Abstract
Immune checkpoint inhibitors (ICIs) are widely used in lung cancer therapy due to their effectiveness and minimal side effects. However, only a few lung cancer patients benefit from ICI therapy, driving the need to develop alternative biomarkers. Programmed death-ligand 1 (PD-L1) molecules expressed in tumor cells and immune cells play a key role in the immune checkpoint pathway. Therefore, PD-L1 expression is a prognostic biomarker in evaluating the effectiveness of programmed death-1 (PD-1)/PD-L1 inhibitors. Nevertheless, adverse predictive outcomes suggest that other factors are implicated in the response. In this review, we present a detailed introduction of existing biomarkers concerning tumor abnormality and host immunity. PD-L1 expression, tumor mutation burden, neoantigens, specific gene mutations, circulating tumor DNA, human leukocyte antigen class I, tumor microenvironment, peripheral inflammatory cells, and microbiome are discussed in detail. To sum up, this review provides information on the current application and future prospects of ICI biomarkers.
Keywords: Biomarker, Immune checkpoint inhibitor, Lung cancer
Introduction
Lung cancer is the most common malignant cancer in China and is the leading cause of cancer-related mortality worldwide. Currently, the 5-year survival rate of lung cancer is less than 20%. Additionally, the 5-year survival rate of half of patients diagnosed with metastatic lung cancer is approximately 5%.[1,2] Poor prognosis of lung cancer is partially attributed to dependence on chemotherapy for the treatment of refractory lesions. Molecular targeted therapy is an alternative approach applied to approximately 30% of lung cancer patients with specific oncogenes; however, drug resistance is observed in this type of therapy.[3] Notably, immune checkpoint inhibitor (ICI), which relies on the immunological function of the patient, is a significant breakthrough in the treatment of lung cancer. ICI is effective and not evidently threatened by resistance and offers patients’ long-term survival. Therefore, this approach is used as first-line, second-line, and maintenance treatment. Nevertheless, not all lung cancer patients could benefit from the efficiency of ICIs considering that some patients develop drug resistance or immune-related adverse events. Hence, determining alternative biomarkers that are effective for this group of patients is required.
Immune checkpoint inhibitors
Mechanisms of the immune checkpoints
The immune system plays a key role in maintaining health, and immune checkpoints regulate the function of immune cells. Basically, immune checkpoints protect normal tissue and cells from being attacked under physiological conditions. Additionally, they enable tumor cells to evade the identification and elimination of antitumor immune factors. Cytotoxic T-lymphocyte antigen 4 (CTLA-4) and programmed death-1 (PD-1)/programmed death-ligand 1 (PD-L1) pathways are the main immune checkpoints adopted by tumor cells. CTLA-4 plays a key role in the initial antigen-presenting phase where it interferes with the activation of lymphocytes. On the contrary, PD-1/PD-L1 impairs the function of the activated T cells, thus providing a favorable microenvironment for tumor growth. Therefore, inhibitors targeting the two pathways would recover and enhance the specific immune response against malignant cells. In recent years, discovery, research, and application of ICIs has significantly contributed to lung cancer therapy.[4]
Agents of immune checkpoint inhibitors
Primary ICIs include PD-1 inhibitors (nivolumab, pembrolizumab, and sintilimab), PD-L1 inhibitors (atezolizumab, durvalumab, and avelumab), and monoclonal antibodies targeting CTLA-4 (ipilimumab and tremelimumab). Nivolumab was approved by the US Food and Drug Administration (FDA) for the treatment of advanced non-small cell lung cancer (NSCLC).[5,6] Currently, nivolumab is used as a third-line therapy for metastatic SCLC.[7] Furthermore, pembrolizumab is used for the treatment of metastatic NSCLC patients with a PD-L1 score of ≥ 50%.[8–10] Pembrolizumab in combination with platinum-based doublet chemotherapy is used as a first-line therapy for advanced-stage patients.[11–13] Additionally, pembrolizumab monotherapy is approved as a third-line therapy for SCLC patients.[14] Sintilimab is currently undergoing clinical trials as a potential NSCLC therapy.[15] Notably, studies have reported success in the application of sintilimab as a neoadjuvant therapy for NSCLC patients.[16] Atezolizumab has been approved as a second-line treatment in patients with advanced stages of NSCLC based on the results of several clinical trials. Previous studies have reported that the high efficacy of atezolizumab is associated with the high expression levels of PD-L1.[17–20] Notably, a combination therapy comprising atezolizumab and chemotherapeutic agents showed high efficacy on NSCLC patients regardless of the expression levels of PD-L1.[21] Although the combination therapy was effective in patients with advanced-stage SCLC, the treatment cost was much higher than chemotherapy.[22,23] Durvalumab was approved for the treatment of stage III unresectable NSCLC.[24] Currently, several researchers are conducting clinical trials on avelumab, a PD-L1 inhibitor.[25,26] Additionally, several clinical trials studying the validation of clinical applications of ICIs such as monotherapy and use in combination therapy with another ICI, chemotherapy, radiotherapy, and targeted therapy are underway.
Despite the promising advancements of ICIs in lung cancer treatment, this therapy is only effective for 15% to 25% of lung cancer patients. Limited efficacy is partially attributed to immunotherapy resistance whereby the mechanisms are unknown.[27] Some oncogenes and anti-oncogenes such as EGFR and STK11 are implicated in ICI resistance.[28,29] Moreover, a previous study reported that the diversity of the gut microbiome, which easily changes upon the administration of antibiotics, may be involved in ICI resistance.[30] On the contrary, approximately 20% to 30% of patients presented immune-related adverse events (irAEs) after PD-1/PD-L1 inhibitor therapy. These adverse effects are attributed to excessive activation of immune system or development of autoimmunity in the endocrine system, skin tissue, cardiovascular system, respiratory tract, and digestive system.[31–33] The limitations of ICI therapy discussed above are an impetus for the identification of effective biomarkers that stratify patients and minimize irAEs in lung cancer.
Biomarkers
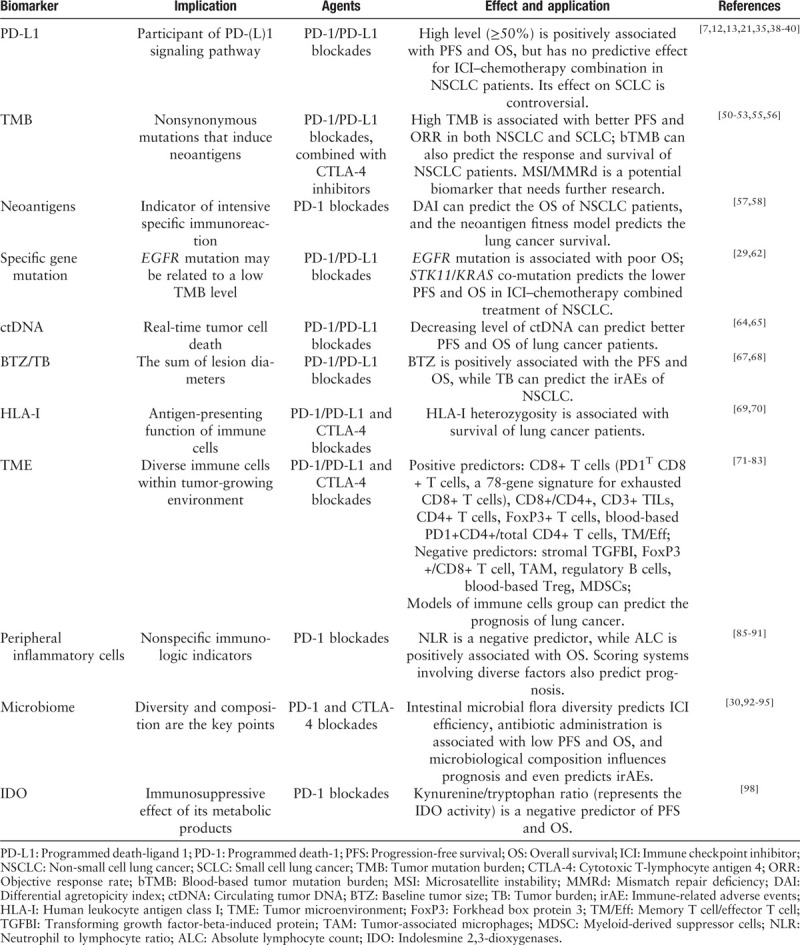
Biomarkers inform the use of a therapeutic approach depending on efficacy, resistance, and toxicity of the approach. PD-1/PD-L1 signal pathway, as mentioned earlier, is a key target of ICIs; therefore, PD-L1 molecules are believed to be biomarkers for PD-1/PD-L1 inhibitors. Although the predictive ability of the high expression levels of PD-L1 has been reported in NSCLC patients,[34,35] several trials have drawn the opposite conclusions.[5,36] The application of ICIs in cancer therapy has several challenges such as determining an accurate interpretation of the absence of PD-L1 expression as a biomarker in some cases and identifying other potential determinants of ICI application. A myriad of research has been conducted to understand the complicated interaction between the tumor and immune system, and factors such as tumor mutation burden (TMB), neoantigens, various immune cells, and gut microorganism have been investigated. In the following section, we discuss major biomarkers including molecules, cells, and genes. Some of these biomarkers result from tumor formation, whereas others result from immune responses [Table 1].
Table 1.
Main biomarkers of immune checkpoint inhibitor treatment in lung cancer.
Tumor abnormality-associated biomarkers or factors
PD-L1 expression
PD-L1 molecule is expressed in tumor cells and immune cells, and its expression levels can be analyzed using immunohistochemistry (IHC). It is reported that NSCLC has significantly higher expression levels of PD-L1 compared with renal cell carcinoma and melanoma.[37] Previous studies have confirmed that the high expression levels of PD-L1 are positively associated with progression-free survival (PFS) and overall survival (OS) after treatment with PD1/PD-L1 inhibitors. However, some studies have reported the high efficacy of PD1/PD-L1 inhibitors in patients expressing low levels of PD-L1. Studies on six stage III clinical trials have reported that ICI therapy is highly effective in NSCLC patients expressing high levels of PD-L1 molecules (≥50%).[38] On the contrary, a meta-analysis has reported that a combination therapy comprising PD-1/PD-L1 inhibitors and chemotherapy is more effective compared with chemotherapy in NSCLC patients with <1% PD-L1 expression.[39] Furthermore, several studies have reported that ICI–chemotherapy combination therapy is effective for NSCLC patients regardless of the expression levels of PD-L1.[12,13,21,40] A study on SCLC has reported that PD-L1 molecules expressed on the stroma are positively associated with the efficacy of pembrolizumab.[41] However, PD-L1 expression is found to be irrelevant to the objective response rate (ORR) in nivolumab-treated SCLC patients.[7,42] Variation in effectiveness can be attributed to the representativeness of the pathological specimen and reliability in detection techniques. First, heterogeneity of PD-L1 distribution in the neoplasm partly results in the inaccuracy in the determination of PD-L1 expression levels from biopsy specimens or resected tissues. A previous study has compared the PD-L1 expression levels of five core biopsy specimens and the whole sections of 268 cases to understand the variation. Out of these, 39% and 10% of the samples showed positive results with a 1% and 50% cutoffs, respectively.[43] Second, PD-L1 expression is not consistent, and early-stage therapeutic regimen alters the expression levels, thus affecting the association between the PD-L1 expression and the therapeutic effect.[44] It is reported that PD-L1 molecules show inducible expression apart from constitutive expression specifically on immune cells. Researchers have investigated the PD-L1 expression profiles in 4549 NSCLC patients taking atezolizumab and have demonstrated that interferon-γ upregulated PD-L1 expression on immune cells, PD-L1 expression levels on tumor cells were implicated in the genetic dysfunction, and the predictive effects of PD-L1 were observed in both tumor and immune cells.[45] Third, five distinct FDA-approved antibodies are used for PD-L1 testing. The Blue Print study reports consistency in staining outcomes among 22C3, 28-8, and SP263 antibodies in tumor cells. However, lower sensitivity on SP142 and higher sensitivity on 73-10 were reported. Notably, PD-L1 scores reported by pathologists are comparable to scores determined through digital images. On the contrary, significant differences are observed for the scores of the antibodies in immune cells.[46] The inconsistency among these antibody clones should be investigated further, and variation of pathologists in assessing the PD-L1 expression levels may also contribute to variation in results. Moreover, the application of ICIs is based on the expression levels of PD-L1 molecules rather than their presence. However, current studies do not define the exact PD-L1 expression cutoff, which determines the application of ICIs as a therapy.
PD-L1 expression is a promising biomarker, and its application can be optimized further. Reports from previous clinical trials indicate that PD-L1 expression should be combined with other factors to effectively determine the effectiveness of ICI as a therapy. Studies have investigated the predictive value of PD-L1 expression in combination with CD8+ tumor-infiltrating lymphocyte (TIL) density in NSCLC patients. Notably, the PD-L1+/CD8low group is associated with high grade and advanced stage of tumor, whereas the PD-L1−/CD8high group is associated with better OS and PFS. However, more studies should be conducted to validate the clinical significance of this biomarker pattern as the study used a small sample size (55 cases).[47] In a recent study, PD-L1 expression levels within the peripheral blood were tested, and the association between PD-L1 expression levels and systemic immune cells and the response rates of PD-1/PD-L1 inhibition in lung cancer patients were investigated. The study reports that patients who express ≥30% of PD-L1+CD11b+ cells before treatment attain a 50% response rate.[48] In another study, positron emission tomography-computed tomography (PET-CT) is used to estimate the PD-1/PD-L1 expression levels in NSCLC patients prior to nivolumab treatment. The study describes the heterogeneity of PD-1/PD-L1 expression between and within patients, shows the consistency between PET-CT and IHC technologies, and demonstrates the association between tumor tracer uptake and ICI therapy.[49] Furthermore, combination therapy comprising immunotherapy and chemotherapy may not be associated with PD-L1 expression. The effectiveness of combination therapy may be attributed to the synergistic effect on the immune system, and further studies are required to understand the mechanism.
TMB
TMB is the total number of nonsynonymous mutations in tumors and is quantified by somatic mutations per megabase (Mb). Lung tissue specimens are used to determine the TMB using the next-generation sequencing (NGS) technology. The application of TMB as a biomarker in immunotherapy is mainly due to increased neoantigens resulting from high levels of gene mutations, which in turn activates specific immunity. A retrospective analysis shows that high TMB (>243 mutations according to whole exome sequencing) is associated with superior PFS and ORR among NSCLC patients receiving nivolumab treatment.[50] In another study, NSCLC patients with high TMB, defined as ≥10 Mut/Mb, are reported to have higher ORR or PFS after nivolumab and ipilimumab therapy. Furthermore, a combination of ICI treatment with chemotherapy has a high response rate.[51] Notably, similar TMB prognostic value is reported in SCLC patients receiving either nivolumab monotherapy or nivolumab plus ipilimumab combination therapy.[52,53]
Some mutated genes are more vulnerable to form neoantigens compared with other genes. Due to this indirect association between TMB and neoantigens, TMB does not always correspond with immunotherapy efficacy. Microsatellite instability (MSI), which is mainly followed by mismatch repair deficiency (MMRd) mutation, results in high neoantigen levels.[54] MMRd represents more insertion and deletion (indel) mutations and undergoes more unnatural frameshifts, thus forming more neoantigens. Although MMRd/MSI accounts for only a small percentage of mutations in NSCLC, its predictive value is worth investigating.
However, different from practical biomarkers for ICI therapy, TMB is time-consuming and requires specimens. To circumvent these shortcomings, some researchers use a more accessible surrogate. Studies report that blood-based TMB, which can be detected with sensitive NGS technique, is effective in estimating the response and survival rate of NSCLC patients receiving ICIs, including atezolizumab and durvalumab + tremelimumab.[55,56] Although the threshold of TMB level is a challenge, it can be overcome with increasing data generated through sequencing techniques and improving the analytical method.
Neoantigens
As mentioned above, neoantigen is an indicator of intensive specific immunoreaction. However, the application of neoantigen as immunotherapy biomarker is dependent not only on the quantity, which can be estimated generally by TMB, but also on its quality, which is affected by three factors. Neoantigens are grouped into two kinds based on whether they stem from clonal mutation or subclonal mutation, videlicet, whether they are distributed over the whole tumor or a part of it. Notably, neoantigens resulting from clonal mutations are more responsive to attack from immune cells in comparison with subclonal mutation; therefore, intratumoral heterogeneity of neoantigens resulting from subclonal mutations may be the first negative predictor to ICI therapy. A combination of major histocompatibility complex class I (MHCI) and T-cell receptor (TCR) is a step of T cell–neoantigen reaction, and binding affinity of MHC1 to TCR is the second factor, which can be measured using the differential agretopicity index (DAI). A study using pembrolizumab-treated cohort reports that DAI can be used to predict the OS of NSCLC patients.[57] Moreover, high sequence homology obtained through similarity analysis on the epitopes of neoantigens and the known immunogenic microbial epitopes is the third characteristic of foreign neoantigen. Researchers construct a neoantigen fitness model based on these factors, use it in patients receiving PD-1 inhibitor therapy, and confirm its survival prediction effect for lung cancer and other tumors.[58]
Specific gene mutations
Studies indicate that some mutated genes, mainly driver genes in lung cancer, are associated with the response of ICIs in lung cancer. A meta-analysis reported that EGFR mutation is not associated with good objective response (OR) in anti-PD-1/PD-L1-treated NSCLC patients.[29] This observation can be attributed to the low TMB state of patients with EGFR mutations.[59] Another study also reports that TMB is associated with the EGFR-wild lung cancers.[60] Interestingly, a study reports that durvalumab improves the objective response (OR) of EGFR/ALK-mutated NSCLC patients with ≥25% of PD-L1 expression.[61] Furthermore, a combination of atezolizumab with tyrosine kinase inhibitor (TKI) is shown to be effective on TKI-invalid patients with EGFR mutation.[40]STK11 gene mutation is also believed to affect the effectiveness of ICI. Mutant STK11 in combination with KRAS is associated with decreased response and survival in lung adenocarcinoma patients.[28] In a non-squamous NSCLC cohort, STK11/KRAS co-mutation patients showed lower PFS and OS after treatment with pembrolizumab plus doublet chemotherapy.[62] On the contrary, STK11/TP53-wild NSCLC patients present longer OS according to a genomic analysis.[63] Most mutated genes display adverse effects on ICI therapy; however, the underlying mechanism and therapeutic strategies have not been fully investigated.
Circulating tumor DNA (ctDNA)
ctDNA detection is a type of “liquid biopsy.” It involves the evaluation of real-time tumor cell death using NGS technique to test mutating gene segments from which immunotherapy effect can be monitored. A small sample study of metastatic NSCLC patients reports comparable outcome of ctDNA change and radiological manifestation in patients under anti-PD-1/PD-L1 treatment. Furthermore, low level of ctDNA level is a positive predictor of PFS and OS.[64] Another cohort showed an average 8.7 weeks of ctDNA earlier response compared with CT imaging in PD-1 inhibitor-treated lung cancer patients.[65] Although this finding provides an effective way to monitor ICI efficiency dynamically, its accuracy should be validated further.
Baseline tumor size and tumor burden
Baseline tumor size (BTZ) is defined as the summation of diameters of all lesions. Studies have reported that BTZ is an effective prognostic biomarker for pembrolizumab therapy in the treatment of melanoma.[66] A retrospective analysis measured BTZ among NSCLC patients and reported that high BTZ (>101 mm) corresponds to lower PFS and OS in PD-1/PD-L1 inhibitor therapy.[67] A study on NSCLC patients receiving anti-PD-1/PD-L1 therapy uses tumor burden as a parameter (means the sum of diameters of up to five lesions), which is confirmed to be a predictive biomarker for anticipating severe irAEs.[68] Tumor size is associated with the stage, grade, and histologic type of lung cancer, and its association with poor prognosis should be investigated further.
Host immunity-associated biomarkers or factors
Human leukocyte antigen class I
Human leukocyte antigen genes encode immunosurveillance function in the body, and human leukocyte antigen class I (HLA-I) gene is principally associated with antigen presentation. This implies that the diversity in HLA-I gene will result in the recognition of several antigens. A study on approximately 1535 cancer patients receiving anti-PD-1 or anti-CTLA-4 therapy reports a positive association between HLA-I heterozygosity and longer survival.[69] On the contrary, impaired antigen-presenting function of HLA-I gene presents a negative effect on PD-1/PD-L1 inhibition therapy in lung cancer patients.[70]
Tumor microenvironment
The tumor microenvironment (TME) plays an important role in tumor growth and comprises diverse immune cells including tumor-associated microphages (TAMs), natural killer cells, dendritic cells, lymphocytes, and myeloid-derived suppressor cells (MDSCs). TILs are responsible for antitumor activity during ICI treatment. Previous studies have reported that the enrichment of TILs, such as cytotoxic T cells, helper T cells, and memory T cells in TME is associated with ICI response in NSCLC patients. Different TILs play distinct roles in tumor–immune interactions. A study analyzing the expression of CD8 and CD4 molecules on NSCLC tissue samples using IHC reports that higher CD8+ T cell count (886–1899/mm2) and higher CD8+/CD4+ ratios (>2) are positively associated with higher response rate of anti-PD-1 therapy.[71] Notably, NSCLC patients with high stromal infiltration of CD8+ and CD4+ immune cells present better OS upon nivolumab treatment.[72] Analysis of stromal transforming growth factor-beta-induced protein (TGFBI) and intertumoral CD8+ T cells in lung cancer patients treated with nivolumab showed that low TGFBI and high CD8 expression levels are positively associated with high tumor response.[73] PD-1-expressing TILs might be a potential predictor, and studies report that CD8+ T cells characterized by highest PD1 expression (PD1T) before anti-PD-1 treatment are positively associated with better drug response.[74] A recent study has investigated a subtype of exhausted CD8+ T cells as a 78-gene signature for exhausted CD8+ T cells based on transcriptional features and reported a positive association with the ICI therapeutic effect in NSCLC patients.[75] A study on the expression of CD3, CD8, CD4, PD1, and forkhead box protein 3 (FoxP3) on TILs reports that high CD3+ TILs (>617.5/mm2) and low FoxP3+/CD8+ T cell ratio (<25%) are both prognostic factors of anti-PD1 therapy response among NSCLC patients.[76] However, FoxP3 is positively associated with therapy response in other situations. A study on EGFR-mutated NSCLC patients receiving nivolumab reports that CD4+ and FoxP3+ T cells are positive prognostic factors, whereas PD-L1 expression would not predict therapy response.[77] In addition to T cells, TAM and regulatory B cells in immune-competent subtype of NSCLC, which is categorized computationally based on gene expression, are reported to reduce the efficacy of ICIs.[78] Notably, TME is complex and cannot conclusively be studied through a few cell types; therefore, studies have developed an immunogram. The TME of lung cancer patients is divided into T cell-rich, T cell-poor, and intermediate regardless of the histological types based on this immunogram, which is a more promising biomarker for personalized ICI therapy.[79] A transcriptome-based model was developed through comparison of messenger RNA (mRNA) sequencing of 188 NSCLC patients and validated by 35 patients. The model showed that a molecular subtype of lung adenocarcinoma characterized by high CD8+ T cells and memory B cells versus low CD4+ Tregs and tumor-associated myeloid cells is an effective predictor of anti-PD-1 therapy.[80]
In attempts to determine an effective alternative for the estimation of antitumor activity of immune system, the concept of liquid biopsy was introduced for immune cell detection. A study on the profile of T lymphocytes in the peripheral blood of NSCLC patients before or just starting anti-PD-1/PD-L1 therapy reports a positive association between high PD1+CD4+/total CD4+ T cell ratio and long PFS.[81] Furthermore, high ratio of CD4+ and CD8+ central memory T cell to effector T cell in the blood is positively associated with high PD-L1 expression levels and PFS of NSCLC patients receiving nivolumab.[82] Additionally, analysis of blood-based subtypes of immune cells among atezolizumab-treated advanced NSCLC patients revealed a reduction of regulatory T cells and MDSCs in the disease-controlled patients.[83] Moreover, hyperprogressive disease (HPD) in patients under ICI therapy can be predicted using peripheral immune cells. A prospective study involving 263 anti-PD-1/PD-L1-treated NSCLC patients reports that HPD is prevalent in patients with a lower proportion of chemokine receptor 7 (CCR7)-CD45RA-/CD8+ T cells and a higher ratio of T-cell immunoreceptor with immunoglobin and ITIM domains protein (TIGIT)+/PD-1+CD8+ T cells.[84] Further studies and development of computer models based on the entire immune system should be developed to fully understand the mechanisms that drive antitumor immune responses.
Peripheral inflammatory cells
The expression levels of peripheral inflammatory cells are applied in the evaluation of ICI efficacy. The neutrophil to lymphocyte ratio (NLR) is a key determinant of ICI efficacy. Previous studies have reported the role of NLR in predicting PFS and OS for lung cancer patients treated with PD-1 inhibitors.[85] ΔNLR>1 is associated with tumor progression and poor OS for NSCLC patients receiving second-line nivolumab treatment.[86] A study on the absolute lymphocyte count (ALC) reports that high level of baseline and 6-week post-therapy ALC is positively associated with increased OS of PD-1 inhibitor-treated NSCLC patients.[87] The study further suggests avoiding the combination of ICIs and radiotherapy in case of subsequent low ALC levels. A recent study reports the application of immune-metabolic-prognostic index (IMPI) to determine the effectiveness of PD-1 inhibitors in NSCLC patients. In this study, complete blood cell count is assessed and 18F-fluoro-2-deoxy-D-glucose-positron emission tomography (18F-FDG PET)-CT is performed, and low levels of two IMPI parameters (NLR <4.9 and total lesion glycolysis <541.5 mL) are found to be positively associated with patients’ PFS and OS.[88] Studies on other scoring systems involving inflammatory, metabolic, and nutritious factors such as lung immune prognostic index,[89] advanced lung cancer inflammation index,[90] Royal Marsden Hospital prognostic score, and MD Anderson Cancer Center prognostic score are underway.[91] Although blood-based factors are currently popular, they have low efficacy.
Microbiome
Preclinical and clinical studies report that the diversity of commensal microbiome, specifically the gut microorganisms, is a key player of immune response against tumors. Therefore, microbiome is a potential biomarker for ICI therapy in lung cancer patients. Analysis of stool samples of patients shows a positive association between intestinal microbial flora diversity and ICI therapy efficacy in lung cancer. Furthermore, the use of antibiotics during PD-1 inhibitor therapy is negatively associated with ICI efficacy, which can be attributed to changes in species diversity of the gut microbiome.[30] Shorter PFS and OS are reported among NSCLC patients who took antibiotics before ICI treatment.[92] A study on nivolumab-treated NSCLC patients and healthy individuals reports that pretreatment composition of microbiome affects anti-PD-1 response.[93] Microbiological diversity is interfered by several factors including physical conditions, dietary patterns, tobacco inhaling, and dwelling environment. A study on Chinese NSCLC patients reports that particular bacterial floras are associated with the response to PD-1 inhibitors.[94] Furthermore, the gut microbiota is a potential predictor for irAEs. High levels of Faecalibacterium and other Firmicutes of the baseline gut microbiota in melanoma patients are associated with better response to ipilimumab and are positively associated with ICI-related colitis.[95] However, these studies are not conclusive, and the predictive role of the gut microbiota in lung cancer should be further validated.
Indolesmine 2,3-dioxygenases (IDOs)
IDO is the rate-limiting enzyme in tryptophan catabolism, and the metabolic products of tryptophan are reported to suppress antitumor immunity.[96] High level of IDO expression enhances tumor growth; therefore, studies investigating the inhibitors targeting IDO pathway in different cancer types including NSCLC are conducted.[97] On the contrary, IDO immunosuppressive effect can be used as a prognosis marker in ICI therapy. A study on the role of IDO in anti-PD-1-treated NSCLC patients reports that lower kynurenine/tryptophan ratio, which suggests low IDO activity, is positively associated with longer PFS and OS.[98]
Other biomarkers or approaches
Recent studies have investigated other biomarkers in ICI-treated lung cancer patients, including red blood cell distribution width,[99] baseline serum sodium concentration,[100] blood-based prolactin,[101] and even skeletal muscle area measured at the level of the third lumbar vertebra (L3).[102] Additionally, PET is an effective approach to predict the PFS and OS in ICI-treated NSCLC patients.[103] After further validation, these means will be indispensable in predicting ICI efficacy.
On the contrary, studies on biomarkers for the prediction of irAEs are limited. Some studies report the positive association between histological, epidemiological, and clinical characteristics of NSCLC patients with higher incidence of irAEs. For instance, women, the elderly, and patients with nonsquamous carcinoma and those with interstitial lung disease (ILD) or radiotherapy history are more likely to develop ICI-related pneumonitis.[104,105] IrAEs result from overactive immunoreactions; therefore, biomarkers implicated in ICI efficacy can be used to predict the occurrence of irAEs. However, current trials report that PD-L1 expression and TMB cannot be used as irAE biomarkers. Moreover, irAE is associated with ICI response. Two retrospective analyses report that irAEs are associated with ORR, PFS, and OS of nivolumab-treated NSCLC patients.[106,107] A prospective study on NSCLC patients receiving anti-PD-1 therapy reports the potential mechanism of autoimmune skin toxic effect by identifying shared antigens and T cells of skin lesions and tumor tissue. Furthermore, the study reports a positive association between skin irAEs and tumor response.[108] On the contrary, checkpoint inhibitor pneumonitis is negatively associated with survival of NSCLC patients under ICI monotherapy or combination therapy.[109]
Conclusion
Lung cancer is a common malignant disease. Notably, the association between tumor cells and immune system is a multifactorial and dynamic process. Therefore, immunotherapy intervention and therapeutic evaluation of lung cancer is challenging. Currently, no biomarkers can solely be used to inform the medication regimen for lung cancer patients; therefore, by conducting several studies, a combination of different factors has been optimized. Studies have investigated the combination of distinct biomarkers, whereas they focus on only a subgroup of biomarkers. A comprehensive model covering gene sequencing, cellular staining, molecular identification, and imageological examination should be investigated in the future. Computer modeling methods and statistical approaches are important in the development of an effective model as the factors involved play diverse roles and have distinct cutoffs. A comprehensive model may contribute to the standardization and predictive accuracy of ICI therapy; however, its timeliness and convenience are uncertain. Currently, liquid biopsy has attracted increased interest among researchers due to its noninvasive nature, widespread application for various stages of patients, and acceptable consistency with gold standard of biopsy. Furthermore, liquid-based detection is easily applicable, enabling the monitoring of the dynamic variation of indicators, and leads to a better comprehension of real-time response to ICIs. Moreover, studies on biomarkers have not sufficiently investigated certain areas such as the following aspects: (1) the recalcitrant trait of SCLC poses an urgent need to stratify patients with appropriate biomarkers; therefore, it requires further research, and (2) although ICI–chemotherapy combination has been shown to be highly effective, it is limited by adverse events; therefore, understanding of underlying mechanisms would help identify better biomarkers or biomarker groups. Moreover, current reports on biomarkers are mostly based on retrospective analysis, which may be biased; therefore, more prospective studies are required to support the effectiveness of ICIs.
In conclusion, promising results have been achieved on biomarkers guiding the clinical application of ICIs for lung cancer patients. PD-L1 expression has been approved by the FDA as a prognostic marker. Furthermore, studies have reported that TMB and TILs are positively associated with ICI treatment. More potential biomarkers have been investigated by conducting several studies on therapeutic response or resistance, including immune-related neoantigens, specific mutated genes, and microbial diversity. Nonetheless, there is no “golden standard” for the determination of immunotherapy efficacy as biomarkers have limitations. Therefore, further studies should be conducted to investigate the advantages of combining different biomarkers.
Conflicts of interest
None.
Footnotes
How to cite this article: Shi WJ, Zhao W. Biomarkers of immune checkpoint inhibitors in lung cancer: achievements and prospective. Chin Med J 2020;20:2466–2475. doi: 10.1097/CM9.0000000000001090
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer Statistics, 2020. CA Cancer J Clin 2020; 70:7–30. doi: 10.3322/caac.21590. [DOI] [PubMed] [Google Scholar]
- 2.Khaltaev N, Axelrod S. Global lung cancer mortality trends and lifestyle modifications: preliminary analysis. Chin Med J 2020; 133:1526–1532. doi: 10.1097/CM9.0000000000000918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Arbour KC, Riely GJ. Systemic therapy for locally advanced and metastatic non-small cell lung cancer: a review. JAMA 2019; 322:764–774. doi: 10.1001/jama.2019.11058. [DOI] [PubMed] [Google Scholar]
- 4.Lim SW, Ahn MJ. Current status of immune checkpoint inhibitors in treatment of non-small cell lung cancer. Korean J Intern Med 2019; 34:50–59. doi: 10.3904/kjim.2018.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brahmer J, Reckamp KL, Baas P, Crinò L, Eberhardt WE, Poddubskaya E, et al. Nivolumab versus docetaxel in advanced squamous-cell non-small-cell lung cancer. N Engl J Med 2015; 373:123–135. doi: 10.1056/NEJMoa1504627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Borghaei H, Paz-Ares L, Horn L, Spigel DR, Steins M, Ready NE, et al. Nivolumab versus Docetaxel In Advanced Nonsquamous Non-Small-Cell Lung Cancer. N Engl J Med 2015; 373:1627–1639. doi: 10.1056/NEJMoa1507643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ready N, Farago AF, de Braud F, Atmaca A, Hellmann MD, Schneider JG, et al. Third-Line Nivolumab Monotherapy in Recurrent SCLC: CheckMate 032. J Thorac Oncol 2019; 14:237–244. doi: 10.1016/j.jtho.2018.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Herbst RS, Baas P, Kim DW, Felip E, Pérez-Gracia JL, Han JY, et al. Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): a randomised controlled trial. Lancet 2016; 387:1540–1550. doi: 10.1016/S0140-6736(15)01281-7. [DOI] [PubMed] [Google Scholar]
- 9.Garon EB, Rizvi NA, Hui R, Leighl N, Balmanoukian AS, Eder JP, et al. Pembrolizumab for the treatment of non-small-cell lung cancer. N Engl J Med 2015; 372:2018–2028. doi: 10.1056/NEJMoa1501824. [DOI] [PubMed] [Google Scholar]
- 10.Reck M, Rodríguez-Abreu D, Robinson AG, Hui R, Csőszi T, Fülöp A, et al. Pembrolizumab versus chemotherapy for PD-L1-positive non-small-cell lung cancer. N Engl J Med 2016; 375:1823–1833. doi: 10.1056/NEJMoa1606774. [DOI] [PubMed] [Google Scholar]
- 11.Langer CJ, Gadgeel SM, Borghaei H, Papadimitrakopoulou VA, Patnaik A, Powell SF, et al. Carboplatin and pemetrexed with or without pembrolizumab for advanced, non-squamous non-small-cell lung cancer: a randomised, phase 2 cohort of the open-label KEYNOTE-021 study. Lancet Oncol 2016; 17:1497–1508. doi: 10.1016/S1470-2045(16)30498-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gandhi L, Rodríguez-Abreu D, Gadgeel S, Esteban E, Felip E, De Angelis F, et al. Pembrolizumab plus chemotherapy in metastatic non-small-cell lung cancer. N Engl J Med 2018; 378:2078–2092. doi: 10.1056/NEJMoa1801005. [DOI] [PubMed] [Google Scholar]
- 13.Paz-Ares L, Luft A, Vicente D, Tafreshi A, Gümüş M, Mazières J, et al. Pembrolizumab plus chemotherapy for squamous non-small-cell lung cancer. N Engl J Med 2018; 379:2040–2051. doi: 10.1056/NEJMoa1810865. [DOI] [PubMed] [Google Scholar]
- 14.Chung HC, Piha-Paul SA, Lopez-Martin J, Schellens J, Kao S, Miller WH, Jr, et al. Pembrolizumab after two or more lines of previous therapy in patients with recurrent or metastatic SCLC: results from the KEYNOTE-028 and KEYNOTE-158 studies. J Thorac Oncol 2020; 15:618–627. doi: 10.1016/j.jtho.2019.12.109. [DOI] [PubMed] [Google Scholar]
- 15.Hoy SM. Sintilimab: first global approval. Drugs 2019; 79:341–346. doi: 10.1007/s40265-019-1066-z. [DOI] [PubMed] [Google Scholar]
- 16.Gao S, Li N, Gao S, Xue Q, Ying J, Wang S, et al. Neoadjuvant PD-1 inhibitor (Sintilimab) in NSCLC. J Thorac Oncol 2020; 15:816–826. doi: 10.1016/j.jtho.2020.01.017. [DOI] [PubMed] [Google Scholar]
- 17.Fehrenbacher L, Spira A, Ballinger M, Kowanetz M, Vansteenkiste J, Mazieres J, et al. Atezolizumab versus docetaxel for patients with previously treated non-small-cell lung cancer (POPLAR): a multicentre, open-label, phase 2 randomised controlled trial. Lancet 2016; 387:1837–1846. doi: 10.1016/S0140-6736(16)00587-0. [DOI] [PubMed] [Google Scholar]
- 18.Peters S, Gettinger S, Johnson ML, Jänne PA, Garassino MC, Christoph D, et al. Phase II trial of atezolizumab as first-line or subsequent therapy for patients with programmed death-Ligand 1-selected advanced non-small-cell lung cancer (BIRCH). J Clin Oncol 2017; 35:2781–2789. doi: 10.1200/JCO.2016.71.9476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Spigel DR, Chaft JE, Gettinger S, Chao BH, Dirix L, Schmid P, et al. FIR: efficacy, safety, and biomarker analysis of a phase II open-label study of atezolizumab in PD-L1-selected patients with NSCLC. J Thorac Oncol 2018; 13:1733–1742. doi: 10.1016/j.jtho.2018.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rittmeyer A, Barlesi F, Waterkamp D, Park K, Ciardiello F, von Pawel J, et al. Atezolizumab versus docetaxel in patients with previously treated non-small-cell lung cancer (OAK): a phase 3, open-label, multicentre randomised controlled trial. Lancet 2017; 389:255–265. doi: 10.1016/S0140-6736(16)32517-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.West H, McCleod M, Hussein M, Morabito A, Rittmeyer A, Conter HJ, et al. Atezolizumab in combination with carboplatin plus nab-paclitaxel chemotherapy compared with chemotherapy alone as first-line treatment for metastatic non-squamous non-small-cell lung cancer (IMpower130): a multicentre, randomised, open-label, phase 3 trial. Lancet Oncol 2019; 20:924–937. doi: 10.1016/S1470-2045(19)30167-6. [DOI] [PubMed] [Google Scholar]
- 22.Horn L, Mansfield AS, Szczęsna A, Havel L, Krzakowski M, Hochmair MJ, et al. First-Line atezolizumab plus chemotherapy in extensive-stage small-cell lung cancer. N Engl J Med 2018; 379:2220–2229. doi: 10.1056/NEJMoa1809064. [DOI] [PubMed] [Google Scholar]
- 23.Li LY, Wang H, Chen X, Li WQ, Cui JW. First-line atezolizumab plus chemotherapy in treatment of extensive small cell lung cancer: a cost-effectiveness analysis from China. Chin Med J 2019; 132:2790–2794. doi: 10.1097/CM9.0000000000000536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Antonia SJ, Villegas A, Daniel D, Vicente D, Murakami S, Hui R, et al. Overall survival with durvalumab after chemoradiotherapy in stage III NSCLC. N Engl J Med 2018; 379:2342–2350. doi: 10.1056/NEJMoa1809697. [DOI] [PubMed] [Google Scholar]
- 25.Gulley JL, Rajan A, Spigel DR, Iannotti N, Chandler J, Wong D, et al. Avelumab for patients with previously treated metastatic or recurrent non-small-cell lung cancer (JAVELIN Solid Tumor): dose-expansion cohort of a multicentre, open-label, phase 1b trial. Lancet Oncol 2017; 18:599–610. doi: 10.1016/S1470-2045(17)30240-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Barlesi F, Vansteenkiste J, Spigel D, Ishii H, Garassino M, de Marinis F, et al. Avelumab versus docetaxel in patients with platinum-treated advanced non-small-cell lung cancer (JAVELIN Lung 200): an open-label, randomised, phase 3 study. Lancet Oncol 2018; 19:1468–1479. doi: 10.1016/S1470-2045(18)30673-9. [DOI] [PubMed] [Google Scholar]
- 27.Jenkins RW, Barbie DA, Flaherty KT. Mechanisms of resistance to immune checkpoint inhibitors. Br J Cancer 2018; 118:9–16. doi: 10.1038/bjc.2017.434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Skoulidis F, Goldberg ME, Greenawalt DM, Hellmann MD, Awad MM, Gainor JF, et al. STK11/LKB1 mutations and PD-1 inhibitor resistance in KRAS-mutant lung Adenocarcinoma. Cancer Discov 2018; 8:822–835. doi: 10.1158/2159-8290.CD-18-0099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lee CK, Man J, Lord S, Links M, Gebski V, Mok T, et al. Checkpoint inhibitors in metastatic EGFR-mutated non-small cell lung cancer-A meta-analysis. J Thorac Oncol 2017; 12:403–407. doi: 10.1016/j.jtho.2016.10.007. [DOI] [PubMed] [Google Scholar]
- 30.Routy B, Le Chatelier E, Derosa L, Duong C, Alou MT, Daillère R, et al. Gut microbiome influences efficacy of PD-1-based immunotherapy against epithelial tumors. Science 2018; 359:91–97. doi: 10.1126/science.aan3706. [DOI] [PubMed] [Google Scholar]
- 31.Baraibar I, Melero I, Ponz-Sarvise M, Castanon E. Safety and tolerability of immune checkpoint inhibitors (PD-1 and PD-L1) in cancer. Drug Saf 2019; 42:281–294. doi: 10.1007/s40264-018-0774-8. [DOI] [PubMed] [Google Scholar]
- 32.Barroso-Sousa R, Barry WT, Garrido-Castro AC, Hodi FS, Min L, Krop IE, et al. Incidence of endocrine dysfunction following the use of different immune checkpoint inhibitor regimens: a systematic review and meta-analysis. JAMA Oncol 2018; 4:173–182. doi: 10.1001/jamaoncol.2017.3064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mekki A, Dercle L, Lichtenstein P, Marabelle A, Michot JM, Lambotte O, et al. Detection of immune-related adverse events by medical imaging in patients treated with anti-programmed cell death 1. Eur J Cancer 2018; 96:91–104. doi: 10.1016/j.ejca.2018.03.006. [DOI] [PubMed] [Google Scholar]
- 34.Gibney GT, Weiner LM, Atkins MB. Predictive biomarkers for checkpoint inhibitor-based immunotherapy. Lancet Oncol 2016; 17:e542–e551. doi: 10.1016/S1470-2045(16)30406-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hersom M, Jørgensen JT. Companion and complementary diagnostics-focus on PD-L1 expression assays for PD-1/PD-L1 checkpoint inhibitors in non-small cell lung cancer. Ther Drug Monit 2018; 40:9–16. doi: 10.1097/FTD.0000000000000460. [DOI] [PubMed] [Google Scholar]
- 36.Hellmann MD, Paz-Ares L, Bernabe Caro R, Zurawski B, Kim SW, Carcereny Costa E, et al. Nivolumab plus ipilimumab in advanced non-small-cell lung cancer. N Engl J Med 2019; 381:2020–2031. doi: 10.1056/NEJMoa1910231. [DOI] [PubMed] [Google Scholar]
- 37.Kluger HM, Zito CR, Turcu G, Baine MK, Zhang H, Adeniran A, et al. PD-L1 studies across tumor types, its differential expression and predictive value in patients treated with immune checkpoint inhibitors. Clin Cancer Res 2017; 23:4270–4279. doi: 10.1158/1078-0432.CCR-16-3146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Melosky B, Chu Q, Juergens RA, Leighl N, Ionescu D, Tsao MS, et al. Breaking the biomarker code: PD-L1 expression and checkpoint inhibition in advanced NSCLC. Cancer Treat Rev 2018; 65:65–77. doi: 10.1016/j.ctrv.2018.02.005. [DOI] [PubMed] [Google Scholar]
- 39.Landre T, Des Guetz G, Chouahnia K, Taleb C, Vergnenègre A, Chouaïd C. First-line PD-1/PD-L1 inhibitor plus chemotherapy vs chemotherapy alone for negative or < 1% PD-L1-expressing metastatic non-small-cell lung cancers. J Cancer Res Clin Oncol 2020; 146:441–448. doi: 10.1007/s00432-019-03070-3. [DOI] [PubMed] [Google Scholar]
- 40.Reck M, Mok T, Nishio M, Jotte RM, Cappuzzo F, Orlandi F, et al. Atezolizumab plus bevacizumab and chemotherapy in non-small-cell lung cancer (IMpower150): key subgroup analyses of patients with EGFR mutations or baseline liver metastases in a randomised, open-label phase 3 trial. Lancet Respir Med 2019; 7:387–401. doi: 10.1016/S2213-2600(19)30084-0. [DOI] [PubMed] [Google Scholar]
- 41.Gadgeel SM, Pennell NA, Fidler MJ, Halmos B, Bonomi P, Stevenson J, et al. Phase II study of maintenance pembrolizumab in patients with extensive-stage small cell lung cancer (SCLC). J Thorac Oncol 2018; 13:1393–1399. doi: 10.1016/j.jtho.2018.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ready NE, Ott PA, Hellmann MD, Zugazagoitia J, Hann CL, de Braud F, et al. Nivolumab monotherapy and nivolumab plus ipilimumab in recurrent small cell lung cancer: results from the checkmate 032 randomized cohort. J Thorac Oncol 2020; 15:426–435. doi: 10.1016/j.jtho.2019.10.004. [DOI] [PubMed] [Google Scholar]
- 43.Munari E, Zamboni G, Lunardi G, Marchionni L, Marconi M, Sommaggio M, et al. PD-L1 expression heterogeneity in non-small cell lung cancer: defining criteria for harmonization between biopsy specimens and whole sections. J Thorac Oncol 2018; 13:1113–1120. doi: 10.1016/j.jtho.2018.04.017. [DOI] [PubMed] [Google Scholar]
- 44.Sheng J, Fang W, Yu J, Chen N, Zhan J, Ma Y, et al. Expression of programmed death ligand-1 on tumor cells varies pre and post chemotherapy in non-small cell lung cancer. Sci Rep 2016; 6:20090.doi: 10.1038/srep20090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kowanetz M, Zou W, Gettinger SN, Koeppen H, Kockx M, Schmid P, et al. Differential regulation of PD-L1 expression by immune and tumor cells in NSCLC and the response to treatment with atezolizumab (anti-PD-L1). Proc Natl Acad Sci U S A 2018; 115:E10119-E10126.doi: 10.1073/pnas.1802166115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tsao MS, Kerr KM, Kockx M, Beasley MB, Borczuk AC, Botling J, et al. PD-L1 immunohistochemistry comparability study in real-life clinical samples: results of blueprint phase 2 project. J Thorac Oncol 2018; 13:1302–1311. doi: 10.1016/j.jtho.2018.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.El-Guindy DM, Helal DS, Sabry NM, Abo El-Nasr M. Programmed cell death ligand-1 (PD-L1) expression combined with CD8 tumor infiltrating lymphocytes density in non-small cell lung cancer patients. J Egypt Natl Canc Inst 2018; 30:125–131. doi: 10.1016/j.jnci.2018.08.003. [DOI] [PubMed] [Google Scholar]
- 48.Bocanegra A, Fernandez-Hinojal G, Zuazo-Ibarra M, Arasanz H, Garcia-Granda MJ, Hernandez C, et al. PD-L1 expression in systemic immune cell populations as a potential predictive biomarker of responses to PD-L1/PD-1 blockade therapy in lung cancer. Int J Mol Sci 2019; 20:1631.doi: 10.3390/ijms20071631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Niemeijer AN, Leung D, Huisman MC, Bahce I, Hoekstra OS, van Dongen G, et al. Whole body PD-1 and PD-L1 positron emission tomography in patients with non-small-cell lung cancer. Nat Commun 2018; 9:4664.doi: 10.1038/s41467-018-07131-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Peters S, Creelan B, Hellmann MD, Socinski MA, Reck M, Bhagavatheeswaran P, et al. Abstract Ct082: Impact of tumor mutation burden on the efficacy of first-line nivolumab in stage iv or recurrent non-small cell lung cancer: an exploratory analysis of Checkmate 026. Cancer Res 2017; 77: 13 suppl: CT082.doi: 10.1158/1538-7445.AM2017-CT082. [Google Scholar]
- 51.Hellmann MD, Ciuleanu TE, Pluzanski A, Lee JS, Otterson GA, Audigier-Valette C, et al. Nivolumab plus ipilimumab in lung cancer with a high tumor mutational burden. N Engl J Med 2018; 378:2093–2104. doi: 10.1056/NEJMoa1801946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ricciuti B, Kravets S, Dahlberg SE, Umeton R, Albayrak A, Subegdjo SJ, et al. Use of targeted next generation sequencing to characterize tumor mutational burden and efficacy of immune checkpoint inhibition in small cell lung cancer. J Immunother Cancer 2019; 7:87.doi: 10.1186/s40425-019-0572-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hellmann MD, Callahan MK, Awad MM, Calvo E, Ascierto PA, Atmaca A, et al. Tumor mutational burden and efficacy of nivolumab monotherapy and in combination with ipilimumab in small-cell lung cancer. Cancer Cell 2019; 35:329.doi: 10.1016/j.ccell.2019.01.011. [DOI] [PubMed] [Google Scholar]
- 54.Campbell BB, Light N, Fabrizio D, Zatzman M, Fuligni F, de Borja R, et al. Comprehensive analysis of hypermutation in human cancer. Cell 2017; 171:1042–1056. e10. doi: 10.1016/j.cell.2017.09.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gandara DR, Paul SM, Kowanetz M, Schleifman E, Zou W, Li Y, et al. Blood-based tumor mutational burden as a predictor of clinical benefit in non-small-cell lung cancer patients treated with atezolizumab. Nat Med 2018; 24:1441–1448. doi: 10.1038/s41591-018-0134-3. [DOI] [PubMed] [Google Scholar]
- 56.Wang Z, Duan J, Cai S, Han M, Dong H, Zhao J, et al. Assessment of blood tumor mutational burden as a potential biomarker for immunotherapy in patients with non-small cell lung cancer with use of a next-generation sequencing cancer gene panel. JAMA Oncol 2019; 5:696–702. doi: 10.1001/jamaoncol.2018.7098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ghorani E, Rosenthal R, McGranahan N, Reading JL, Lynch M, Peggs KS, et al. Differential binding affinity of mutated peptides for MHC class I is a predictor of survival in advanced lung cancer and melanoma. Ann Oncol 2018; 29:271–279. doi: 10.1093/annonc/mdx687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Łuksza M, Riaz N, Makarov V, Balachandran VP, Hellmann MD, Solovyov A, et al. A neoantigen fitness model predicts tumour response to checkpoint blockade immunotherapy. Nature 2017; 551:517–520. doi: 10.1038/nature24473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Tsakonas G, Ekman S. Oncogene-addicted non-small cell lung cancer and immunotherapy. J Thorac Dis 2018; 10: suppl 13: S1547–S1555. doi: 10.21037/jtd.2018.01.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ozaki Y, Muto S, Takagi H, Watanabe M, Inoue T, Fukuhara M, et al. Tumor mutation burden and immunological, genomic, and clinicopathological factors as biomarkers for checkpoint inhibitor treatment of patients with non-small-cell lung cancer. Cancer Immunol Immunother 2020; 69:127–134. doi: 10.1007/s00262-019-02446-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Garassino MC, Cho BC, Kim JH, Mazières J, Vansteenkiste J, Lena H, et al. Durvalumab as third-line or later treatment for advanced non-small-cell lung cancer (ATLANTIC): an open-label, single-arm, phase 2 study. Lancet Oncol 2018; 19:521–536. doi: 10.1016/S1470-2045(18)30144-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Skoulidis F, Arbour KC, Hellmann MD, Patil PD, Marmarelis ME, Awad MM, et al. Association of STK11/LKB1 genomic alterations with lack of benefit from the addition of pembrolizumab to platinum doublet chemotherapy in non-squamous non-small cell lung cancer. J Clin Oncol 2019; 37: 15_suppl: 102.doi: 10.1200/JCO.2019.37.15_suppl.102. [Google Scholar]
- 63.Willard MD, Smyth ENN, Tiu R, Beyrer J, Zhu YE, Bowman L, et al. Genomic characterization of lung tumors and metastatic (Met) sites in advanced (Adv) NSCLC. J Clin Oncol 2019; 37: 15_suppl: 2014.doi: 10.1200/JCO.2019.37.15_suppl.2014. [Google Scholar]
- 64.Goldberg SB, Narayan A, Kole AJ, Decker RH, Teysir J, Carriero NJ, et al. Early assessment of lung cancer immunotherapy response via circulating tumor DNA. Clin Cancer Res 2018; 24:1872–1880. doi: 10.1158/1078-0432.CCR-17-1341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Anagnostou V, Forde PM, White JR, Niknafs N, Hruban C, Naidoo J, et al. Dynamics of tumor and immune responses during immune checkpoint blockade in non-small cell lung cancer. Cancer Res 2019; 79:1214–1225. doi: 10.1158/0008-5472.CAN-18-1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Joseph RW, Elassaiss-Schaap J, Kefford R, Hwu WJ, Wolchok JD, Joshua AM, et al. Baseline tumor size is an independent prognostic factor for overall survival in patients with melanoma treated with pembrolizumab. Clin Cancer Res 2018; 24:4960–4967. doi: 10.1158/1078-0432.Ccr-17-2386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Katsurada M, Nagano T, Tachihara M, Kiriu T, Furukawa K, Koyama K, et al. Baseline tumor size as a predictive and prognostic factor of immune checkpoint inhibitor therapy for non-small cell lung cancer. Anticancer Res 2019; 39:815–825. doi: 10.21873/anticanres.13180. [DOI] [PubMed] [Google Scholar]
- 68.Sakata Y, Kawamura K, Ichikado K, Shingu N, Yasuda Y, Eguchi Y, et al. The association between tumor burden and severe immune-related adverse events in non-small cell lung cancer patients responding to immune-checkpoint inhibitor treatment. Lung Cancer 2019; 130:159–161. doi: 10.1016/j.lungcan.2019.02.011. [DOI] [PubMed] [Google Scholar]
- 69.Chowell D, Morris L, Grigg CM, Weber JK, Samstein RM, Makarov V, et al. Patient HLA class I genotype influences cancer response to checkpoint blockade immunotherapy. Science 2018; 359:582–587. doi: 10.1126/science.aao4572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Gettinger S, Choi J, Hastings K, Truini A, Datar I, Sowell R, et al. Impaired HLA Class I antigen processing and presentation as a mechanism of acquired resistance to immune checkpoint inhibitors in lung cancer. Cancer Discov 2017; 7:1420–1435. doi: 10.1158/2159-8290.CD-17-0593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Uryvaev A, Passhak M, Hershkovits D, Sabo E, Bar-Sela G. The role of tumor-infiltrating lymphocytes (TILs) as a predictive biomarker of response to anti-PD1 therapy in patients with metastatic non-small cell lung cancer or metastatic melanoma. Med Oncol 2018; 35:25.doi: 10.1007/s12032-018-1080-0. [DOI] [PubMed] [Google Scholar]
- 72.Niemeijer AN, Sahba S, Smit EF, Lissenberg-Witte BI, de Langen AJ, Thunnissen E. Association of tumour and stroma PD-1, PD-L1, CD3, CD4 and CD8 expression with DCB and OS to nivolumab treatment in NSCLC patients pre-treated with chemotherapy. Br J Cancer 2020; 123:392–402. doi: 10.1038/s41416-020-0888-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Nakazawa N, Yokobori T, Kaira K, Turtoi A, Baatar S, Gombodorj N, et al. High Stromal TGFBI in lung cancer and intratumoral CD8-Positive T cells were associated with poor prognosis and therapeutic resistance to immune checkpoint inhibitors. Ann Surg Oncol 2020; 27:933–942. doi: 10.1245/s10434-019-07878-8. [DOI] [PubMed] [Google Scholar]
- 74.Thommen DS, Koelzer VH, Herzig P, Roller A, Trefny M, Dimeloe S, et al. A transcriptionally and functionally distinct PD-1+ CD8+ T cell pool with predictive potential in non-small-cell lung cancer treated with PD-1 blockade. Nat Med 2018; 24:994–1004. doi: 10.1038/s41591-018-0057-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Cai MC, Zhao X, Cao M, Ma P, Chen M, Wu J, et al. T-cell exhaustion interrelates with immune cytolytic activity to shape the inflamed tumor microenvironment. J Pathol 2020; 251:147–159. doi: 10.1002/path.5435. [DOI] [PubMed] [Google Scholar]
- 76.Kim H, Kwon HJ, Han YB, Park SY, Kim ES, Kim SH, et al. Increased CD3+ T cells with a low FOXP3+/CD8+ T cell ratio can predict anti-PD-1 therapeutic response in non-small cell lung cancer patients. Mod Pathol 2019; 32:367–375. doi: 10.1038/s41379-018-0142-3. [DOI] [PubMed] [Google Scholar]
- 77.Sato M, Watanabe S, Tanaka H, Nozaki K, Arita M, Takahashi M, et al. Retrospective analysis of antitumor effects and biomarkers for nivolumab in NSCLC patients with EGFR mutations. PLoS One 2019; 14:e0215292.doi: 10.1371/journal.pone.0215292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Seo JS, Kim A, Shin JY, Kim YT. Comprehensive analysis of the tumor immune micro-environment in non-small cell lung cancer for efficacy of checkpoint inhibitor. Sci Rep 2018; 8:14576.doi: 10.1038/s41598-018-32855-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Karasaki T, Nagayama K, Kuwano H, Nitadori JI, Sato M, Anraku M, et al. An immunogram for the cancer-immunity cycle: towards personalized immunotherapy of lung cancer. J Thorac Oncol 2017; 12:791–803. doi: 10.1016/j.jtho.2017.01.005. [DOI] [PubMed] [Google Scholar]
- 80.Jang HJ, Lee HS, Ramos D, Park IK, Kang CH, Burt BM, et al. Transcriptome-based molecular subtyping of non-small cell lung cancer may predict response to immune checkpoint inhibitors. J Thorac Cardiovasc Surg 2020; 159:1598–1610. e3. doi: 10.1016/j.jtcvs.2019.10.123. [DOI] [PubMed] [Google Scholar]
- 81.Inomata M, Kado T, Okazawa S, Imanishi S, Taka C, Kambara K, et al. Peripheral PD1-positive CD4 T-lymphocyte count can predict progression-free survival in patients with non-small cell lung cancer receiving immune checkpoint inhibitor. Anticancer Res 2019; 39:6887–6893. doi: 10.21873/anticanres.13908. [DOI] [PubMed] [Google Scholar]
- 82.Manjarrez-Orduño N, Menard LC, Kansal S, Fischer P, Kakrecha B, Jiang C, et al. Circulating T cell subpopulations correlate with immune responses at the tumor site and clinical response to PD1 inhibition in non-small cell lung cancer. Front Immunol 2018; 9:1613.doi: 10.3389/fimmu.2018.01613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Zhuo M, Chen H, Zhang T, Yang X, Zhong J, Wang Y, et al. The potential predictive value of circulating immune cell ratio and tumor marker in atezolizumab treated advanced non-small cell lung cancer patients. Cancer Biomark 2018; 22:467–476. doi: 10.3233/CBM-171089. [DOI] [PubMed] [Google Scholar]
- 84.Kim CG, Kim KH, Pyo KH, Xin CF, Hong MH, Ahn BC, et al. Hyperprogressive disease during PD-1/PD-L1 blockade in patients with non-small-cell lung cancer. Ann Oncol 2019; 30:1104–1113. doi: 10.1093/annonc/mdz123. [DOI] [PubMed] [Google Scholar]
- 85.Jiang T, Qiao M, Zhao C, Li X, Gao G, Su C, et al. Pretreatment neutrophil-to-lymphocyte ratio is associated with outcome of advanced-stage cancer patients treated with immunotherapy: a meta-analysis. Cancer Immunol Immunother 2018; 67:713–727. doi: 10.1007/s00262-018-2126-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Dusselier M, Deluche E, Delacourt N, Ballouhey J, Egenod T, Melloni B, et al. Neutrophil-to-lymphocyte ratio evolution is an independent predictor of early progression of second-line nivolumab-treated patients with advanced non-small-cell lung cancers. PLoS One 2019; 14:e0219060.doi: 10.1371/journal.pone.0219060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Karantanos T, Karanika S, Seth B, Gignac G. The absolute lymphocyte count can predict the overall survival of patients with non-small cell lung cancer on nivolumab: a clinical study. Clin Transl Oncol 2019; 21:206–212. doi: 10.1007/s12094-018-1908-2. [DOI] [PubMed] [Google Scholar]
- 88.Castello A, Toschi L, Rossi S, Mazziotti E, Lopci E. The immune-metabolic-prognostic index and clinical outcomes in patients with non-small cell lung carcinoma under checkpoint inhibitors. J Cancer Res Clin Oncol 2020; 146:1235–1243. doi: 10.1007/s00432-020-03150-9. [DOI] [PubMed] [Google Scholar]
- 89.Varga A, Bernard-Tessier A, Auclin E, Mezquita Pérez L, Baldini C, Planchard D, et al. Applicability of the lung immune prognostic index (Lipi) in patients with metastatic solid tumors when treated with immune checkpoint inhibitors (Ici) in early clinical trials. Ann Oncol 2019; 30: Suppl 1: i2.doi: 10.1093/annonc/mdz027.001. [Google Scholar]
- 90.Shiroyama T, Suzuki H, Tamiya M, Tamiya A, Tanaka A, Okamoto N, et al. Pretreatment advanced lung cancer inflammation index (ALI) for predicting early progression in nivolumab-treated patients with advanced non-small cell lung cancer. Cancer Med 2018; 7:13–20. doi: 10.1002/cam4.1234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Maymani H, Hess K, Groisberg R, Hong DS, Naing A, Piha-Paul S, et al. Predicting outcomes in patients with advanced non-small cell lung cancer enrolled in early phase immunotherapy trials. Lung Cancer 2018; 120:137–141. doi: 10.1016/j.lungcan.2018.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Schett A, Rothschild SI, Curioni-Fontecedro A, Krähenbühl S, Früh M, Schmid S, et al. Predictive impact of antibiotics in patients with advanced non small-cell lung cancer receiving immune checkpoint inhibitors: antibiotics immune checkpoint inhibitors in advanced NSCLC. Cancer Chemother Pharmacol 2020; 85:121–131. doi: 10.1007/s00280-019-03993-1. [DOI] [PubMed] [Google Scholar]
- 93.Botticelli A, Vernocchi P, Marini F, Quagliariello A, Cerbelli B, Reddel S, et al. Gut metabolomics profiling of non-small cell lung cancer (NSCLC) patients under immunotherapy treatment. J Transl Med 2020; 18:49.doi: 10.1186/s12967-020-02231-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Jin Y, Dong H, Xia L, Yang Y, Zhu Y, Shen Y, et al. The diversity of gut microbiome is associated with favorable responses to anti-programmed death 1 immunotherapy in Chinese patients with NSCLC. J Thorac Oncol 2019; 14:1378–1389. doi: 10.1016/j.jtho.2019.04.007. [DOI] [PubMed] [Google Scholar]
- 95.Chaput N, Lepage P, Coutzac C, Soularue E, Le Roux K, Monot C, et al. Baseline gut microbiota predicts clinical response and colitis in metastatic melanoma patients treated with ipilimumab. Ann Oncol 2017; 28:1368–1379. doi: 10.1093/annonc/mdx108. [DOI] [PubMed] [Google Scholar]
- 96.Hornyák L, Dobos N, Koncz G, Karányi Z, Páll D, Szabó Z, et al. The role of indoleamine-2,3-dioxygenase in cancer development, diagnostics, and therapy. Front Immunol 2018; 9:151.doi: 10.3389/fimmu.2018.00151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Brochez L, Chevolet I, Kruse V. The rationale of indoleamine 2,3-dioxygenase inhibition for cancer therapy. Eur J Cancer 2017; 76:167–182. doi: 10.1016/j.ejca.2017.01.011. [DOI] [PubMed] [Google Scholar]
- 98.Botticelli A, Cerbelli B, Lionetto L, Zizzari I, Salati M, Pisano A, et al. Can IDO activity predict primary resistance to anti-PD-1 treatment in NSCLC. J Transl Med 2018; 16:219.doi: 10.1186/s12967-018-1595-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Kiriu T, Yamamoto M, Nagano T, Koyama K, Katsurada M, Tamura D, et al. Prognostic value of red blood cell distribution width in non-small cell lung cancer treated with anti-programmed cell death-1 antibody. In Vivo 2019; 33:213–220. doi: 10.21873/invivo.11462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Fucà G, Galli G, Poggi M, Lo Russo G, Proto C, Imbimbo M, et al. Low baseline serum sodium concentration is associated with poor clinical outcomes in metastatic non-small cell lung cancer patients treated with immunotherapy. Target Oncol 2018; 13:795–800. doi: 10.1007/s11523-018-0599-5. [DOI] [PubMed] [Google Scholar]
- 101.Caponnetto S, Iannantuono GM, Barchiesi G, Magri V, Gelibter A, Cortesi E. Prolactin as a potential early predictive factor in metastatic non-small cell lung cancer patients treated with nivolumab. Oncology 2017; 93:62–66. doi: 10.1159/000464328. [DOI] [PubMed] [Google Scholar]
- 102.Takada K, Yoneshima Y, Tanaka K, Okamoto I, Shimokawa M, Wakasu S, et al. Clinical impact of skeletal muscle area in patients with non-small cell lung cancer treated with anti-PD-1 inhibitors. J Cancer Res Clin Oncol 2020; 146:1217–1225. doi: 10.1007/s00432-020-03146-5. [DOI] [PubMed] [Google Scholar]
- 103.Wu Q, Liu J, Zhang Y, Wu S, Xie X. Predictive value of positron emission tomography for the prognosis of immune checkpoint inhibitors (ICIs) in malignant tumors. Cancer Immunol Immunother 2020; 69:927–936. doi: 10.1007/s00262-020-02515-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Cho JY, Kim J, Lee JS, Kim YJ, Kim SH, Lee YJ, et al. Characteristics, incidence, and risk factors of immune checkpoint inhibitor-related pneumonitis in patients with non-small cell lung cancer. Lung Cancer 2018; 125:150–156. doi: 10.1016/j.lungcan.2018.09.015. [DOI] [PubMed] [Google Scholar]
- 105.Suresh K, Voong KR, Shankar B, Forde PM, Ettinger DS, Marrone KA, et al. Pneumonitis in non-small cell lung cancer patients receiving immune checkpoint immunotherapy: incidence and risk factors. J Thorac Oncol 2018; 13:1930–1939. doi: 10.1016/j.jtho.2018.08.2035. [DOI] [PubMed] [Google Scholar]
- 106.Ricciuti B, Genova C, De Giglio A, Bassanelli M, Dal Bello MG, Metro G, et al. Impact of immune-related adverse events on survival in patients with advanced non-small cell lung cancer treated with nivolumab: long-term outcomes from a multi-institutional analysis. J Cancer Res Clin Oncol 2019; 145:479–485. doi: 10.1007/s00432-018-2805-3. [DOI] [PubMed] [Google Scholar]
- 107.Naqash AR, Ricciuti B, Owen DH, Florou V, Toi Y, Cherry C, et al. Outcomes associated with immune-related adverse events in metastatic non-small cell lung cancer treated with nivolumab: a pooled exploratory analysis from a global cohort. Cancer Immunol Immunother 2020; 69:1177–1187. doi: 10.1007/s00262-020-02536-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Berner F, Bomze D, Diem S, Ali OH, Fässler M, Ring S, et al. Association of checkpoint inhibitor-induced toxic effects with shared cancer and tissue antigens in non-small cell lung cancer. JAMA Oncol 2019; 5:1043–1047. doi: 10.1001/jamaoncol.2019.0402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Suresh K, Psoter KJ, Voong KR, Shankar B, Forde PM, Ettinger DS, et al. Impact of checkpoint inhibitor pneumonitis on survival in NSCLC patients receiving immune checkpoint immunotherapy. J Thorac Oncol 2019; 14:494–502. doi: 10.1016/j.jtho.2018.11.016. [DOI] [PubMed] [Google Scholar]


