Abstract
Climate change affects individual life-history characteristics and species interactions, including predator-prey interactions. While effects of warming on Aedes aegypti adults are well known, clarity the interactive effects of climate change (temperature and CO2 concentration) and predation risk on the larval stage remains unexplored. In this study, we performed a microcosm experiment simulating temperature and CO2 changes in Manaus, Amazonas, Brazil, for the year 2100. Simulated climate change scenarios (SCCS) were in accordance with the Fourth Assessment Report of Intergovernmental Panel on Climate Change (IPCC). Used SCCS were: Control (real-time current conditions in Manaus: average temperature is ~25.76°C ± 0.71°C and ~477.26 ± 9.38 parts per million by volume (ppmv) CO2); Light: increase of ~1,7°C and ~218 ppmv CO2; Intermediate: increase of ~2.4°C and ~446 ppmv CO2; and Extreme: increase of ~4.5°C and ~861 ppmv CO2, all increases were relative to a Control SCCS. Light, Intermediate and Extreme SCCS reproduced, respectively, the B1, A1B, and A2 climatic scenarios predicted by IPCC (2007). We analyzed Aedes aegypti larval survivorship and adult emergence pattern with a factorial design combining predation risk (control and predator presence–Toxorhynchites haemorrhoidalis larvae) and SCCS. Neither SCCS nor predation risk affected Aedes aegypti larval survivorship, but adult emergence pattern was affected by SCCS. Accordingly, our results did not indicate interactive effects of SCCS and predation risk on larval survivorship and emergence pattern of Aedes aegypti reared in SCCS in western Amazonia. Aedes aegypti is resistant to SCCS conditions tested, mainly due to high larval survivorship, even under Extreme SCCS, and warmer scenarios increase adult Aedes aegypti emergence. Considering that Aedes aegypti is a health problem in western Amazonia, an implication of our findings is that the use of predation cues as biocontrol strategies will not provide a viable means of controlling the accelerated adult emergence expected under the IPCC climatic scenarios.
Introduction
Climate change is among the main environmental concerns of this century [1]. Its effects on arthropod vectors have been stimulating intense research, due to the risks that such vectors may pose to human health. Mosquitoes are one of the main vectors of human diseases, globally causing more than 17% of all infectious diseases [2]. Worldwide, the geographic range of mosquitoes is expanding [3], and the number of vector-borne diseases has also increased in recent years [4]. The main mosquito-borne diseases, such as dengue and malaria cause some 700,000 deaths annually, and the large numbers of people infected often overloads health systems [2]. Aedes aegypti (Ae. aegypti) mosquitoes are one of the main disease vectors, being responsible for the transmission of dengue, yellow fever, Zika, and chikungunya viruses. Around the world, some 390 million people are infected with dengue virus each year [5]. The effects of climate change on adult disease vectors are well known [6, 7], because the virus is transmitted during this stage. However, few studies focus on larval life-history, despite it being well known that changes that occur in the environment of the larval stage, such as climate change, may shape the development and behavior of adults (this being known as a carry-over effect) [8].
Climate change affects biodiversity at multiple levels. It may cause shifts on biomass [9], metabolism and behavior [10], at the individual level and, at population level, it can alter species distribution via changes in local conditions. Consequently, community composition can be altered by climate change [11], changing ecosystems and food webs [12–14]. There are two ways in which predator-prey interactions are influenced by climate change. First, it can increase the metabolic rates of individuals, as a consequence of higher temperatures [12], affecting the ability of predators to forage, capture and handle prey. In this way, climate change may modify prey density (density-mediated interactions) [15]; Second, besides direct predation, climate change alter predator-prey interactions via production, transmission, and reception of chemical cues. Under such circumstances, both predator and prey may suffer reduction in their abilities to detect each other [16]. In predation risk situations, releases of chemical cues is common, and the detection of predator by prey through them can modify feeding behavior and/or development rates (trait-mediated interactions) [15].
Temperature increases can influence the metabolism, behavior and life-history traits of adult mosquitoes [17, 18], including speeding up development [19–21]. This may result in enhanced offspring production and, consequently, increase the number of people infected by etiologic agents transmitted by mosquitoes. For example, Ryan et al. [3] showed that warming increases the transmission risk of diseases caused by Ae. aegypti and Aedes albopictus. Meanwhile, predation risk can cause different responses in mosquitoes; adult female Culex pipiens increase dispersal distance in the presence of predators [22], while predation risk does not alter Ae. aegypti survivorship [23], even though it decreases adults lifespan [24]. In natural systems, individuals, populations and community dynamics are all affected by both abiotic and biotic factors [25]. Thus, it is essential to better understand the unexplored interactive effects of simulated climate change scenarios (SCCS) and predation risk on Ae. aegypti larval stage. Understanding the efficacy of predation risk in the development of a disease vector species under different SCCS can provide information on carry-over effects, and a perspective into the efficacy of using predation cues as biocontrol strategies.
Our overall goal was to understand the single and interactive outcomes of SCCS and predation risk on larval survivorship and adult emergence pattern of Ae. aegypti. It is important to consider this interaction in an environment that favors the development of this species, such as western Amazonia, where the climate is hot and humid throughout the year. Accordingly, we conducted an experiment in a microcosm simulating real-time climatic condition in Manaus (Control) and gradual increase in temperature and CO2 in other three SCCS for this city in the year 2100. We used as a predator Toxorhynchites haemorrhoidalis (T. haemorrhoidalis Diptera: Culicidae) larvae to investigate the effect of predation risk on Ae. aegypti. This microcosm simulates four climate change scenarios predicted by the Fourth Assessment Report (AR4) of Intergovernmental Panel on Climate Change (IPCC) [26].
It is widely known that temperature increases, within thermal tolerance, affects development and behavior of Ae. aegypti [27]. Predation risk alone would not affect directly prey survivorship, but might lead to changes in prey development and behavior, as well as phenotypic alterations [15, 28]. Although, effects of predation risk under climate change are uncertain, they can either accelerate, decrease or cause no change in prey behavior and life-history characteristics [16]. Accordingly, we hypothesized that the single or interaction effects of both ecological factors, SCCS and predation risk, would not affect Ae. aegypti larval survivorship, mainly since SCCS lie within the thermal tolerance of Ae. aegypti [27]. Our second hypothesis was that increase in climatic variables (temperature and CO2) under SCCS would accelerate adult emergence of Ae. aegypti, and interactive effects of SCCS and predation risk would lead to earlier emergence. In this context, we discuss implications of our findings concerning the impact of predation risk on Ae. aegypti larvae reared under different SCCS to western Amazonia.
Methods
Simulated Climate Change Scenarios (SCCS)
The SCCS (microcosm) comprised of four chambers (4.05m x 2.94m), designed in accordance with the AR4-IPCC [26] recommendations to simulate temperature and CO2 concentrations for the year 2100 in Manaus. The microcosm is located in the Center for Studies of Adaptations of Aquatic Biota of the Amazon (long-term project ADAPTA), installed in the Laboratory of Ecophysiology and Molecular Evolution at the National Institute for Amazon Research (LEEM/INPA), Manaus, Amazonas, Brazil.
The SCCS include: i) Control: real-time current conditions in Manaus, Amazonas, Brazil, the average temperature is 25.76 ± 0.71°C and the CO2 concentration is 477.26 ± 9.38 parts per million by volume (ppmv). The other three scenarios reproducing respectively B1, A1B and A2 climatic conditions predicted by AR4-IPCC (2007) are: ii) Light: increase of ~1.7°C and ~218 ppmv CO2, iii) Intermediate: increase of ~2.4°C and ~446 ppmv CO2, and iv) Extreme: increase of ~4.5°C and ~861 ppmv CO2. Temperature and CO2 concentration of the Control SCCS varied instantaneously according to external values to capture real-time daily variation in Manaus. Values of temperature and CO2 concentration for the Control SCCS were used to estimate values for Light, Intermediate and Extreme SCCS (S1 and S2 Figs). The SCCS was monitored automatically every 2 min to maintain temperature and CO2 concentration values. Photoperiod was 12h light:12h dark, and humidity was approximately 80% in all chambers.
Predator and prey
To study the effect of predation risk on Ae. aegypti larval survivorship and adult emergence pattern, we designed two treatments: control (without predation) and predation risk using T. haemorrhoidalis larvae as the predator. This species frequently coexists in natural and artificial environments with Ae. aegypti [29]. Aedes aegypti larvae use different strategies to avoid predators, such as seeking shelter in macrophytes roots [30]. In such habitats, they are under predation risk effects, which can lead prey to decrease the search for food resources and allocate energy in defense instead of development [31, 32]. Larvae of T. haemorrhoidalis were collected in Manaus and, prior to the experiment, were housed individually in cups with water and fed daily with Ae. aegypti larvae until reaching the 3rd instar. Based on a pilot study, we estimated that each predator consumes one Ae. aegypti larva per day, and set this as the number to feed to the predators during the experiment. Predators were acclimatized in each SCCS for two days prior to the beginning of the experiment. We also acclimatized an additional two individuals for replacement purposes (in case a predator died).
Eggs of Ae. aegypti were obtained from colonies held by the Malaria and Dengue Laboratory at INPA. These colonies were established with wild-caught eggs collected in Manaus, using oviposition traps. Aedes aegypti eggs were collected with authorization and approval of the Brazilian Biodiversity Authorization and Information System (SISBIO; Permit 61563). We install multiple traps to obtain eggs in different private properties. Each owner gave us permission to install the traps. Fieldwork did not involve endangered or protected species. We placed filter paper to collect eggs of 4th and 5th generation adults from these colonies, and used these in the experiment. In each SCCS, the filter paper containing the eggs were placed in plastic containers until hatching occurred (± 17 hours). Following hatching, 60 first instar larvae were placed in each replication (see below).
Experimental design
We designed a factorial experiment to test the effect of predation risk and SCCS on Ae. aegypti larval survivorship and adult emergence pattern (Fig 1). Survivorship was defined as larvae that survived until the adult stage, to calculate this we used the number of emerged adults divided by the initial number of larvae in each replicate. Adult emergence pattern was estimated via counting the number of adults emerged in each replicate on a daily basis, time to adult emergence was considered from hatching to adult emergence. We included four replicates for each combination of factors (predation risk and SCCS). The experimental units were plastic containers (20x30x6 cm) with distilled water and fish food TetraMin™ to provide Ae. aegypti feed.
Fig 1. Experimental design.
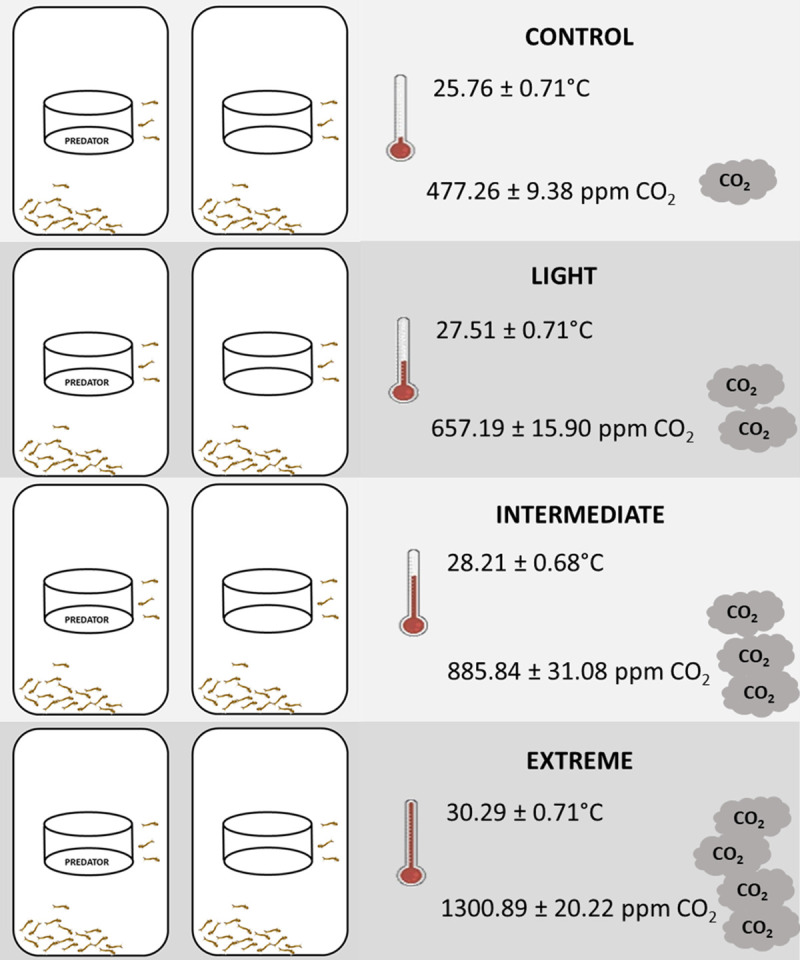
Experiment with two predation risk levels (predation risk–first column; and control–second column) in the four SCCS (Control, Light, Intermediate and Extreme—rows). Four replicates were used in the experiment. For each SCCS, the mean temperature and CO2 concentration are shown. Humidity was 83.91 ± 2.10% in all scenarios.
At the beginning of the experiment, we filled the plastic containers with 600 mL of distilled water and placed the predator cage in the center of each container. The predator cage was a circular plastic container, 10.1 cm in diameter, sealed with nylon mesh to ensure water circulation, but still prevent entrance of Ae. aegypti into the predator enclosure (see Fig 1). The predator cage was placed in all experimental replicates (predation risk or control) to avoid any effect caused by the presence of the cage itself.
Then, to each replicate, we added one predator and 60 randomly selected Ae. aegypti first instar larvae. Each replicate received 0.0264g of food every two days. The density 0.1 larvae/mL and the amount of food were calculated to avoid effects related to intraspecific competition [33]. If evaporation occurred, water was added to the plastic containers to maintain the original water level. The 3rd instar T. haemorrhoidalis larvae were fed daily with one 4th instar Ae. aegypti larva, other than those used in the experiment. Predation of Ae. aegypti larva by T. haemorrhoidalis larva releases chemical cues that could be perceived by the other larvae in the container [23].
Aedes aegypti were maintained in the replicates until adult emergence, we recorded the daily adult emergence and total survivorship in each replicate.
Statistical analysis
To evaluate whether the SCCS (Control, Light, Intermediate and Extreme), predation risk (predator and control), and their interaction, affected Ae. aegypti larval survivorship and adult emergence pattern, we performed a two-way ANOVA, while a post hoc least square means test was carried out if any of the tested factors tested were significant.
Adult emergence pattern was evaluated using two complementary approaches. We used linear mixed model considering the replicates as a random factor, an autocorrelation function to control repetition across days, and SCCS and predation risk as fixed factors. This model is equivalent to a two-way ANOVA with repeated measures, and was used to detect if SCCS, predation and their interaction affected the response variable, i.e. number of emergences, controlling the repeated measures and time autocorrelation. Second, to better understand the effect of these predictors on emergence patterns without including time in our models as fixed factor (keeping enough degrees of freedom), we evaluated the distribution of emergence time through the estimation of skewness and kurtosis of the adult emergence per day for each replicate. Skewness measures horizontal asymmetry in data distribution relative to normal curve. A symmetrical distribution is indicated by a skewness coefficient of zero; positive and negative skewness values indicate the data are right-skewed and have a longer right tail, and left-skewed with a longer left tail, respectively. For example, a right-skewed would indicate an early emergence of Ae. aegypti, whereas a left-skewed would indicate a late emergence. Kurtosis measures vertical asymmetry in data distribution relative to a normal curve. Zero values indicate a normal curve (mesokurtic); positive values (leptokurtic) indicate that the shape of the curve is more peaked than the normal distribution; negative values (platykurtic) indicate that the shape of the curve is flatter than the normal curve. A positive kurtosis would indicate that most larvae emerge at the same time, whereas a negative kurtosis would indicate a more evenly emergence distribution. Then, we applied a two-way ANOVA and a post hoc least square means test using skewness and kurtosis as response variables and SCCS and predation risk as predictors. Finally, we plotted emergence patterns per day. All analyses were carried out in the R environment [34].
Results
Survivorship
Mean Ae. aegypti larval survivorship was higher than 78% for all SCCS. Larval survivorship was not affected by SCCS (F = 2.77, P = 0.0638), predation risk (F = 1.14, P = 0.2957), or by interaction between them (F = 1.45, P = 0.2517) (Fig 2).
Fig 2. Aedes aegypti larval survivorship.
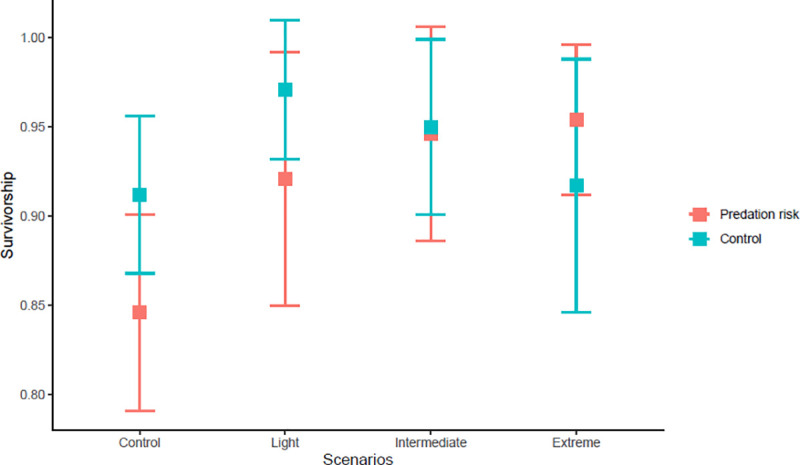
Mean (confidence interval of 95%) of total larval survivorship of Aedes aegypti larvae after 14 days in four SCCS (Control; Light- increase of ~1.7°C; Intermediate- increase of ~2.4°C; and Extreme- increase of ~4.5°C) in the presence (predation risk) and absence (control) of Toxorhynchites haemorrhoidalis predatory larva.
Emergence patterns
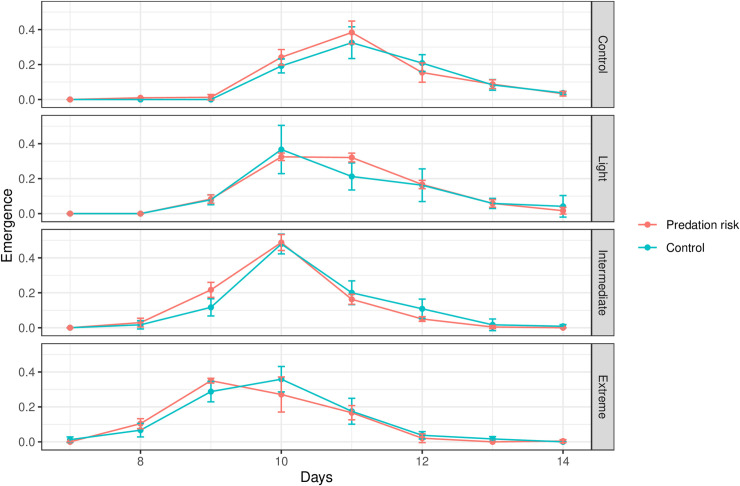
The first Ae. aegypti adult emergence was on day seven in the Extreme SCCS (n = 3 individuals), on day eight in the Intermediate SCCS (n = 11 individuals), while in the Light and Control SCCS the first adult emerged on day nine (n = 39 individuals; n = 3 individuals, respectively), with higher emergence rate in the Light SCCS. Adult Ae. aegypti emergence was not influenced by fixed factors SCCS (F = 0.0536; P = 0.9836), predation risk (F = 0.0221; P = 0.8819), or their interaction (F = 0.0282; P = 0.9936). However, adult emergence pattern measured by skewness and kurtosis revealed interesting results. SCCS significantly affected both skewness (F = 4.287; P = 0.0147) and kurtosis (F = 4.905; P = 0.0085). Emergence pattern in the Intermediate SCCS was not significantly different from the Control SCCS (df = 24; P = 0.0584), but was significantly higher (right skewness) than the Light (df = 24; P = 0.0269) and Extreme (df = 24; P = 0.0291) SCCS (Fig 3). This indicates that most individuals emerged earlier in the Intermediate SCCS. Furthermore, emergence pattern estimated by kurtosis in the Intermediate SCCS was different from the other three SCCS (Control: df = 24, P = 0.0449; Light: df = 24, P = 0.0286; and Extreme: df = 24, P = 0.0107) (Fig 4). The Intermediate SCCS was the only one with positive kurtosis (peak of frequency distribution), indicating that most individuals emerged on day ten, while in the other SCCS the adult emergence was more evenly distributed across several days (Fig 5).
Fig 3. Least squares mean (confidence interval of 95%) for skewness.
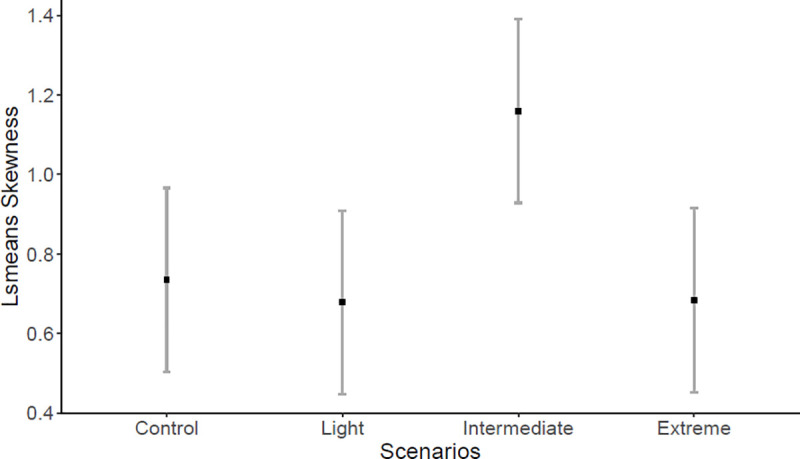
Emergence pattern estimated by skewness of Aedes aegypti between four SCCS (Control; Light- increase of ~1.7°C; Intermediate- increase of ~2.4°C; and Extreme- increase of ~4.5°C).
Fig 4. Least squares mean (confidence interval of 95%) for kurtosis.
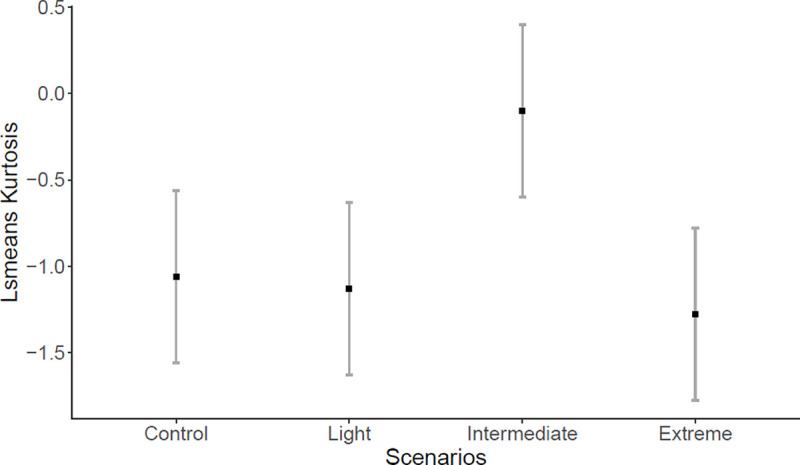
Emergence pattern estimated by kurtosis of Aedes aegypti between four SCCS (Control; Light- increase of ~1.7°C; Intermediate- increase of ~2.4°C; and Extreme- increase of ~4.5°C).
Fig 5. Mean (±SE) adult emergence of Aedes aegypti.
Mean adult emergence in four SCCS (Control; Light- increase of ~1.7°C; Intermediate- increase of ~2.4°C; and Extreme- increase of ~4.5°C), and in two predation treatments: control and under predation risk (predator: Toxorhynchites haemorrhoidalis larva), from the first (day 7) until the last day (day 14) when all individuals reached adulthood.
Discussion
We showed that simulated climate change scenarios accelerate development time of Ae. aegypti larvae, which agrees with previous studies in western Amazonia [21], with a emergence peak on a single day in Intermediate SCCS. We also found that Ae. aegypti larval survivorship was not affected by SCCS and predation risk, with larval survivorship rates being greater than 78% in all replicates, indicating the resilience of this species. We observed only SCCS did affect adult emergence pattern of Ae. aegypti, indicating that, in this study, climatic variables effects (temperature and CO2 concentration) are stronger ecological driver than predation risk, particularly those related to chemical and visual cues.
Other studies have also reported that predation risk did not affect development time or survivorship Ae. aegypti larvae [23, 35], although, in some cases, an effect was perceived in the adult stage. For example, blood feeding success was higher in Ae. aegypti females exposed to predator risk during the larval stage [23]. Similarly, Chandrasegaran et al. [35] found interactive effects of predation risk, competition and food availability on teneral reserves in Ae. aegypti males. Also, interaction between predation risk and high intraspecific competition reduced the egg production of Ae. aegypti females [23]. In our study, we did not find any effect of predation risk on Ae. aegypti larval survivorship or adult emergence pattern, even in interaction with SCCS. These findings have implications for human health, as the impact of predation risk may not keep pace with the accelerated development of Ae. aegypti larvae under SCCS. Consequently, a warmer world will have more mosquitoes and an increase in vector-borne diseases [36].
Toxorhynchites haemorroidalis larvae, here used as predation risk, is a natural predator of other immature culicids and share the same oviposition habitats as Ae. aegypti. Accordingly, Toxorhynchites larvae have the potential to be used as a biocontrol agent, especially against disease vector species [37]. The presence of T. haemorrhoidalis larvae in our experiment could broadcast to chemical cues that, if detected by the Ae. aegypti larvae, could change the adult emergence pattern. Many studies on pest control use predation cues to manage pests. For example, beetle larvae consumed fewer leaves when these leaves were previously exposed to predators [38]. Chemical trails of ladybird on plants repel aphids that are cereal pest [39]. However, our finding suggests that biocontrol strategies based on predation cues are not effective in the control of Ae. aegypti larvae. However, it is important to highlight that it does not mean the direct predation of Toxorhynchites is not efficient as a biocontrol agent.
Some non-exclusive explanations can account for the absence of the interaction effect between SCCS and predation risk on Ae. aegypti larvae survivorship and adult emergence pattern. This absence may be due to an alteration caused by climatic variables in the release of chemical cues or decreasing prey sensitivity to chemical signals of predator presence [16, 40]. In addition, the presence of Toxorhynchites does not seem to be a real threat to some mosquito species. For example, species of Aedes select sites for oviposition based on levels of available organic matter, and they do not avoid areas where predators are present. As a result, females prioritize sites with abundant resources for their progeny, regardless of predator presence [41, 42]. Although the ability to detect predation risk varies among Diptera, Romero et al. [43] observed that predation risk did not affect Diptera flower visitation rates, contrary to many other insect orders assessed by them. Thus, as with other dipterous insects, Ae. aegypti larvae may not be able to detect predation risk.
Our results revealed that predation risk does not modify Ae. aegypti adult emergence patterns, but also showed that an increase in climatic variables (temperature and CO2 concentration) caused distinct effects on emergence distribution of Ae. aegypti. We observed that the Intermediate and not the Extreme SCCS sped up the emergence of adult Ae. aegypti, agreeing with previous studies showing that warmer environments increase development [19–21]. Typically, the relationship between temperature and life history traits is non-linear [44], and species have a thermal optimum to complete their development. Temperatures above the thermal optimum decrease species performance (e.g. immature survival). Although emergencies started earlier in the Extreme SCCS, the Intermediate SCCS revealed a pronounced emergence on a single day (10th day). This suggests that the larval response to predation risk does not change the phenological patterns of Ae. aegypti larval development, but reveals that individual SCCS can alter patterns of emergence, a result with consequences and implications for human health.
Considering that Ae. aegypti is the vector of several diseases, and that the Intermediate SCCS is not so far from becoming a reality given the nature of mitigation measures taking place and the speed of their implementation, it is important to carry out this species might behave in the face of SCCS. As the climate change patterns across Amazonia will not be homogeneous [45], it is important to carried out such experiments in ways that incorporate the nature of regional differences. There are many uncertainties in how combined effects of biotic and abiotic factors may influence Ae. aegypti larval life-history characteristics; our results add new pieces to this puzzle. Western Amazonia and regions with similar climatic conditions, will probably suffer increases in mosquito populations, partly as a result of the intensive urbanization process (the main driver to their establishment), and partly as a result of climate change, since, as we showed here, Ae. aegypti larvae develops faster under SCCS. Our study showed that biocontrol methods simulating predation risk using T. haemorrhoidalis larvae are unlikely to be effective for Ae. aegypti control, because these signals have no effect on this species. Though the use of T. haemorrhoidalis in direct biological control should not be discounted. Therefore, in the near future, shorter life cycles will result in high numbers of mosquitoes, with potential increase in cases of diseases caused by this vector.
Supporting information
(TIF)
(TIF)
(TIF)
(TIF)
(TIF)
Acknowledgments
We are grateful to Mohamed F. Sallam, Rachel Sippy, and two other reviewers for their useful comments and advice. We also thank Dr. Adalberto Val for microcosm use, MSc. Raquel Telles de Moreira Sampaio and Ulysses Barbosa for creation rooms of Toxorhynchites, and MSc. William Ribeiro da Silva for support in mosquitoes breeding. Universidade Federal de Mato Grosso do Sul (UFMS) and Instituto Nacional de Pesquisas da Amazônia (INPA) for the support in experiments.
Data Availability
All relevant data are within the manuscript and its Supporting Information files.
Funding Statement
ACPB received part of fellowship from Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES) and part from Fundação de Apoio ao Desenvolvimento do Ensino, Ciência e Tecnologia do Estado de Mato Grosso do Sul (Fundect). ACPB was supported by Fundo Brasileiro para Biodiversidade (FUNBIO) and Instituto Humanize grant. FVN received a Post-doctoral fellowship #88882.317337/2019-01 from CAPES; NH (307849/2014-7; 308970/ 2019-5) and FOR are Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPQ) research fellow. This study was also, partially, financed by CAPES - Finance Code 001. The views expressed in the present article are those of the authors and not necessarily those of any funding agencies. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.IPCC. Climate Change 2014: Synthesis Report. Contribution of Working Groups I, II and III to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change [Core Writing Team, R.K. Pachauri and L.A. Meyer (eds.)]. IPCC, Geneva, Switzer. 2014.
- 2.WHO. World Health Organization. Mosquito borne disease. 2017. Available: https://www.who.int/neglected_diseases/vector_ecology/mosquito-borne-diseases/en/
- 3.Ryan SJ, Carlson CJ, Mordecai EA, Johnson LR. Global expansion and redistribution of Aedes-borne virus transmission risk with climate change. PLoS Negl Trop Dis. 2019;13: 1–20. 10.1371/journal.pntd.0007213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Watts N, Amann M, Ayeb-Karlsson S, Belesova K, Bouley T, Boykoff M, et al. The Lancet Countdown on health and climate change: from 25 years of inaction to a global transformation for public health. Lancet. 2018;391: 581–630. 10.1016/S0140-6736(17)32464-9 [DOI] [PubMed] [Google Scholar]
- 5.Bhatt S, Gething PW, Brady OJ, Messina JP, Farlow AW, Moyes CL, et al. The global distribution and burden of dengue. Nature. 2013;496: 504–507. 10.1038/nature12060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Campbell-Lendrum D, Manga L, Bagayoko M, Sommerfeld J. Climate change and vector-borne diseases: What are the implications for public health research and policy? Philos Trans R Soc B Biol Sci. 2015;370: 1–8. 10.1098/rstb.2013.0552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Franklinos LHV, Jones KE, Redding DW, Abubakar I. The effect of global change on mosquito-borne disease. Lancet Infect Dis. 2019;19: e302–e312. 10.1016/S1473-3099(19)30161-6 [DOI] [PubMed] [Google Scholar]
- 8.O’Connor CM, Orris D, Rossin GLTC. Biological carryover effects: linking common concepts and mechanisms in ecology and evolution. 2014;5: 1–11. 10.1890/ES13-00388.1 [DOI] [Google Scholar]
- 9.Dossena M, Yvon-Durocher G, Grey J, Montoya JM, Perkins DM, Trimmer M, et al. Warming alters community size structure and ecosystem functioning. 2012;279: 3011–3019. 10.1098/rspb.2012.0394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Laws AN. Climate change effects on predator–prey interactions. Curr Opin Insect Sci. 2017;23: 28–34. 10.1016/j.cois.2017.06.010 [DOI] [PubMed] [Google Scholar]
- 11.Gruner DS, Bracken MES, Berger SA, Eriksson BK, Gamfeldt L, Matthiessen B, et al. Effects of experimental warming on biodiversity depend on ecosystem type and local species composition. Oikos. 2017;126: 8–17. 10.1111/oik.03688 [DOI] [Google Scholar]
- 12.Vucic-Pestic O, Ehnes RB, Rall BC, Brose U. Warming up the system: Higher predator feeding rates but lower energetic efficiencies. Glob Chang Biol. 2011;17: 1301–1310. 10.1111/j.1365-2486.2010.02329.x [DOI] [Google Scholar]
- 13.Bellard C, Bertelsmeier C, Leadley P, Thuiller W, Courchamp F. Impacts of climate change on the future of biodiversity. Ecol Lett. 2012;15: 365–377. 10.1111/j.1461-0248.2011.01736.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Boukal DS, Bideault A, Carreira BM, Sentis A. Species interactions under climate change: connecting kinetic effects of temperature on individuals to community dynamics. Curr Opin Insect Sci. 2019;35: 88–95. 10.1016/j.cois.2019.06.014 [DOI] [PubMed] [Google Scholar]
- 15.Preisser EL, Bolnick DI, Benard ME. Scared to death? The effects of intimidation and consumption in predator-prey interactions. Ecology. 2005;86: 501–509. 10.1890/04-0719 [DOI] [Google Scholar]
- 16.Draper AM, Weissburg MJ. Impacts of global warming and elevated CO2 on sensory behavior in predator-prey interactions: A review and synthesis. Front Ecol Evol. 2019;7 10.3389/fevo.2019.00072 [DOI] [Google Scholar]
- 17.Mordecai EA, Cohen JM, Evans M V., Gudapati P, Johnson LR, Lippi CA, et al. Detecting the impact of temperature on transmission of Zika, dengue, and chikungunya using mechanistic models. PLoS Negl Trop Dis. 2017;11: 1–18. 10.1371/journal.pntd.0005568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ezeakacha NF, Yee DA. The role of temperature in affecting carry-over effects and larval competition in the globally invasive mosquito Aedes albopictus. Parasites and Vectors. 2019;12: 1–11. 10.1186/s13071-018-3256-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Carrington LB, Armijos MV, Lambrechts L, Barker CM, Scott TW. Effects of Fluctuating Daily Temperatures at Critical Thermal Extremes on Aedes aegypti Life-History Traits. PLoS One. 2013;8 10.1371/journal.pone.0058824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Couret J, Dotson E, Benedict MQ. Temperature, larval diet, and density effects on development rate and survival of Aedes aegypti (Diptera: Culicidae). PLoS One. 2014;9 10.1371/journal.pone.0087468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Leonel BF, Koroiva R, Hamada N, Ferreira-Keppler RL, Roque FO. Potential effects of climate change on ecological interaction outcomes between two disease-vector mosquitoes: A mesocosm experimental study. J Med Entomol. 2015;52: 866–872. 10.1093/jme/tjv068 [DOI] [PubMed] [Google Scholar]
- 22.Alcalay Y, Tsurim I, Ovadia O. Female mosquitoes disperse further when they develop under predation risk. Behav Ecol. 2018;29: 1402–1408. 10.1093/beheco/ary113 [DOI] [Google Scholar]
- 23.Chandrasegaran K, Juliano SA. How do trait-mediated non-lethal effects of predation affect population-level performance of mosquitoes? Front Ecol Evol. 2019;7: 1–12. 10.3389/fevo.2019.00025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bellamy SK, Alto BW. Mosquito responses to trait- and density-mediated interactions of predation. Oecologia. 2018;187: 233–243. 10.1007/s00442-018-4107-5 [DOI] [PubMed] [Google Scholar]
- 25.Huston MA, McBride AC. Evaluating the relative strenghts of biotic versus abiotic controls on ecosystem processes In: Loreau M, Naeem S, Inchausti P, editors. Biodiversity and Ecosystem functioning: Synthesis and Perspectives. Oxford, UK: Oxford University Press; 2002. pp. 47–60. [Google Scholar]
- 26.IPCC. Climate Change 2007: Synthesis Report. Contribution of Working Groups I, II and III to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change [Core Writing Team, Pachauri, R.K and Reisinger, A. (eds.)]. IPCC, Geneva, Swi. 2007. 10.1038/446727a [DOI]
- 27.Reinhold JM, Lazzari CR, Lahondère C. Effects of the environmental temperature on Aedes aegypti and Aedes albopictus mosquitoes: A review. Insects. 2018;9 10.3390/insects9040158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Stoks R, De Block M, McPeek M. Alternative growth and energy storage responses to mortality threats in damselflies. Ecol Lett. 2005;8: 1307–1316. 10.1111/j.1461-0248.2005.00840.x [DOI] [Google Scholar]
- 29.Steffan W, Evenhuis N. Biology of Toxorhynchites. Annu Rev Entomol. 1981;26: 159–181. 10.1146/annurev.en.26.010181.001111 [DOI] [Google Scholar]
- 30.Ofulla A, Karanja D, Omondi R, Okurut T, Matano A, Jembe T, et al. Relative abundance of mosquitoes and snails associated with water hyacinth Relative abundance of mosquitoes and snails associated with water hyacinth and hippo grass in the Nyanza gulf of Lake Victoria. Lakes Reserv Res Manag. 2010;15: 255–271. 10.1111/j.1440-1770.2010.00434.x [DOI] [Google Scholar]
- 31.Lima SL. Stress and Decision Making under the Risk of Predation: Recent Developments from Behavioral, Reproductive, and Ecological Perspectives. Adv Study Behav. 1998;27: 215–290. 10.1016/S0065-3454(08)60366-6 [DOI] [Google Scholar]
- 32.Werner EE, Peacor SD. A review of trait-mediated indirect interactions in ecological communities. Ecology. 2003;84: 1083–1100. 10.1890/0012-9658(2003)084[1083:AROTII]2.0.CO;2 [DOI] [Google Scholar]
- 33.Roberts D, Kokkinn M. Larval crowding effects on the mosquito Culex quinquefasciatus: Physical or chemical? Entomol Exp Appl. 2010;135: 271–275. 10.1111/j.1570-7458.2010.00993.x [DOI] [Google Scholar]
- 34.R Core Team. R: A language and environment for statistical computing. R Foundation for Statistical Computing; Vienna, Austria; 2016. Available: https://www.r-project.org/. [Google Scholar]
- 35.Chandrasegaran K, Rao Kandregula S, Quader S, Juliano SA. Context-dependent interactive effects of nonlethal predation on larvae impact adult longevity and body composition. PLoS One. 2018;13: 1–19. 10.1371/journal.pone.0192104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Iwamura T, Guzman-holst A, Murray KA. Accelerating invasion potential of disease vector Aedes aegypti under climate change. Nat Commun. 2020. 10.1038/s41467-020-16010-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Focks DA. Toxorhynchites as biocontrol agents. J Am Mosq Control Assoc. 2007;23: 118–127. 10.2987/8756-971X(2007)23[118:TABA]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- 38.Hermann SL, Thaler JS. Prey perception of predation risk: volatile chemical cues mediate non ‑ consumptive effects of a predator on a herbivorous insect. 2014. 10.1007/s00442-014-3069-5 [DOI] [PubMed] [Google Scholar]
- 39.Ninkovic V, Feng Y, Olsson U, Pettersson J. Ladybird footprints induce aphid avoidance behavior. Biol Control. 2013;65: 63–71. 10.1016/j.biocontrol.2012.07.003 [DOI] [Google Scholar]
- 40.Harley CDG. Climate change, keystone predation, and biodiversity loss. Science (80-). 2011;334: 1124–1127. 10.1126/science.1210199 [DOI] [PubMed] [Google Scholar]
- 41.Juliano SA, Lounibos LP, Nishimura N, Greene K. Your worst enemy could be your best friend: Predator contributions to invasion resistance and persistence of natives. Oecologia. 2010;162: 709–718. 10.1007/s00442-009-1475-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Vonesh JR, Blaustein L. Predator-induced shifts in mosquito oviposition site selection: A meta-analysis and implications for vector control. Isr J Ecol Evol. 2010;56: 263–279. [DOI] [Google Scholar]
- 43.Romero GQ, Antiqueira PAP, Koricheva J. A Meta-Analysis of Predation Risk Effects on Pollinator Behaviour. 6 10.1371/journal.pone.0020689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mordecai EA, Caldwell JM, Grossman MK, Lippi CA, Johnson LR, Neira M, et al. Thermal biology of mosquito-borne disease. Ecol Lett. 2019;22: 1690–1708. 10.1111/ele.13335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Marengo J, Nobre CA, Betts RA, Cox PA, Sampaio G, Salazar L. Global Warming and Climate Change in Amazonia: Climate-Vegetation Feedback and Impacts on Water Resources In: Kelle M, Bustamante M, Gash J, Dias P, editors. Amazonia and Global Ghange. AGU Books Board; 2009. p. 566. [Google Scholar]