ABSTRACT
Background:
In contrast to static stretching (SS), previous research has demonstrated increases in flexibility after an acute bout of self-myofascial release (SMR) without any subsequent decreases in force output. Previous research has utilized measures of surface electromyography (sEMG) and mechanomyography (MMG) to examine the influence of SS on the electrical and mechanical processes of muscle activation, respectively. However, there is a lack of research examining the potential changes in electro-mechanical muscle activation post-SMR.
Purpose:
To examine the influence of SMR, via an acute bout of foam rolling (FR) to the vastus lateralis (VL), on the expression of knee extension force output and the inter-muscular electro-mechanical activation of the quadriceps musculature.
Study Design:
Randomized crossover trial.
Methods:
Twenty (10 males, 10 females) recreationally-active participants with prior FR experience completed both SMR and control (CON) testing protocols during separate testing sessions that were conducted in a randomized order 48 hours apart. During the SMR protocol, participants performed 3 sets of 60 seconds of FR over the VL portion of their quadriceps musculature, with 60 seconds of rest between sets. During the CON protocol, participants quietly sat upright for 10 minutes. Peak knee extension force output (Forcepeak) data, as well as sEMG and MMG data from the VL and the rectus femoris (RF) were collected during maximal voluntary isometric contractions (MVICs) before and after both testing protocols. Root mean square sEMG and MMG amplitudes were calculated to represent electro-mechanical muscle activation of the VL (VL–sEMGRMS, VL–MMGRMS) and RF (RF–sEMGRMS, RF–MMGRMS) musculature.
Results:
Repeated measures analyses of variance (RM ANOVAs) identified a significant (p < 0.05) increase in Forcepeak within the SMR protocol among males, but no change among females. No statistically significant changes in any electro-mechanical muscle activation measures were identified pre-to-post-SMR within either sex.
Conclusion:
In contrast to the SS literature body, these results suggest that SMR does not influence the electro-mechanical aspects of muscle activation during MVICs. These results provide support for the absence of decreases in force output post-SMR, but further examination regarding the potential muscle mass influence of SMR on electro-mechanical muscle function remains warranted.
Level of Evidence:
2c
Keywords: electro-mechanical efficiency, foam rolling, mechanomyography, surface electromyography
INTRODUCTION
Self-myofascial release (SMR), applied via foam rolling (FR), is commonly utilized by individuals as a method of increasing joint range of motion (ROM).1-3 The prescription of SMR has also grown in popularity among practitioners,4-5 with 81% of allied health professionals utilizing FR within their practice.6 One of the potential factors associated with the increased utilization of SMR has been the fact that previous research has demonstrated increases in joint ROM after an acute bout of SMR, theoretically due to an increase in muscle tissue extensibility, without any subsequent decreases in force output.7-9 The lack of influence on force output after SMR-related increases in joint ROM also differs from the static stretching (SS) literature, which has routinely demonstrated a decrease in force and power output after an acute bout of SS.10-12 As such, an emerging body of evidence suggests that practitioners can prescribe SMR interventions without detrimentally influencing subsequent athletic performance.13
The physiological mechanisms in which SS and SMR influence muscle tissue extensibility have been largely associated with either neural and/or morphological mechanisms. Specifically, potential neural mechanisms of both SS14 and SMR1 include activation of Golgi tendon organs (GTOs) and/or mechanoreceptors (e.g., group III/IV afferents), which decrease the efferent α-motorneuron drive, creating a relaxation effect on the target musculature and an increase in muscle tissue extensibility. Potential morphological mechanisms of SS include changes in viscoelastic properties of the muscle-tendon unit (MTU), thereby increasing the compliance of the MTU, and the subsequent extensibility of the target musculature.15 Since these mechanisms may also inhibit the neural activation and viscoelastic properties of the target musculature, these are often the mechanisms used to describe the decrease in force-generating capacity of the musculature after SS.16 In contrast, potential morphological mechanisms of SMR include a reduction in fascial adhesions and changes in fluid mechanics of the fascia surrounding the target musculature due to a thixotropy-like effect,17-18 rendering the fascia more malleable and increasing the extensibility of the target musculature.1 While these may be slightly different mechanisms from those for SS, these processes theoretically influence the viscoelastic properties of the fascia, and in turn, increase the pliability of the muscle tissue resulting in increased muscle extensibility.
Unlike the SMR literature body, previous SS research has utilized non-invasive methodologies to examine both the neural and mechanical properties of muscle function pre- and post-SS. Specifically, surface electromyographic (sEMG) measures, which represent the electrical processes of muscle activation, and mechanomyographic (MMG) measures, which represent the mechanical processes of muscle activation, have been simultaneously collected to assess electro-mechanical muscle function in a variety of capacities,19-20 including to examine potential changes as a result of SS.21-26 For example, previous research has demonstrated a decrease in sEMG amplitude,27-29 but an increase in MMG amplitude,25 during isometric muscle actions after an acute bout of SS. These results suggest that SS is capable of creating both electrical and mechanical changes in muscle function, providing evidence to the previously described neural and morphological alterations that theoretically occur as a result of SS.14-16
In contrast to the SS literature body, there is a lack of research examining the potential changes in electro-mechanical function as a result of SMR. Currently, there are equivocal findings in the literature regarding the influence of SMR on sEMG activity, with previous research demonstrating a decrease in sEMG measures post-SMR,30-31 as well as no changes in sEMG measures post-SMR.7-9,32-33 These conflicting results could be due to changes in inter-muscular activation post-SMR, as Cavanaugh et al.31 demonstrated a significant decrease in sEMG measures of the hamstrings after FR was applied to the quadriceps musculature, but further investigation of potential muscle coordination changes is still required. In addition, the influence of SMR on MMG measures has yet to be examined and no previous research has simultaneously collected both sEMG and MMG measures. Thus, the examination of the influence of SMR on the mechanical aspects of electro-mechanical muscle function remain largely unexplored. Furthermore, previous research has also suggested that MMG signal may be particularly sensitive to the tissue stiffness of the vastus lateralis given the longitudinal covering of the iliotibial band along this aspect of the quadriceps musculature.34 Therefore, the purpose of this study was to examine the influence of SMR, via an acute bout of FR to the vastus lateralis (VL), on the expression of knee extension force output and the inter-muscular electro-mechanical activation of the quadriceps musculature.
METHODS
Participants
Twenty (10 males, 10 females) recreationally-active35 participants with prior FR experience volunteered to participate in the current study (mean ± SD, age: 24.2 ± 2.6 yrs; height: 173.1 ± 9.4 cm; body mass: 70.7 ± 17.6 kg). This study was approved by the Institutional Review Board at the University of Wisconsin-Milwaukee. Before any data were collected, all participants provided written informed consent to the study protocols. The functionally dominant leg of each participant was determined based upon the leg in which the participant would prefer to kick a ball in order to achieve maximal distance.36
Testing Protocols
To ensure a relative similar starting point in terms of blood flow and tissue warmth, all participants first completed a 5-minute warm-up on a bicycle ergometer (Ergomedic 828E, Monark Exercise AB, Vansbro, Sweden), at 0.5 kp of resistance and a cadence of 50 rpm, at the beginning of each testing period. Each participant completed both the SMR and control (CON) testing protocols (i.e., crossover design). Testing protocols were completed in a research laboratory 48 hours apart during separate testing sessions. Participants were instructed to not perform any resistance training and/or vigorous physical activity or exercise in-between testing sessions. The order these protocols were completed was counterbalanced in a randomized fashion across all participants.
SMR Protocol
During the SMR protocol, participants performed 3 sets of 60 seconds of FR, with 60 seconds of rest between sets, in a similar fashion to the protocol previously utilized by MacDonald et al.8 Specifically, participants performed FR using a high-density foam roller (Black Mountain Products, McHenry, IL) over the entire length of the VL portion (i.e., proximal to distal aspects of femur) of their respective quadriceps musculature of their dominant leg.37 Participants were instructed to perform the FR in the plank position, with their opposite leg crossed over the kicking leg and as much of their body weight as tolerable placed on the foam roller (Figure 1). Although all participants reported prior FR experience, proper FR technique37 was monitored by the researchers during both testing sessions. Beyond instructing the participants to perform the FR in a slow and controlled manner, the speed of the FR was not regulated for by the researchers.
Figure 1.
Foam rolling of the vastus lateralis (VL) musculature during the self-myofascial release (SMR) protocol.
CON Protocol
During the CON protocol, participants were instructed to quietly sit upright for 10 minutes. Participants were not allowed to walk, stand up, or stretch during this period.
Maximal Voluntary Isometric Contractions
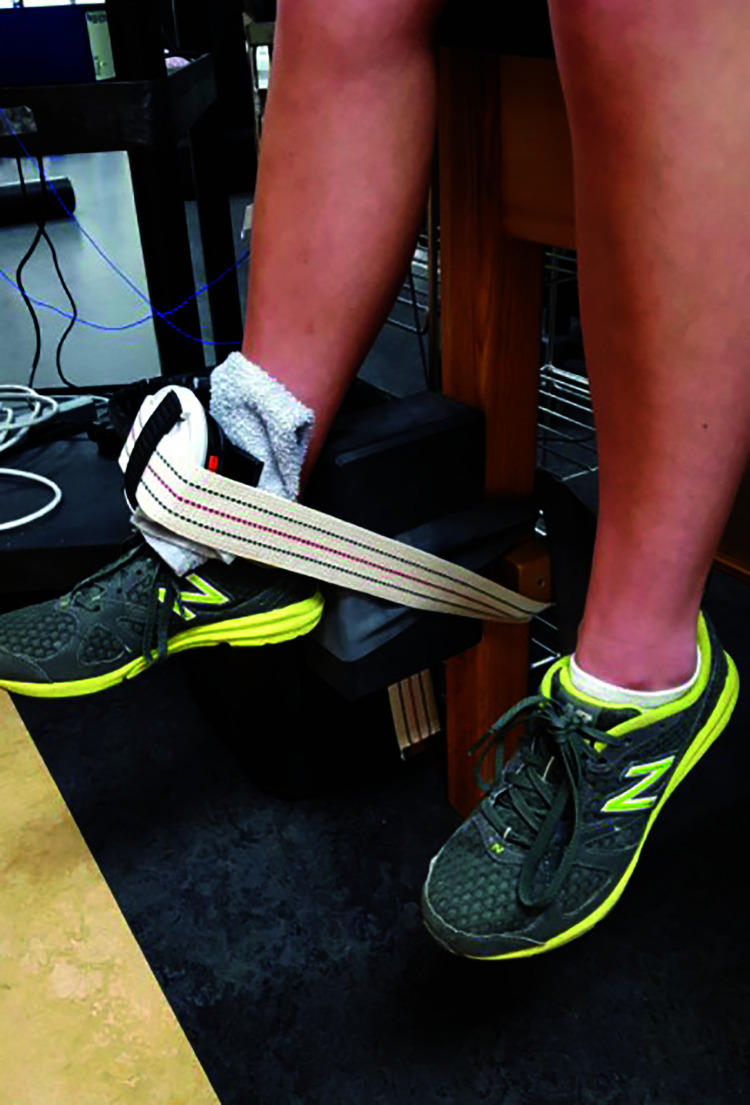
Pre- and post- each testing protocol, participants performed five maximal isometric voluntary contractions (MVICs) of knee extension consistent with standard manual muscle testing techniques38 and according to muscle testing protocols previously utilized in the literature.39 Specifically, the dominant leg of each participant was secured to a treatment table at 60 ° of knee flexion and 90 ° of concurrent hip flexion (Figure 2).40 Participants were instructed to sit upright with their arms across their chest and to maintain each MVIC of knee extension for five seconds. Verbal encouragement during each MVIC, as well as 60 seconds of rest between each MVIC, were provided by the researchers.
Figure 2.
Collection of knee extension force data utilizing a handheld dynamometer.
Knee Extension Force Data
The expression of peak knee extension force output (kg) during each MVIC was measured using a handheld dynamometer (MicroFET 2, Hoggan Health Industries, Salt Lake City, UT) that was secured with an immovable strap (Figure 2).41 The handheld dynamometer was placed on the anterior aspect of the tibia and just proximal to the malleoli of the dominant leg of each participant.40 Previous research has reported adequate validity (r = 0.894),42 as well as excellent intrasession (ICCs = 0.82–0.93) and good-to-excellent intersession (ICCs = 0.70–0.92) test-retest reliability,43 of the assessment of peak knee extension force via handheld dynamometry. Only force data from the MVIC resulting in the greatest peak knee extension muscular force output (Forcepeak) for each participant were used in all subsequent data processing and statistical analyses.
Electro-Mechanical Muscle Activation Data
All electro-mechanical muscle activation data (i.e., sEMG, MMG, & EME) were collected, and are subsequently reported, according to commonly utilized sEMG and MMG standards.44 Specifically, sEMG and MMG data were collected from the VL and rectus femoris (RF) musculature associated with quadriceps of the dominant leg of each participant. All sEMG and MMG signals were collected using the Noraxon Telemyo 2G System at a sampling frequency of 1,500 Hz and processed and analyzed using MyoResearch XP software (Noraxon U.S.A., Inc., Scottsdale, AZ). Only electro-mechanical muscle activation data from the Forcepeak MVIC for each participant were used in all subsequent signal processing and statistical analyses.
sEMG Data
All sEMG data were collected using bipolar Ag/AgCl surface electrodes (MeshTrode A10040-60, Vermed, Buffalo, NY) placed along the longitudinal axis of the VL and RF quadriceps muscle bellies, with the reference electrode placed over the patella of each participant.45 To reduce any impedance of the sEMG signals, the skin of each participant was first shaved with a disposable razor and abraded using isopropyl alcohol and sterile gauze before electrode placement. All sEMG data were differentially amplified (gain = 2000×) and digitally bandpass filtered (fourth-order Butterworth) at 10–500 Hz. Root mean square amplitudes (μV) were calculated from sEMG signals collected from the VL (VL–sEMGRMS) and RF (RF–sEMGRMS) musculature using a smoothing window of 50 milliseconds during three second epochs corresponding to seconds 1.0–4.0 of the five-second knee extension MVIC. Black permanent marker was used to outline electrode placement to ensure the same electrode placement during both SMR and CON protocols.46 Previous research has demonstrated excellent intrasession test-retest reliability of sEMGRMS data collected during MVICs from the quadriceps musculature (ICCs = 0.95–0.97),47 as well as good intersession test-retest reliability of sEMG amplitude data collected during MVICs in general (ICCs = 0.68–0.74) (Yang & Winter, 1983).48 In addition, previous research within both the SS21-24,26 and SMR9 literature bodies have examined sEMG data in a similar manner.
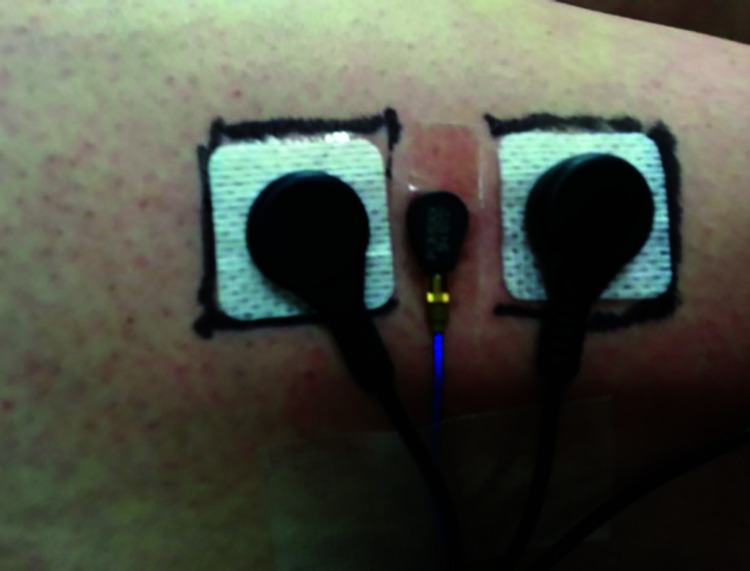
MMG Data
All MMG data were collected using tri-axial piezoelectric accelerometers (352A24, PCB Piezotronics, Depew, NY). Each accelerometer (dimensions = 0.5 cm × 0.5 cm × 1.0 cm; sensitivity = 100 mVžg-1) was placed between the previously placed proximal and distal bipolar sEMG electrodes using double-sided adhesive tape,49 resulting in an inter-electrode distance of 2.5 cm between sEMG electrodes (Figure 3). All MMG data were digitally bandpass filtered at 5-150 Hz.50 Similar to the sEMG signals, root mean square amplitudes (mžs-2) were calculated from MMG signals collected from the VL (VL–MMGRMS) and RF (RF–MMGRMS) musculature using a smoothing window of 50 milliseconds during three second epochs corresponding to seconds 1.0–4.0 of the 5-second knee extension MVIC. Previous research has demonstrated excellent intrasession test-retest reliability (ICCs = 0.84–0.96)51 and excellent intersession test-retest reliability (ICC = 0.81)52 of MMGRMS data collected during MVICs from the quadriceps musculature, as well as among MMGRMS data collected during MVICs in general (ICCs = 0.94–0.97).46 In addition, previous research within the SS literature body has examined MMG data in a similar manner.21-24,26
Figure 3.
Arrangement of surface electromyographic (sEMG) electrodes and mechanomyographic (MMG) sensors.
Statistical Analyses
All statistical analyses were conducted using the IBM SPSS 25 software package (IBM Corp., Armonk, NY). An alpha of p < 0.05 determined statistical significance for all analyses. Previous research has demonstrated differences in both electrical53 and mechanical54 muscle function between sexes during MVICs. In particular, differences in MMGRMS data have been attributed to potential differences in muscle stiffness between males and females due to sex differences in muscle mass.54-56 Due to this, independent t-tests were first conducted to identify potential differences in Forcepeak, VL–sEMGRMS, RF–sEMGRMS, VL–MMGRMS, and RF–MMGRMS measures at baseline between sexes. Since statistically significant differences were identified within all measures (Table 1), all subsequent statistical analyses were split by sex.
Table 1.
Baseline sex differences in participant characteristics and measures of interest (data presented as mean ± SE).
| Measure | Males | Females |
|---|---|---|
| Age (yrs) | 24.20 ± 0.71 | 24.10 ± 0.95 |
| Height (cm) | 180.33 ± 2.22* | 165.80 ± 1.54 |
| Body Mass (kg) | 82.08 ± 4.96* | 59.30 ± 3.71 |
| Forcepeak (kg) | 33.94 ± 2.86* | 21.61 ± 1.01 |
| VL–sEMGRMS (μV) | 371.57 ± 72.22* | 180.24 ± 26.51 |
| RF–sEMGRMS (μV) | 205.67 ± 22.30* | 115.02 ± 13.37 |
| VL–MMGRMS (m·s-2) | 0.42 ± 0.04* | 0.32 ± 0.04 |
| RF–MMGRMS (m·s-2) | 0.59 ± 0.04* | 0.36 ± 0.03 |
Forcepeak = peak knee extension force output; VL = vastus lateralis musculature; RF = rectus femoris musculature; sEMGRMS = root mean square amplitude of the surface electromyographic data; MMGRMS = root mean square amplitude of the mechanomyographic data. *Males significantly greater than Females (p < 0.05)
In order to examine the potential influence of an acute bout of SMR on the expression of knee extension force output and the electro-mechanical activation of the VL and RF quadriceps musculature, separate 2 × 2 (protocol × time) within-between repeated measure analyses of variance (RM ANOVAs) and follow-up main effects within each protocol were conducted for each measure of interest: Forcepeak, VL–sEMGRMS, RF–sEMGRMS, VL–MMGRMS, and RF–MMGRMS. In addition, if significant differences in any measure were identified, the analysis was repeated with body mass included as a covariate in order to identify the potential influence of muscle mass on that measure of interest.
Finally, standardized effect size statistics were calculated to determine potential practical pre-to-post changes in the expression of knee extension force output and the electro-mechanical activation of the VL and RF quadriceps musculature. Given the small sample size, Hedge's g effect size statistics were chosen57 and were interpreted using the following criteria58: trivial (g ≤ 0.19), small (0.20 ≤ g ≤ 0.49), medium (0.50 ≤ g ≤ 0.79), large (0.80 ≤ g ≤ 1.29), and very large (1.30 ≤ g).
RESULTS
Descriptive statistics (mean ± SE) within sexes are reported in Table 2 for all measures of knee extension force output and electro-mechanical muscle activation collected during each testing protocol (i.e., SMR & CON).
Table 2.
Influence of an acute bout of SMR on the expression of knee extension force output and electro-mechanical muscle activation.
| SMR | CON | |||||||
|---|---|---|---|---|---|---|---|---|
| Measure | Pre (mean ± SE) | Post (mean ± SE) | Significance | Effect Size* | Pre (mean ± SE) | Post (mean ± SE) | Significance | Effect Size* |
| Males (n = 10) | ||||||||
| Forcepeak (kg) | 34.2 ± 3.7 | 37.6 ± 4.7 | p = 0.039 | g = 0.24 small | 33.7 ± 4.6 | 34.7 ± 4.3 | p = 0.468 | g = 0.07 trivial |
| VL–sEMGRMS (μV) | 370.5 ± 103.4 | 345.1 ± 86.0 | p = 0.480 | g = 0.08 trivial | 372.6 ± 106.4 | 360.5 ± 102.5 | p = 0.513 | g = 0.04 trivial |
| RF–sEMGRMS (μV) | 207.0 ± 29.0 | 190.3 ± 28.0 | p = 0.378 | g = 0.18 trivial | 204.4 ± 35.5 | 201.3 ± 34.9 | p = 0.761 | g = 0.03 trivial |
| VL–MMGRMS (m·s-2) | 0.4 ± 0.1 | 0.4 ± 0.1 | p = 0.939 | g < 0.01 trivial | 0.4 ± 0.1 | 0.4 ± 0.1 | p = 0.226 | g = 0.17 trivial |
| RF–MMGRMS (m·s-2) | 0.6 ± 0.1 | 0.6 ± 0.1 | p = 0.215 | g = 0.33 small | 0.6 ± 0.1 | 0.7 ± 0.1 | p = 0.578 | g = 0.13 trivial |
| Females (n = 10) | ||||||||
| Forcepeak (kg) | 21.7 ± 1.3 | 21.1 ± 1.8 | p = 0.530 | g = 0.11 trivial | 21.5 ± 1.6 | 22.2 ± 1.8 | p = 0.170 | g = 0.13 trivial |
| VL–sEMGRMS (μV) | 189.6 ± 44.8 | 174.1 ± 40.3 | p = 0.420 | g = 0.11 trivial | 170.9 ± 30.6 | 180.8 ± 32.1 | p = 0.161 | g = 0.10 trivial |
| RF–sEMGRMS (μV) | 116.5 ± 21.0 | 112.6 ± 18.5 | p = 0.597 | g = 0.06 trivial | 113.5 ± 17.7 | 118.5 ± 20.0 | p = 0.283 | g = 0.08 trivial |
| VL–MMGRMS (m·s-2) | 0.3 ± 0.1 | 0.32 ± 0.1 | p = 0.689 | g = 0.08 trivial | 0.3 ± 0.03 | 0.3 ± 0.04 | p = 0.912 | g = 0.10 trivial |
| RF–MMGRMS (m·s-2) | 0.4 ± 0.1 | 0.35 ± 0.04 | p = 0.896 | g = 0.07 trivial | 0.4 ± 0.03 | 0.4 ± 0.04 | p = 0.914 | g < 0.01 trivial |
SMR = self-myofascial release protocol; CON = control protocol; Forcepeak = peak knee extension force output; VL = vastus lateralis musculature; RF = rectus femoris musculature; sEMGRMS = root mean square amplitude of the surface electromyographic data; MMGRMS = root mean square amplitude of the mechanomyographic data.
Effect size interpretations are based on guidelines provided by Sullivan and Feinn58
Forcepeak Data
Among males, no significant protocol × time interaction effect on Forcepeak was identified (F1,18 = 1.577, p = 0.225). Although a significant and small main effect of time on Forcepeak was identified within the SMR protocol (p = 0.039, g = 0.24), when body mass was controlled for, no significant effect of time was identified within either the SMR (p = 0.920) or CON (p = 0.518) protocols. A non-significant and trivial main effect of time on Forcepeak was identified within the CON protocol (p = 0.468, g = 0.07). Among females, no significant protocol × time interaction effect on Forcepeak was identified (F1,18 = 1.630, p = 0.218). In addition, non-significant and trivial main effects of time on Forcepeak were identified within both the SMR (p = 0.530, g = 0.11) and CON (p = 0.170, g = 0.13) protocols. Collectively, these results suggest that the SMR protocol significantly influenced the Forcepeak data among males, and that SMR may influence Forcepeak via muscle mass, as this influence was no longer statistically significant when body mass was controlled for as a covariate. In addition, the effect of SMR on Forcepeak was small, and did not create a change that was significantly different between the SMR and CON protocols. Finally, neither the SMR and CON protocols significantly influenced Forcepeak data among females (Table 2).
sEMG Data
Among males, no significant protocol × time interaction effects on VL–sEMGRMS (F1,18 = 0.116, p = 0.737) and RF–sEMGRMS (F1,18 = 0.448, p = 0.512) were identified. In addition, non-significant and trivial main effects of time on VL–sEMGRMS and RF–sEMGRMS were identified within both the SMR (p = 0.480, g = 0.08; p = 0.378, g = 0.18, respectively) and CON (p = 0.513, g = 0.04; p = 0.761, g = 0.03, respectively) protocols. Among females, no significant protocol × time interaction effects on VL–sEMGRMS (F1,18 = 1.698, p = 0.209) and RF–sEMGRMS (F1,18 = 1.138, p = 0.300) were identified. In addition, non-significant and trivial main effects of time on VL–sEMGRMS and RF–sEMGRMS were identified within both the SMR (p = 0.420, g = 0.11; p = 0.597, g = 0.06, respectively) and CON (p = 0.161, g = 0.10; p = 0.283, g = 0.08, respectively) protocols. Collectively, these results suggest that the SMR and CON protocols did not significantly influence the VL–sEMGRMS and RF–sEMGRMS data among both males and females (Table 2).
MMG Data
Among males, no significant protocol × time interaction effects on VL–MMGRMS (F1,18 = 0.432, p = 0.519) and RF–MMGRMS (F1,18 = 0.174, p = 0.681) were identified. In addition, non-significant and trivial-to-small main effects of time on VL–MMGRMS and RF–MMGRMS were identified within both the SMR (p = 0.939, g < 0.01; p = 0.215, g = 0.33, respectively) and CON (p = 0.226, g = 0.17; p = 0.578, g = 0.13, respectively) protocols. Among females, no significant protocol × time interaction effects on VL–MMGRMS (F1,18 = 0.145, p = 0.707) and RF–MMGRMS (F1,18 = 0.010, p = 0.932) were identified. In addition, non-significant and trivial main effects of time on VL–MMGRMS and RF–MMGRMS were identified within both the SMR (p = 0.689, .g = 0.08; p = 0.896, g = 0.07, respectively) and CON (p = 0.912, g = 0.10; p = 0.914, g < 0.01, respectively) protocols. Collectively, these results suggest that the SMR and CON protocols did not significantly influence the VL–MMGRMS and RF–MMGRMS data among both males and females (Table 2).
DISCUSSION
The purpose of this study was to examine the influence of SMR, via an acute bout of FR to the VL, on the expression of knee extension force output and the inter-muscular electro-mechanical activation of the quadriceps musculature. The results of the current study suggest that SMR may result in a small, but significant increases in knee extension Forcepeak among males, but no significant change in Forcepeak within the SMR group was identified among females. This potential sex-specific influence on the expression of knee extension force output may be due to differences in muscle mass between males and females, as no significant changes in Forcepeak were identified among males after controlling for body mass. However, although this pre-to-post change in Forcepeak was significant with the SMR group, the post-Forcepeak within the SMR group was not significantly different from the post-Forcepeak within the CON group. These findings differ with the existing body of literature indicating a lack of influence of SMR on the expression of force output.7-9 Nevertheless, due to the resulting increase in force production (vs. decrease), these findings still provide further support for the use of SMR, instead of SS, as a method of increasing joint ROM without any subsequent decrements in force production that may hinder sport performance.
Similarly, the results of the current study also indicate that SMR does not influence the electrical processes associated with the electro-mechanical activation of the quadriceps musculature, as significant changes in sEMGRMS amplitudes were not identified within either sex. Given the fact that a decrease in the efferent α-motorneuron drive due to activation of Golgi tendon organs (GTOs) and/or mechanoreceptors (e.g., group III/IV afferents) have been proposed as neural mechanisms of increasing muscle tissue flexibility via SMR,1 it is surprising that decreases in sEMGRMS amplitudes were not identified in the current study. These results are further surprising given the fact that an increase in Forcepeak was identified among males, which suggests that this increase in force output was not attributed to the electrical aspects of muscle activation. That said, these results further contribute to the body literature that has previously identified no changes in sEMG measures post-SMR,7-9,32-33 as well as decreases in sEMG measures post-SMR.30-31
It has been hypothesized that these conflicting results in the previous literature could be due to changes in inter-muscular activation, as significant decreases in sEMG measures of the hamstring musculature has been observed in the literature after SMR was applied to the quadriceps musculature.31 However, even though the SMR was applied specifically to the VL musculature in the current study, no changes in the RF–sEMGRMS data were identified, and Killen et al.32 did not identify post-SMR changes in sEMG measures of the contralateral musculature as well. Therefore, it is possible that SMR only creates electrical changes in electro-mechanical muscle activation within the antagonist musculature (vs. the agonist or contralateral musculature). These changes could be due to reciprocal inhibition of the antagonist musculature (i.e., hamstrings) as a result of the SMR applied to the agonist musculature (i.e., quadriceps), which is a phenomenon that has been demonstrated after both SS and proprioceptive neuromuscular facilitation (PNF) stretching.59 In addition, both studies that have previously identified significant decreases in sEMG measures post-SMR examined muscle activity during dynamic muscle contractions, such as a lunge30 and a single-leg landing from a hurdle jump,31 whereas the current study utilized an isometric contraction. Since this is the first study to simultaneously examine the influence of SMR on both the electrical and mechanical processes associated with muscle activation, an isometric contraction was chosen to better isolate the potential influence of SMR on these parameters be removing the influence of motion artifact that is associated with dynamic muscle contractions. That said, it is possible that the influence of SMR on the electrical processes associated with the electro-mechanical muscle activation is also specific to the type of muscle action.
In concurrence with the lack of change in the electrical processes associated with muscle activation, the results of the current study indicate that SMR does not influence the mechanical processes associated with activation of the quadriceps musculature either, as significant changes in MMGRMS amplitudes were also not identified. While this was the first known study to examine the influence of SMR on measures of MMG, previous research has identified increases in MMG amplitude post-SS.25 Since it has been suggested that MMG amplitude may be inversely related to muscle stiffness,19,34 the previously identified increases in MMG amplitude post-SS have been attributed to the potential increase in compliance of the non-contractile tissues (i.e., a decrease in muscle stiffness), which in turn, may allow for greater lateral oscillations of the muscle fibers during contraction (i.e., increase in MMG amplitude). Therefore, given the fact that adaptations to the viscoelastic properties of the fascia have been proposed as morphological mechanisms of increasing muscle tissue flexibility via SMR,1 it is surprising that changes in MMGRMS amplitudes were not identified in the current study.
When taken together, the results of the current study indicate that SMR does not influence the electro-mechanical aspects of quadriceps muscle activation, which provides further mechanistic rationale regarding the maintenance in force output post-SMR. However, these results suggest that although previous research has routinely demonstrated that SMR has the ability to increase muscle tissue flexibility,1-3 the physiological mechanisms in which this increase in muscle tissue flexibility are achieved remain unclear. Although the lack of change in MMGRMS amplitude suggests these mechanisms do not influence the mechanical aspects of muscle activation, changes in fluid mechanics of the fascia surrounding the target musculature due to the previously described thixotropy-like effect17-18 remain possible. For example, recent research has suggested that increases in arterial blood flow,60 potentially due to increases in nitric oxide, and subsequent changes in endothelial function,61 are created after an acute bout of FR. These potential physiological changes may ultimately render the fascia more malleable and the increase the extensibility of the target musculature, but in a manner that does not influence the electrical or mechanical aspects of muscle activation. Furthermore, such changes may be responsible for the potential sex-specific changes in Forcepeak observed in the current study. As such, future research should utilize other methodologies (e.g., diagnostic ultrasound, heart rate variability, etc.) to further investigate potential changes in physiological processes, both local and systemic, that occur as a result of SMR.
However, it should be noted that the current study only examined the influence of SMR on electro-mechanical muscle activation during isometric muscle actions at one joint position (60 ° of knee flexion). It is possible that SMR may influence the mechanical processes associated with electro-mechanical muscle activation (i.e., MMG measures) during isometric muscle actions differently based on joint position, which is a phenomenon that has been previously observed within the SS literature body.25 In addition, based on the previously identified discrepancies in the literature regarding post-SMR changes in sEMG measures between isometric and dynamic muscle actions, it is possible that post-SMR changes in MMG measures may also differ between isometric and dynamic muscle contractions. Furthermore, the influence of SMR on electro-mechanical muscle activation may be specific to muscle action type (i.e., concentric vs. eccentric) as well. While this has yet to be examined in the SMR literature, the SS literature body has demonstrated significant increases in MMG amplitude,24,26 as well as no changes in MMG amplitude,21-23 during isokinetic muscle actions post-SS. Such conflicting results in the SS literature have not only been attributed to differences between muscle actions types,22 but could be due to inherent differences in muscle architecture as well.25 For example, Herda et al.25 proposed that the post-SS observed increases in MMG amplitude during concentric knee flexion muscle actions, and the lack of change in MMG amplitude during concentric knee extension muscle actions,21,23 may be related to the fusiform nature of the hamstring musculature (vs. the pennate nature of the quadriceps musculature). As a result, SS may have a greater influence on MTU compliance, and thus, a greater influence on measures of MMG, within muscles comprised of a fusiform architecture (e.g., hamstrings, biceps brachi, etc.) in comparison to muscles comprised of a pennate architecture (e.g., quadriceps, deltoid, etc.). Therefore, it is also possible that differences in muscle architecture influence the underlying mechanisms in which SMR creates changes in muscle flexibility. In contrast, given the influence of muscle mass on force output,62 it is possible that muscle mass may mediate the influence of SMR on the physiological processes of electro-mechanical function. Such influence may also explain the potential sex-specific changes in Forcepeak observed in the current study. Thus, further research examining potential changes in both sEMG measures and MMG measures at a variety of joint angles, during various muscle actions, within different muscle architecture types, while controlling for sex differences in muscle mass is warranted.
It is also possible that the type of foam roller and the duration of FR may influence the acute physiological adaptations associated with SMR. Although the body of literature is currently limited, research suggests that different types of foam rollers exert differing level of pressure to the target musculature.63 In addition, recent research also suggests that vibrating foam rollers may create differing (or enhanced) effects than non-vibrating foam rollers.64-65 Furthermore, a recent commentary on the state of the SMR literature has highlighted the fact that there appear to be a large variety in FR methods, including FR durations, examined in the SMR literature,66 which has also created a large variety in the SMR methodologies utilized by practitioners.6 It is possible that this large variety in SMR methodologies utilized by researchers has influenced the conflicting findings in the literature regarding the influence of SMR on the electrical aspects of electro-mechanical function. Notably, the duration in which FR is applied may create differing changes in subsequent electro-mechanical activation post-SMR. Thus, although the results of the current study did not identify an influence of SMR on subsequent electro-mechanical aspects of muscle activation, further examination on the influence of differing types and durations of SMR on electro-mechanical muscle activation remains warranted.
Several limitations of the current study should be acknowledged. First, the amount of force applied to the foam roller due to bodyweight was not controlled beyond researchers ensuring proper FR form. It is possible that subtle weight shifting across the participants could have resulted in differing amounts of relative force being applied to the foam roller, which could theoretically influence the SMR mechanisms. In addition, the speed of the FR was not controlled for by the researchers. However, recent research also suggests that the amount of force applied to the quadriceps musculature during FR did not influence the observed changes in ROM or result in differing MVICs pre-to-post-SMR67 and that the speed at which FR was conducted did not influence the observed changes in ROM or myofascial stiffness pre-to-post-SMR.68 Thus, while the amount of force applied during FR, and the speed at which the FR was conducted, were not controlled across participants, this previous research suggests these methodological factors did not likely influence the results of the current study.
CONCLUSIONS
The results of the current study indicate that SMR does not influence the electro-mechanical aspects of quadriceps muscle activation. These results provide further mechanistic rationale regarding the lack of any subsequent decreases in force output observed in the current study, as well as previously identified in the literature. Therefore, clinicians and practitioners should recommend the use of SMR, instead of SS, as a method of increasing flexibility, due to a lack of decrements in force production that may acutely hinder sport performance. Given the potential for SMR-related changes in the expression of force to be mediated by body mass, it is also possible that clinicians may choose to utilize SMR (or SS) based on the size of the individual. However, further research examining the potential sex-specific and/or muscle mass influence of different SMR types and protocols on electro-mechanical muscle activation at a variety of joint angles, during various muscle actions, and within different muscle architecture types remains warranted.
REFERENCES
- 1.Beardsley C Škarabot J. Effects of self-myofascial release: a systematic review. J Bodyw Mov Ther. 2015;19(4):342-348. [DOI] [PubMed] [Google Scholar]
- 2.Cheatham SW Kolber MJ Cain M et al. The effects of self-myofascial release using a foam roll or roller massager on joint range of motion, muscle recovery, and performance: a systematic review. Int J Sports Phys Ther. 2015;10(6):827-838. [PMC free article] [PubMed] [Google Scholar]
- 3.Schroeder AN Best TM. Is self myofascial release an effective preexercise and recovery strategy? A literature review. Curr Sports Med Rep. 2015;14(3):200-208. [DOI] [PubMed] [Google Scholar]
- 4.Cheatham SW Stull KR. Roller massage: a commentary on clinical standards and survey of physical therapy professionals – part 1. Int J Sports Phys Ther. 2018;13(4):763-772. [PMC free article] [PubMed] [Google Scholar]
- 5.Thompson WR. Worldwide survey of fitness trends for 2019. ACSMs Health Fit J. 2018;22(6):10-17. [Google Scholar]
- 6.Cheatham SW. Roller massage: a descriptive survey of allied health professionals. J Sport Rehabil. 2019;28(6):640-649. [DOI] [PubMed] [Google Scholar]
- 7.Halperin I Aboodarda SJ Button DC et al. Roller massager improves range of motion of plantar flexor muscles without subsequent decreases in force parameters. Int J Sports Phys Ther. 2014;9(1):92-102. [PMC free article] [PubMed] [Google Scholar]
- 8.MacDonald GZ Penney MDH Mullaley ME et al. An acute bout of self-myofascial release increases range of motion without a subsequent decrease in muscle activation or force. J Strength Cond Res. 2013;27(3):812-821. [DOI] [PubMed] [Google Scholar]
- 9.Sullivan KM Silvey DBJ Button DC et al. Roller-massager application to the hamstrings increases sit-and-reach range of motion within five to ten seconds without performance impairments. Int J Sports Phys Ther. 2013;8(3):228-236. [PMC free article] [PubMed] [Google Scholar]
- 10.Behm DG Chaouachi A. A review of the acute effects of static and dynamic stretching on performance. Eur J Appl Physiol. 2011;111(11):2633-2651. [DOI] [PubMed] [Google Scholar]
- 11.Peck E Chomko G Gaz DV et al. The effects of stretching on performance. Curr Sports Med Rep. 2014;13(3):179-185. [DOI] [PubMed] [Google Scholar]
- 12.Simic L Sarabon N Markovic G. Does pre-exercise static stretching inhibit maximal muscular performance? A meta-analytical review. Scand J Med Sci Sports. 2013;23(2):131-148. [DOI] [PubMed] [Google Scholar]
- 13.Freiwald J Baumgart C Kühnemann M et al. Foam-rolling in sport and therapy – potential benefits and risks: Part 2 – positive and adverse effects on athletic performance. Sports Orthop Traumatol. 2016;32(3):267-275. [Google Scholar]
- 14.Trajano GS Nosaka K Blazevich AJ. Neurophysiological mechanisms underpinning stretch-induced force loss. Sports Med. 2017;47(8):1531-1541. [DOI] [PubMed] [Google Scholar]
- 15.McHugh MP Cosgrave CH. To stretch or not to stretch: the role of stretching in injury prevention and performance. Scand J Med Sci Sports. 2010;20(2):169-181. [DOI] [PubMed] [Google Scholar]
- 16.Rubini EC Costa ALL Gomes PSC. The effects of stretching on strength performance. Sports Med. 2007;37(3):213-224. [DOI] [PubMed] [Google Scholar]
- 17.Barnes MF. The basic science of myofascial release: morphological change in connective tissue. J Bodw Mov Ther. 1997;1(4):231-238. [Google Scholar]
- 18.Schleip R. Fascial plasticity – a new neurobiological explanation: part 1. J Bodw Mov Ther. 2003;7(1):11-19. [Google Scholar]
- 19.Beck TW Housh TJ Cramer JT et al. Mechanomyographic amplitude and frequency responses during dynamic muscle actions: a comprehensive review. Biomed Eng Online. 2005;19(4):67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Malek MH Coburn JW. The utility of electromyography and mechanomyography for assessing neuromuscular function: a noninvasive approach. Phys Med Rehabil Clin N Am. 2012;23(1):23-32. [DOI] [PubMed] [Google Scholar]
- 21.Cramer JT Beck TW Housh TJ et al. Acute effects of static stretching on characteristics of the isokinetic angle-torque relationship, surface electromyography, and mechanomyography. J Sports Sci. 2007;25(6):687-698. [DOI] [PubMed] [Google Scholar]
- 22.Cramer JT Housh TJ Johnson GO et al. An acute bout of static stretching does not affect maximal eccentric isokinetic peak torque, the joint angle at peak torque, mean power, electromyography, or mechanomyography. J Orthop Sports Phys Ther. 2007;37(3):130-139. [DOI] [PubMed] [Google Scholar]
- 23.Cramer JT Housh TJ Weir JP et al. The acute effects of static stretching on peak torque, mean power output, electromyography, and mechanomyography. Eur J Appl Physiol. 2005;93(5-6):530-539. [DOI] [PubMed] [Google Scholar]
- 24.Evetovich TK Nauman NJ Conley DS et al. Effect of static stretching of the biceps brachii on torque, electromyography, and mechanomyography during concentric isokinetic muscle actions. J Strength Cond Res. 2003;17(3):484-488. [DOI] [PubMed] [Google Scholar]
- 25.Herda TJ Cramer JT Ryan ED et al. Acute effects of static versus dynamic stretching on isometric peak torque, electromyography, and mechanomyography of the biceps femoris muscle. J Strength Cond Res. 2008;22(3):809-817. [DOI] [PubMed] [Google Scholar]
- 26.Marek SM Cramer JT Fincher AL et al. Acute effects of static and proprioceptive neuromuscular facilitation stretching on muscle strength and power output. J Athl Train. 2005;40(2):94-103. [PMC free article] [PubMed] [Google Scholar]
- 27.Avela J Kyröläinen H Komi PV. Altered reflex sensitivity after repeated and prolonged passive muscle stretching. J Appl Physiol. 1999;86(4):1283-1291. [DOI] [PubMed] [Google Scholar]
- 28.Behm DG Button DC Butt JC. Factors affecting force loss with prolonged stretching. Can J Appl Physiol. 2001;26(3):262-272. [PubMed] [Google Scholar]
- 29.Fowles JR Sale DG MacDougall JD. Reduced strength after passive stretch of the human plantarflexors. J Appl Physiol. 2000;89(3):1179-1188. [DOI] [PubMed] [Google Scholar]
- 30.Bradbury-Squires DJ Noftall JC Sullivan KM et al. Roller-massager application to the quadriceps and knee-joint range of motion and neuromuscular efficiency during a lunge. J Athl Train. 2015;50(2):133-140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cavanaugh MT Aboodarda SJ Hodgson DD et al. Foam rolling of quadriceps decreases biceps femoris activation. J Strength Cond Res. 2017;31(8):2238-2245. [DOI] [PubMed] [Google Scholar]
- 32.Killen BS Zelizney KL Ye X. Crossover effects of unilateral static stretching and foam rolling on contralateral hamstring flexibility and strength. J Sport Rehabil. 2019;28(6):533-539. [DOI] [PubMed] [Google Scholar]
- 33.Madoni SN Costa PB Coburn JW et al. Effects of foam rolling on range of motion, peak torque, muscle activation, and the hamstrings-to-quadriceps strength ratios. J Strength Cond Res. 2018;32(7):1821-1830. [DOI] [PubMed] [Google Scholar]
- 34.Ebersole KT Housh TJ Johnson GO et al. MMG and EMG responses of the superficial quadriceps femoris muscles. J Electromyogr Kinesiol. 1999;9(3):219-227. [DOI] [PubMed] [Google Scholar]
- 35.Garber CE Blissmer B Deschenes MR et al. American College of Sports Medicine position stand. Quantity and quality of exercise for developing and maintaining cardiorespiratory, musculoskeletal, and neuromotor fitness in apparently healthy adults: guidance for prescribing exercise. Med Sci Sports Exerc. 2011;43(7):1334-1359. [DOI] [PubMed] [Google Scholar]
- 36.Hoffman M Schrader J Applegate T et al. Unilateral postural control of the functionally dominant and nondominant extremities of health subjects. J Athl Train. 1998;33(4):319-322. [PMC free article] [PubMed] [Google Scholar]
- 37.Robertson M. Self-Myofascial Release: Purpose, Methods and Techniques. Indianapolis, IN: Robertson Training Systems; 2008. [Google Scholar]
- 38.Kendall FP McCreary EK Provance PG et al. Muscles: Testing and Function with Posture and Pain. 5th ed. Baltimore, MD: Lippincott Williams & Wilkins; 2005. [Google Scholar]
- 39.Bolgla LA Earl-Boehm J Emery C et al. Comparison of hip and knee strength in males with and without patellofemoral pain. Phys Ther Sport. 2015;16(3):215-221. [DOI] [PubMed] [Google Scholar]
- 40.Bolgla LA Malone TR Umberger BR et al. Comparison of hip and knee strength and neuromuscular activity in subjects with and without patellofemoral pain syndrome. Int J Sports Phys Ther. 2011;6(4):285-296. [PMC free article] [PubMed] [Google Scholar]
- 41.Bohannon RW Kindig J Sabo G et al. Isometric knee extension force measured using a handheld dynamometer with and without belt-stabilization. Physiother Theory Pract. 2012;28(7):562-567. [DOI] [PubMed] [Google Scholar]
- 42.Lesnak J Anderson D Farmer B et al. Validity of hand-held dynamometry in measuring quadriceps strength and rate of torque development. Int J Sports Phys Ther. 2019;14(2):180-187. [PMC free article] [PubMed] [Google Scholar]
- 43.Kelln BM McKeon PO Gontkof LM et al. Hand-held dynamometry: reliability of lower extremity muscle testing in healthy, physically active, young adults. J Sport Rehabil. 2008;17(2):160-170. [DOI] [PubMed] [Google Scholar]
- 44.Merletti R. Standards for reporting EMG data. J Electromyogr Kinesiol. 1999;9(1):III-IV. [Google Scholar]
- 45.Hermens HJ Freriks B Merletti R et al. European Recommendations for Surface Electromyography: Results of the SENIAM Project. Enschede, The Netherlands: Roessingh Research and Development; 1999. [Google Scholar]
- 46.Ebersole KT Housh TJ Johnson GO et al. Mechanomyographic and electromyographic responses to unilateral isometric training. J Strength Cond Res. 2002;16(2):192-201. [PubMed] [Google Scholar]
- 47.Fauth ML Petushek EJ Feldmann CR et al. Reliability of surface electromyography during maximal voluntary isometric contractions, jump landings, and cutting. J Strength Cond Res. 2010;24(4):1131-1137. [DOI] [PubMed] [Google Scholar]
- 48.Yang JF Winter DA. Electromyography reliability in maximal and submaximal isometric contractions. Arch Phys Med Rehabil. 1983;64(9):417-420. [PubMed] [Google Scholar]
- 49.Camic CL Housh TJ Zuniga JM et al. Electromyographic and mechanomyographic responses across repeated maximal isometric and concentric muscle actions of the leg extensors. J Electromyogr Kinesiol. 2013;23(2):342-348. [DOI] [PubMed] [Google Scholar]
- 50.Beck TW. Processing the surface mechanomyographic signal. In: Beck TW. Applications of Mechanomyography for Examining Muscle Function. Kerala, India: TransWorld Research Network; 2010:109-116. [Google Scholar]
- 51.Al-Zahrani E Gunasekaran C Callaghan M et al. Within-day and between-days reliability of quadriceps isometric muscle fatigue using mechanomyography on healthy subjects. J Electromyogr Kinesiol. 2009;19(4):695-703. [DOI] [PubMed] [Google Scholar]
- 52.Herda TJ Ryan ED Beck TW et al. Reliability of mechanomyographic amplitude and mean power frequency during isometric step and ramp muscle actions. J Neurosci Methods. 2008;171(1):104-109. [DOI] [PubMed] [Google Scholar]
- 53.Pincivero DM Green RC Mark JD et al. Gender and muscle differences in EMG amplidue and median frequency, and variability during maximal voluntary contractions of the quadriceps femoris. J Electromyogr Kinesiol. 2000;10(3):189-196. [DOI] [PubMed] [Google Scholar]
- 54.Beck TW Housh TJ Johnson GO et al. Gender comparisons of mechanomyographic amplitude and mean power frequency versus isometric torque relationships. J Appl Biomech. 2005;21(1):96-190. [DOI] [PubMed] [Google Scholar]
- 55.Evetovich TK Housh TJ Johnson GO et al. Gender comparisons of the mechanomyographic responses to maximal concentric and eccentric muscle actions. Med Sci Sports Sci. 1998;30(12):1697-1702. [DOI] [PubMed] [Google Scholar]
- 56.Nonaka H Mita K Akataki K et al. Sex differences in mechanomyographic responses to voluntary isometric contractions. Med Sci Sports Sci. 2006;38(7):1311-1316. [DOI] [PubMed] [Google Scholar]
- 57.Ferguson CJ. An effect size primer: a guide for clinicians and researchers. Prof Psychol Res Pr. 2009;40(5):532-538. [Google Scholar]
- 58.Sullivan GM Feinn R. Using effect size–or why the P value is not enough. J Grad Med Educ. 2012;4(3):179-182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sharman MJ Cresswell AG Riek S. Proprioceptive neuromuscular facilitation stretching. Sports Med. 2006;36(11):929-939. [DOI] [PubMed] [Google Scholar]
- 60.Hotfiel T Swoboda B Krinner S et al. Acute effects of lateral thigh foam rolling on arterial tissue perfusion determined by spectral doppler and power doppler ultrasound. J Strength Cond Res. 2017;31(4):893-900. [DOI] [PubMed] [Google Scholar]
- 61.Okamoto T Masuhara M Ikuta K. Acute effects of self-myofascial release using a foam roller on arterial function. J Strength Cond Res. 2014;28(1):69-73. [DOI] [PubMed] [Google Scholar]
- 62.Lieber RL Bodine-Fowler SC. Skeletal muscle mechanics: implications for rehabilitation. Phys Ther. 1993;73(12):844-856. [DOI] [PubMed] [Google Scholar]
- 63.Curran PF Fiore RD Crisco JJ. A comparison of the pressure exerted on soft tissue by 2 myofascial rollers. J Sport Rehabil. 2008;17(4):432-442. [DOI] [PubMed] [Google Scholar]
- 64.Cheatham SW Stull KR Kolber MJ. Comparison of a vibrating foam roller and a non-vibrating foam roller intervention on knee range of motion and pressure pain threshold: A randomized controlled trial. J Sport Rehabil. 2019;28(1):39-45. [DOI] [PubMed] [Google Scholar]
- 65.Lee CL Chu IH Lyu BJ et al. Comparison of vibration rolling, nonvibration rolling, and static stretching as a warm-up exercise on flexibility, joint proprioception, muscle strength, and balance in young adults. J Sports Sci. 2018;36(22):2575-2582. [DOI] [PubMed] [Google Scholar]
- 66.Cheatham SW Stull KR Ambler-Wright T. Roller massage: survey of physical therapy professionals and a commentary on clinical standards – part II. Int J Sports Phys Ther. 2018;13(5):920-930. [PMC free article] [PubMed] [Google Scholar]
- 67.Grabow L Young JD Alcock LR et al. Higher quadriceps roller massage forces do not amplify range-of-motion increases nor impair strength and jump performance. J Strength Cond Res. 2018;32(11):3059-3069. [DOI] [PubMed] [Google Scholar]
- 68.Wilke J Niemeyer P Niederer D et al. Influence of foam rolling velocity on knee flexibility and tissue stiffness: a randomized, controlled crossover trial. J Sport Rehabil. 2019;28(7):711-715. [DOI] [PubMed] [Google Scholar]