ABSTRACT
Life-threatening infections (sepsis) are usually associated with co-morbidities, among which obesity deserves attention. Here, we evaluated whether and how obesity affects the switch from fever to hypothermia that occurs in the most severe cases of sepsis, which is thought to provide physiological support for a change in host defense strategy from resistance to tolerance. Obesity was induced by keeping rats on a high-fat diet for 32–34 weeks. The hypothermia induced by a high dose of bacterial lipopolysaccharide (LPS, 300 μg/animal, i.a.) was attenuated in the obese rats, as compared to their low-fat diet counterparts. Surprisingly, such attenuation occurred in spite of an enhancement in the circulating level of TNF-α, the most renowned mediator of LPS-induced hypothermia. Hence, it seems that factors counteracting not the production, but rather the action of TNF-α are at play in rats with diet-induced obesity. One of these factors might be IL-1β, a febrigenic mediator that also had its circulating levels augmented in the obese rats challenged with LPS. Taken together with previous reports of diet-induced obesity enhancing the fever induced by lower doses of LPS, the results of the present study indicate that obesity biases host defense toward a fever/resistance strategy, in lieu of a hypothermia/tolerance strategy.
KEYWORDS: Host defense, immune response, inflammation, LPS, fever, hypothermia, resistance, tolerance, fat, adipose
Introduction
Fever is an evolutionarily conserved response thought to aid immunity in animals ranging from invertebrates to mammals and birds [1–3]. Not surprisingly, at least in some studies, an increased body core temperature (Tc) has been shown to favorably impact the outcome of infectious diseases in humans [4–6] and experimental animals [7–9]. However, the relationship between Tc and infectious diseases is still incompletely understood, and, in this context, it is important to consider that life-threatening infections (sepsis) usually occur in the setting of comorbidities such as obesity, which could impact immune and thermoregulatory functions 2.
In rats, obesity has been shown to enhance and prolong the fever induced by mild-to-moderate doses of bacterial lipopolysaccharide (LPS), regardless of whether the obesity was induced by high-fat diet (HFD) [10,11], fructose [12] or neonatal overfeeding [13]. It should be considered, however, that fever is only one side of a dichotomous host defense strategy, being replaced by a brain-driven, regulated form of hypothermia when the immune challenge is too strong or when host fitness is threatened [14]. To our knowledge, there is only one published study in which immune-triggered hypothermia was assessed in a model of diet-induced obesity [15]. In that study, the hypothermia associated with septic peritonitis was evident in lean controls and absent in the obese group. Whether this observation can be extended to other models of systemic inflammation is unknown, as are the mechanisms by which obesity impacts the hypothermic response 5.
In the present study, we evaluated whether and how obesity affects the hypothermia induced by a high dose of LPS in rats, as well as the relationship of hypothermia with early cytokines. The following cytokines were assessed: TNF-α (the most renowned mediator of hypothermia [16–20]); IL-1β (a mediator of fever, with little or no role in hypothermia [20–22]); and IL-10 (an anti-inflammatory cytokine that can counteract the production and effects of both TNF-α and IL-1β[23]).
Methods
Animals and diet-induced obesity
The study was conducted in male Wistar rats from the same litter, originated from the specific pathogen-free animal facility of the University of São Paulo. From the day of weaning (21 days afterbirth), the rats were put on a HFD (60% of calories from fat, 5.1 kcal/g; Rhoster, Araçoiaba da Serra, SP, Brazil) or on a low-fat diet (LFD: 12% of calories from fat, 3.7 kcal/g; Nuvilab, Curitiba, PR, Brazil). The diets were similar in terms of protein content (23% in HFD and 22% in LFD). Water was available ad libitum. The animal room was on a 12:12 h light-dark cycle, with lights on at 7:00 h. With the aim of providing a thermally comfortable environment for the grouped rats [24], room temperature was kept at 24–27ºC. The rats were maintained under these dietary and housing conditions for 32–34 weeks. Body weight and caloric intake were assessed at least three times a week. All protocols were approved by the Animal Care and Use Committee at the Institute of Biomedical Sciences of the University of São Paulo 8.
Surgical preparation
One week before an experiment, the rats were chronically instrumented with an arterial (carotid) catheter and an abdominal telemetry temperature transmitter. The surgery was performed under anesthesia with inhaled isoflurane (2.0–2.5%) and antibiotic prophylaxis with enrofloxacin (5 mg/kg, s.c.). The rats were maintained on a heated pad during surgery. For the catheterization, the left common carotid artery was accessed via the ventral aspect of the neck, and isolated from the adjacent nerve. Using an occlusive technique, a 3-Fr polyurethane catheter was inserted into the artery at this site, and then advanced until its tip reached the descending thoracic aorta. The catheter was then passed under the skin and exteriorized at the nape, after which it was locked with heparinized glycerol (500 U/ml). The telemetry transmitter (model TA-F40; Data Sciences International, St. Paul, MN, USA) was implanted via a midline laparotomy, after which the incision site was sutured in layers. Ketoprofen (5 mg/kg, s.c.) was administered at the end of surgery and on the next day. On the first and fourth days post-surgery, the arterial catheters were flushed with 0.5 ml of saline and re-locked with heparinized glycerol 17.
Experimental setup and LPS challenge
The experiment was conducted 1 week after surgery, when the rats had been under HFD or LFD for 32–34 weeks. Early on the day of the experiment, the freely moving rats were transferred individually caged to an environmental chamber (NQ1 model; Environmental Growth Chambers, Chagrin Falls, OH, USA). In the chamber, the rats were maintained at an ambient temperature of 22.0ºC, which is preferred by rats challenged with high doses of LPS [25–27] and adequate to reveal the hypothermic component of the response to LPS [28–30]. Each cage was placed on top of a PhysiolTel RPC-1 receiver (Data Sciences), which captured the radio waves emitted by the telemetry transmitter, and conveyed temperature and locomotor activity data to a computer. The data were acquired in the Dataquest ART software, for later processing in the Ponemah software. The arterial catheter was extended with PE50 tubing filled with saline, and the extension was passed via a swivel system (Instech Laboratories, Plymouth Meeting, PA USA) to the outside of the environmental chamber, where it was connected to a syringe. An infusion harness worn by the rat and a spring coil protected the arterial extension from bites and scratches 18.
At approximately 12:00 h, when the rats were well habituated to the experimental conditions, LPS from E. coli O127:B8 (Sigma-Aldrich, St. Louis, MO, USA) was bolus injected via the extensions of the arterial catheters. The dose of LPS was 300 μg/rat in both dietary groups, targeting a dose that would be equivalent in relation to lean body mass (see Discussion for more details). Tc and gross locomotor activity were monitored by telemetry from 60 min pre-LPS to 360 min post-LPS. Blood samples were collected at three time points: 60 min pre-LPS; 90 min post-LPS; and 360 min post-LPS. With this sampling schedule, ~1 ml of blood could be collected at each time point without inadvertently impacting the Tc of the rats. Immediately after the last blood draw, the rats were euthanized with an overdose of sodium thiopental (100 mg/kg, i.a.).
Cytokine assay
Blood samples were collected into heparinized tubes and immediately centrifuged at 6,000 × g for 10 min at 4°C. The resulting plasma was stored at −80°C for not longer than 3 months. The levels of TNF-α, IL-1β and IL-10 were quantified by sandwich ELISA using reagents from R&D Systems (Minneapolis, MN, USA). All samples were run simultaneously, in duplicate. Detection ranges were 31–2,000 pg/ml for all cytokines assayed 19.
Statistical analyses
Statistical comparisons were performed using Statistica Advanced 8.0 (StatSoft, Tulsa, OK, USA), with the level of significance set at p < 0.05. The body mass of the rats was compared across dietary groups by Student’s t-test. Tc, gross locomotor activity and cytokine levels were evaluated across dietary groups and time points by two-way ANOVA followed, as necessary, by the Fisher Least Significant Difference test 21.
Results
On the day of the LPS challenge, rats of the LFD and HFD groups weighed 479 ± 26 g and 611 ± 41 g, respectively. This difference in body mass was statistically significant (p = 0.009), and is consistent with the increased adiposity reported for rats under the same HFD regimen [31]. At the ambient temperature of 22ºC, the rats responded to 300 μg of LPS with a biphasic drop in Tc (Figure 1). The first phase had a nadir at 110 min post-LPS, whereas the second phase was still in progress when the rats were euthanized at 360 min post-LPS. This hypothermic response was significantly attenuated in the HFD group, as compared to the LFD group (Figure 1). Gross locomotor activity was low throughout the daytime experiment and did not differ between the two dietary groups (Figure 1). Although one could argue that a trend toward an increase in locomotor activity might have occurred at 30 min post-LPS, this was not close to reaching statistical significance (p = 0.197).
Figure 1.
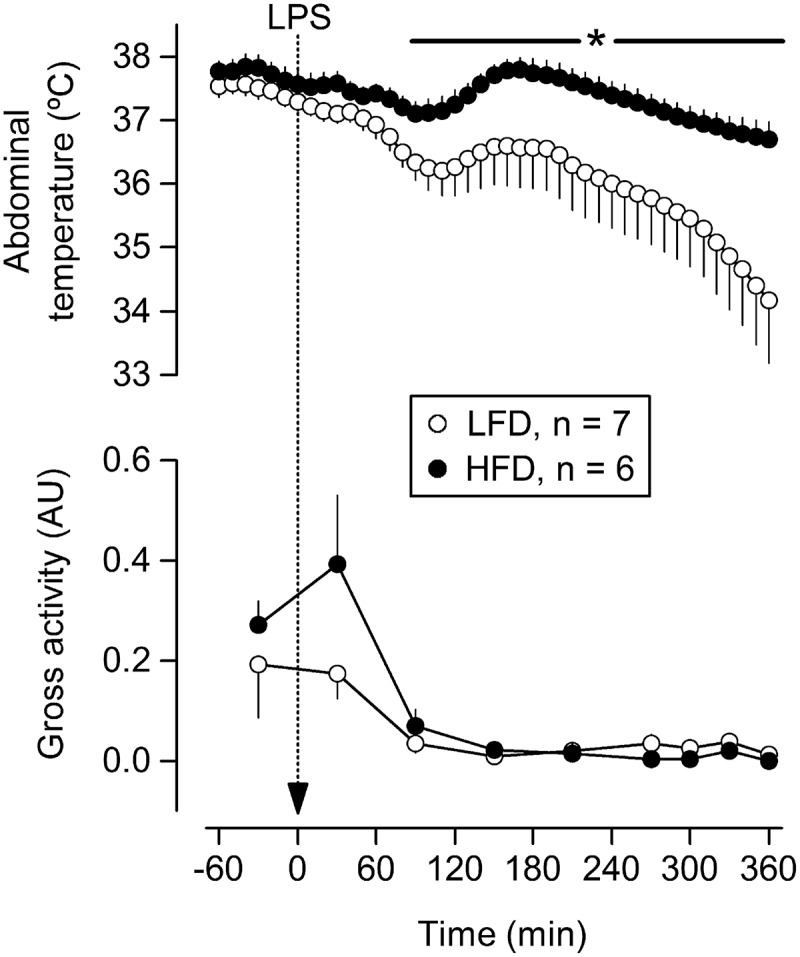
Effects of LPS at a high dose (300 μg, i.a.) on the abdominal temperature and gross locomotor activity of rats with HFD-induced obesity or their LFD-fed counterparts. The rats were kept at an ambient temperature of 22ºC. LPS was administered via extensions of pre-implanted carotid catheters, without handling the rats. Data are plotted as means ± SEM. The number of animals (n) is indicated. *, statistically significant difference between the HFD and LFD groups. AU, arbitrary units 26.
The analysis of circulating cytokines revealed that TNF-α was much more abundant than IL-1β or IL-10 at the time corresponding to the first phase of hypothermia (90 min), but this was no longer the case at the time corresponding to the second phase (360 min); see Figure 2. Most importantly, this analysis revealed that attenuation of LPS-induced hypothermia by HFD was not associated with a reduction in plasma TNF-α, but rather with an enhancement. Such an enhancement was statistically significant at 90 min post-LPS, when HFD similarly augmented the levels IL-1β and IL-10 (Figure 2). At the 360-min time point, nonetheless, none of the cytokines evaluated was influenced by diet. The results of this experiment further show that the balance between the pro-inflammatory cytokines (TNF-α and IL-1β) and the anti-inflammatory cytokine (IL-10) was not affected by HFD-induced obesity, regardless of time (Table 1).
Figure 2.
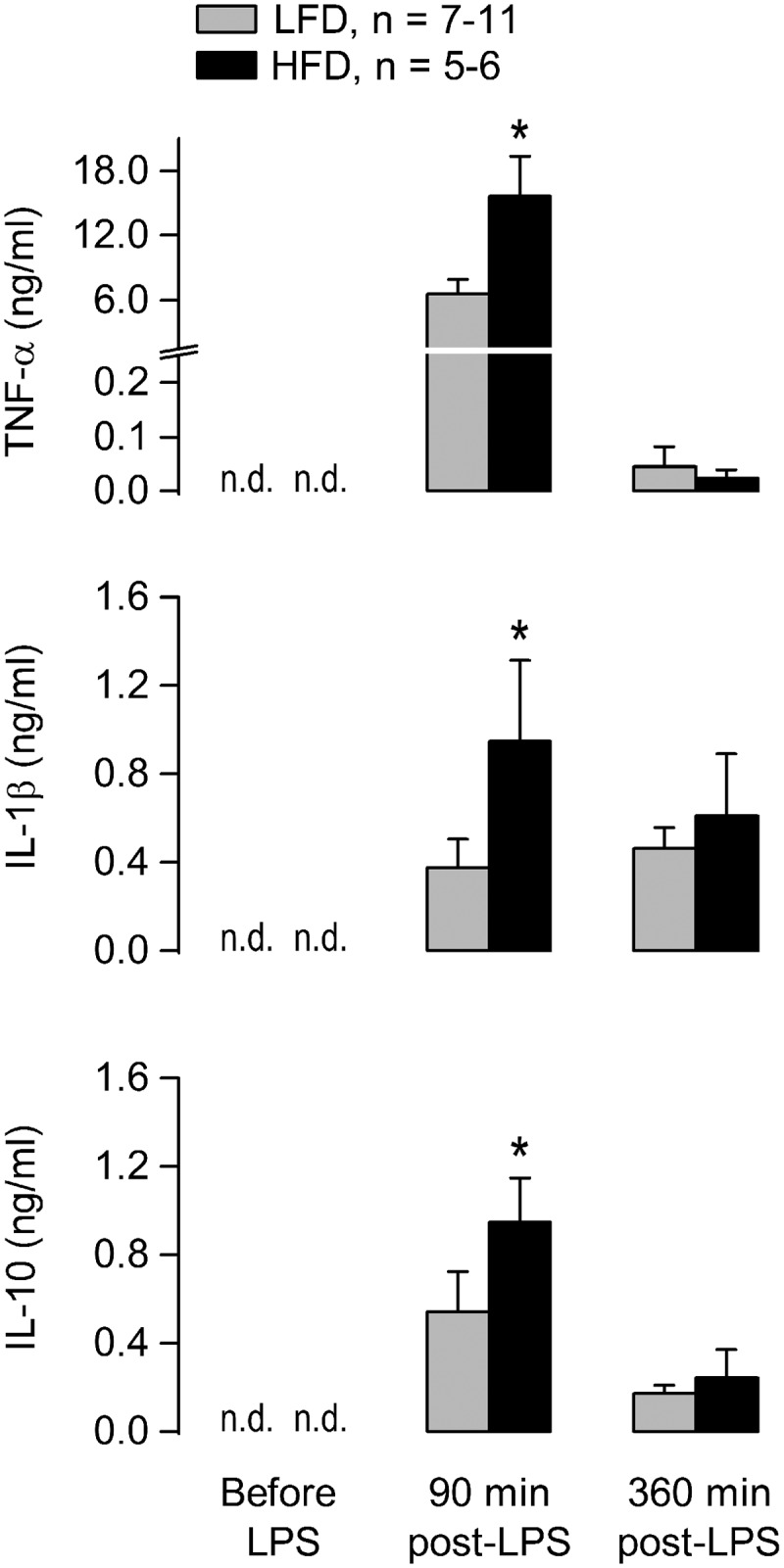
Plasma levels of cytokines during the course of the hypothermic response to a high dose of LPS (300 μg, i.a.). Experimental conditions as in Figure 1. Data are plotted as means ± SEM. The number of animals (n) is indicated. *, statistical difference between the HFD and LFD groups. n.d., not detectable.
Table 1.
Effects of diet on cytokine ratios at 90 or 360 min post-LPS.
| Cytokine ratio | Dietary groups | Time post-LPS |
|
|---|---|---|---|
| 90 min | 360 min | ||
| TNF-α/IL-10 | LFD | 31 ± 14 | 0.3 ± 0.2 |
| HFD | 19 ± 5 | 0.4 ± 0.4 | |
| p value | 0.42 | 0.99 | |
| IL-1β/IL-10 | LFD | 0.3 ± 0.2 | 4 ± 1 |
| HFD | 0.4 ± 0.4 | 19 ± 15 | |
| p value | 0.94 | 0.09 | |
Data shown as means ± SEM.
Discussion
The present study shows that HFD-induced obesity attenuates the hypothermic component of the systemic inflammatory response to LPS in rats, even though it enhances the circulating levels of its most renowned mediator, viz., TNF-α. This was the case for the first, most studied phase of hypothermia. In the second phase, LPS-induced hypothermia was associated with low circulating levels of TNF-α, and its attenuation in the obese group occurred in the absence of altered TNF-α levels. In either case, there was dissociation between the effects of obesity on hypothermia vs. TNF-α. In our view, these findings do not challenge the notion that TNF-α is an early mediator of LPS-induced hypothermia [16–20], but rather indicate that factors counteracting not the production, but the action of TNF-α are at play in the obese rats. The identity of such factors remains a matter for future investigation, but it is conceivable to speculate that IL-1β may be a putative candidate on the bases of the following facts: (i) IL-1β is a predominantly febrigenic cytokine [20–22]; (ii) by working in opposition to hypothermia-inducing signals, febrigenic mediators may limit the magnitude of LPS-induced hypothermia [32]; and (iii) an enhancement in plasma IL-1β accompanies the attenuation of LPS-induced hypothermia in obesity (present study) 41.
Another point that deserves attention is that obesity not only enhanced the LPS-induced rises in pro-inflammatory cytokines (TNF-α and IL-1β), but also the rise in an anti-inflammatory cytokine (IL-10), in a way that the balance between them remained unchanged. This can be seen as evidence that the anti-inflammatory effect of IL-10 was not heightened in obesity, in which case it would be unlikely to have accounted for the attenuation of LPS-induced hypothermia. It should be considered, though, that global changes in cytokine ratios do not necessarily reflect what happens at the tissue level. Indeed, a recent study [31] has revealed that obesity reprograms the cytokine responses of macrophages to LPS in a site-specific manner, with the responses of adipose tissue and peritoneal macrophages shifting in opposite directions. How important this site-specific reprogramming is to LPS-induced thermoregulatory responses remains a question for future investigations.
It should also be considered that cytokines are not the only class of mediators that operate the fever-hypothermia switch in systemic inflammation. Lipid-derived mediators appear to be of major importance, with prostaglandin E2 derived from cyclooxygenase-2 being an established mediator of fever [33,34], and a yet unidentified product of cyclooxygenase-1 being a putative mediator of hypothermia [32,35]. Leukotrienes might also play a role in LPS-induced hypothermia [36,37]. Unfortunately, limited sample volume precluded an analysis of these lipid-derived mediators in the present study.
Dosing is always a point that deserves discussion in obesity-related research, because although obese animals on a HFD are typically heavier than their LFD-fed controls, there is usually no difference between these dietary groups in terms of lean body mass [38,39]. Hence, the question as to whether dosing should be made in relation to total body mass or lean body mass has no easy answer. In the present study, we chose to administer the same amount of LPS (300 μg) in both dietary groups, targeting a dose that would be equivalent in relation to lean body mass. This approach does not seem to have resulted in underdosing of the obese animals, given that their cytokine responses were not diminished, but rather enhanced. Hence, underdosing does not pose as a plausible explanation for the attenuation of LPS-induced hypothermia in the obese rats.
The results of the present study also provide insights into the mechanisms by which LPS-induced hypothermia is affected in a genetic model of obesity, the Koletsky f/f rats. These mutant rats have been shown to display a prolonged hypothermic response to LPS [40], but it remained to be determined whether this response pattern was a reflection of their primary defect in leptin receptor signaling or their secondary obese phenotype. Now, in view of the present findings, it seems unlikely that the secondary obesity of the Koletsky f/f rats could account for prolongation of LPS-induced hypothermia. By exclusion, the primary defect in leptin receptor signaling stands out as a more likely cause of their prolonged hypothermic response to LPS. This mechanistic inference is consistent with another study in which physiologically relevant doses of recombinant leptin were shown to be inversely related to the magnitude of LPS-induced hypothermia [29]. Therefore, genetic and dietary models of obesity appear to be quite distinct when it comes to immunity and host defense. Such a distinction may also underlie controversies about fever being enhanced in dietary models of obesity [10–13], but not in certain genetic models of obesity [40–42].
In conclusion, the present study provides evidence that diet-induced obesity attenuates the hypothermic response to a high dose of LPS independently of changes in the levels of its most renowned mediator, TNF-α. Taken together with previous reports of diet-induced obesity exaggerating the febrile responses induced by low-to-moderate doses of LPS [10–13], this finding prompts us to propose that obesity biases the immune response toward a fever-associated strategy, as opposed to a hypothermia-associated strategy. The relevance of this shift may extend beyond thermoregulation and energy balance, since there is reason to believe that the fever vs. hypothermia theory is intimately intertwined with the immunology concept of host resistance vs. tolerance [14]. From this broader perspective, fever appears to provide physiological support to disease resistance (the ability of a host to clear pathogens), whereas hypothermia seems to provide physiological support to disease tolerance (the ability of a host to withstand damage inflicted by pathogens or by the immune response). A bias toward fever/resistance might provide the basis for the so-called obesity paradox, according to which obesity may improve the outcome of acute infections under at least some circumstances, despite being a risk factor for so many chronic diseases [43]. Further studies are needed to put this broader idea to the test.
Acknowledgments
The authors thank Silvana Silva for technical assistance.
Funding Statement
The study was supported by grants from Fundação de Amparo à Pesquisa do Estado de São Paulo to AAS (12/03831-8, 16/04921-1 & 18/03418-0), as well as by postdoctoral fellowships from this agency to ENK and MTF (14/03719-9 & 17/13350-0). MTF also received graduate and postdoctoral fellowships from Coordenação de Aperfeiçoamento de Pessoal de Nível Superior – Brasil (CAPES) – finance code 001.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- [1].Kluger MJ. Fever: role of pyrogens and cryogens. Physiol Rev. 1991;71:93–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Hasday JD, Thompson C, Singh IS.. Fever, immunity, and molecular adaptations. Compr Physiol. 2014;4:109–148. [DOI] [PubMed] [Google Scholar]
- [3].Evans SS, Repasky EA, Fisher DT.. Fever and the thermal regulation of immunity: the immune system feels the heat. Nat Rev Immunol. 2015;15:335–349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Plaisance KI, Kudaravalli S, Wasserman SS, et al. Effect of antipyretic therapy on the duration of illness in experimental influenza A, Shigella sonnei, and Rickettsia rickettsii infections. Pharmacotherapy. 2000;20:1417–1422. [DOI] [PubMed] [Google Scholar]
- [5].Stanley ED, Jackson GG, Panusarn C, et al. Increased virus shedding with aspirin treatment of rhinovirus infection. JAMA. 1975;231:1248–1251. [PubMed] [Google Scholar]
- [6].Schulman CI, Namias N, Doherty J, et al. The effect of antipyretic therapy upon outcomes in critically ill patients: a randomized, prospective study. Surg Infect. 2005;6:369–375. [DOI] [PubMed] [Google Scholar]
- [7].Jiang Q, Cross AS, Singh IS, et al. Febrile core temperature is essential for optimal host defense in bacterial peritonitis. Infect Immun. 2000;68:1265–1270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Kurosawa S, Kobune F, Okuyama K, et al. Effects of antipyretics in rinderpest virus infection in rabbits. J Infect Dis. 1987;155:991–997. [DOI] [PubMed] [Google Scholar]
- [9].Vaughn LK, Veale WL, Cooper KE. Antipyresis: its effect on mortality rate of bacterially infected rabbits. Brain Res Bull. 1980;5:69–73. [DOI] [PubMed] [Google Scholar]
- [10].Pohl J, Woodside B, Luheshi GN. Changes in hypothalamically mediated acute-phase inflammatory responses to lipopolysaccharide in diet-induced obese rats. Endocrinology. 2009;150:4901–4910. [DOI] [PubMed] [Google Scholar]
- [11].Pohl J, Sheppard M, Luheshi GN, et al. Diet-induced weight gain produces a graded increase in behavioral responses to an acute immune challenge. Brain Behav Immun. 2014;35:43–50. [DOI] [PubMed] [Google Scholar]
- [12].Orlandi L, Fonseca WF, Enes-Marques S, et al. Sickness behavior is accentuated in rats with metabolic disorders induced by a fructose diet. J Neuroimmunol. 2015;289:75–83. [DOI] [PubMed] [Google Scholar]
- [13].Clarke MA, Stefanidis A, Spencer SJ. Postnatal overfeeding leads to obesity and exacerbated febrile responses to lipopolysaccharide throughout life. J Neuroendocrinol. 2012;24:511–524. [DOI] [PubMed] [Google Scholar]
- [14].Steiner AA, Romanovsky AA. Energy trade-offs in host defense: immunology meets physiology. Trends Endocrinol Metab. 2019;30:875–878. [DOI] [PubMed] [Google Scholar]
- [15].Siegl D, Annecke T, Johnson BL 3rd, et al. Obesity-induced hyperleptinemia improves survival and immune response in a murine model of sepsis. Anesthesiology. 2014;121:98–114. [DOI] [PubMed] [Google Scholar]
- [16].Derijk RH, Berkenbosch F. Hypothermia to endotoxin involves the cytokine tumor necrosis factor and the neuropeptide vasopressin in rats. Am J Physiol. 1994;266:R9–14. [DOI] [PubMed] [Google Scholar]
- [17].Kozak W, Conn CA, Klir JJ, et al. TNF soluble receptor and antiserum against TNF enhance lipopolysaccharide fever in mice. Am J Physiol. 1995;269:R23–9. [DOI] [PubMed] [Google Scholar]
- [18].Tollner B, Roth J, Storr B, et al. The role of tumor necrosis factor (TNF) in the febrile and metabolic responses of rats to intraperitoneal injection of a high dose of lipopolysaccharide. Pflugers Arch. 2000;440:925–932. [DOI] [PubMed] [Google Scholar]
- [19].Leon LR, White AA, Kluger MJ. Role of IL-6 and TNF in thermoregulation and survival during sepsis in mice. Am J Physiol. 1998;275:R269–77. [DOI] [PubMed] [Google Scholar]
- [20].Long NC, Otterness I, Kunkel SL, et al. Roles of interleukin 1 beta and tumor necrosis factor in lipopolysaccharide fever in rats. Am J Physiol. 1990;259:R724–8. [DOI] [PubMed] [Google Scholar]
- [21].Smith BK, Kluger MJ. Human IL-1 receptor antagonist partially suppresses LPS fever but not plasma levels of IL-6 in Fischer rats. Am J Physiol. 1992;263:R653–5. [DOI] [PubMed] [Google Scholar]
- [22].Kozak W, Kluger MJ, Soszynski D, et al. IL-6 and IL-1 beta in fever. Studies using cytokine-deficient (knockout) mice. Ann N Y Acad Sci. 1998;856:33–47. [DOI] [PubMed] [Google Scholar]
- [23].Howard M, Muchamuel T, Andrade S, et al. Interleukin 10 protects mice from lethal endotoxemia. J Exp Med. 1993;177:1205–1208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Maloney SK, Fuller A, Mitchell D, et al. Translating animal model research: does it matter that our rodents are cold? Physiology (Bethesda). 2014;29:413–420. [DOI] [PubMed] [Google Scholar]
- [25].Almeida MC, Steiner AA, Branco LG, et al. Cold-seeking behavior as a thermoregulatory strategy in systemic inflammation. Eur J Neurosci. 2006;23:3359–3367. [DOI] [PubMed] [Google Scholar]
- [26].Almeida MC, Steiner AA, Branco LG, et al. Neural substrate of cold-seeking behavior in endotoxin shock. PLoS ONE. 2006;1:e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Wanner SP, Almeida MC, Shimansky YP, et al. Cold-induced thermogenesis and inflammation-associated cold-seeking behavior are represented by different dorsomedial hypothalamic sites: a three-dimensional functional topography study in conscious rats. J Neurosci. 2017;37:6956–6971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Steiner AA, Flatow EA, Brito CF, et al. Respiratory gas exchange as a new aid to monitor acidosis in endotoxemic rats: relationship to metabolic fuel substrates and thermometabolic responses. Physiol Rep. 2017;5:e13100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Flatow EA, Komegae EN, Fonseca MT, et al. Elucidating the role of leptin in systemic inflammation: a study targeting physiological leptin levels in rats and their macrophages. Am J Physiol Regul Integr Comp Physiol. 2017;313:R572–82. [DOI] [PubMed] [Google Scholar]
- [30].Corrigan JJ, Fonseca MT, Flatow EA, et al. Hypometabolism and hypothermia in the rat model of endotoxic shock: independence of circulatory hypoxia. J Physiol. 2014;592:3901–3916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Komegae EN, Fonseca MT, da Silveira Cruz-machado S, et al. Site-specific reprogramming of macrophage responsiveness to bacterial lipopolysaccharide in obesity. Front Immunol. 2019;10:1496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Steiner AA, Hunter JC, Phipps SM, et al. Cyclooxygenase-1 or −2 – which one mediates lipopolysaccharide-induced hypothermia? Am J Physiol Regul Integr Comp Physiol. 2009;297:R485–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Roth J, Blatteis CM. Mechanisms of fever production and lysis: lessons from experimental LPS fever. Compr Physiol. 2014;4:1563–1604. [DOI] [PubMed] [Google Scholar]
- [34].Saper CB, Romanovsky AA, Scammell TE. Neural circuitry engaged by prostaglandins during the sickness syndrome. Nat Neurosci. 2012;15:1088–1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Akarsu ES, Mamuk S. Escherichia coli lipopolysaccharides produce serotype-specific hypothermic response in biotelemetered rats. Am J Physiol Regul Integr Comp Physiol. 2007;292:R1846–50. [DOI] [PubMed] [Google Scholar]
- [36].Paul L, Fraifeld V, Kaplanski J. Evidence supporting involvement of leukotrienes in LPS-induced hypothermia in mice. Am J Physiol. 1999;276:R52–8. [DOI] [PubMed] [Google Scholar]
- [37].Brus R, Krzeminski T, Juraszczyk Z, et al. Central effects of leukotriene C4 and D4 in rats and mice. Biomed Biochim Acta. 1986;45:1153–1158. [PubMed] [Google Scholar]
- [38].Rutter K, Hennoste L, Ward LC, et al. Bioelectrical impedance analysis for the estimation of body composition in rats. Lab Anim. 1998;32:65–71. [DOI] [PubMed] [Google Scholar]
- [39].Yang Y, Smith DL Jr., Keating KD, et al. Variations in body weight, food intake and body composition after long-term high-fat diet feeding in C57BL/6J mice. Obesity (Silver Spring). 2014;22:2147–2155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Steiner AA, Dogan MD, Ivanov AI, et al. A new function of the leptin receptor: mediation of the recovery from lipopolysaccharide-induced hypothermia. Faseb J. 2004;18:1949–1951. [DOI] [PubMed] [Google Scholar]
- [41].Ivanov AI, Romanovsky AA. Fever responses of Zucker rats with and without fatty mutation of the leptin receptor. Am J Physiol Regul Integr Comp Physiol. 2002;282:R311–6. [DOI] [PubMed] [Google Scholar]
- [42].Ivanov AI, Kulchitsky VA, Romanovsky AA. Does obesity affect febrile responsiveness? Int J Obes Relat Metab Disord. 2001;25:586–589. [DOI] [PubMed] [Google Scholar]
- [43].Roth J, Sahota N, Patel P, et al. Obesity paradox, obesity orthodox, and the metabolic syndrome: an approach to unity. Mol Med. 2016;22:873–885. [DOI] [PMC free article] [PubMed] [Google Scholar]


