Abstract
Background:
Large-scale normative studies of pancreatic stiffness and potential influences have yet to be pursued via magnetic resonance elastography (MRE).
Purpose:
To determine normative MRE-based pancreatic stiffness values and to examine related influential factors.
Study Type:
Prospective.
Subjects:
In all, 361 volunteers (men, 199; women, 162) with a median age of 54.0 years and a median body mass index (BMI) of 22.86 kg/m2 were prospectively recruited. Those with no histories of smoking, alcohol abuse, and diabetes mellitus (DM) were grouped as healthy volunteers, designating all others as positive controls.
Field Strength/Sequence:
Each volunteer underwent 3.0T pancreatic MRI at a frequency of 40 Hz.
Assessment:
Pancreatic stiffness values, pancreatic width and volume, waist circumference, and wave distance were measured in all subjects.
Statistical Tests:
Multiple linear regression analyses were performed to determine variables that influence MRE-determined stiffness.
Results:
The mean pancreatic stiffness in all volunteers was 1.20 ± 0.16 kPa. Stiffness levels in positive control volunteers proved significantly greater than levels in healthy volunteers (1.29 ± 0.17 kPa vs. 1.14 ± 0.13 kPa; P < 0.001). In multiple linear regression analysis, sex (P = 0.004), BMI (P < 0.001), pancreatic width (P = 0.005), smoking (P < 0.001), alcohol abuse (P < 0.001), and DM (P = 0.001) emerged as significant independent factors impacting pancreatic stiffness. Smoking, alcohol abuse, DM, and wide pancreas were associated with greater pancreatic stiffness (coefficients = 0.202, 0.183, 0.149, and 0.160, respectively), while reduced pancreatic stiffness corresponded with female sex and larger BMI (coefficient = −0.155 and −0.192, respectively).
Data Conclusion:
MRE-based pancreatic stiffness values are impacted by sex, BMI, pancreatic width, smoking, alcohol abuse, and DM. Reference values are essential for future clinical studies.
Level of Evidence:
1
Technical Efficacy:
Stage 2
PANCREATIC DISEASES in aggregate, including primary cancers, cystic lesions, and acute or chronic pancreatitis, affect >10% of the world’s population, imposing significant burdens on healthcare systems worldwide.1–3 Early diagnosis of these disorders is extremely important, especially the screening of pancreatic cancer and its precursor lesions.4 However, current imaging modalities and screening methods often fall short, failing to detect early disease in a highly sensitive and specific manner.5,6
Magnetic resonance elastography (MRE) is a newly emergent MR-based functional technique for quantifying mechanical properties of tissues in vivo. In a number of pancreatic diseases, particularly those involving solid tumors, fibrosis, and inflammation, pancreatic stiffness is significantly altered. This has been documented by prior small-scale clinical studies (all <100 samples), even in the early phases of chronic pancreatitis.7–11 To accurately gauge abnormal stiffness, a range of normal pancreatic stiffness values must first be established. At present, there are few articles on MRE-based pancreatic stiffness addressing the distribution of pancreatic stiffness in a normal population, and their sample sizes have been relatively small (14–22 subjects).12–14 A recent systematic review3 has also shown that tobacco use, obesity-related diabetes mellitus (DM), and alcohol abuse (all common in populations at-large), are associated with significantly heightened risks of pancreatic disease worldwide.15–17 Whether these factors impact pancreatic stiffness and thus may be viewed as confounders of so-called “normal pancreatic stiffness” is still unclear.
Thus, this study aimed to determine the distribution of pancreatic stiffness on a large scale, assessing the impact of risk factors (smoking, alcohol abuse, DM), demographics (sex, age, body mass index [BMI]), and morphologic parameters (pancreatic width and volume, waist circumference [WC], wave distance) on pancreatic stiffness.
Materials and Methods
Study Participants
This prospective study complied with the Health Insurance Portability and Accountability Act (HIPAA) and was approved by the Institutional Review Board. Once details were fully explained, each participant granted written informed consent. A total of 397 volunteers >18 years old, all nearby community members with urban population accounting for 65% (n = 258) and semiurban population of 35% (n = 139), were recruited between December 2016 and November 2018. Initially, no preexisting abdominal conditions (other than diabetes) were evident. Those with evidence of abdominal disease (including but not limited to pancreatitis, cystic fibrosis, and neoplasms), surgical interventions involving pancreas or biliary ducts, or abnormal hepatic or pancreatic laboratory tests (eg, liver function tests and serum CA 199, lipase, or amylase levels) were subsequently disqualified. Another 34 were excluded on the following grounds: 1) pregnancy (n = 1); 2) cystic (n = 9) or solid (n = 2) masses as incidental MRI findings (detected by a senior radiologist with 21 years of experience in abdominal imaging); 3) MRE failures in 13 volunteers (~3.5%) including intolerance to vibration (n = 2), poor breath holding (n = 5), or insufficient wave penetration (n = 6); 4) severe pancreatic atrophy, maximum parenchymal width <1 cm (n = 2) at each subregion, without any intention to exclude those with lobulated pancreatic atrophy and fat infiltration; and 5) contraindications to MRI (n = 3); and (6) refusal to grant informed consent (n = 4).
Questionnaire and Data Collection
A self-administered standardized questionnaire was completed by each volunteer to collect social and demographic data (ie, sex, age, current weight, body size, smoking status, alcohol consumption, etc.), and past medical records of participants were screened for pertinent facts (ie, medical history of DM). Smoking was defined as a habit of at least one cigarette on average per day for at least 3 months18 or had smoked at least 100 cigarettes during their life-time.19 Alcohol abuse was equated with intake >50 g/day in male and >30 g/day in female on average for >1 year.20 This study was confined to type 2 DM (T2DM), although not intentionally. Diagnostic criteria of DM were fasting plasma glucose (FPG) >7.0 mmol/L and/or 2-hour plasma glucose (2hPG) >11.1 mmol/L after a 75-g oral glucose tolerance test (OGTT).21 Subjects with any of these three qualifiers (smoking, alcohol abuse, or DM) above were grouped as positive controls, designating all others as healthy volunteers.
Ultimately, 361 volunteers (men, 199; women, 162) qualified for the study. The median age was 54.0 years (range, 19–82 years), and median BMI was 22.86 kg/m2 (range, 15.75–42.12 kg/m2). Demographic characteristics of the volunteer population are shown in Table 1.
TABLE 1.
Baseline Characteristics and Morphologic Parameters of Study Volunteers
| Parameters | n or Median (IQR) | n or Median (IQR) in groups | P value | |
|---|---|---|---|---|
| Healthy volunteers | Positive controls | |||
| Sex (male/female) | 199/162 | 87/126 | 112/36 | <0.001 |
| Age (years) | 54 (31–64) | 42 (24–61) | 59 (48–65) | <0.001 |
| BMI (kg/m2) | 22.857 (20.488–25.301) | 23.246 (20.004–25.858) | 22.857 (20.984–24.763) | 0.771 |
| Lipase level (U/L) | 32.140(22.050–37.675) | 31.250 (22.050–36.240) | 33.195 (23.033–39.858) | 0.163 |
| Amylase level (U/L) | 60.290 (44.670–72.775) | 57.110 (44.303–72.758) | 61.790 (44.680–72.875) | 0.818 |
| A-P Width (cm) | 1.830 (1.670–2.010) | 1.800 (1.620–1.980) | 1.910 (1.730–2.120) | <0.001 |
| Waist circumference (cm) | 83.533 (75.618–89.444) | 81.733 (73.552–88.961) | 84.979 (79.448–90.285) | 0.001 |
| Pancreatic volume (cm3) | 65.369 (52.407–75.565) | 65.230 (52.045–73.413) | 66.358 (53.963–79.052) | 0.106 |
| Distance (cm) | 6.178 (4.910–7.715) | 5.900 (4.840–7.430) | 6.480 (4.999–7.860) | 0.096 |
| Smoking history (yes/no) | 99/262 | 0/213 | 99/49 | <0.001 |
| Alcohol abuse (yes/no) | 59/302 | 0/213 | 59/89 | <0.001 |
| Diabetes mellitus (yes/no) | 43/318 | 0/213 | 43/105 | <0.001 |
Continuous data presented as median (25th–75th percentiles). Positive control group includes volunteers with any of the three conditions including alcohol abuse, smoking, and type II diabetes. Healthy volunteer group has no known conditions other than overweight or obese volunteers. P values in bold denote statistical significance.
Imaging Acquisition
All examinations were performed using a 3.0T MR system (Signa HDX; GE Healthcare, Milwaukee, WI) equipped with an eight-channel phased-array body coil. All volunteers were instructed to fast for 6–8 hours beforehand. During examinations they were placed in supine position. An active pneumatic driver system situated outside the scan room generated mechanical vibrations at a fixed frequency of 40 Hz for delivery to the upper abdomen via a plastic tube. The frequency of 40 Hz was chosen based on previous preclinical and clinical studies, proving that the wave images at 40 Hz showed a significantly higher amplitude of wave motion and better wave pattern than those obtained at 60 Hz.10,14 The flat passive driver at its terminus served to propagate waves deep into the pancreas. Volunteers used a rectangular flexible soft driver (19 × 14 cm), and a rigid round passive driver (19 cm in diameter) was optionally used in case of insufficient wave data via semi-soft driver, as described else-where.7,10 Both drivers were developed at the Mayo Clinic (MR Touch, Resoundant, Rochester, MN) and supplied to us through service agreements. The acquisition included 4 × 22 sec and 1 × 11 sec of breath-holding. Settings of the imaging parameters were as follows: repetition time (TR), 1375 msec; echo time (TE), 39.4 msec; phase offsets, 3; field of view (FOV), 350–430 mm; acquisition matrix, 96 × 96; parallel imaging acceleration factor, 3; slice total, 32; slice thickness, 3.5 mm; and pixel size, 1.4 × 1.4 mm to 1.7 × 1.7 mm.10 To ensure consistent positioning of the pancreas, care was taken to monitor the level of expiration for each acquisition. In addition to MRE sequences, we also obtained routine plain pancreatic MRI studies, including respiratory-triggered T2-weighted imaging with/without fat suppression and T1-weighted imaging (T1WI), to screen for pancreatic lesions. The total time in the scanner (MRE + routine pancreatic MRI) was ~15 minutes.
Image Analysis
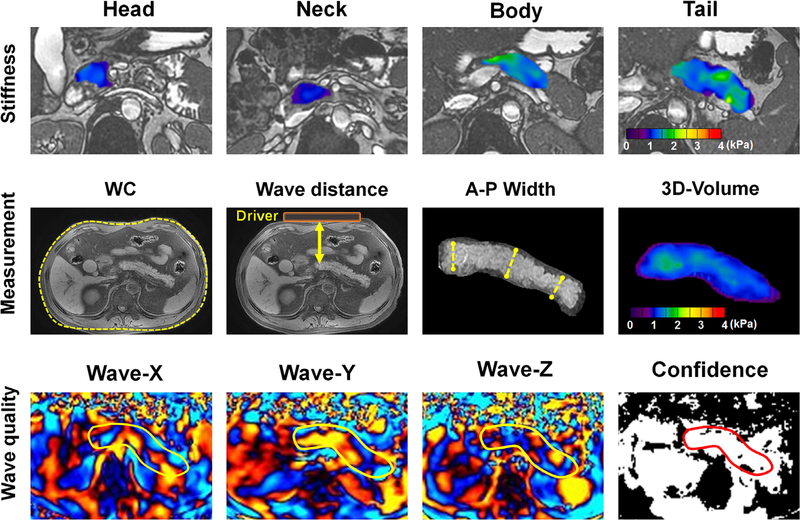
A direct inversion algorithm supplied by the Mayo Clinic enabled automated within-scanner processing of 3D original complex data into elastograms (stiffness maps).14 XYZ curled wave images, magnitude images, and local frequency estimation (LFE) confidence maps were also automatically generated. The wave images and confidence maps were used to control image quality (Fig. 1).
FIGURE 1:
Regions of interest drawn on pancreatic head, neck, body, and tail (first row); representative images for measuring waist circumference (WC), wave distance, anterior–posterior (A-P) width. and 3D volume estimate (left to right) (second row); the XYZ wave images and the confidence map for wave quality control (left to right) (third row). The pancreatic neck was not discussed separately due to the small size of this area.
Volumetric regions of interest (ROIs) were drawn slice-by-slice for head, body, tail, and the entirety of pancreatic parenchyma, using an in-house MatLab script (v. 2018a; MathWorks, Natick, MA) to define 3D stiffness voxels. The mean value of each bulk was calculated by averaging all voxel values within the volumetric ROIs. Care was taken for pancreatic boundaries, avoiding artifacts, pancreatic/biliary ducts, large vessels, and surrounding tissues. For each ROI, stiffness was displayed by a colorimetric scale (0–4 kPa).14 Interreader reproducibility was directed at two radiologists (first and second readers) with 7 and 4 years of experience in MRE, respectively. Intrareader reproducibility reflected performance by each radiologist at 1-month intervals, separated in time to avoid memory bias. Overall pancreatic stiffness was ultimately calculated as the mean of these four measurements. Anterior–posterior (A-P) pancreatic width was recorded as the average of maximum dimensions at head, body, and tail of pancreas on axial T2-weighted images. Since the driver was fastened firmly to the anterior abdominal wall, the minimum perpendicular distance between anterior abdominal wall and the body of the pancreas was estimated as the distance of wave propagation (ie, wave distance), which was also obtained from axial T2-weighted images, and pancreatic volume estimates were calculated as the product of voxel count and voxel size by our MatLab script. The voxel size was calculated as the product of slice thickness (3.5 mm) and the MRE pixel size. All measurements logged are shown in Fig. 1.
Statistical Analysis
After testing the normality of continuous variables by a Shapiro–Wilk test, normally distributed variables, such as pancreatic stiffness, were expressed as means ± SD; whereas abnormally distributed variables, such age and BMI, were expressed as median with first and third interquartile range (IQR). Categorical variables were expressed as counts. The Mann-Whitney U-test was used for groupwise comparisons of continuous variables (stiffness, age, BMI, etc.), applying the chi-square test to categorical variables (eg, sex) and invoking Bonferroni post hoc analysis as needed for multiple comparisons. LOESS (locally weighted scatterplot smoother) curve analysis, showing the relation between stiffness and age or BMI, was used to fit a smooth curve and suggest cutpoints for age (<25 years, 25–60 years, 60–75 years, and >75 years) and BMI (<24 kg/m2, 24–30 kg/m2, >30 kg/m2) (Figure S1 in the Supplemental Material). Levels of inter- and intrareader agreement in gauging stiffness were assessed via the intraclass correlation coefficient (ICC) and Bland-Altman test. The within-subject coefficient of variation (CV) represented the mean intrinsic variability within ROI measurements of each volunteer, calculated as the ratio of SD to average within ROIs. Between-subject CV signified the variability among subjects within each group, calculated as the ratio of SD to average within individual groups.
Multiple linear regression analysis with stepwise selection of variables served to evaluate the impact of demographics (sex, age, BMI), morphologic pancreatic parameters (A-P width, volume, WC, wave distance), and other risk factors (smoking, alcohol abuse, DM) on stiffness, using variables of significance (P < 0.10) in univariate analysis (Spearman correlation analysis for continuous variables, t-test, or Mann–Whitney U-test [sex] for binary variables). For stiffness levels not normally distributed, nonlinear transformation (eg, log-transformation) was implemented to assume a linear model. Multicollinearity was also tested to avoid strong intercorrelation among variables (tolerance >0.2; variance inflation factor <5.0).22 Strengths of associations were expressed as standardized coefficients with 95% confidence intervals (CIs).
All computations were driven by standard software (R freeware v. 3.6.0 [R Foundation for Statistical Computing, Vienna, Austria] and SPSS v. 22.0 [IBM, Armonk, NY]), constructing bar graphs separately (Prism v. 7.00; GraphPad Software, San Diego, CA). Statistical significance was set at P < 0.05.
Results
Volunteer Characteristics
Overall, 36.8% of volunteers (133/361) were with BMI >24 kg/m2; 10.5% (38/361) were obese (BMI >30 kg/m2); 27.4% (99/361) were smokers; 16.3% (59/361) appeared to abuse alcohol; and 11.9% (43/361) had histories of T2DM. There were 34 concurrent alcohol and tobacco users (9.4%), accounting for 34.3% of smokers and 57.6% of alcohol abusers. Smoking and alcohol abuse differed dramatically by sex (both P < 0.001) and were largely restricted to men (smoking: 82.8% vs. 17.2%; alcohol: 84.7% vs. 15.3%), although diabetes failed to exhibit such disparity (P = 0.818). As indicated in Table 1, healthy volunteers and positive controls differed significantly by sex, age, A-P width, and WC (all P < 0.001).
Pancreatic Stiffness Measurements by Readers and Subregion
The overall agreement in MRE readings was excellent at both interreader (ICC = 0.862, 95% CI: 0.843–0.879; Bland–Altman bias: [−16.7] to 11.9%) and intrareader (ICC = 0.900, 95% CI: 0.886–0.913; Bland–Altman bias: [–12.0] to 11.7%) levels across timepoints (Table 2).
TABLE 2.
Reproducibility of Pancreatic Stiffness Measurements Between Readers and at Two Points in Time
| ICC (95% CI) | Mean bias (%) | BALA (%) | Within-subject CV (%) | Between-subject CV (%) | ||
|---|---|---|---|---|---|---|
| Interreader | Reader 1 | 0.867 (0.845–0.886) | −6.8 | (−14.2) – 0.6 | 6.792 | 5.425 |
| Reader 2 | 0.864 (0.842–0.883) | 2.0 | (−12.3) – 16.3 | 2.026 | 5.351 | |
| Overall | 0.862 (0.843–0.879) | −2.4 | (−16.7) – 11.9 | 0.138 | 5.388 | |
| Intrareader | 1st reading | 0.887 (0.868–0.904) | 4.3 | (−4.1) – 12.6 | 4.271 | 4.521 |
| 2nd reading | 0.914 (0.899–0.926) | −4.5 | (−12.3) – 3.2 | 4.547 | 4.614 | |
| Overall | 0.900 (0.886–0.913) | −0.1 | (−12.0) – 11.7 | 2.383 | 4.567 |
ICC = intraclass correlation coefficient; BALA = Bland–Altman limits of agreement (%); CV = coefficient of variation (%).
Mean pancreatic stiffness in all volunteers was 1.20 ± 0.16 kPa, with a median value of 1.19 (IQR: 1.08–1.31 kPa; range: 0.84–1.70 kPa). Mean stiffness readings at the pancreatic head, body, and tail were 1.20 ± 0.14 kPa, 1.19 ± 0.13 kPa, and 1.22 ± 0.16 kPa, respectively, showing no significant difference (P = 0.233). Regional stiffness determinations of both readers in healthy and positive control volunteers were likewise consistent across subregions (all P >0.05) (Table 3). The overall mean stiffness (kPa) in healthy pancreas was significantly lower than that of positive control (kPa) across readers and subregions.
TABLE 3.
Main Pancreatic Segments Stiffness Values (kPa) Measured by Two Readers
| Reader 1 | Subregions | Healthy volunteers | P | Positive control | P | ||
|---|---|---|---|---|---|---|---|
| First Reading | Second Reading | First Reading | Second Reading | ||||
| Head | 1.182 ± 0.145 | 1.173 ± 0.150 | 0.551 | 1.243 ± 0.129 | 1.220 ± 0.131 | 0.343 | |
| Body | 1.182 ± 0.119 | 1.191 ± 0.121 | 0.410 | 1.227 ± 0.143 | 1.229 ± 0.141 | 0.922 | |
| Tail | 1.158 ± 0.124 | 1.198 ± 0.134 | 0.025 | 1.266 ± 0.161 | 1.283 ± 0.157 | 0.534 | |
| Total | 1.174 ± 0.130 | 1.188 ± 0.136 | 0.194 | 1.246 ± 0.145 | 1.244 ± 0.142 | 0.852 | |
| P | 0.596 | 0.208 | — | 0.283 | 0.079 | — | |
| Reader 2 | Head | 1.198 ± 0.142 | 1.202 ± 0.153 | 0.833 | 1.254 ± 0.126 | 1.191 ± 0.133 | 0.019 |
| Body | 1.157 ± 0.131 | 1.164 ± 0.106 | 0.538 | 1.217 ± 0.147 | 1.233 ± 0.145 | 0.516 | |
| Tail | 1.178 ± 0.117 | 1.167 ± 0.132 | 0.504 | 1.234 ± 0.156 | 1.265 ± 0.158 | 0.491 | |
| Total | 1.177 ± 0.129 | 1.178 ± 0.131 | 0.968 | 1.237 ± 0.141 | 1.230 ± 0.147 | 0.723 | |
| P | 0.114 | 0.261 | — | 0.318 | 0.091 | — | |
Data expressed as mean ± standard deviation. P was calculated by Kruskal–Wallis test. P values in bold denote statistical significance.
Pancreatic Stiffness in Healthy Volunteers
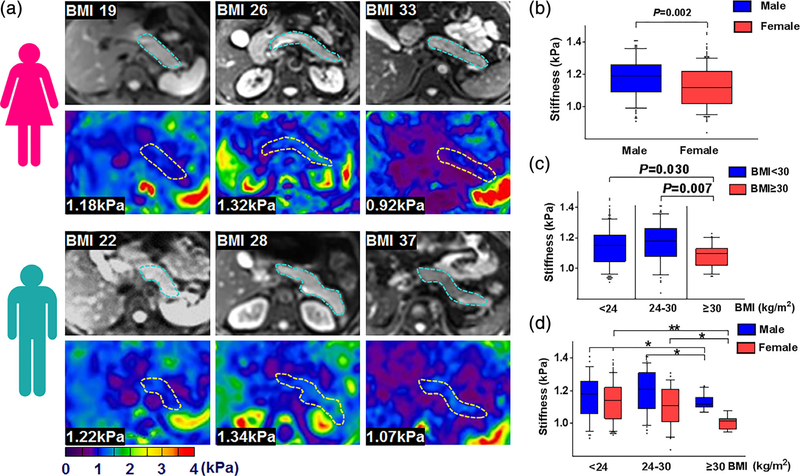
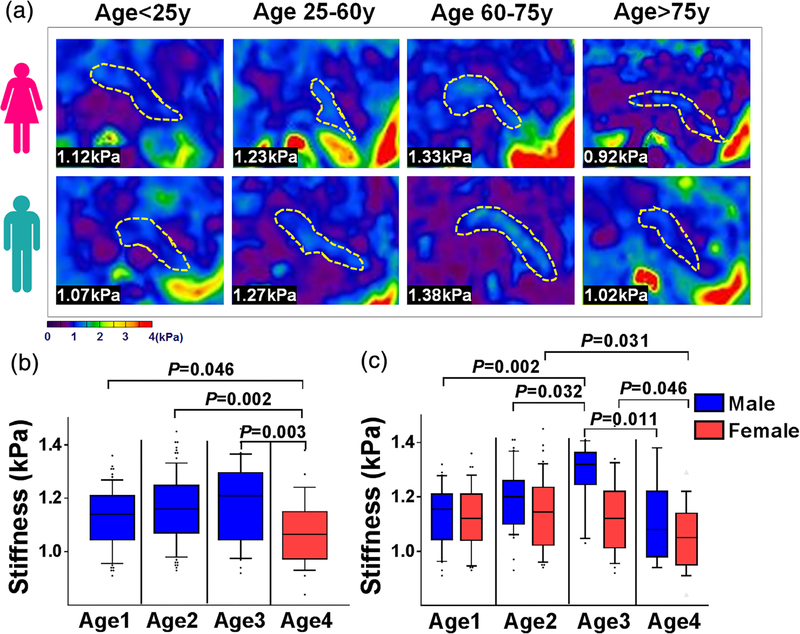
As illustrated in Table 4 and Fig. 2, the mean pancreatic stiffness in men significantly exceeded that in women (1.18 kPa vs. 1.12 kPa; P = 0.002) (Fig. 2b). Although pancreatic stiffness at lower BMI levels (<24 kg/m2 and 24–30 kg/m2) did not differ significantly, both subsets differed significantly from the highest BMI group (P = 0.030 and P = 0.007, respectively) (Fig. 2c), men and women demonstrating similar trends by BMI group (Fig. 2d). When stratifying subjects by age (<25, 25–60, 60–75, >75 years) (Fig. 3), pancreatic stiffness beyond age 75 was significantly less than in the other age groups (<25 years: P = 0.046; 25–60 years: P = 0.002; 60–75 years: P = 0.003). However, pairwise comparisons of three lower age groups showed no significant differences (Fig. 3b). In men, pancreatic stiffness increased marginally with age, peaking at 60–75 years, declining significantly thereafter. Pancreatic stiffness in women remained consistent until age 75, again waning significantly thereafter (Fig. 3c). Mean A-P width peaked in years 25–60 and declined thereafter (1.83 cm, 1.86 cm, 1.73 cm, and 1.56 cm, respectively; P < 0.001), as did pancreatic volume (63.74 cm3, 70.23 cm3, 58.76 cm3, and 45.22 cm3, respectively; P < 0.001). Men significantly surpassed women in mean A-P width (1.93 cm vs. 1.70 cm; P < 0.001) but showed no significant difference in pancreatic volume (65.58 cm3 vs. 61.84 cm3; P = 0.223).
TABLE 4.
Pancreatic Stiffness Measurements (kPa) in Healthy and Positive Control Volunteers
| Male | Healthy volunteer group | Total | P | Positive control group | P | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Age | BMI < 24 | 24 ≤ BMI < 30 | BMI ≥30 | BMI < 24 | 24 ≤ BMI < 30 | BMI ≥30 | Total | ||||
| <25y | 1.11 ± 0.13 | 1.15 ± 0.13 | 1.14 ± 0.05 | 1.13 ± 0.11 | 0.698 | 1.145* | 1.34 ± 0.14 | 1.18* | 1.28 ± 0.14 | 0.163 | |
| 25–60 | 1.18 ± 0.13 | 1.23 ± 0.10 | 1.11 ± 0.05 | 1.19 ± 0.12 | 0.102 | 1.25 ± 0.17 | 1.28 ± 0.21 | 1.20 ± 0.18 | 1.25 ± 0.18 | 0.582 | |
| 60–75 | 1.31 ± 0.06 | 1.29 ± 0.12 | — | 1.29 ± 0.11 | 0.711 | 1.37 ± 0.13 | 1.40 ± 0.07 | 1.22 ± 0.21 | 1.37 ± 0.13 | 0.357 | |
| >75 | 1.20 ± 0.14 | 1.00 ± 0.07 | — | 1.11 ± 0.15 | 0.114 | 1.28 ± 0.06 | — | — | 1.28 ± 0.06 | — | |
| Total | 1.17 ± 0.13 | 1.20 ± 0.13 | 1.13 ± 0.05 | 1.18 ± 0.13 | 0.087 | 1.31 ± 0.16 | 1.33 ± 0.17 | 1.20 ± 0.17 | 1.30 ± 0.16 | 0.105 | |
| P | 0.141 | 0.014 | 0.372 | 0.005 | — | 0.021 | 0.163 | 0.885 | 0.002 | — | |
| Female | <25 | 1.12 ± 0.11 | 1.15 ± 0.08 | 1.03* | 1.12 ± 0.11 | 0.583 | — | 1.03* | 1.01* | 1.03, 1.01* | — |
| 25–60 | 1.15 ± 0.14 | 1.12 ± 0.11 | 1.05 ± 0.06 | 1.14 ± 0.13 | 0.332 | 1.31 ± 0.22 | 1.06 ± 0.07 | — | 1.25 ± 0.23 | 0.032 | |
| 60–75 | 1.15 ± 0.15 | 1.11 ± 0.12 | 1.08* | 1.13 ± 0.14 | 0.814 | 1.35 ± 0.18 | 1.30 ± 0.12 | 1.08* | 1.32 ± 0.17 | 0.467 | |
| >75 | 1.09 ± 0.95 | 1.06 ± 0.16 | 0.98 ± 0.03 | 1.06 ± 0.12 | 0.218 | 1.20 ± 0.12 | — | — | 1.20 ± 0.12 | — | |
| Total | 1.14 ± 0.13 | 1.11 ± 0.12 | 1.01 ± 0.04 | 1.12 ± 0.13 | 0.012 | 1.29 ± 0.18 | 1.20 ± 0.17 | 1.06 ± 0.04 | 1.26 ± 0.18 | 0.083 | |
| P | 0.673 | 0.715 | 0.104 | 0.129 | — | 0.213 | 0.071 | 1.006 | 0.072 | — | |
Data are expressed as mean ± standard deviation. P was calculated by Kruskal–Wallis test. P values in bold denote statistical significance.
Denotes: only one or two cases.
FIGURE 2:
Pancreatic axial magnitude imaging (upper row, pancreas outlined in blue) and elastograms (lower row, pancreas outlined in yellow) in three BMI groups, stratified by (a) female and male gender, with bar graphs of (b) M/F subsets, (c) BMI groups, and (d) M/F subsets in each BMI group. *P < 0.05, **P < 0.01, ***P < 0.001.
FIGURE 3:
Pancreatic elastograms of (a) four age groups in women (upper row) and men (lower row), pancreas outlined in yellow; bar graphs of (b) four age groups and (c) M/F subsets in each age group.
Effects of Smoking, Alcohol Abuse, and Diabetes on Pancreatic Stiffness
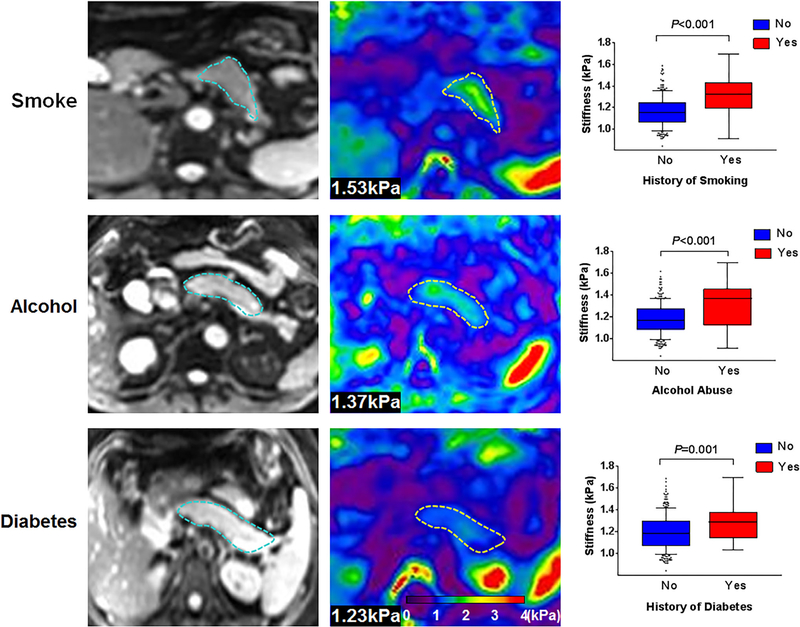
As Fig. 4 indicates, smokers displayed significantly greater stiffness than nonsmokers (1.31 ± 0.17 kPa vs. 1.16 ± 0.14 kPa; P < 0.001). Alcohol abuse (1.32 ± 0.20 kPa vs. 1.18 ± 0.14 kPa; P < 0.001) and diabetes (1.28 ± 0.16 vs. 1.19 ± 0.13 kPa; P = 0.001) also increased pancreatic stiffness significantly, compared with healthy volunteers. As opposed to singular use of tobacco (1.28 ± 0.15 kPa; P = 0.009) or alcohol (1.27 ± 0.20 kPa; P = 0.018), concurrent alcohol and tobacco use corresponded with dramatic stiffening (1.36 ± 0.19 kPa), yielding the three highest levels (1.6–1.7 kPa) in this cohort.
FIGURE 4:
Representative pancreatic axial magnitude images (first column, pancreas outlined in blue) and elastograms (second column, pancreas outlined in yellow) grouped by smoking, alcohol abuse, and diabetes, with bar graphs of healthy (No) and positive control (Yes) volunteers.
Multiple Linear Regression Analysis
Upon performing natural log transformation, pancreatic stiffness appeared normally distributed (Shapiro–Wilk test, P = 0.20). In multiple linear regression analysis, logged pancreatic stiffness was independently related to sex (coefficient = −0.155; P = 0.004), BMI (coefficient = −0.192; P < 0.001), A-P width (coefficient = 0.160; P = 0.005), smoking (coefficient = 0.202; P < 0.001), alcohol abuse (coefficient = 0.183, P < 0.001), and diabetes (coefficient = 0.149; P = 0.001) (Table 5). Hence, 60% of total stiffness variability in this model was attributable to these variables (adjusted R2 = 0.276; F = 23.874; P < 0.001). In healthy volunteers, the width of the pancreas was the sole significant factor associated with pancreatic stiffness (coefficient = 0.197; P = 0.012) (Table 6).
TABLE 5.
Univariate and Multivariate Linear Analysis of Log-transformed Pancreatic Stiffness in All Volunteers
| Parameter | Univariate | Multivariate | ||||
|---|---|---|---|---|---|---|
| Coefficient | 95% CI | P | Coefficient | 95% CI | P | |
| Sex (male/female) | −0.290 | −0.389 to −0.191 | <0.001 | −0.155 | −0.259 to −0.051 | 0.004 |
| Age (years) | 0.144 | 0.042–0.247 | 0.006 | 0.088 | −0.010 to 0.186 | 0.080 |
| BMI (kg/m2) | −0.106 | −0.209 to −0.003 | 0.044 | −0.192 | −0.288 to −0.097 | <0.001 |
| A-P Width (cm) | 0.267 | 0.167–0.367 | <0.001 | 0.160 | 0.050–0.270 | 0.005 |
| Waist circumference (cm) | 0.063 | −0.041 to 0.166 | 0.234 | — | — | — |
| Pancreatic volume (cm3) | 0.181 | 0.079–0.283 | 0.001 | 0.088 | −0.017 to 0.193 | 0.102 |
| Distance (cm) | −0.034 | −0.137 to 0.070 | 0.524 | — | — | — |
| Smoking | 0.385 | 0.289–0.481 | <0.001 | 0.202 | 0.098–0.305 | <0.001 |
| Alcohol abuse | 0.309 | 0.210–0.408 | <0.001 | 0.183 | 0.087–0.278 | <0.001 |
| Diabetes | 0.182 | 0.080–0.284 | <0.001 | 0.149 | 0.059–0.238 | 0.001 |
Multivariate analyses achieved via linear regression model including all parameters with P < 0.10 in the univariate analysis. Anterior–posterior (A-P) width denotes the average pancreatic width across subregions. Distance denotes the minimum perpendicular distance between anterior abdominal wall and pancreas. P values in bold denote statistical significance. BMI = body mass index; Coefficient = standardized regression coefficient.
TABLE 6.
Univariate and Multivariate Linear Regression Analysis of Pancreatic Stiffness in Healthy Volunteers
| Parameter | Univariate | Multivariate | ||||
|---|---|---|---|---|---|---|
| Coefficient | 95% CI | P | Coefficient | 95% CI | P | |
| Sex (male/female) | −0.212 | −0.345 to −0.080 | 0.002 | −0.128 | −0.266 to 0.009 | 0.068 |
| Age (years) | −0.084 | −0.219 to 0.051 | 0.222 | — | — | — |
| BMI (kg/m2) | −0.062 | −0.198 to 0.073 | 0.364 | — | — | — |
| A-P Width (cm) | 0.301 | 0.172–0.430 | <0.001 | 0.197 | 0.043–0.350 | 0.012 |
| Waist circumference (cm) | 0.018 | −0.118 to 0.153 | 0.797 | — | — | — |
| Pancreatic volume (cm3) | 0.231 | 0.099–0.363 | 0.001 | 0.131 | −0.013 to 0.274 | 0.074 |
| Distance (cm) | −0.068 | −0.203 to 0.067 | 0.323 | — | — | — |
Multivariate analyses achieved via linear regression model including all parameters with P < 0.10 in the univariate analysis. Anterior–posterior (A-P) width denotes the average pancreatic width across subregions. Distance denotes the minimum perpendicular distance between anterior abdominal wall and pancreas. P values in bold denote statistical significance. BMI = body mass index; Coefficient = standardized regression coefficient.
Discussion
To our knowledge, the present study incorporates the largest population as yet investigated by either MRE or ultrasound (US)-based elastography for factors impacting pancreatic stiffness. Our analysis indicates excellent inter- and intrareader agreement in gauging pancreatic stiffness by MRE. Men generally exhibited greater stiffness than female counterparts, whereas softening was apparent in overtly obese subjects (BMI >30 kg/m2) and those of advanced age (>75 years). Smoking, alcohol abuse, and diabetes were identified as factors significantly linked to pancreatic stiffening. Joint use of tobacco and excessive alcohol culminated in the highest levels of stiffness within this population.
A few earlier MRE studies of pancreas have reported stiffness values of ~1.12 kPa at 40 Hz in volunteers.7,8,10,14 Despite higher values determined by Itoh et al12 and Kolipaka et al13 at 60 Hz, we recorded similar levels at 40 Hz (~1.1–1.3 kPa) in our healthy subjects. Pancreatic head, body, and tail showed no regional differences. However, our findings depart from US-based elastography outcomes23–25 that show variation in shear-wave velocities at head, body, and tail. Inconsistencies of this sort may well be rooted in the technique limitation in US-based elastography. Most US-based elastography methods are 2D instead of 3D. That is to say, the ultrasound only collects motion travels along the probe axis, not all three orthogonal directions. Complex geometry and the boundary condition of pancreas causes apparent inhomogeneous measurements. MRE enables imaging of the entire pancreas and production of volumetric elastograms. Thus, the homogeneity of pancreatic stiffness is better demonstrated.
Age-related changes in the pancreas, such as fibrosis, lymphoplasmacytic infiltration, fatty replacement, and lobulocentric atrophy26,27 may cause difficulties in discriminating between true pathology and degenerative phenomena. In theory, the aging pancreas should stiffen due to ongoing fibrosis and lymphoplasmacytic influx.28,29 On the other hand, parenchymal atrophy or fatty deposits may cause it to soften. Studies of the aging pancreas are therefore imbued with controversy. It has been determined by ultrasound strain elastography that pancreatic stiffness gradually declines after 40 years of age.30 However, another study based on shear-wave velocity has demonstrated significant stiffening of the pancreas after age 60. These contradictory findings might be due to the age distributions bias within cohorts. The former analysis involved a limited number of patients <40 years old, and there were few subjects past age 60 in the latter. In assessing subjects 20–64 years of age, Kolipaka et al13 reported that MRE-determined pancreatic stiffness in older adults (>45 years) significantly exceeded that of younger group members (<45 years). Nonetheless, sex was not addressed in any of these efforts. To some extent, then, our results are consistent with the above data, showing peak stiffness at age 60–75 in men but not in women, and declines in both genders after age 75. The peak at age 60–75 ostensibly indicates a preponderance of degenerative stiffness, with atrophy or deposition of fat after age 75 accounting for later softening.
Although the obese subjects (>30 kg/m2) we examined, men and women alike, displayed a significantly low stiffness, our data failed to establish a negative correlation between increments of BMI and stiffness, thus aligning with the results of Puttmann et al31 and Saglam et al,32 but conflicting with other sources.25 A higher BMI presumptively signals increased risk of fatty deposition in various organs. Fat is softer than normal pancreatic parenchyma (<1 kPa at 40 Hz)14 and its presence may lead to a subsequent reduction in the pancreatic stiffness. Yet there is still debate over apparent fat deposition in overweight subjects (24–30 kg/m2), in whom we found no loss of pancreatic stiffness, compared with those of normal BMI. The softening effect of infiltrating fat is seemingly confined to states of overt obesity. Our data additionally confirmed earlier US elastography results showing no difference in volunteers grouped as BMI <25 or BMI >25.25
Another perceived influence in US study reports is wave propagating distance. Healthy volunteers with higher BMIs have thicker abdominal walls that further separate organ and driver, potentially weakening wave energy and reducing stiffness readings. However, wave distance and pancreatic stiffness were unrelated in our study, refuting its importance in the realm of MRE. Moreover, we checked wave amplitudes and confidence maps to ensure quality control. Six subjects with poor wave propagation were subsequently excluded.
Gender is clearly a significant contributor to pancreatic stiffness, reportedly playing a role in liver as well.33 Aside from innate hormonal differences, the significantly broader dimensions of pancreas in men might account for the greater stiffness observed.
Our investigation into the effects of smoking, alcohol, and diabetes on stiffness reading revealed significant associations. Alcohol and smoking contribute greatly to the development of chronic pancreatitis, and the risks are likely multiplicative.34 As several studies have corroborated, smoking stimulates inflammation and fibrosis in the pancreas, increasing its stiffness.35,36 However, the impact of alcohol intake is more contentious. Although Stumpf et al25 found no significant relation between stiffness and alcohol, pancreatic stiffness correlated significantly with active alcohol consumption and alcoholic liver disease in Conti et al’s study.37
Consistent with Conti et al’s study, we similarly observed alcohol-related increases in stiffness, and alcohol abuse is known to incite chronic inflammation and fibrosis in the pancreas. Moreover, many of the alcohol abusers (34/59, 57.6%) we studied were also smokers. Voluminous consumers of alcohol tend to smoke more cigarettes.38 This cooccurrence of smoking and alcoholism conferred the highest stiffness within our cohort, underscoring the synergistic impact of these two risk factors.
Limitations
Certain limitations of the present study should be acknowledged. First, despite excluding pancreatic pathology detectable by routine MR sequences and laboratory testing, we could not offer histologic proof that mild pancreatitis or other subliminal disorders were absent. However, this approach would be untenable in volunteers. Second, smoking, alcohol abuse, and diabetes were the only risk factors we assessed. The duration of these variables and other lifestyle habits (diet, exercise, etc.) were not addressed. Third, younger volunteers outnumbered older ones, particularly those beyond age 75. Future studies should include a more balanced mix of elderly participants. Fourth, we did not quantify pancreatic fat and examine the relationship of pancreatic fat with stiffness. Finally, we did not carry out a long-term follow-up of our volunteers to confirm the volunteers’ backgrounds.
Conclusion
We have compiled normative pancreatic stiffness values determined by MRE. MRE-based pancreatic stiffness values are impacted by sex, BMI, pancreatic width, DM, smoking, and alcohol abuse. Reference values are essential for future studies on quantitative evaluation of abnormal pancreatic mechanical properties signaling both diffuse and focal pancreatic diseases.
Supplementary Material
Acknowledgment
Grant Support: This project was funded by the National Natural Science Foundation of China (81771802 and 81771893), Support Program for Innovative Talents in Universities of Liaoning Province (LR2016020), and Foundation for the National Institutes of Health (grant EB001981).
We thank Richard L. Ehman and Jun Chen of the Mayo Clinic for providing the MRE system and the tailored pancreatic MRE drivers.
Footnotes
Conflict of Interest
None of the authors have conflicts of interest or specific financial interests with respect to this subject matter.
Additional supporting information may be found in the online version of this article
References
- 1.Dimastromatteo J, Brentnall T, Kelly KA. Imaging in pancreatic disease. Nat Rev Gastroenterol Hepatol 2017;14(2):97–109. [DOI] [PubMed] [Google Scholar]
- 2.Xiao AY, Tan ML, Wu LM, et al. Global incidence and mortality of pancreatic diseases: A systematic review, meta-analysis, and meta-regression of population-based cohort studies. Lancet Gastroenterol Hepatol 2016;1(1):45–55. [DOI] [PubMed] [Google Scholar]
- 3.Alsamarrai A, Das SL, Windsor JA, Petrov MS. Factors that affect risk for pancreatic disease in the general population: A systematic review and meta-analysis of prospective cohort studies. Clin Gastroenterol Hepatol. 2014;12(10): 1635–1644.e1635. quiz e1103. [DOI] [PubMed] [Google Scholar]
- 4.Chhoda A, Lu L, Clerkin BM, Risch H, Farrell JJ. Current approaches to pancreatic cancer screening. Am J Pathol 2019;189(1):22–35. [DOI] [PubMed] [Google Scholar]
- 5.Chung YS, Ho JJ, Kim YS, et al. The detection of human pancreatic cancer-associated antigen in the serum of cancer patients. Cancer 1987;60(7):1636–1643. [DOI] [PubMed] [Google Scholar]
- 6.Madhani K, Farrell JJ. Autoimmune pancreatitis: An update on diagnosis and management. Gastroenterol Clin North Am 2016;45(1):29–43. [DOI] [PubMed] [Google Scholar]
- 7.Wang M, Gao F, Wang X, et al. Magnetic resonance elastography and T1 mapping for early diagnosis and classification of chronic pancreatitis. J Magn Reson Imaging 2018;48:837–845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shi Y, Liu Y, Gao F, et al. Pancreatic stiffness quantified with MR Elastography: Relationship to postoperative pancreatic fistula after pancreaticoenteric anastomosis. Radiology 2018;288(2):476–484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shi Y, Gao F, Li Y, et al. Differentiation of benign and malignant solid pancreatic masses using magnetic resonance elastography with spinecho echo planar imaging and three-dimensional inversion reconstruction: A prospective study. Eur Radiol 2018;28(3):936–945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ji R, Li J, Yin Z, et al. Pancreatic stiffness response to an oral glucose load in obese adults measured by magnetic resonance elastography. Magn Reson Imaging 2018;51:113–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liu Y, Wang M, Ji R, Cang L, Gao F, Shi Y. Differentiation of pancreatic ductal adenocarcinoma from inflammatory mass: Added value of magnetic resonance elastography. Clin Radiol 2018;73(10):865–872. [DOI] [PubMed] [Google Scholar]
- 12.Itoh Y, Takehara Y, Kawase T, et al. Feasibility of magnetic resonance elastography for the pancreas at 3T. J Magn Reson Imaging. 2016;43 (2) :384–390. [DOI] [PubMed] [Google Scholar]
- 13.Kolipaka A, Schroeder S, Mo X, Shah Z, Hart PA, Conwell DL. Magnetic resonance elastography of the pancreas: Measurement reproducibility and relationship with age. Magn Reson Imaging 2017;42:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shi Y, Glaser KJ, Venkatesh SK, Ben-Abraham EI, Ehman RL. Feasibility of using 3D MR elastography to determine pancreatic stiffness in healthy volunteers. J Magn Reson Imaging. 2015;41(2):369–375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Foitzik T, Fernandez-del Castillo C, Rattner DW, Klar E, Warshaw AL. Alcohol selectively impairs oxygenation of the pancreas. Arch Surg 1995;130(4):357–360. discussion 361. [DOI] [PubMed] [Google Scholar]
- 16.Edderkaoui M, Thrower E. Smoking and pancreatic disease. J Cancer Ther 2013;4(10A):34–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Meier JJ, Giese A. Diabetes associated with pancreatic diseases. Curr Opin Gastroenterol 2015;31(5):400–406. [DOI] [PubMed] [Google Scholar]
- 18.Hardy CJ, Palmer BP, Muir KR, Sutton AJ, Powell RJ. Smoking history, alcohol consumption, and systemic lupus erythematosus: A case-control study. Ann Rheum Dis 1998;57(8):451–455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Garrett BE, Dube SR, Trosclair A, Caraballo RS, Pechacek TF. Cigarette smoking — United States, 1965–2008. MMWR Suppl 2011;60(1): 109–113. [PubMed] [Google Scholar]
- 20.Korean Association for the Study of the L. KASL clinical practice guide-lines: Management of alcoholic liver disease . Clin Mol Hepatol 2013;19 (3) :216–254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kuzuya T, Nakagawa S, Satoh J, et al. Report of the Committee on the Classification and Diagnostic Criteria of Diabetes Mellitus. Diabetes Res Clin Pract 2002;55(1):65–85. [DOI] [PubMed] [Google Scholar]
- 22.Hsieh FY, Lavori PW, Cohen HJ, Feussner JR. An overview of variance inflation factors for sample-size calculation. Eval Health Prof 2003;26(3): 239–257. [DOI] [PubMed] [Google Scholar]
- 23.Gallotti A, D’Onofrio M, Pozzi Mucelli R. Acoustic radiation force impulse (ARFI) technique in ultrasound with virtual touch tissue quantification of the upper abdomen. Radiol Med 2010;115(6):889–897. [DOI] [PubMed] [Google Scholar]
- 24.Yashima Y, Sasahira N, Isayama H, et al. Acoustic radiation force impulse elastography for noninvasive assessment of chronic pancreatitis. J Gastroenterol 2012;47(4):427–432. [DOI] [PubMed] [Google Scholar]
- 25.Stumpf S, Jaeger H, Graeter T, et al. Influence of age, sex, body mass index, alcohol, and smoking on shear wave velocity (p-SWE) of the pancreas. Abdom Radiol (NY) 2016;41(7):1310–1316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tariq H, Nayudu S, Akella S, Glandt M, Chilimuri S. Non-alcoholic fatty pancreatic disease: A review of literature. Gastroenterology Res 2016;9 (6):87–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Matsuda Y Age-related pathological changes in the pancreas. Front Biosci (Elite Ed) 2018;10:137–142. [DOI] [PubMed] [Google Scholar]
- 28.Akkaya HE, Erden A, Kuru Oz D, Unal S, Erden I. Magnetic resonance elastography: Basic principles, technique, and clinical applications in the liver. Diagn Interv Radiol 2018;24(6):328–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Venkatesh SK, Wells ML, Miller FH, et al. Magnetic resonance elastography: Beyond liver fibrosis-a case-based pictorial review. Abdom Radiol (NY) 2018;43(7):1590–1611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chantarojanasiri T, Hirooka Y, Kawashima H, et al. Age-related changes in pancreatic elasticity: When should we be concerned about their effect on strain elastography? Ultrasonics 2016;69:90–96. [DOI] [PubMed] [Google Scholar]
- 31.Puttmann S, Koch J, Steinacker JP, et al. Ultrasound point shear wave elastography of the pancreas: Comparison of patients with type 1 diabetes and healthy volunteers - results from a pilot study. BMC Med Imaging 2018;18(1):52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Saglam D, Bilgici MC, Kara C, Yilmaz GC, Camlidag I. Acoustic radiation force impulse elastography in determining the effects of type 1 diabetes on pancreas and kidney elasticity in children. AJR Am J Roentgenol 2017;209(5):1143–1149. [DOI] [PubMed] [Google Scholar]
- 33.Roulot D, Czernichow S, Le Clesiau H, Costes JL, Vergnaud AC, Beaugrand M. Liver stiffness values in apparently healthy subjects: Influence of gender and metabolic syndrome. J Hepatol 2008;48(4): 606–613. [DOI] [PubMed] [Google Scholar]
- 34.Conwell DL, Lee LS, Yadav D, et al. American pancreatic association practice guidelines in chronic pancreatitis: Evidence-based report on diagnostic guidelines. Pancreas 2014;43(8):1143–1162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Andriulli A, Botteri E, Almasio PL, Vantini I, Uomo G, Maisonneuve P. Smoking as a cofactor for causation of chronic pancreatitis: A meta-analysis. Pancreas 2010;39(8):1205–1210. [DOI] [PubMed] [Google Scholar]
- 36.Setiawan VW, Pandol SJ, Porcel J, et al. Prospective study of alcohol drinking, smoking, and pancreatitis: The multiethnic cohort. Pancreas 2016;45(6):819–825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Conti CB, Weiler N, Casazza G, et al. Feasibility and reproducibility of liver and pancreatic stiffness in patients with alcohol-related liver disease. Dig LiverDis 2019;51(7):1023–1029. [DOI] [PubMed] [Google Scholar]
- 38.Cross SJ, Lotfipour S, Leslie FM. Mechanisms and genetic factors underlying co-use of nicotine and alcohol or other drugs of abuse. Am J Drug Alcohol Abuse 2017;43(2):171–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.