each of the surgical modalities.
Our study has shown the feasibility of investigating robot-assisted vitreoretinal surgery using the Eyesi simulator. Furthermore, our study shows that robot-assisted surgery may hold a significant advantage to the novice surgeon by increasing precision and limiting tissue damage. However, the results of this study cannot directly be transferred to a clinical setting, because there may be differences in the application of robot-assisted surgery in a simulated setting compared with real-life. Consequently, future studies should focus on investigating the effects of robot-assisted surgery on patient outcomes in human trials.
Footnotes
The study and the corresponding author/first author is funded by Velux fonden, Søborg, Denmark (Grant number: 16627, grant provider for PhD project). The funding organization had no role in the design or conduct of this research.
None of the authors has any conflicting interests to disclose.
References
- 1.Singhy SP, Riviere CN. Physiological tremor amplitude during retinal microsurgery. InBioengineering Conference, 2002 Proceedings of the IEEE 28th Annual Northeast 2002. Philadelphia, PA: 2002: pp. 171–172. [Google Scholar]
- 2.de Smet MD, de Jonge N, Iannetta D, et al. Human/robotic interaction: vision limits performance in simulated vitreoretinal surgery. Acta Ophthalmol 2019;97:672–678. [DOI] [PubMed] [Google Scholar]
- 3.Molaei A, Abedio E, de Smet MD, et al. Toward the art of robotic-assisted vitreoretinal surgery. J Ophthalmic Vis Res 2017;12:212–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bergeles C, Yang GZ. From passive tool holders to microsurgeons: safer, smaller, smarter surgical robots. IEEE Trans Biomed Eng 2014;61:1565–1576. [DOI] [PubMed] [Google Scholar]
- 5.Bourcier T, Chammas J, Becmeur PH, et al. Robot-assisted simulated cataract surgery. J Cataract Refract Surg 2017;43:552–557. [DOI] [PubMed] [Google Scholar]
- 6.Chammas J, Sauer A, Pizzuto J, et al. Da Vinci Xi robot-assisted penetrating keratoplasty. Transl Vis Sci Technol 2017;6:21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bourcier T, Chammas J, Becmeur PH, et al. Robotically assisted pterygium surgery: first human case. Cornea 2015;34:1329–1330. [DOI] [PubMed] [Google Scholar]
- 8.Bourla DH, Hubschman JP, Culjat M, et al. Feasibility study of intraocular robotic surgery with the da Vinci surgical system. Retina 2008;28:154–158. [DOI] [PubMed] [Google Scholar]
- 9.Fine HF, Wei W, Goldman R, Simaan N. Robot-assisted ophthalmic surgery. Can J Ophthalmol 2010;45:581–584. [DOI] [PubMed] [Google Scholar]
- 10.de Smet MD, Stassen JM, Meenink TC, et al. Release of experimental retinal vein occlusions by direct intraluminal injection of ocriplasmin. Br J Ophthalmol 2016;100:1742–1746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.de Smet MD, Meenink TC, Janssens T, et al. Robotic assisted cannulation of occluded retinal veins. PLoS One 2016;11:e0162037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Edwards TL, Xue K, Meenink HCM, et al. First-in-human study of the safety and viability of intraocular robotic surgery. Nat Biomed Eng 2018;2:649–656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Thomsen AS, Subhi Y, Kiilgaard JF, et al. Update on simulation-based surgical training and assessment in ophthalmology: a systematic review. Ophthalmology 2015;122:1111–1130.e1111. [DOI] [PubMed] [Google Scholar]
- 14.Thomsen AS, Kiilgaard JF, la Cour M, et al. Is there inter-procedural transfer of skills in intraocular surgery? A randomized controlled trial. Acta Ophthalmol 2017;95:845–851. [DOI] [PubMed] [Google Scholar]
- 15.Konge L, Ringsted C, Bjerrum F, et al. The simulation centre at rigshospitalet, copenhagen, Denmark. J Surg Educ 2015;72:362–365. [DOI] [PubMed] [Google Scholar]
- 16.Cheng A, Kessler D, Mackinnon R, et al. Reporting guidelines for health care simulation research: extensions to the CONSORT and STROBE statements. Adv Simul (Lond) 2016;1:25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.van der Meijden OA, Schijven MP. The value of haptic feedback in conventional and robot-assisted monimal invasive surgery and virtual reality training: a current review. Surg Endosc 2009;23:1180–1190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Anders S, Rabe-Hesketh S. Generalized Latent Variable Modeling: Multilevel, Longitudinal, and Structural Equation Models. Boca Raton, FL: Chapman and Hall/CRC; 2004. [Google Scholar]
- 19.Cohen J. Statistical Power Analysis for the Behavioral Sciences. New Jersey: Lawrence Erlbaum Associates; 1988. [Google Scholar]
- 20.de Smet MD, Naus GJL, Faridpooya K, Mura M. Robotic-assisted surgery in ophthalmology. Curr Opin Ophthalmol 2018;29:248–253. [DOI] [PubMed] [Google Scholar]
- 21.Ahmad A, Ahmad ZF, Carleton JD, Agarwala A. Robotic surgery: current perceptions and the clinical evidence. Surg Endosc 2017;31:255–263. [DOI] [PubMed] [Google Scholar]
- 22.Eshner AA. A graphic study of tremor. J Exp Med 1897;2:301–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Harwell RC, Ferguson RL. Physiologic tremor and microsurgery. Microsurgery 1983;4:187–192. [DOI] [PubMed] [Google Scholar]
- 24.Dogramaci M, Steel DH. Unintentional movements during the use of vitreoretinal forceps. Translational Vis Sci Technol 2018;7:28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jensen K, Hansen HJ, Petersen RH, et al. Evaluating competency in video-assisted thoracoscopic surgery (VATS) lobectomy performance using a novel assessment tool and virtual reality simulation. Surg Endosc 2019;33:1465–1473. [DOI] [PubMed] [Google Scholar]
- 26.Jacobsen MF, Konge L, Bach-Holm D, et al. Correlation of virtual reality performance with real-life cataract surgery performance. J Cataract Refract Surg 2019; 45:1246–1251. [DOI] [PubMed] [Google Scholar]
- 27.Tadayoni R, Paques M, Massin P, et al. Dissociated optic nerve fiber layer appearance of the fundus after idiopathic epiretinal membrane removal. Ophthalmology 2001;108:2279–2283. [DOI] [PubMed] [Google Scholar]


