Abstract
Background.
Whole pancreas transplantation (Tx) is a successful treatment for type 1 diabetes resulting in independence from antidiabetic therapies. Transplant-related factors contributing to pancreatic islet failure are largely unknown; both recurring insulitis and pancreatitis have been implicated. The aim was to determine if cellular changes in islets and exocrine tissue are evident early in Tx, which could contribute to eventual graft failure using well-preserved tissue of grafts explanted from largely normoglycemic recipients.
Methods.
Histological specimens of explants (n = 31), Tx duration 1 day–8 years (median 29 d), cold ischemia time 7.2–17.3 hours (median 11.1 h), donor age 13–54 years (median 38 y) were examined; sections were labeled for inflammation, islet amyloidosis, and tissue fibrosis, and morphometry performed on immunolabeled insulin and glucagon positive islet cells. Data were related to clinical details of donor, recipient, and features of Tx.
Results.
Islet inflammation consistent with recurrent insulitis was not seen in any sample. Insulin-labeled islet cell proportion decreased with donor age (P < 0.05) and cold ischemia (P < 0.01) in explants from 26 normoglycemic patients; glucagon-labeled area proportion increased with cold ischemia (P < 0.05). Clinical pancreatitis was the explant reason in 12 of 28 normoglycemic cases. Exocrine fibrotic area/pancreas was variable (0.7%–55%) and unrelated to clinical/pathological features. Islet amyloid was present in 3 normoglycemic cases (donor ages 58, 42, and 31 y; Tx duration 8 y, 31 and 33 d, respectively). In 1 patient receiving antidiabetic therapy, the insulin-labeled area was reduced but with no evidence of islet inflammation.
Conclusions.
Explant histological changes after short-term Tx are similar to those seen in type 2 diabetes and occur in the absence of immunologic rejection without causing hyperglycemia. This suggests that factors associated with Tx affect islet stability; persistent deterioration of islet integrity and exocrine tissue fibrosis could impact on sustainability of islet function.
INTRODUCTION
Whole pancreas transplantation (Tx) is an effective treatment for patients with type 1 diabetes (T1D) and insulin-dependent type 2 diabetes (T2D). It is the only therapy known to restore normoglycemia in the long-term, reduce/reverse diabetes-associated complications without the need for insulin therapy1,2 and provide a more successful sustained insulin independence compared with islet Tx.3 Simultaneous pancreas-kidney transplant is the most common form (accounting for 80%–90% of all pancreas transplants) and is offered to insulin-dependent patients diagnosed with T1D or T2D with end-stage renal disease.1
The success of pancreas Tx (defined by independence from antidiabetic therapy) is high; current 5-year graft survival is >70% for simultaneous pancreas-kidney transplant.4 Long-term insulin independence is more predictable in patients with good glucose tolerance at 1 year postoperation compared with those with poor glucose tolerance.5 It is unclear why impaired graft function occurs and has been attributed to different factors including recurrence of autoimmune insulitis, decreased beta-cell mass, and loss of insulin sensitivity/obesity.1,3 Early graft failures occur in approximately 8% of pancreas transplants as a result of localized thrombosis, acute rejection, pancreatitis, or bleeding resulting in surgical graft removal. Late graft failure resulting in explantation is uncommon; histology of some grafts removed >1 year of Tx have shown evidence of insulitis6 (a feature of T1D) and islet amyloidosis (a feature of T2D).7
In Oxford, over 1000 transplants have been performed in the last 18 years, of which 93 were explanted. Technical and immunotherapy improvements are continuously being developed; however, investigations of the condition of donor pancreatic islets and how this relates to recipient endocrine function are few and rarely quantitative.8 Needle biopsies have been used to examine pancreas Tx,9,10 but these, very small samples, obtained at some risk to pancreas integrity, are insufficient for a representative estimation of pathological processes. Our collection of well-preserved explant material provided an unique opportunity to examine effects of Tx.
The aim was to examine explant pancreas to determine if islet and exocrine cellular changes occur in the early stages of Tx, which could inform on the mechanisms that underlie chronic graft failure.
MATERIALS AND METHODS
Samples
A database of all pancreas explants performed in Oxford 2003–2017 was constructed, and 93 explants were identified. These samples had been collected from 699 pancreatic transplants performed at that time. Records were screened for pathological notes and research permissions. Histological sections of formalin-fixed pancreas from explants were obtained from the Oxford Center for Histopathology Research and the Oxford Radcliffe Biobank with appropriate ethical approval for research (Oxford Regional Ethics committee; REC #09/H0606/5 + 5), compliance with the National Human Tissue Authority and with the Declaration of Helsinki. Only samples with well preserved and adequate pancreatic volume as seen on hematoxylin and eosin–stained slides were included; 41 explants were examined, of which 31 were suitable for inclusion. Pathology reports rarely indicated pancreatic sample location; samples obviously from the pancreatic head with pancreatic polypeptide-rich islets were not included. Since there are few differences in islet morphology in the body and tail regions, these were considered together when known.11
Histology
Sections were immunolabelled for insulin (Ins) and glucagon (Ggn) and stained for islet amyloid with thioflavin S (a fluorescent compound specifically binding to amyloid) (SigmaAldrich, Welwyn Garden City, United Kingdom). Explants were additionally immunolabelled for somatostatin, lymphocytes (CD45), chromogranin A (CGA), caspase 3, smooth muscle actin (SMA), and vimentin (vim). Immunolabeling was visualized using fluorescently labeled secondary antibodies (antibody information Table S1, SDC, http://links.lww.com/TXD/A288). Fibrosis was quantified (%fibrosis/exocrine area) on sections stained for collagen with Sirius Red. Immunolabelled sections were mounted in Vectashield (Vector Laboratories, Peterborough, United Kingdom). Fluorescent images were collected using a Biorad, Radiance 2100 confocal microscope.
Morphometry of Fluorescently Labelled Cells/Tissue
For quantification, random sequential fields of the section were viewed, and images collected of all islets in a section (25–70 islets/pancreas). Islets were defined as endocrine clusters containing >10 cells labeled for insulin and/or glucagon; small endocrine cell clusters associated with ducts (so-called nesidioblastosis) were not included. Image processing was performed with Fiji/ImageJ (version 2.0-rc-65/1.51 µ) and was automated using Image J’s endogenous language. Each image was segmented into background, whole islet and red (glucagon), blue (insulin), and green (amyloid) label-positive areas. This method was developed to accurately measure labeled and unlabeled regions of images and was validated against a set of manually assessed images using a random-pixel validation technique. Briefly, the user was presented with regions of interest corresponding to candidate islets in each field. Selection of the requisite islet was made, and the subsegmentation was computed using a unique threshold derived from the area to perimeter ratio of the label-positive region. A visual representation of the segmentation for each islet was produced, and the associated areas were reported.
Labeling for Other Cell Types and Endocrine Cell Characteristics
The lymphocytic marker (anti-CD45) and marker for dedifferentiation (anti-vimentin) were used with a fluorophore-labeled, tyramide amplification system (Life Technologies, Carlsbad, CA); at least 25 islets/case were examined for inflammatory cells in islets and vimentin in insulin+ve or glucagon+ve cells. SMA and the apoptotic marker caspase 3 was used to determine dedifferentiation and cell death, respectively. The frequency of bihormonal (Ins plus Ggn +ve) cells was determined from at least 25 high magnification images in each of the 3 patients with diabetes.
Statistics
%insulin positive area (%beta) and % glucagon positive area (%alpha)/islet area (see %beta, percentage by area of islet immunolabelled for insulin; %alpha, percentage by area of islet immunolabelled for glucagon) were calculated for each islet; these data were not normally distributed and were expressed as a median for each sample. To determine if changes could be attributed to patient-related variables, data were compared with a beta regression in R.12
RESULTS
Clinical Information
Clinical parameters (Table 1), including donor and recipient age, body mass index (BMI), cold ischemic time (CIT), type of the transplant, and diabetic status, were noted. The explants were removed for different, clinically identified reasons, including vascular complications and pancreatitis (defined as a clinical syndrome with congruent radiographic features and confirmed by pathological examination) (Table 1). Twenty-nine of 31 recipients had been diagnosed with T1D at Tx and 2 with T2D. Cohort 1 included patients with explants <1000 days post-Tx who were normoglycemic (n = 26). For some analyses, this was subdivided into cohort 2 (explanted for clinically defined pancreatitis, n = 12) and cohort 3 those explanted for other reasons (bleeding, thrombosis, enteric leak, n = 14). Of the remaining 5 cases (which were examined separately), 1 patient had been receiving treatment for hyperglycemia for 1 month before explant (islet cell antibody negative; case no 69); 2 patients (cases no. 14 and 18) remained hyperglycemic and hospitalized following Tx (Table 1); 1 patient was explanted at 1050 days (normoglycemic no. 66); 1 was explanted at 2802 days (no. 58).
TABLE 1.
Explant patient details
| No. | Donor | Recipient | Tx type | Immunosuppres | %Ggn median | %Ins median | Fibrosis %exo | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Days in situ | FPG/DM mM/L | Reason for explant | BMI | Age (y) | CIT (h) | Age | BMI (kg/m2) | DM type | ||||||
| 2 | 36 | ND | GI bleed | 27.55 | 36 | 14.0 | 37 | 1 | SPK | tac/mmf | 16.34 | 28.67 | 2.99 | |
| 4 | 14 | ND | Pancreatitis | 27.13 | 38 | 14.8 | 51 | 32.00 | 1 | SPK | tac/mmf | 35.30 | 30.27 | n/a |
| 5 | 17 | 5.40 | Pancreatitis | 30.86 | 34 | 9.5 | 56 | 30.40 | 2 | SPK | tac/mmf | 14.22 | 39.60 | 0.72 |
| 7 | 17 | 6.50 | Pancreatitis | 30.67 | 33 | 10.5 | 49 | 28.90 | 2 | SPK | tac/mmf | 14.22 | 59.13 | 10.54 |
| 8 | 33 | ND | Pancreatitis | 28.40 | 58 | 13.8 | 58 | 24.09 | 1 | SPK | tac/mmf | 6.66 | 60.48 | 3.01 |
| 9 | 32 | 6.60 | Pancreatitis | 24.69 | 43 | 14.8 | 44 | 20.50 | 1 | SPK | tac/mmf | 21.17 | 52.86 | 11.11 |
| 10 | 70 | ND | Pancreatitis | 21.13 | 40 | 10.9 | 37 | 19.00 | 1 | PTA | tac/mmf | 9.34 | 27.16 | 6.66 |
| 14 | 27 | DM | Pancreatitis | 25.00 | 48 | n/a | 35 | 24.20 | 1 | PTA | tac/mmf | 32.28 | 39.37 | 5.28 |
| 18 | 23 | DM | Pancreatitis | 22.89 | 42 | 8.8 | 48 | 27.00 | 1 | SPK | tac/mmf | 28.51 | 29.19 | 18.11 |
| 20 | 12 | ND | Thrombosis | 24.00 | 18 | 7.4 | 38 | 25.00 | 1 | PTA | tac/mmf | 9.69 | 61.25 | 5.28 |
| 22 | 17 | ND | Enteric leak | n/a | 45 | 12.3 | 37 | 22.20 | 1 | SPK | tac/mmf | 23.10 | 48.74 | 18.00 |
| 23 | 24 | ND | Enteric leak | 24.00 | 31 | 8.9 | 65 | 21.50 | 1 | PTA | tac/mmf | 19.27 | 53.45 | 18.74 |
| 24 | 31 | 6.50 | Thrombosis | 21.93 | 34 | 9.4 | 47 | 25.00 | 1 | SPK | tac/mmf | 13.50 | 47.20 | 19.89 |
| 25 | 24 | ND | Enteric leak | 27.00 | 41 | 14.5 | 50 | 22.30 | 1 | PTA | tac/mmf | 22.70 | 42.66 | n/a |
| 28 | 32 | 6.50 | Enteric leak | 25.40 | 45 | 11.0 | 40 | 24.00 | 1 | SPK | tac/mmf | 16.83 | 49.88 | 10.47 |
| 29 | 48 | 11.90 | Bleeding | 24.97 | 54 | 10.0 | 54 | 33.00 | 1 | SPK | tac/mmf | 12.57 | 35.39 | 1.43 |
| 31 | 17 | ND | Pancreatitis | 26.40 | 34 | 8.6 | 58 | 28.00 | 1 | SPK | tac/mmf | 15.10 | 53.72 | 11.14 |
| 32 | 139 | 9.80 | Thrombosis | 22.00 | 53 | 11.6 | 50 | 29.98 | 1 | SPK | tac/mmf | 22.03 | 31.97 | 7.30 |
| 33 | 1 | ND | Thrombosis | 24.09 | 44 | 12.4 | 49 | 31.00 | 1 | PASPK | tac/mmf | 19.11 | 41.72 | 4.40 |
| 34 | 14 | 7.10 | Pancreatitis | 23.00 | 59 | 11.4 | 41 | 26.00 | 1 | SPK | tac/mmf | 13.96 | 41.97 | 12.99 |
| 35 | 59 | 6.10 | Pancreatitis | 25.00 | 52 | 10.1 | 35 | 35.00 | 1 | SPK | tac/mmf | 17.56 | 33.74 | 15.05 |
| 36 | 15 | 4.40 | Pancreatitis | 27.00 | 37 | 10.2 | 47 | 23.00 | 1 | SPK | tac/mmf | 9.96 | 58.09 | n/a |
| 37 | 25 | ND | Pancreatitis | n/a | n/a | 11.4 | 40 | 24.00 | 1 | SPK | tac/mmf | 12.87 | 54.89 | n/a |
| 38 | 12 | 5.20 | Pancreatitis | 20.56 | 29 | 12.4 | 57 | 34.00 | 1 | SPK | tac/mmf | 20.20 | 48.16 | n/a |
| 39 | 16 | 6.30 | Thrombosis | 21.13 | 38 | 14.0 | 54 | 23.00 | 1 | SPK | tac/mmf | 14.17 | 50.93 | n/a |
| 58 | 2802 | 5.10 | Bowel cancer | 24.50 | 42 | 17.3 | 56 | 27.00 | 1 | PAK | cyc/mmf | 15.17 | 55.60 | 12.02 |
| 66 | 1050 | ND | Aneurysm | 20.30 | 13 | 9.9 | 37 | 29.90 | 1 | SPK | tac/mmf | 14.38 | 56.65 | 2.32 |
| 67 | 131 | 5.70 | Lymphoma | 22.30 | 20 | 7.2 | 48 | 28.00 | 1 | SPK | tac/mmf | 9.70 | 63.37 | 7.31 |
| 68 | 116 | 6.60 | Enteric leak | 18.80 | 52 | 10.2 | 29 | 25.00 | 1 | SPK | tac/mmf | 20.39 | 49.33 | 8.47 |
| 69 | 521 | diabetic | Pancreatitis | 19.30 | 22 | 11.5 | 28 | 19.00 | 1 | PTA | tac/mmf | 27.26 | 0.29 | 9.10 |
| 82 | 67 | 6.70 | GI bleed | n/a | 30 | 12.5 | 45 | n/a | 1 | PTA | tac/mmf | 24.62 | 50.27 | 11.22 |
Light shading indicates cases that remained hyperglycemic postexplant, and darker shading indicates patient receiving antidiabetic therapy at time of explant.
BMI, body mass index, kg/m2; CIT, cold ischemia time; Column 1, case number; Cyc/mmf, cyclosporin and mycophenolate mofetil; DM, diagnosed diabetes mellitus; FPG, recipient fasting plasma glucose recorded immediately before explant procedure, mM/L; PASPK, pancreas after simultaneous pancreas and kidney; PTA, pancreas transplant alone; SPK, simultaneous pancreas and kidney; T1D, DM-type recipient 1 Tx type; T2D, DM-type recipient 2 Tx type; Tac/mmf, tacrolimus and mycophenolate mofetil; Tx, transplantation.
The original pathology reports were examined for all patients. No evidence of rejection was noted in 25 of 26 cases in cohort 1; an inflammatory cellular infiltrate in the media of some arteries was noted in case no. 29. Case no. 69 (hyperglycemic for 1 mo) was noted to have evidence of chronic cellular rejection.
Quantitative Proportions of Islet Cells
Distribution of alpha and beta cells in islets was similar to that previously observed in normoglycemic subjects11,13 (Figure 1A). No islet inflammation (CD45+ve cell infiltrate) was found in any explant; CD45+ve cells were observed in ductal areas, in the exocrine interlobular spaces (Figure 1B) and areas of trauma. Pancreatic weight was not available therefore, beta-cell mass was not calculated. Beta-cell proportion of islet area (%beta) decreased with donor age in cohort 1 (P < 0.05) (Figure 1C). In cohort 3, %beta had a negative relationship with CIT (P < 0.01) (Figure 1D) and %alpha was positively related to CIT (P < 0.05) (Figure 1E). %beta and %alpha were unrelated to each other in any cohort and were unrelated to Tx duration, age or BMI of recipient, BMI of donor or the type of Tx. Islet cell proportions in the 2 Tx >1000 days were in the normal range (%beta 56.65%, 55.60%; %alpha, 14.38%, 15.17%, respectively) (Table 1) and in 2 patients who remained hospitalized postoperatively (Tx duration, 23 and 27 d) (Table 1). Islets in the sample retrieved from a subject who had been receiving diabetes therapy for 1 month (no. 69) had very low %beta (0.3%) but normal %alpha (27.6%).
FIGURE 1.
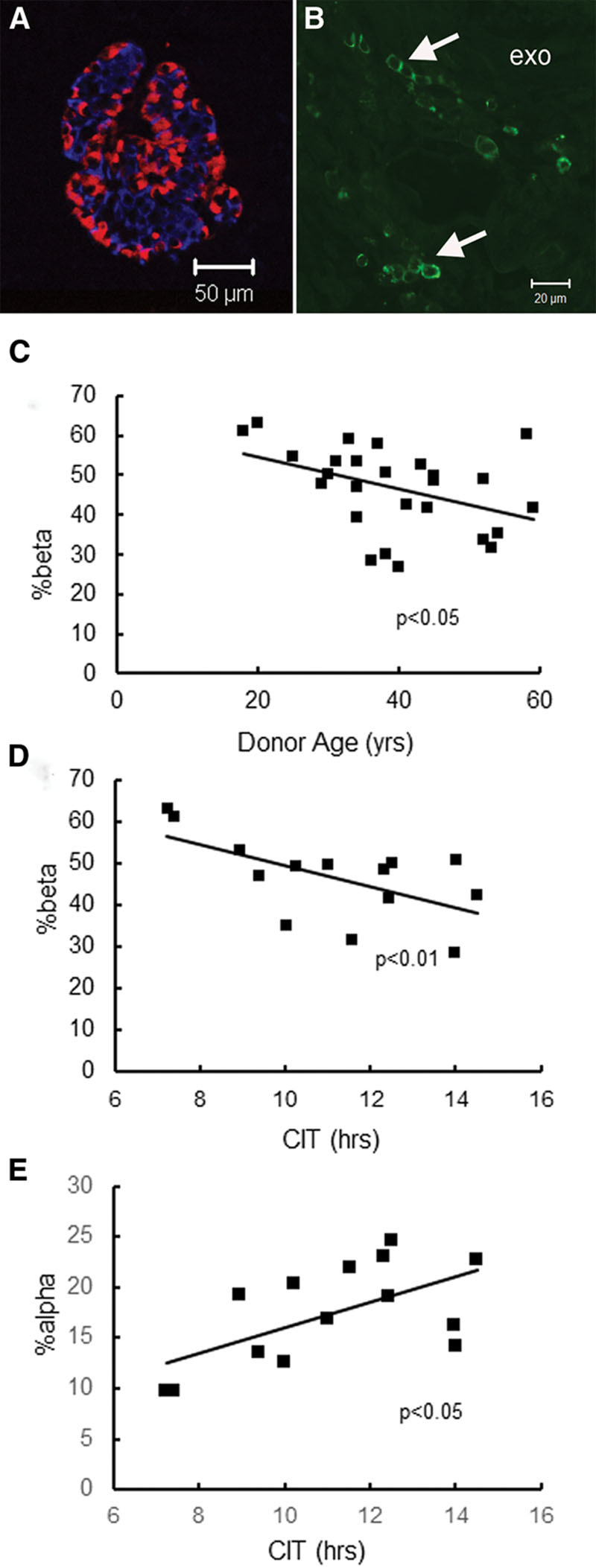
Images of labeled islets from explants and relationship of islet cell proportions to clinical variables: (A) labeled for insulin (blue), glucagon (red) showing normal distribution of islet cells; (B) labeling for lymphocyte marker CD45. No positive cells were found in, or adjacent to, islets, but many clusters of positive cells were present between exocrine lobules (exo). C, beta%islet area declined with increasing donor age (P < 0.05) cohort 1. D, beta%islet area declined with increased CIT (P < 0.01). This analysis included explants from cohort 3; this relationship was also significant (P < 0.05) in cohort 1. E, alpha%islet area increased with increased CIT (P < 0.01) (cohort 3). CIT, cold ischemic time.
Fibrosis, Pancreatitis, Islet Amyloid and Changes of Cellular Identity
Exocrine fibrosis was a prominent feature of several explants (Figure 2A); collagen deposition was present in intralobular and interlobular areas and surrounded islets. Quantitation of Sirius red positive areas indicated variable degrees of fibrosis both within a pancreas and between cases (median 9.1%; range 0.7%–19.9%) (Table 1). Fibrosis% was similar in patients diagnosed with pancreatitis compared with cohort 3 (pancreatitis, 8.8% ± 5.4; other, 8.6% ± 5.8; mean ± SD) and was unrelated to duration of Tx and all other variables. Cells between exocrine lobules and the fibrotic areas in the exocrine tissue were positive for SMA (Figure 2B and C).
FIGURE 2.
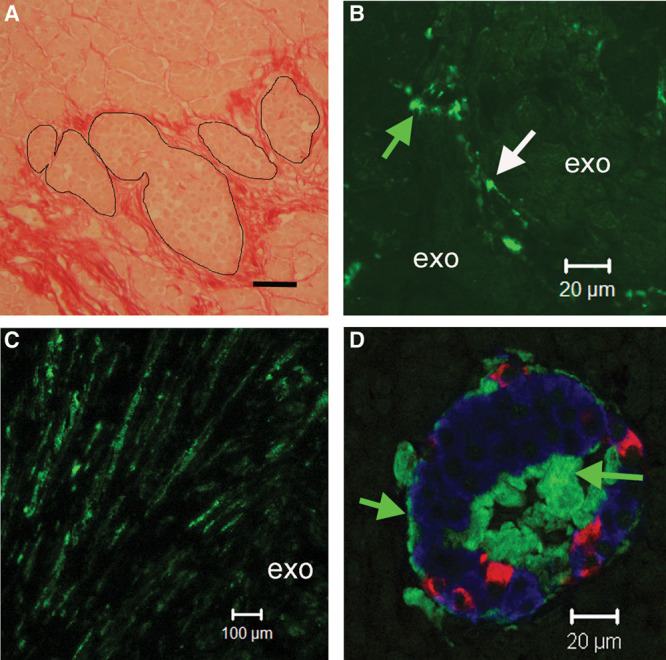
A, Explant stained (red) with Sirius red for collagen. Fibrotic tissue surrounded islets (outlined) and accumulated in interlobular and intralobular spaces; scale bar 50 µm. B, Cells (possibly pericytes) labeled for SMA (arrows) were present between exocrine lobules. C, SMA positive cells in interlobular fibrotic tracts between exocrine lobules (exo). D, Islet labeled for insulin (blue) glucagon (red) and islet amyloid (green). Islet amyloid was localized adjacent to islet capillaries at the islet periphery and in the center of the islet (green arrows). SMA, smooth muscle actin.
Islet amyloidosis was present in 3 explants from normoglycaemic subjects (Figure 2D). Amyloid-containing islets were distributed in an exocrine lobular pattern (as seen in T2D).11,14 Two patients (cases nos 24 and 8; Table 1) had explants 31 and 33 days posttransplant from donors aged 31 and 58 years, respectively; the prevalence of islets affected was 6.2% and 12%, and the areas of the islets affected (severity) were 21.6% and 19.2%, respectively. Amyloid was detected (20% prevalence and 10% severity) in an explant (case no. 58) with a longer duration of transplant (8 y) and a donor aged 42 years. There was no amyloidosis in the explant from the patient receiving therapy for diabetes.
The somatostatin-containing delta-cell islet proportion (somatostatin+ve) was unrelated to the changes in %beta or %alpha or any other variables. Isolated alpha- and beta-cells or cell clusters were present in areas of ductular proliferation associated with pancreatitis.
To examine the possibility of endocrine cell dedifferentiation or transdifferentiation, islet cells were examined for colabeling for vimentin (a potential marker for dedifferention of endocrine cells to fibroblasts). There was no evidence for vimentin+ve alpha or beta cells or for bihormonal (insulin+ve and glucagon+ve) cells. Some explants were also labeled for the secretory granule marker CGA15 to identify neogenic/regenerating primitive endocrine cells. Insulin and glucagon+ve cells were CGA+ve as expected, but no islet cells insulin- or glucagon–ve but CGA+ve were detected. Occasional caspase 3 positive cells were detected in the exocrine tissue but not in islets.
DISCUSSION
Explant pancreatic tissue provides an opportunity to examine how a normal human donor pancreas responds to Tx. We sought to detect histological changes in islets/exocrine tissue at early stages following Tx that could inform causes of later chronic functional deterioration. Our data from cohort 1 (normoglycaemic before explant) suggest that pancreatic changes in islets or exocrine tissue occurring post-Tx in these patients were insufficiently severe to affect maintenance of normal glucose tolerance; exocrine fibrosis (extensive in some cases) can occur without affecting the islet cellular composition. Transplant duration (up to 8 y) was unrelated to any of the morphometric data collected or other variables. The proportions of islet cells (mean %beta 44.5%, %alpha 17.5%) are in the range of published data for islets in nondiabetic subjects, (mean %beta 47.5%,16 %beta 50%, %alpha 10%11).
All explant patients were receiving immunosuppression, which has been considered to be deleterious for islet function8,17; however, it is evident that graft function can be maintained despite immunosuppression. Recurrence of T1D and insulitis (determined from biopsy data) at a frequency of 5.8% of patients occurring after >1 year post-Tx has been reported with immunosuppression therapy.18 We found no evidence of lymphocytic infiltration in any islets examined (>25/case) of our grafts most of which were from Tx periods <1 year.
The significant reduction in %beta associated with donor age was a feature persisting after several weeks of Tx. This suggests that either younger donors have a higher proportion of beta cells (not described in the literature) or that organs from younger donors are more resilient to anoxia and trauma associated with Tx. An alternative scenario is that islet cell proliferation occurs posttransplant in patients aged under 35 years; the existing evidence from human islets suggests that this is unlikely since islet cell proliferation/differentiation is low or zero after the age of about 25 years.19
Fibrosis and replacement of exocrine parenchyma are a feature of pancreatitis20 and has been reported in allografts.10 The acinar inflammation generated as a result of local release of cytokines and activation of stellate cells results in increased extracellular matrix proteins including collagen.21,22 SMA-labeled cells were present in fibrotic areas and interlobular spaces consistent with activated stellate cells and myofibroblasts.20,22 As seen previously, distribution of fibrotic areas varied both within and between cases and some of the explants had areas of fatty tissue necrosis and calcified deposits.22,23 Sequential explant biopsies over 5 years have demonstrated progressively increased fibrosis and some predictive value for loss of graft function.10 Banff grading of pancreas graft rejection2 lists graft fibrosis as a rejection feature; grade I includes fibrosis occupying <30% pancreatic tissue and grade II fibrosis occupying 30%–60% of the pancreas. Ten of our cases had >10% fibrotic area, but this was not associated with immunologic rejection and did not affect the islet cellular composition or glycemia. An accurate estimation of fibrosis associated with pancreatitis and its role in tissue rejection and graft survival would require a larger sample size than a single biopsy or that examined in this study.
The reduction of %beta with extended CIT provides histological support for physiological data showing a relationship between longer CIT and abnormal postoperative oral glucose tolerance/graft failure.24 The increased %alpha cell population was statistically independent of changes in %beta and is similar to that occurring in islets in T2D11 but is of unknown cause. There were no significant changes of islet cell populations in the 2 patients who did not achieve normoglycemia post-Tx suggesting that short-term exposure to intermittent periods of hyperglycemia does not have obvious effects on islets.
The presence of islet amyloid in 3 explants from nondiabetic subjects suggest that development of this pathology is not restricted to older nondiabetic subjects and those with T2D.11,14,16,25 Extensive islet amyloidosis (>95% of islets affected) has been identified in 2 pancreatic explants from diabetic patients 5 and 8 years posttransplant.7 Islet amyloid has also been observed in islets transplanted into the liver.26 The number of islets affected and severity of the amyloidosis in our explants is similar to that observed in T2D and associated with beta-cell loss.11,16,27 However, in our cohort, amyloidosis did not affect glycemic control. The question remains whether the amyloidosis was present in the nondiabetic donors’ pancreas (aged 42, 31, and 58 y) or had developed subsequent to Tx. The latter case would suggest that some local trauma in the transplanted organ had initiated islet amyloid polypeptide fibrillogenesis28 and that amyloid deposition in vivo can occur within weeks. Since detection of islet amyloidosis is not possible in vivo from serum markers, the effect of this pathology on graft survival cannot be easily estimated.
Changes in islet cell identity, cell proliferation, and dedifferentiation can be induced by different types of trauma, including hyperglycemia29 and pancreatitis.15,30 No CGA+ve/insulin or glucagon negative cells were found in the explants, suggesting that endocrine neogenesis was not occurring. Transdifferentation and dedifferentiation of islet cells are associated with bihormonal expression of insulin and glucagon and appearance of vimentin in hormone-positive cells, respectively.29 These features were not found in our cohort suggesting that the islet cell population was relatively stable.
The changes in proportions of islet cells (decreased %beta and increased %alpha cells), islet amyloidosis, and increased fibrosis are associated with and are considered by some to be casual factors for T2D.31 It has been proposed that >60% reduction of beta-cell proportion is required for induction of hyperglycemia assuming that function is not compromised.32 Thus, the relatively small changes of these features in explants from normoglycemic patients suggest that some degree of islet pathology is present but insufficient to cause hyperglycemia.
In conclusion, Tx results in small changes in islet cell proportions, which are not sufficient to affect glycemia. However, the processes of organ selection, retrieval, and Tx impacts on islet and exocrine integrity which, in the absence of immunologic rejection, could have lasting effects on graft survival
ACKNOWLEDGMENTS
We thank Oxford Centre for Histopathology Research for retrieval of archival samples and for the Biomedical Research Centre (NIHR, Oxford) for financial support for this activity.
Footnotes
Published online 19 October, 2020.
R.D. and H.L.-S. shared first coauthorship to this work.
R.D., H.L.-S., J.B., and A.C. participated in the performance of the research, research design, writing of the article, and analysis of the data. R.D. developed a new analytic tool. S.M. and E.S. contributed with patient data. P.F. initiated the idea for the research.
The authors declare no conflicts of interest.
The authors thank the Biomedical Research Centre (NIHR, Oxford) for financial support for retrieval of the specimens.
REFERENCES
- 1.Sharples EJ, Mittal SM, Friend PJ. Challenges in pancreas transplantation. Acta Diabetol. 2016; 53:871–878 [DOI] [PubMed] [Google Scholar]
- 2.Loupy A, Haas M, Solez K, et al. The Banff 2015 Kidney Meeting Report: current challenges in rejection classification and prospects for adopting molecular pathology. Am J Transplant. 2017; 17:28–41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dean PG, Kudva YC, Larson TS, et al. Posttransplant diabetes mellitus after pancreas transplantation. Am J Transplant. 2008; 8:175–182 [DOI] [PubMed] [Google Scholar]
- 4.Girman P, Lipár K, Kočík M, et al. Sirolimus vs mycophenolate mofetil (MMF) in primary combined pancreas and kidney transplantation. Results of a long-term prospective randomized study. Am J Transplant. 2020; 20:779–787 [DOI] [PubMed] [Google Scholar]
- 5.Mittal S, Nagendran M, Franklin RH, et al. Postoperative impaired glucose tolerance is an early predictor of pancreas graft failure. Diabetologia. 2014; 57:2076–2080 [DOI] [PubMed] [Google Scholar]
- 6.Burke GW, III, Vendrame F, Virdi SK, et al. Lessons from pancreas transplantation in type 1 diabetes: recurrence of islet autoimmunity. Curr Diab Rep. 2015; 15:121. [DOI] [PubMed] [Google Scholar]
- 7.León Fradejas M, Kandil D, Papadimitriou JC, et al. Islet amyloid in whole pancreas transplants for type 1 diabetes mellitus (DM): possible role of type 2 DM for graft failure. Am J Transplant. 2015; 15:2495–2500 [DOI] [PubMed] [Google Scholar]
- 8.Drachenberg CB, Klassen DK, Weir MR, et al. Islet cell damage associated with tacrolimus and cyclosporine: morphological features in pancreas allograft biopsies and clinical correlation. Transplantation. 1999; 68:396–402 [DOI] [PubMed] [Google Scholar]
- 9.Drachenberg CB, Papadimitriou JC, Klassen DK, et al. Distribution of alpha and beta cells in pancreas allograft biopsies: correlation with rejection and other pathologic processes. Transplant Proc. 1998; 30:665–666 [DOI] [PubMed] [Google Scholar]
- 10.Papadimitriou JC, Drachenberg CB, Klassen DK, et al. Histological grading of chronic pancreas allograft rejection/graft sclerosis. Am J Transplant. 2003; 3:599–605 [DOI] [PubMed] [Google Scholar]
- 11.Clark A, Wells CA, Buley ID, et al. Islet amyloid, increased A-cells, reduced B-cells and exocrine fibrosis: quantitative changes in the pancreas in type 2 diabetes. Diabetes Res. 1988; 9:151–159 [PubMed] [Google Scholar]
- 12.Ferrari SLP, Cribari-Neto F. Beta regression for modelling rates and proportions. Journal of Applied Statistics. 2004; 31:799–815 [Google Scholar]
- 13.Butler AE, Janson J, Bonner-Weir S, et al. Beta-cell deficit and increased beta-cell apoptosis in humans with type 2 diabetes. Diabetes. 2003; 52:102–110 [DOI] [PubMed] [Google Scholar]
- 14.Bell ET. Hyalinization of the islet of Langerhans in diabetes mellitus. Diabetes. 1952; 1:341–344 [DOI] [PubMed] [Google Scholar]
- 15.Md Moin AS, Dhawan S, Shieh C, et al. Increased hormone-negative endocrine cells in the pancreas in type 1 diabetes. J Clin Endocrinol Metab. 2016; 101:3487–3496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jurgens CA, Toukatly MN, Fligner CL, et al. β-cell loss and β-cell apoptosis in human type 2 diabetes are related to islet amyloid deposition. Am J Pathol. 2011; 178:2632–2640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kloster-Jensen K, Sahraoui A, Vethe NT, et al. Treatment with tacrolimus and sirolimus reveals no additional adverse effects on human islets in vitro compared to each drug alone but they are reduced by adding glucocorticoids. J Diabetes Res. 2016; 2016:4196460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vendrame F, Hopfner YY, Diamantopoulos S, et al. Risk factors for type 1 diabetes recurrence in immunosuppressed recipients of simultaneous pancreas-kidney transplants. Am J Transplant. 2016; 16:235–245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cnop M, Igoillo-Esteve M, Hughes SJ, et al. Longevity of human islet alpha- and beta-cells. Diabetes Obes Metab. 2011; 13:39–46 [DOI] [PubMed] [Google Scholar]
- 20.Klöppel G, Bommer G, Commandeur G, et al. The endocrine pancreas in chronic pancreatitis. Immunocytochemical and ultrastructural studies. Virchows Arch A Pathol Anat Histol. 1978; 377:157–174 [DOI] [PubMed] [Google Scholar]
- 21.Xue R, Jia K, Wang J, et al. A rising star in pancreatic diseases: pancreatic stellate cells. Front Physiol. 2018; 9:754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Detlefsen S, Sipos B, Feyerabend B, et al. Fibrogenesis in alcoholic chronic pancreatitis: the role of tissue necrosis, macrophages, myofibroblasts and cytokines. Mod Pathol. 2006; 19:1019–1026 [DOI] [PubMed] [Google Scholar]
- 23.Schrader H, Menge BA, Schneider S, et al. Reduced pancreatic volume and beta-cell area in patients with chronic pancreatitis. Gastroenterology. 2009; 136:513–522 [DOI] [PubMed] [Google Scholar]
- 24.Mittal S, Page SL, Friend PJ, et al. De novo donor-specific HLA antibodies: biomarkers of pancreas transplant failure. Am J Transplant. 2014; 14:1664–1671 [DOI] [PubMed] [Google Scholar]
- 25.Westermark P, Grimelius L. The pancreatic islet cells in insular amyloidosis in human diabetic and non-diabetic adults. Acta Pathol Microbiol Scand A. 1973; 81:291–300 [DOI] [PubMed] [Google Scholar]
- 26.Westermark GT, Westermark P, Berne C, et al. ; Nordic Network for Clinical Islet Transplantation. Widespread amyloid deposition in transplanted human pancreatic islets. N Engl J Med. 2008; 359:977–979 [DOI] [PubMed] [Google Scholar]
- 27.Westermark P, Wilander E. The influence of amyloid deposits on the islet volume in maturity onset diabetes mellitus. Diabetologia. 1978; 15:417–421 [DOI] [PubMed] [Google Scholar]
- 28.Raleigh D, Zhang X, Hastoy B, et al. The β-cell assassin: IAPP cytotoxicity. J Mol Endocrinol. 2017; 59:R121–R140 [DOI] [PubMed] [Google Scholar]
- 29.Roefs MM, Carlotti F, Jones K, et al. Increased vimentin in human α- and β-cells in type 2 diabetes. J Endocrinol. 2017; 233:217–227 [DOI] [PubMed] [Google Scholar]
- 30.Beamish CA, Gaber AO, Afshar SF, et al. Variability in endocrine cell identity in patients with chronic pancreatitis undergoing islet autotransplantation. Am J Transplant. 2019; 19:1568–1576 [DOI] [PubMed] [Google Scholar]
- 31.Matveyenko AV, Butler PC. Relationship between beta-cell mass and diabetes onset. Diabetes Obes Metab. 2008; 10:23–31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Meier JJ, Breuer TG, Bonadonna RC, et al. Pancreatic diabetes manifests when beta cell area declines by approximately 65% in humans. Diabetologia. 2012; 55:1346–1354 [DOI] [PubMed] [Google Scholar]


