Abstract
Castleman disease (CD) is a rare lymphoproliferative disorder with variable presentation and prognosis. Most CD cases are unicentric and correspond to the hyaline-vascular variant, a histopathological classification associated with better outcomes, which commonly presents as an enhancing hypervascular mediastinal mass. CD is often asymptomatic and surgically resectable. Nonetheless, surgical resection can be difficult when the lymphoid mass is causing compression of vital structures. We discuss a rare case of hyaline-vascular unicentric CD presenting as an incidental pericardial mass.
Keywords: Castleman disease, lymphoid disorder, mediastinal mass, pericardial mass
Castleman disease (CD) is a benign lymphoid disorder that affects <200,000 people in the United States. 1 It can be classified clinically into unicentric and multicentric and can be divided by histologic subtypes, including hyaline-vascular and plasma cell variants. 1 , 2 Most CD cases are unicentric, with 75% of these belonging to the hyaline-vascular variant. 1 The unicentric type is well circumscribed, involving one or more lymph nodes in the same anatomic site and predominantly above the diaphragm. Thus, it is often asymptomatic and amenable to surgical resection. Unicentric CD usually becomes symptomatic when the mass compresses adjacent organs. On the other hand, the multicentric variant is a systemic lymphoproliferative disorder encompassing groups of lymph nodes in different locations. It has a higher risk of malignancy and is frequently associated with a state of immunosuppression, secondary to HIV or human herpesvirus–8 infection. 1 We discuss a rare case of hyaline-vascular unicentric CD presenting as an incidental pericardial mass.
CASE PRESENTATION
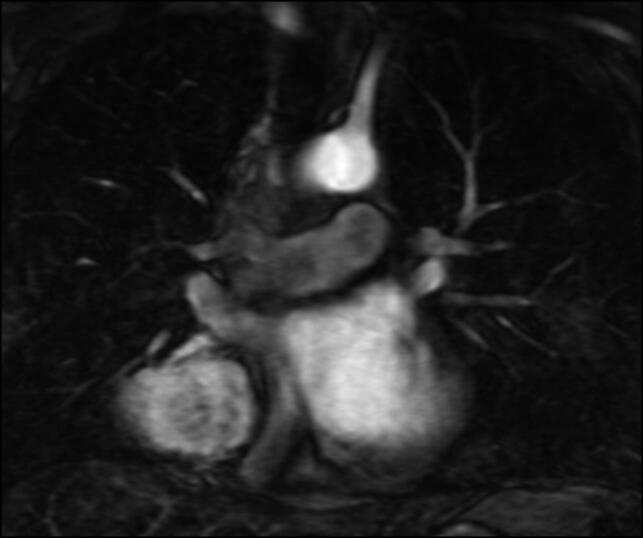
A 43-year-old man with known asthma and hypertension was found to have an asymptomatic incidental mediastinal mass. He denied constitutional symptoms, chest pain, and dyspnea and reported only a mild nocturnal cough. Chest computed tomography (CT), magnetic resonance imaging, and magnetic resonance angiography showed a right posterior pericardial mass (6.9 × 6.4 × 5.1 cm) compressing the right atrium and partially encasing the right lower lobe bronchi and pulmonary vasculature with associated subcarinal and paratracheal lymphadenopathy. It did not show evidence of arteriovenous malformation (Figure 1). Positron emission tomography (PET) showed a mild increase in fluorodeoxyglucose uptake in the right mediastinal mass abutting the right atrium. An endobronchial ultrasound bronchoscopy noted extrinsic compression at the right upper and right middle lobe from the mass. Pathology results were negative for malignancy.
Figure 1.
Magnetic resonance angiography of the chest with contrast showing a large right pericardial mass with mild subcarinal and paratracheal lymphadenopathy and no evidence of vascular malformations.
Due to the mass size, cardiothoracic surgery was consulted. A median sternotomy was performed with resection of the right lower lobe mediastinal mass. The mass was attached to the pericardium, inferior bronchus of the right lower lobe, and inferior pulmonary vein. Surgical pathology confirmed the diagnosis of Castleman lymphadenopathy, hyaline-vascular type (Figure 2). The patient was negative for HIV and human herpesvirus-8.
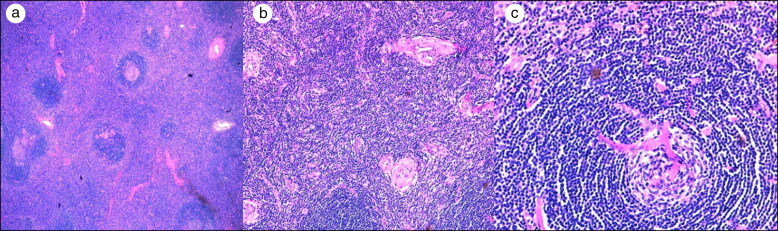
Figure 2.
(a) Numerous blood vessels with hyperplastic endothelial cells surrounded by cuffs of collagen (hematoxylin and eosin [H&E] 10×). (b) Lymph node follicle with regressed germinal center (H&E 10×). (c) Lymphoid follicle with partially involuted germinal center transfixed by penetrating arteriole and surrounded by mantle small lymphocytes, resembling a lollipop (H&E 20×).
The postoperative course was complicated by a persistent right hemothorax that required thoracotomy for evacuation. Intraoperative bronchoscopy revealed >90% stenosis of the anterior and medial segments of the right lower lobe, requiring further balloon dilation with improvement in lumen size. Additionally, imaging studies showed persistent atelectasis of the right middle lobe. Despite these findings, the patient has had a favorable clinical course; he has good exercise tolerance (walks 2.5 miles/day) and remains asymptomatic. An annual CT scan/PET scan will be performed to monitor disease.
DISCUSSION
The pathogenesis of CD involves increased levels of cytokines, particularly IL-6, responsible for the B symptoms that are more frequent in multicentric CD. 1 The excessive cytokine levels stimulate reactive proliferative changes in the lymph nodes with the potential of malignant transformation. 3 , 4
CD is initially detected on imaging studies, and the diagnosis is confirmed with histopathology. More than 70% of cases occur in the chest as a well-defined enhancing hypervascular mediastinal mass. 1 Masses involving the pleura, pericardium and chest wall, pulmonary nodules, and nonspecific airspace opacification are less frequent manifestations. 1 Excisional biopsy is required to rule out other pathologies, including lymphoproliferative disorders, metastatic disease, and hypervascular masses. 1 Autoimmune and infectious etiologies need to be considered in the differential diagnosis and should include IgG4-related disease. 5
The prognosis for unicentric CD vs multicentric CD is considerably different and is independent of the therapeutic approach. A systematic analysis of 416 cases showed that the 3-year disease-free survival rate for patients with unicentric hyaline vascular disease was 92.5% vs 45.7% for those with multicentric plasma cell disease and 78% for those with any combination (P < 0.0001). 3 It is suggested that the histopathologic classification of CD helps predict long-term outcome.
Compared with patients with multicentric CD, patients with unicentric CD have improved outcomes when undergoing resective as opposed to diagnostic surgery. 6 Although a surgical approach is feasible in most patients with unicentric CD, resection of visceral lymph nodes has many challenges, like those seen in our patient. Several cases have been reported in which resection was unsuccessful due to the large size of the mass, high vascularity, and attachment to organs such as the aortic arch and the pulmonary artery. 1
Unicentric and multicentric disease differ significantly in patient population, clinical manifestations, management, and prognosis. Increasing our understanding of the multiple manifestations of this rare disease and its subsequent management will lead to improved outcomes.
References
- 1. Madan R, Chen JH, Trotman-Dickenson B, et al. . The spectrum of Castleman’s disease: mimics, radiologic pathologic correlation and role of imaging in patient management. Eur J Radiol. 2012;81(1):123–131. doi: 10.1016/j.ejrad.2010.06.018. [DOI] [PubMed] [Google Scholar]
- 2. Wang W, Medeiros LJ.. Castleman disease. Surg Pathol Clin. 2019;12(3):849–863. doi: 10.1016/j.path.2019.03.003. [DOI] [PubMed] [Google Scholar]
- 3. Talat N, Schulte KM.. Castleman’s disease: systematic analysis of 416 patients from the literature. Oncologist. 2011;16(9):1316–1324. doi: 10.1634/theoncologist.2011-0075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Liu AY, Nabel CS, Finkelman BS, et al. . Idiopathic multicentric Castleman’s disease: a systematic literature review. Lancet Haematol. 2016;3(4):e163–e175. doi: 10.1016/S2352-3026(16)00006-5. [DOI] [PubMed] [Google Scholar]
- 5. Wang HW, Pittaluga S, Jaffe ES.. Multicentric Castleman disease: where are we now? Semin Diagn Pathol. 2016;33(5):294–306. doi: 10.1053/j.semdp.2016.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Talat N, Belgaumkar AP, Schulte KM.. Surgery in Castleman’s disease: a systematic review of 404 published cases. Ann Surg. 2012;255(4):677–684. doi: 10.1097/SLA.0b013e318249dcdc. [DOI] [PubMed] [Google Scholar]