ABSTRACT
Core body temperature changes across the ovulatory menstrual cycle, such that it is 0.3°C to 0.7°C higher in the post-ovulatory luteal phase when progesterone is high compared with the pre-ovulatory follicular phase. This temperature difference, which is most evident during sleep or immediately upon waking before any activity, is used by women as a retrospective indicator of an ovulatory cycle. Here, we review both historical and current literature aimed at characterizing changes in core body temperature across the menstrual cycle, considering the assessment of the circadian rhythm of core body temperature and thermoregulatory responses to challenges, including heat and cold exposure, exercise, and fever. We discuss potential mechanisms for the thermogenic effect of progesterone and the temperature-lowering effect of estrogen, and discuss effects on body temperature of exogenous formulations of these hormones as contained in oral contraceptives. We review new wearable temperature sensors aimed at tracking daily temperature changes of women across multiple menstrual cycles and highlight the need for future research on the validity and reliability of these devices. Despite the change in core body temperature across the menstrual cycle being so well identified, there remain gaps in our current understanding, particularly about the underlying mechanisms and microcircuitry involved in the temperature changes.
KEYWORDS: Circadian rhythm, progesterone, estradiol, thermoregulation, luteal, follicular, exercise, thermometer, wearable temperature sensor, ovulation
Introduction
A biphasic rhythm in basal core body temperature across the menstrual cycle, with body temperature higher in the luteal phase after ovulation, was described more than 100 years ago by Squire in 1868 and van de Velde in 1928 [cited in 1]. Women have used this information to track ovulatory cycles and menstrual cycle phases through daily measurement of their body temperature, and continue to do so with novel temperature sensors that are now available to continuously track 24-h body temperature rhythms 152. Similarly, researchers have relied on body temperature measurements to determine the menstrual cycle phase of reproductive-age women in their studies. There also is a substantial body of work investigating whether the thermoregulatory changes across the menstrual cycle have consequences for exercise performance and coping with heat stress. Despite a biphasic rhythm in core body temperature being such a well-established and recognized feature of the ovulatory menstrual cycle, the neural pathways that link the female reproductive and thermoregulatory systems remain incompletely understood 5.
Here, we review the literature that has investigated changes in daily core body temperature rhythms and temperature regulation across the different phases of the menstrual cycle. First, we provide an overview of the thermoregulatory system and menstrual cycle. Next, we provide a detailed review of studies in humans that have recorded daily (24 h) temperature rhythms at different phases of the menstrual cycle in women. We consider relationships between menstrual cycle-related changes in core body temperature and cognitive function, sleep, and heart rate 124. We then consider how menstrual cycle phase may influence temperature regulation at rest, during exercise, heat and cold exposure, and fever 111. We also draw on comparative and human studies to discuss mechanisms underlying the effects of the principal gonadal steroid hormones, progesterone, and estradiol, on body temperature regulation, and consider the effects of exogenous hormones (e.g. oral contraceptives) on core body temperature. Finally, we summarize recent literature investigating the usefulness of tracking daily body temperature with wearable temperature sensors for women, an approach that is reinvigorating the idea of using body temperature to determine ovulatory cycles 112. We focus here on thermoregulatory function across the menstrual cycle in reproductive-age women. The reader is referred to other excellent reviews by Charkoudian and Stachenfeld on thermoregulation in women across their lifespan, including pregnancy and post-menopause, and also sex differences in thermoregulation [2,3].
Methods
Using the electronic databases, PubMed and Google Scholar, comprehensive literature searches were conducted on clinical and experimental studies of the menstrual cycle and body temperature 96. In combination with the phrases “menstrual cycle” and “body temperature,” one or more of the following search terms were used to obtain articles published in English-language, peer-reviewed journals only (published conference abstracts were not included): circadian rhythm, progesterone, estrogen or estradiol, luteal phase, follicular phase, ovulation, vasodilation, hypothalamus, oral contraceptives, exercise, heating, heat, fever, cooling, cold, wearable sensors. Full-text manuscripts were reviewed for relevancy and reference lists were cross-checked for additional relevant studies 87.
Body temperature regulation
A brief overview of the thermoregulatory system is provided here; the reader is referred to comprehensive reviews elsewhere [4–7]. Internal core body temperature reflects the temperature within deep body tissues. The peripheral shell temperature is influenced by the external environment and blood flow to the skin [7,8]. Thermoregulation involves a balance between heat gain (metabolic heat production and dry heat gain from the environment) and heat loss (dry and evaporative heat loss to the environment) such that a constant core body temperature can be maintained [8]. In most placental mammals, core body temperature is regulated around 37°C [9,10]. Core body temperature is regulated in mammals through both physiological and behavioral mechanisms, ensuring it remains relatively constant over a range of environmental temperatures, at least in mammals with access to sufficient food energy and water [see 11]. There are, however, both spatial (between various regions of the body) and temporal variations in body temperature [6]. Shell or surface temperature is typically around 4°C lower than core temperature; however, this difference varies as skin blood flow varies [7] and in response to sweating [8].
The thermoregulatory network consists of a central integrative circuit in the preoptic area of the hypothalamus (POA), which orchestrates the activation of thermoeffector mechanisms in response to input from peripheral and central thermoreceptors [4]. To defend core body temperature in environments where environmental temperature is lower than their surface temperature, mammals reduce heat loss to the environment through cutaneous vasoconstriction, increase metabolic heat production through shivering and non-shivering thermogenesis, and engage in behaviors that reduce heat loss to or increase heat gain from the environment (such as huddling, wearing clothing and sun-basking). To defend body temperature from rising, mechanisms include cutaneous vasodilation (if the environmental temperature is less than body surface temperature), suppression of thermogenesis and evaporative heat loss (e.g. sweating, which is particularly important in humans) [6], together with thermoregulatory behaviors such as shade-seeking [12].
Core body temperature is normally maintained within a narrow range in the interthreshold zone of shivering and sweating by relying on the autonomic thermoregulatory defenses of peripheral vasoconstriction and vasodilatation [10]. Unlike sweating or panting, cutaneous vasodilation does not directly result in water loss and is therefore a first-line autonomic defense against heat [13]. Human non-glabrous (hairy) skin is innervated by two distinct branches of the sympathetic nervous system: an adrenergic vasoconstrictor system and a cholinergic active vasodilator system [14]. As an initial response to heat stress, there is a withdrawal of tonic adrenergic vasoconstriction, resulting in increased skin blood flow bringing heat to the surface, from where heat is dissipated to the environment. As core body temperature increases, the cholinergic vasodilator system is activated, leading to a more substantial increase in skin blood flow, mediated by acetylcholine and likely other multiple local co-transmitter vasodilators, including nitric oxide [14]. Conversely, during cold exposure, the adrenergic vasoconstrictor system becomes more active, reducing skin blood flow, thus reducing heat transfer from the core to periphery. Glabrous (non-hairy) skin (e.g. palms of humans, tail of the rat, the ear of the rabbit) also plays a critical role in thermoregulation. It does not have an active vasodilator system such that changes in skin blood flow are mediated entirely by alterations in sympathetic adrenergic vasoconstriction [14]. Glabrous skin is densely vascularized and equipped with arteriovenous anastomoses and a large surface-to-volume ratio, enabling a rapid and dramatic increase in blood flow to instigate heat loss or, conversely, a decrease in blood flow to conserve heat, as needed [13]. An increase in sweating in humans is temporally linked with increased skin blood flow as mediated by the active cholinergic vasodilator system [14]. Sweating is the only heat loss mechanism available to humans once ambient temperature exceeds surface temperature [7,8], but it is also implemented even in conditions where dry heat loss is possible [8].
The thermoregulatory neural circuits, including details of the preoptic area microcircuitry, are still not completely defined. However, it is postulated that temperature-sensitive neurons in the POA integrate ascending peripheral thermosensory signals (e.g. from thermoreceptors in the skin) with information about brain temperature [15] to regulate outputs of brown adipose tissue and shivering thermogenesis-promoting neurons in the dorsomedial hypothalamus, and of cutaneous vasoconstriction-promoting neurons in the median preoptic nucleus [4]. In peripheral tissues, cold sensation activates TRPM8 channels while warmth activates TRP channels [6]. In the brain, the POA is the most thermally sensitive region, with about 30% of neurons warm-sensitive, 10% cold-sensitive and the remainder temperature-insensitive [16]. A substantial percentage of POA neurons (25–50%) that are activated by local brain warming are also activated by warming of the skin or spinal cord [6], showing the importance of peripheral temperature inputs in influencing thermoregulatory responses. After thermal information is integrated in the POA, appropriate thermoeffector mechanisms are activated, to either generate or dissipate heat, via the rostral medulla in the brain [6]. Medullary output neurons activate the peripheral sympathetic branch of the autonomic nervous system to modify cutaneous blood flow and evaporative heat loss, or they activate somatic motor neurons that induce shivering. The neural circuity underlying the activation of thermoregulatory behaviors is not clear, with the role of the POA unclear [6].
In addition to regulation resulting from activation of peripheral and central thermoreceptors, body temperature is modulated by other neural circuits, including that arising from the central pacemaker located in the suprachiasmatic nucleus (SCN), which regulates circadian rhythms. The endogenous molecular clock within the SCN receives inputs from the retina to synchronize its activity, and thus synchronize internal physiological processes, with the environmental light-dark cycle. It conveys this time of day information to subordinate extra-SCN tissue clocks to coordinate circadian function throughout the body [17], including that of core body temperature, which declines across the sleep phase (the nocturnal period in humans) and increases as the active phase approaches.
Core body temperature has an endogenously generated circadian rhythm with a period close to 24-h, which is evident even when environmental conditions are kept constant, free of influences from sleep-wake patterns, activity, meals, or light exposure [18,19]. In such constant conditions (i.e., a constant routine protocol), subjects undergo a regime of semi-recumbent wakefulness in dim light while energy intake is controlled [20]. The core body temperature curve recorded under constant conditions is one of the gold standard methods to quantify or demonstrate circadian phase, or the output of the central clock (along with melatonin). Core body temperature continues to oscillate independently of other physiological rhythms or external time cues between a maximum in the active period (daytime for humans) and a minimum during the inactive period (nocturnal for humans), with an amplitude of 0.8°C to 1.0°C [21]. A cosinor curve can be fitted to the rhythm in order to determine its circadian mesor (average temperature across 24 h), phase (time of maximum temperature [acrophase], or time of minimum temperature [nadir]) and amplitude (mesor – minimum temperature). Both heat production and heat loss contribute to the circadian variation in heat content, leading to a circadian rhythm in core body temperature [22]. Indeed, under constant conditions, the circadian rhythms in heat production (measured with indirect calorimetry) and distal skin (hands and feet) temperature, reflecting heat loss, are phase-advanced relative to that of core body temperature [22].
When not living under constant conditions, the 24-h rhythm in core body temperature is still apparent, however, in addition to circadian influences, it is influenced by other factors, including sleep, which lowers nocturnal core body temperature further, and activity and meals, which raise core body temperature. In addition to these factors, the amplitude of the circadian rhythm of core body temperature can change as a result of the season, photoperiod, energy intake, water balance, disease, and reproductive status [23]. A lower minimum daily core body temperature serves to conserve energy stores, while a higher maximum daily core body temperature in hot environments saves body water that otherwise would be lost by evaporation [11]. Minimum resting energy expenditure coincides with minimum core body temperature [24]. In women and non-human primates with menstrual cycles [for example, see 25], core body temperature also fluctuates in accordance with the menstrual cycle rhythm.
Measurement of core body temperature
Accurate measurement of core body temperature is crucial for investigating its variation across the menstrual cycle under different conditions. For a detailed review of the factors that affect temperature measurement at various sites, see Taylor et al. [26] and Childs [9]. The general thermal status of the body is best represented by central arterial or mixed venous blood temperature [27], which is technically difficult to measure. Surrogate measures of core body temperature that agree relatively well with blood temperature include esophageal temperature, rectal temperature, vaginal temperature, and gastrointestinal temperature [9]. Of those, esophageal is considered the best approximate of blood temperature [28], and it has been used in some of the key early studies demonstrating changes in body temperature regulation across the menstrual cycle [for example, 29-31]. However, many subjects do not tolerate the probe or struggle with the nasopharyngeal insertion [28]. For example, the esophageal probe could not be inserted in 3 out of 12 participants in a recent study of whole-body heat loss across the menstrual cycle [32]. Rectal temperature, with the probe carefully inserted to the correct depth [26], is the next best measure, with the drawback being that it is slow to respond to changes in the thermal status of the body [for example, see 29]. Vaginal temperature provides very similar values to rectal temperature [34]. The temperature of the gastrointestinal tract is also similar to that of the rectum (on average, about 0.1°C higher), but it does change as the sensor (typically an ingested telemetry pill) moves along the gastrointestinal tract, and is influenced by drinking liquids [26]. Detection of changes in the circadian rhythm of core body temperature across the menstrual cycle requires continuous data recording, which can be achieved accurately and relatively noninvasively through the use of ingestible telemetry pills [35].
Other commonly used sites of body temperature measurement, including oral, axilla, and auditory canal or tympanic temperature, provide a much poorer index of core body temperature, primarily because they are contaminated by environmental temperatures [26,28]. Nevertheless, by insulating the auditory canal from the influence of ambient air, Hessemer and Bruck [29] were able to demonstrate very similar changes in tympanic and esophageal temperatures across the menstrual cycle. A post-ovulatory temperature rise also can be detected from the measurement of oral and tympanic temperature [36].
Skin temperature does not reflect core body temperature, but rather can vary anywhere between body core temperature and the environmental wet-bulb temperature [28]. The temperature of the skin depends largely on the extent of dilatation of the peripheral vasculature, with skin temperature approaching body core temperature if the vessels are maximally dilated, but approaching ambient air temperature when the vessels are maximally constricted. Skin temperature is also influenced by sweating. Once the water starts evaporating from a fully wet skin surface, the temperature of that surface remains constant, clamped at about 36°C in humans [8]. Despite the skin offering an attractive, easy to access and noninvasive site, its temperature is not a good approximation of core body temperature. In some investigations of changes in body temperature across the menstrual cycle [for example, 29-31], researchers have reported core body temperature as mean body temperature, an index that can estimates average temperature of human tissues by weighting skin and core temperature into a single measure. Such a measure, however, may be difficult to interpret in dynamic conditions when skin temperature can change rapidly and in different directions relative to core body temperature.
As described above, studies that have investigated skin and core body temperature under constant routine conditions when ambient temperature and behavior are controlled, have shown that the circadian rhythm of distal skin (hands and feet) temperature is phase-advanced, by approximately 100 min, and with a larger amplitude than that of core body temperature [10]. As such, not only is distal skin temperature typically lower but it is also out of phase with core body temperature. The circadian rhythm of proximal skin temperature (e.g. forehead, thigh), on the other hand, follows that of core body temperature; the distal – proximal skin temperature gradient, therefore, is useful in determining body heat loss via the extremities [10].
The menstrual cycle
Duration and endocrinology
The human menstrual cycle, defined as the interval between the first day of bleeding of one cycle and first day of bleeding of the next cycle, typically lasts 25 to 35 days, with an average of 28 days for women in their twenties, and 26 days for women in their forties [37]. The follicular phase has an interval of between 10 and 20 days and the luteal phase of between 9 and 17 days, with greater inter-individual variance for the follicular phase [38]. Consistent with established clinical research, prospective data collected from multiple menstrual cycles in over 120,000 women (~612,000 cycles) using the Natural Cycle app, which included daily basal oral temperature measurement, dates of menstruation, and results of an ovulation prediction kit for some cycles, revealed a mean cycle length of 29.3 days, with a mean follicular phase length of 16.9 days (95%CI: 10 to 30 days) and a mean luteal phase length of 12.4 days (95%CI: 7 to 17 days) [39]. Mean cycle length decreased significantly with age, by 3.2 days from age 25 to 45 years. Also, evident from this “big data” set is the variability in menstrual cycle duration between women and within menstrual cycles of the same woman, mostly as a result of variability in the duration of the follicular phase [39]. Similarly, in a cohort of ~98,000 women (~225,000 cycles), in whom data were collected using a mobile app period tracker (Ovia Fertility) and day of ovulation was confirmed with an ovulation prediction kit, average cycle length was 29.6 days, and average follicular and luteal phase lengths were 15.8 days and 13.7 days, respectively [40].
Over its duration of 25 to 35 days, the menstrual cycle is characterized by cyclical changes in hormones across the hypothalamic-pituitary-ovarian system (Figure 1), which are regulated by feedback mechanisms. Estradiol (the most potent estrogen) is the main secretory product of the ovarian follicle during the early follicular phase of the cycle and, together with inhibin, is primarily responsible for inducing a negative feedback regulation on the release of the gonadotrophins, follicle-stimulating hormone (FSH) and luteinizing hormone (LH), from the pituitary [41,42]. In the late follicular phase, as ovarian follicles develop and mature, estradiol is secreted in large amounts; when the estradiol concentration has exceeded a certain threshold for a certain amount of time (>200 pg.ml−1 for ~48 h [43]), it switches to exerting positive feedback on the hypothalamic-pituitary system, sensitizing the pituitary to the hypothalamic peptide gonadotrophin-releasing hormone (GnRH), leading to the mid-cycle LH surge [41,42]. Progesterone, on the other hand, which rises early in the luteal phase, inhibits pulsatile GnRH and, consequently, LH release; it also prevents the positive feedback effect of elevated estrogen on GnRH and LH [44]. Kisspeptin and neurokinin B, neuropeptides secreted by the ventral hypothalamus, play a key role in regulating GnRH and subsequent gonadotropin secretion [45].
Figure 1.
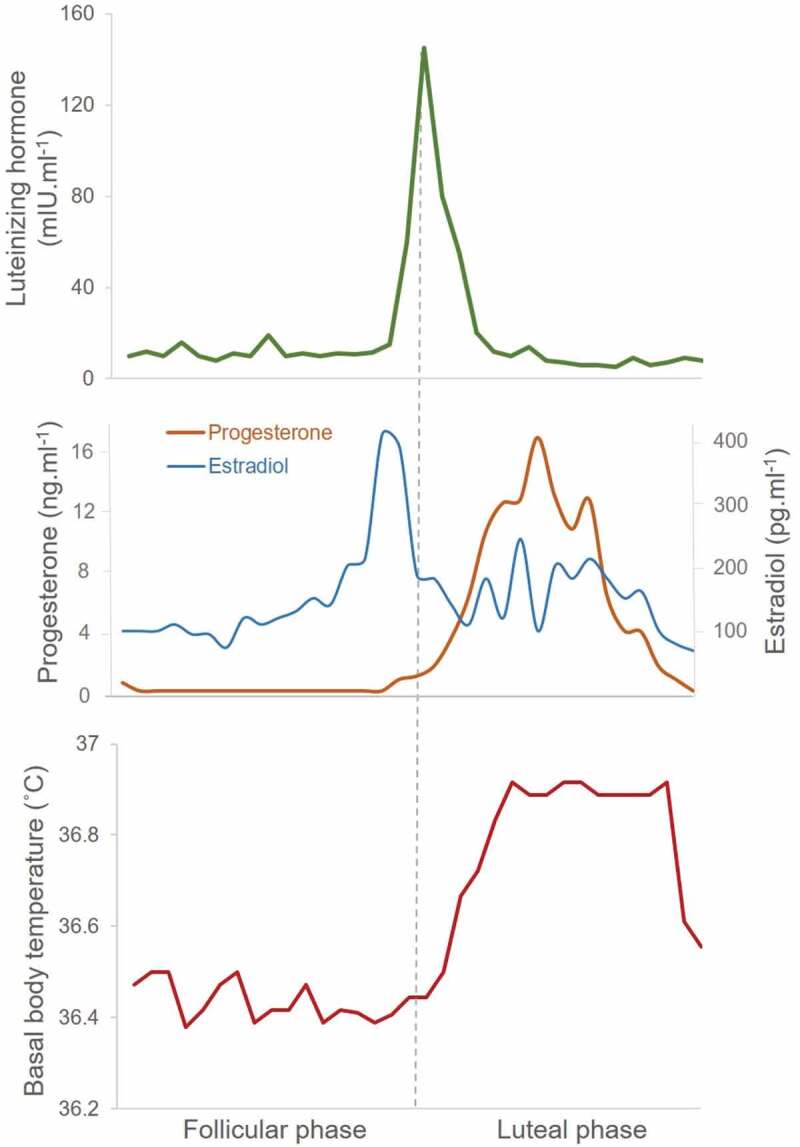
Reproductive hormone and basal body temperature changes across an ovulatory menstrual cycle. Top panel shows the profile for serum luteinizing hormone (LH) that peaks just before ovulation (dotted line). Middle panel shows fluctuations in serum levels of the ovarian hormones, estradiol, and progesterone across the menstrual cycle. Bottom panel indicates the biphasic curve in morning basal body temperature (oral) across the menstrual cycle. Data from 10 women with ovulatory menstrual cycles [58].
Just as for regulation of core body temperature, there are also powerful temporal influences from the SCN on reproductive regulation. A good example of this influence is the LH surge, which relies on the integration of temporal and metabolic information [46]. Temporal information from the SCN is integrated with sex steroid influences from the periphery to target GnRH neurons to induce the preovulatory LH surge. In turn, the SCN receives feedback from the neurons involved in this process in the hypothalamus [46].
The mean LH peak occurs at 14.7 ± 2.4 days [38]. Detection of LH in urine using an over-the-counter device is considered a highly accurate marker of impending ovulation [43]. Approximately 16 h after the peak LH surge, an oocyte is released from the follicle (ovulation) and the corpus luteum then evolves from the ruptured follicle and secretes progesterone and estradiol, marking the start of the luteal phase. About 7 days after ovulation, should fertilization and implantation of the conceptus not occur, the corpus luteum degenerates and hormone production begins to decline. The reduction in ovarian steroid hormones (along with reduced inhibin A concentrations) toward the end of the luteal phase leads to a reduced negative feedback mechanism on the hypothalamic-pituitary system, and there is an inter-cycle rise in FSH [42].
The hormonal changes across the menstrual cycle not only directly control reproductive events in women but also exert effects on other physiological systems, including thermoregulation. These effects may represent a systematic approach to creating an environment conducive to implantation, survival, and development of an embryo. The POA of the hypothalamus is centrally important for the regulation of both temperature and reproductive function, and there is a linkage between these two systems. Indeed, given the substantial investment of energy required for fertility, pregnancy, and lactation, it is not surprising that there is a reciprocal interaction between neural circuits that regulate energy balance, body temperature, and reproduction. This interaction ensures an adequate energy reserve to meet the demands of reproduction [47].
Determining menstrual cycle phase
The length of the menstrual cycle, timing of ovulation, and hormonal concentrations at any particular timepoint in the menstrual cycle vary between individuals and even from month to month within an individual. In studies of the menstrual cycle, it is therefore important to obtain detailed information from participants, before enrollment, concerning typical menstrual cycle duration and regularity. The variability in menstrual cycles makes it challenging to match physiological changes (including core body temperature) to hormone variations across the menstrual cycle. Given these constraints, most studies describing core body temperature changes in detail across the menstrual cycle have focused on pre-menopausal women with regular menstrual cycles, and there are very little data in peri-menopausal women. Women approaching menopause start to experience changes in their menstrual cycles, including irregularity; however, it is still feasible to track their cycles, which can be aided with an app that provides a convenient tool for women, and can be helpful for researchers to schedule visits around specific menstrual phases. However, hormonal measurements are critical at each visit to confirm the accuracy of the menstrual cycle phase.
Given the duration of the menstrual cycle, of approximately 28 days, it is impractical to record physiological measurements along with hormone samples daily or more frequently across the menstrual cycle to track the time course of change. As a result, most studies compare two time-points in the menstrual cycle when the hormone milieu is most distinct: early-mid follicular phase (low estrogen and progesterone) versus the mid-luteal phase (dominant progesterone levels). Some studies have targeted the pre-ovulatory phase, when there is a surge in estrogen; this phase is challenging to target, being of short duration (2–3 days) and requiring either daily tracking of estrogen levels or prediction of the day of ovulation based on previous menstrual cycles, with scheduling a couple of days before expected ovulation. Such studies, while challenging, have provided critical data about the effects of unopposed estrogen on physiological measures, including body temperature.
Anovulatory cycles, in which there is no increase in progesterone, occur about 28% of the time in women aged 20 to 24 years [49], and there can also be month-to-month variability in menstrual cycle duration or day of ovulation in a particular woman. It, therefore, is imperative that studies of the menstrual cycle confirm ovulation (e.g. through ultrasound of the ovaries, or measuring the LH surge), and also preferably measure progesterone and estrogen levels (or metabolites) as part of the study protocol.
Effects of exogenous hormones (e.g. oral contraceptives)
Research designs using the administration of exogenous steroid hormones relative to placebo can provide important information about the effects of a particular hormone, over and above those that may occur in observational studies of changes across the menstrual cycle. Ideally, these hormones should be administered to women in a cross-over placebo design; however, practically, most studies have investigated differences in women taking hormonal contraceptives compared to women with natural menstrual cycles, or have taken advantage of the typical 7-day placebo window within most oral contraceptive packs, to serve as the hormone-free condition. Hormonal contraceptives are exogenous steroid hormones that inhibit GnRH, thus suppressing the secretion of gonadotropins; during the placebo interval, the hypothalamic–pituitary–ovarian axis slowly regains activity, however, endogenous estrogen levels remain suppressed until Day 6 [50]. Researchers, therefore, are advised to consider the placebo (withdrawal) phase as a transient hormone phase [51].
Combined oral contraceptives typically contain ethinyl estradiol and a synthetic progestin taken for 21 days, and a placebo taken for 7 days. There are also progestin-only contraceptives. Women taking oral contraceptives have low endogenous hormone levels but high levels of synthetic hormones [52]. As such, some effects observed in women taking oral contraceptives could reflect suppression of the endogenous hormones and/or effects of the exogenous hormones. Hormones contained in contraceptive preparations also are synthetic so may exert different effects to endogenous hormones. For example, ethinyl estradiol has a high affinity for estrogen (estradiol) receptors and is more potent [52] and may be bioactive for longer [51] than the endogenous hormone. They also may have different actions on progesterone/estrogen receptors as a consequence of differences in structure, type, and concentration, and are taken once per day as a single dose, whereas endogenous hormones are continuously secreted from the ovary in varying amounts across the menstrual cycle [51]. It should be noted that the assays used to measure endogenous progesterone and estrogen (typically estradiol) do not measure synthetic hormones and there is a large variation between individuals in the pharmacokinetics of contraceptive steroids [53].
New oral contraceptive formulations have been developed to contain the minimum steroid doses necessary to inhibit ovulation, and current formulations contain less than 20% of the earliest preparations [54]. Thus, their effects on core body temperature and other physiological responses may differ from those of older formulations. The type of progestin has also varied with generations of oral contraceptive: second-generation progestins (e.g. norgesterel) are more androgenic, and third-generation progestins are anti-androgenic [52]. Progestins also differ in their selectivity for the progesterone receptor, with newer progestins having selectivity ratios seven to eight times higher than those of older progestins [54]. Finally, oral contraceptive brands differ in the hormonal dose delivered across the 21-day period, with monophasic pills containing a constant dose of progestin and estrogen, and triphasic pills containing a varying dose of hormones designed to mimic the natural menstrual cycle. Given the wide range of hormonal contraceptive agents available, it is critical that researchers report the types used by participants, including amounts of estrogen and progestin contained in the contraceptive.
Effect of the menstrual cycle on core body temperature
Resting-state (basal) core body temperature, typically measured in the early morning when waking up before any activity, is 0.3°C to 0.7°C higher in the luteal phase compared with the follicular phase [55–57], providing a simple method for retrospectively detecting ovulatory cycles [55,58]. The higher temperature after ovulation can be detected from the measurement of basal oral, tympanic, rectal, or vaginal temperature [36]; it may also be detectable from 5-min oral temperature measurements taken at a consistent time in the afternoon or at bedtime [59]. The increase in body temperature has been attributed to the thermogenic effect of progesterone, with a rise in core body temperature being evident about 24 h after a detectable increase in plasma progesterone levels [60], reaching a plateau within 48 h [61], and remaining high until the approach to menstruation, when it declines as progesterone levels decline. If pregnancy ensues, core body temperature is maintained at the level of the luteal phase throughout the life of the corpus luteum [62]. In anovulatory cycles, which lack a rise in progesterone, there is no increase in basal core body temperature, although the opposite it not necessarily true, since there are documented cases when ovulatory cycles are hormonally evident yet there is no rise in basal core body temperature [58], which could reflect a lack of sensitivity to progesterone [63].
Baboons, like humans, exhibit a true menstrual cycle with cyclic changes in reproductive hormones. In baboons, abdominal temperature increases from minima recorded during the periovulatory phase to maxima in the luteal phase, however, this higher body temperature persists into the menstruation phase, despite the drop in progesterone [25]. These findings suggest that the higher body temperature is unlikely to be a result from the actions of only progesterone [25].
While tracking core body temperature is a relatively simple and inexpensive way to monitor ovulatory menstrual cycles, it is subject to limitations including measurement and interpretation errors, inconvenience, and the impact of other factors on body temperature variance [64]. Even if basal core body temperature is measured on awakening before any activity, it is still influenced by internal (e.g. fever, sleep disturbance, change of waking time) and external (e.g. environmental temperature) factors, which can confound the measurement of a menstrual cycle effect on body temperature [43]. The impact of confounding factors can be somewhat lessened by following the “three-over-six” rule, which requires that three consecutive daily readings are higher than the six preceding daily readings, reflecting an upward trend in core body temperature [1].
In the last few decades, with the availability of ambulatory core body temperature measuring devices, it has been possible to track body temperature for long periods of time, leading to a more complete picture of how daily core body temperature rhythms are influenced by the menstrual cycle. Studies in which at least 24 h of core body temperature were recorded are reviewed in detail in Table 1. Table 1 also includes studies that applied constant routine or similar protocols to isolate the endogenous circadian rhythm of core body temperature in women at different phases of the menstrual cycle.
Table 1.
Summary of studies, in chronological order, that sampled core body temperature over 24-h in women at different phases of the menstrual cycle.
| Authors, Year [Ref] | Participants | Methodology | Findings | Comment |
|---|---|---|---|---|
| Lee 1988 [70] | 13 women (age: 25–35 y); regular MC (25–35 days) |
|
LP vs FP
|
- Shows for the first time the impact of menstrual cycle phase on 24-h CBT, with a substantial increase in Tmin relative to a smaller increase in peak CBT in LP vs FP. |
| Severino et al 1991 [72] | 22 women (age: 23–44 y); regular MC (26–32 days): 10 PMS; 6 chronic dysphoria; 6 controls (complete data from 6 PMS and 6 controls) |
|
LP vs FP
Group differences
|
- Study shows a raised and flattened 24-h curve in the LP relative to FP. |
| Kattapong et al 1995 [69] | 50 men (age: 25 ± 5.9 y]; 21 women, 10 in FP (age: 24 ± 3.1 y) and 11 in LP (age: 26 ± 7 y); 14 women taking OC, 6 in pseudo-FP (age: 25 ± 2.7 y) and 8 in pseudo-LP (age: 22 ± 3.7 y) |
|
LP vs FP (between group)
Effect of sex
Effect of OCs
|
- Ovulation was not confirmed, however, raised 24-h CBT and blunted amplitude in the LP is consistent with other findings. - OC raised 24-h CBT compared to naturally-cycling women and men, with the effect evident even in pseudo-FP [placebo pills). - women in the FP have similar CBT curves to men. |
| Cagnacci et al 1996 [66] | 7 women (age: 25–35 y); regular MC (26–32 days) |
|
LP vs FP
Daytime melatonin administration relative to placebo
|
- CBT curve was raised with a dampened nocturnal decline and delayed phase [Tmin) by ~90 min in LP. Onset of melatonin secretion was also delayed. Authors suggest that reduced CBT amplitude in LP could result from an absence of the nocturnal hypothermic action of melatonin. |
| Cagnacci et al 1997 [67] | 19 women (age: 28–35 y]; normal MC (28 ± 2 days) but subfertile patterns, 11 studied over spontaneous cycles and 8 during a cycle of multiple follicular development induced with FSH injections (intra uterine insemination, IUI) |
|
Spontaneous cycles, LP vs FP
IUI cycles, early FP vs pre-ovulatory vs LP
Group comparison (IUI vs spontaneous)
Relationships with E2 and P
|
- Uses artificially prolonged pre-ovulatory high E2 environment to show for the first time that in an E2-dominated environment, CBT is shifted downwards uniformly across 24 h. - Confirms other studies showing a raised CBT curve with a blunted nocturnal decline in the LP of spontaneous cycles. - CBT changes in cycles with multiple follicle development were not as robust as for spontaneous cycles. - The ratio of P:E2 rather than absolute levels of each hormone seems to be important for CBT changes. |
| Parry et al 1997 [71] | Original sample (n = 41) with regular MCs (26–32 days), 26 of whom provided complete data, 13 with premenstrual dysphoric disorder and 13 healthy controls |
|
Baseline, LP vs FP (menstrual phase effect)
PMDD vs control, baseline
Sleep deprivation and recovery effects
|
- Confirms other studies showing a raised CBT curve with a blunted nocturnal decline. - Low statistical power for interaction effects, however, suggests that differences in 24-h CBT rhythms in premenstrual depression vs health controls may be revealed with SD. |
| Wright and Badia, 1999 [78] | 25 women (age: 18–28 y), with regular MCs (25–32 days) [8 in mid-FP and 9 in mid-LP] or taking OCs [n = 8] |
|
LP vs FP (between group)
Effect of OCs
Melatonin
Performance and alertness
|
- While T was sampled at tympanic membrane, and only every 30 min, this study is the first to use a constant routine to examine the unmasked circadian T rhythm according to menstrual phase and OC use. Findings show higher T in LP. Amplitude was not significantly blunted in the LP, a finding authors attribute to low statistical power. - Findings suggest a relationship between body T and cognitive performance in the context of female hormonal states. |
| Coyne et al 2000 [35] | 4 women (age: 22–44 y) with regular MCs (26–31 days) |
|
LP vs FP
Pre-ovulatory phase
|
- Sample size is very small, however, CBT is monitored over a few days in each menstrual phase. Data show a raised CBT curve with blunted amplitude in the LP and a lower CBT curve in the pre-ovulatory phase, 24–48 hours before the LH surge. |
| Shibui et al 2000 [73] | 8 women (age: 20–23 years) with regular MCs (28–32 days) |
|
LP vs FP
Melatonin
|
- Under controlled (unmasked) conditions, CBT is higher in the LP, especially at night, with no change in circadian phase (Tmin). – Authors suggest that reduced area-under-the-curve for melatonin, reduced amplitude of cortisol, TSH and CBT represent a reduced amplitude of the endogenous oscillation of the circadian pacemaker in the LP. |
| Baker et al 2001b [79] | 8 men (22 ± 1 years); 8 women with natural MCs (22 ± 4 years); 8 women taking OCs (21 ± 1 years) |
|
LP vs FP
Effect of sex
Effect of OCs
|
- Findings show increased CBT in LP than FP. Amplitude not blunted, possibly as a consequence of small sample size and influence of activities on daytime T leading to unclear Tmax peak. - Findings show men and women in the natural FP have the most similar 24-h T curves, however, women reach Tmin later than men and have a blunted nocturnal T drop. - Women taking OC have similar 24-h T curves to women in the natural LP. |
| Baker et al 2002 [65] | 15 women (22 ± 4 years], 7 with and 8 without primary dysmenorrhea (combined) with complete data |
|
LP vs FP
Effect of acetaminophen
Dysmenorrhea vs. controls
|
- Findings show increased CBT with a blunted nocturnal drop in the LP. - Inhibiting central cyclooxygenase activity with acetaminophen had no effect on the LP-increase in CBT suggesting that the post-ovulatory CBT increase is not a result of increased brain prostaglandin synthesis. |
| Cagnacci et al 2002 [68] | 8 women (24–32 years] |
|
LP vs FP
Effect of salicylate in LP
Relationships with E2 and P
|
- CBT curve was raised with a dampened nocturnal decline and delayed phase (acrophase) in LP. - Salicylates did not antagonize LP modifications in CBT, suggesting prostaglandins are not involved in LP CBT changes. - The increase in CBT with salicylates could result from a slight increase in metabolic rate. |
| Shechter et al 2010 [74], and Shechter et al 2011 [83] | 8 women (26 ± 2.7 years), with regular MCs (26–32 days) |
|
LP vs FP
Melatonin
Sleep
Cross-correlation between measures
|
- In constant conditions with minimal masking effects, nocturnal decline in CBT is lower in the LP [blunted amplitude), with no change in circadian phase. - Normal relationships between CBT, melatonin, and sleep are maintained in FP and LP. - Absence of a change in circadian rhythms of melatonin, DT, and DCG suggest that there is not a change in the circadian system in the LP, rather, changes in CBT likely reflect a thermoregulatory response to P. |
| Vidafar et al 2018 [75] | 40 women with regular menstrual cycles, 19 in FP and 21 in LP, and 84 men (18–30 years). |
|
LP vs FP
Performance on psychomotor vigilance task
|
- Large sample, using constant conditions shows CBT amplitude is lower in LP women than FP women. Weaker effects for Tmin and overall CBT could arise from limitations of MC phase being based on self-report, with some women potentially misassigned or in LP without ovulatory cycles. - Sex differences in effects of sleep deprivation on reaction time depend on MC phase: men and LP women are comparable, and with better night performance than FP women. - The strongest difference in performance between FP and LP women is at night, when CBT is higher in LP than FP, which may protect from attentional failure. |
| Grant et al 2019 [76] | 8 women in FP and 8 women in LP (19–29 years) with regular MCs (26–35 days), who were included in Vidafar et al. [75] |
|
LP vs FP
Performance on psychomotor vigilance task
Melatonin
Performance, CBT, and hormones
With light-induced melatonin suppression
|
- Confirms using constant routine that CBT is higher in LP vs FP, especially during habitual night, but melatonin is not different. - FP women have worse performance with prolonged sleep loss than LP women. Light that suppresses melatonin can improve performance in FP. - Trend relationship suggests that P/E2 ratio indirectly influences neurobehavioral performance through impact on CBT. |
Acrophase = circadian peak from fitted curve; CBT = core body temperature; DCG = distal core temperature gradient; DLMOn/DLMOff = dim light melatonin onset/offset; DT = distal temperature; E2 = estradiol; FP = follicular phase; IUI = Intrauterine Insemination; LH = luteinizing hormone; LP = luteal phase; MC = menstrual cycle; mesor = mean value from fitted curve; P = progesterone; OC = oral contraceptive; REM = rapid eye movement, ROL = rem onset latency; SD = sleep deprivation; T = temperature; Tmax = maximum temperature; Tmin = minimum body temperature; TSH = thyroid-stimulating hormone.
Most studies that confirmed the presence of ovulatory cycles found that the mean or mesor core body temperature is increased and the nocturnal decline in core body temperature is blunted, reducing the amplitude of the daily temperature rhythm in the luteal phase compared with the follicular phase (Figure 2; 35,65-75). Core body temperature rhythms in women in the follicular phase are most similar to those of men. Studies that have applied constant routine protocols or rapid ultradian nap protocols to expose the endogenous circadian core body temperature rhythm have confirmed that the circadian amplitude of core body temperature in the luteal phase is blunted [73–76]. Cagnacci and colleagues [67] found that the progesterone:estrogen ratio, rather than progesterone levels alone, correlated with the high 24-h mean core body temperature and the reduced core body temperature amplitude, suggesting that the balance of these two hormones is critical for modulating core body temperature. However, Shechter and colleagues [74] found a significant negative correlation between urinary progesterone and the amplitude of the core body temperature rhythm in women under controlled conditions. Using a constant routine protocol, Grant, and colleagues [76] found that both the area under the curve for progesterone and for the progesterone:estrogen ratio correlated with the core body temperature area under the curve. The blunted core body temperature amplitude in the luteal phase possibly could be attributed to a direct effect of progesterone on the SCN pacemaker, or could be indirect, for example, as a result of modulation of the hypothermic effect of melatonin. Melatonin, which increases during the night, has a hypothermic effect, contributing to the normal nocturnal decline in core body temperature [77]. In support of this effect, administration of melatonin provokes an increase in distal skin temperature (potentially via a direct effect on blood vessels to induce vasodilation and/or indirectly via modulation of sympathetic nervous system activity) and a decrease in core body temperature [10], as heat is shifted from the core to the shell. Mean circulating melatonin levels and patterns are similar in the follicular and luteal phases [66,78]; however, melatonin seems to have a reduced hypothermic effect in the luteal phase. Melatonin administered during the day elevated circulating melatonin levels to a similar extent in the follicular and luteal phases, and was associated with a decline in core body temperature in the follicular phase (of 0.31°C), but no associated decline in core body temperature in the luteal phase [66]. The authors reasoned that progesterone may inhibit the nocturnal hypothermic action of melatonin in the luteal phase, reducing the nocturnal fall in core body temperature [66]. In support of a role for melatonin in the blunted amplitude of the core body temperature rhythm in the luteal phase, a recent constant routine study showed in a small sample that when environmental light exposure was used to suppress melatonin in the follicular phase, core body temperature was higher and similar to that of women in the luteal phase [76].
Figure 2.
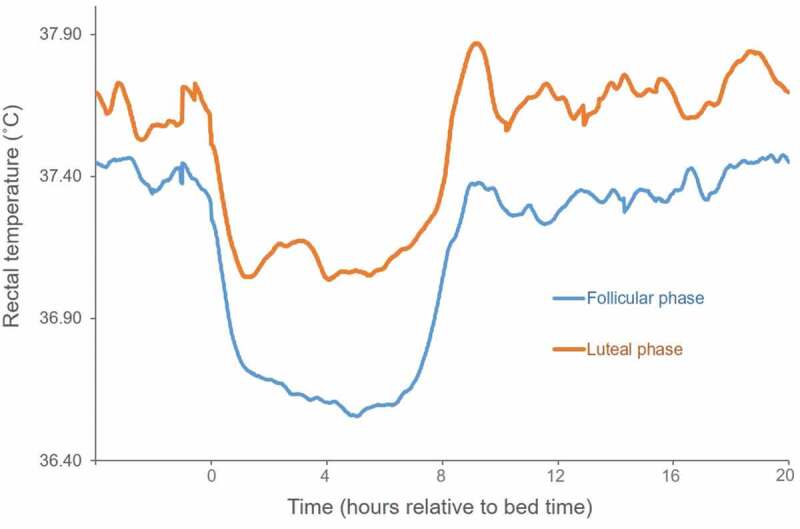
Data represent rectal temperatures recorded every minute for 4 h before bedtime (time 0) and 20 h after from fifteen young women (age: 22 ± 4 years) in the mid-follicular and mid-luteal phases of their menstrual cycles. Women had serum progesterone levels in the luteal phase reflective of ovulatory menstrual cycles. Subjects followed their usual daytime schedules and spent the night in a sleep laboratory. Modified from [65].
While a blunted nocturnal amplitude of the core body temperature rhythm in the luteal phase is evident even in studies with uncontrolled environmental conditions, studies of core body temperature rhythms in ambulatory conditions or in semi-controlled conditions have found variable effects of the menstrual cycle on phase (indicated by the time of maximum or minimum core body temperature) of the circadian temperature rhythm. Some investigators [66–68,80] found a phase delay in the temperature rhythm (sometimes by as much as 90 min) in the luteal phase compared with the follicular phase, while others have found no difference [35,69,71,79]. This variability in findings could be explained by masking of the endogenous core body temperature rhythm by factors such as environmental light, activity, sleep, and posture [78,81], as well as by the application of different methods and indicators of phase. For example, acrophase as a phase marker may be inaccurate in individuals who are free-living and engaging in daytime activity. After controlling for many of the masking effects, by restricting subjects to 24-h bedrest and regulating the sleep period, environmental temperature, and food intake, Cagnacci, and colleagues [67,68] found a significant phase delay in the luteal phase. However, more recent studies that applied a full or modified constant routine [75,76,78] or ultradian nap protocol [73,74] found no difference in the circadian rhythm of body temperature phase between the follicular and luteal phases of the menstrual cycle. Shechter and colleagues [74] argue that the delay found by Cagnacci and colleagues in the luteal phase could be a consequence of the effects of bright light on the core body temperature rhythm, since dim light conditions were not maintained across the experiment. Overall, current evidence supports the concept that the amplitude of the circadian core body temperature rhythm is blunted in the luteal phase, but the circadian core body temperature phase is unchanged in the luteal phase compared with the follicular phase of the menstrual cycle.
Few research studies have examined daily skin temperature rhythms at different phases of the menstrual cycle. Krauchi and colleagues [82] monitored 24-h ambulatory skin temperature using wireless temperature sensors at eleven sites including wrists and feet (distal skin temperature) and infraclavicular regions and sternum (proximal skin temperature) in women across the menstrual cycle under normal living conditions. They showed a change in mean daily skin temperatures with maximum values at the end of the self-reported luteal phase and minimum values at the estimated end of the follicular phase. There was no menstrual cycle phase effect on the distal-proximal skin temperature gradient, suggesting no change in internal heat conduction across the menstrual cycle [82]. In contrast to these findings, Shechter and colleagues [83] found no difference in distal skin temperature (hands and feet) across all circadian phases between the mid-follicular and mid-luteal phases in a highly controlled study using the ultradian sleep-wake cycle procedure in women with ovulatory cycles confirmed with hormone measurements. As shown in earlier constant routine studies [22], distal skin temperature was out of phase with core body temperature, with minimum core body temperature recorded at a similar time (follicular: 0434 h ± 0025; luteal: 0420 h ± 0022) as maximum distal skin temperature (follicular: 0314 h ± 0057; luteal: 0423 h ± 0104) in both menstrual phases [83]. Studies that have measured skin temperature for shorter time intervals either at rest or during exercise have produced mixed results for menstrual cycle effects, with some showing increased skin temperature and others no difference in the luteal relative to the follicular phase [reviewed in 36]. These authors suggest that since the onset of sweating is delayed (which would be expected to be associated with an increase in skin temperature) yet cutaneous vasodilation is increased (increasing convective heat loss) during the luteal phase, the net result may be no change, or little change, in skin temperature [36].
Most studies have compared core body temperature rhythms in the luteal and follicular phases, but a change in body temperature also may occur around the time of ovulation, albeit a smaller change that is not always detected. In 1945, Halbrecht observed that the “progestin phase” (luteal phase) was preceded by a slight but constant fall in basal core body temperature (oral or rectal) in some of the women in which body temperature was monitored [56]. In a small study of four women in the preovulatory phase, in whom high levels of estradiol were documented, resting esophageal temperature was lower and the threshold for sweating was decreased relative to the early follicular phase [31]. In a study of women with multiple follicle development induced with FSH injections from day 8 onwards, which results in a prolonged estrogen surge, the entire 24-h core body temperature curve was shifted lower, with no change in amplitude or phase, in association with a low progesterone:estrogen ratio just before ovulation [67]. The temperature-lowering effect of estrogen is also evident in ambulatory women with normal ovulatory menstrual cycles; mean and minimum 24-h core body temperature were at their lowest during the preovulatory phase, coincident with the estrogen surge, in women tracked across several phases of the menstrual cycle [35]. Together, current data suggest that in the presence of unopposed estrogen, core body temperature is lowered uniformly across 24 h, whereas in the presence of estrogen and progesterone in the luteal phase, the curve is shifted upwards but with a blunted amplitude.
A question best addressed by studies with controlled conditions is whether there is a change to circadian core body temperature regulation overall, or just the rhythm in the luteal phases relative to the follicular phase. Shibui and colleagues [73] investigated multiple circadian hormone and temperature rhythms in women in the follicular versus luteal phase and found a blunted amplitude of thyroid-stimulating hormone and a trend for a lower area under the melatonin curve, in addition to a blunted temperature rhythm, leading them to conclude that there is an overall change in the circadian pacemaker. However, other studies using either constant routine or ultradian naps, did not find any menstrual phase associated difference or change in melatonin phase or amplitude. Based on their findings of no change in circadian amplitude, phase, or level of melatonin, distal skin temperature, or in the distal-core temperature gradient, Shechter et al. [83],concluded that the changes in amplitude and level of core body temperature in the luteal phase likely reflect a thermoregulatory response to progesterone rather than a change in function of the circadian system.
Association between changes in body temperature over the menstrual cycle and cognitive performance, sleep, and heart rate
The consequences of changes in core body temperature over the menstrual cycle for other physiological functions are not well understood. In studies conducted to date, researchers have found that cognitive performance on a simple reaction task differed in association with core body temperature across the circadian cycle in women on a constant routine, with better performance in the luteal phase when core body temperature was higher [75,76,78]. While these results are correlational, it is possible that a higher core body temperature in the luteal phase may explain the better performance; a relationship between core body temperature and cognitive performance, where the poorest performance coincides with the lowest body temperature, has been shown repeatedly in men [78]. In a study of cognitive performance overnight, in which neurobehavioral performance was worse in the follicular phase, Grant and colleagues [76],found a significant inverse correlation between core body temperature and attentional failures in the naturally-cycling women.
Several studies have investigated menstrual cycle-related changes in sleep in parallel with core body temperature, and have suggested that some changes in sleep may be a consequence of changes in body temperature. For example, Driver and colleagues [84],found that slow-wave activity (electroencephalogram power density 0.625–4.625 Hz) was higher in the first sleep cycle of the luteal phase relative to the follicular phase, which they suggest may be attributed to the higher body temperature since slow-wave activity is increased with elevated body temperature in the rat [85]. The well-characterized increase in spindle frequency activity in the luteal phase [86–88] may also be, in part, a result of increased core body temperature [89], although progesterone and its metabolites likely also have a direct effect on spindle generation [84]. Some studies have found a small reduction in REM sleep in the luteal phase relative to the follicular phase [reviewed in 86], particularly evident in the first and fourth sleep cycles [84], which could be related to the higher body temperature in this phase. In their ultradian nap study in the follicular and luteal phases, Shechter, and colleagues (2010)[74] also found reduced REM sleep in naps during the habitual nocturnal sleep period in the luteal phase compared with the follicular phase, which they suggest may reflect the coupling between body temperature and REM sleep. REM sleep has a circadian variation, peaking soon after the nadir in core body temperature [90], and may be inhibited in the presence of a higher core body temperature, as it is under a thermal challenge [91]; thermoregulatory responses (e.g. sweating rate) are reduced in REM sleep [92]. Overall, correlational data suggest some relationship between the rise in body temperature and sleep changes in the luteal phase, although reproductive steroids also act directly on sleep regulatory centers [93], so it is likely that both direct and indirect effects may be in play.
An increase in heart rate (3% to 5%) in the luteal phase relative to the follicular phase is evident, particularly during sleep [84,86,94], and is associated with a decrease in vagal activity [94]. There is also an increase in sympathetic activity (reflected by measurement of muscle sympathetic nerve activity) in the mid-luteal phase compared to the follicular phase [95–97]. The increase in heart rate in the luteal phase, therefore, may result from altered sympathovagal balance, reflecting the actions of progesterone (and possibly balanced with effects of estrogen) on the autonomic nervous system. It has been argued, however, that this increase could be secondary to the increase in body temperature of ~0.4 C (Q10 effect) [29]; increased heart rate in the luteal phase is correlated with the increase in body temperature [84].
Effects of exogenous hormones on core body temperature in women
Studies in women with natural menstrual cycles, which are observational, point strongly to the effects of progesterone and estrogen on body temperature regulation, with progesterone being responsible for the increase in core body temperature in the luteal phase. These findings are supported by studies that have investigated the direct effects of exogenous hormone administration on core body temperature, to determine the separate and combined effects of progesterone and estrogen. Studies of women taking hormonal contraceptives that contain synthetic hormones have also been informative about the effects of progesterone and estrogen on body temperature regulation.
Effects of estrogen and progesterone
The thermogenic action of progesterone was shown in early studies in which progesterone was administered to men [98] (Figure 3) or to women with anovulatory cycles or amenorrhea, or in the follicular phase or luteal phase, or post-menopause [62,63,99]. Statistical analyzes were not undertaken in these early studies, however tests were made in multiple women and the effects reported were large enough to be visually identified. The thermogenic effect of progesterone is also evident in rats [100–102]. Progesterone’s thermogenic effect is evident even at low doses (5 mg) in men [98] (Figure 3) but higher doses are more likely to induce a consistent response [60,98]. Barton and Wiesner [63] showed that daily injections or intramuscular administration of progesterone caused a rise in waking temperature within 24 h in women with amenorrhea. When administered in the presumed luteal phase (when waking temperature was already high), an additional rise in body temperature was observed in some but not all women. In their series of studies, Israel and Schneller [60] found that oral administration of a progestogen alone (pregneninolone, high dose: 4 daily doses of 20 mg for a total dose of 80 mg) in ovariectomized women increased basal rectal temperature by 0.2°C to 0.3°C within 24 h in 19 of 22 trials; temperatures declined within 48 h after progestogen withdrawal. These results suggest that the thermogenic effect of progesterone does not require estrogen priming of progesterone receptors for the response to occur. The rise in core body temperature was still evident if progestogen followed ethinyl estradiol administration, and if estradiol and progestogen were administered simultaneously (although a higher progestogen dose was required to obtain the effect) [60]. Also, while a high dose of estradiol benzoate (1.5 mg) administered to women during the luteal phase lowered basal core body temperature, the effect was temporary. Results are consistent with studies that administered estrogen alone, resulting in a small (<0.25°C) and temporary depression of waking core body temperature [60,63]. Also, studies of the effects of estrogen replacement therapy in postmenopausal women show a reduction in resting rectal temperature (by ~0.5°C) [103,104]. Addition of progestins blocked the temperature-lowering effect of estrogen such that core body temperatures were similar to those of women not taking any hormone replacement therapy [103]. In summary, estrogen has a temperature-lowering effect that can temporarily oppose the effect of progesterone, although progesterone’s hyperthermic effect is dominant [60].
Figure 3.
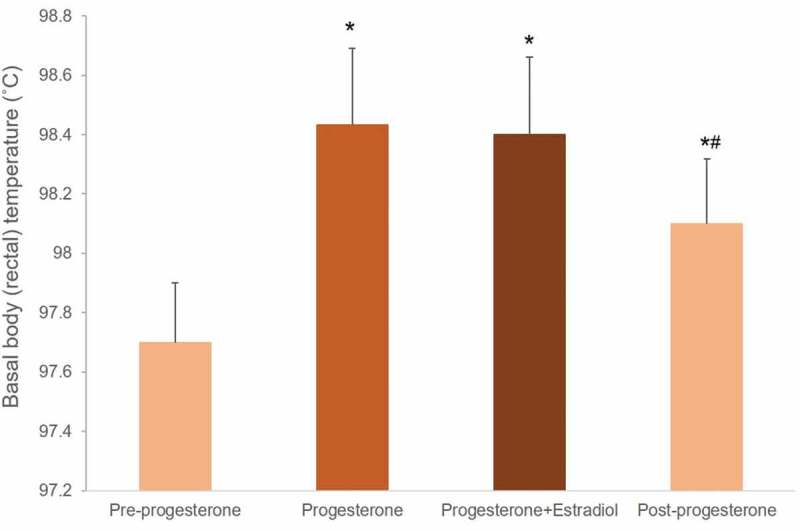
Basal body temperatures (5-min rectal temperature taken in the early morning) averaged over 5 days before any hormones were administered (pre-progesterone), during daily progesterone injections (5 mg or 10 mg, intramuscular), for 3 days when estradiol dipropionate (1.0 mg) was injected together with progesterone, and for 5 days after ending hormone administration, in 6 men. *p < 0.01 compared with the pre-progesterone condition; # p = 0.04, compared with progesterone condition. Data from [98].
There is not always a correlation between the progesterone rise and the core body temperature rise. For example, Forman and colleagues [61],found that the mean temperature rise (oral waking temperature) was almost identical over each of the first 4 days in patients treated with human menopausal gonadotropin therapy compared with patients treated with other forms of follicular stimulation despite progesterone levels being 2–4 times higher in the former group. However, estrogen levels in the patients treated with human menopausal gonadotropin were also higher (more than double that of the other treatment group), and estrogen may have depressed the thermogenic action of progesterone [61].
Effects of oral contraceptives
Studies of women taking oral contraceptives containing an estrogen and progestin (active phase) have shown they have elevated core body temperatures while awake at rest compared with the placebo phase [105,106]. Further, studies of 24-h body temperature rhythms have shown that women taking oral contraceptives in the active phase of the contraceptive regime have body temperatures similar to those of women in the luteal phase, which are higher than those of men and women in the follicular phase [69,78,79]. In contrast to recordings of short-term resting temperatures, two studies that have recorded 24-h core body temperature rhythms did not find a difference in 24-h mean or minimum body temperatures between the active and placebo phases of the oral contraceptive regime [69,80], although visual inspection of the curves shows the placebo phase core body temperatures appeared slightly lower during inactivity (sleep) than during the active phase of the day. Given the variability between studies in timing of when the women were studied during the 7-day placebo phase, oral contraceptive formulations used, and variability in the pharmacokinetics of oral contraceptives, there is likely variability in the hormonal clearance rates, and thus the duration of effect of the exogenous hormones on core body temperature.
Based on findings from the older studies, described above, that administered progesterone, the core body temperature elevation in women taking combined oral contraceptives is most likely caused by the thermogenic action of progestins dominating the temperature-lowering effect of ethinyl estradiol contained in the contraceptive pill. A relevant study in this context is one of the few studies to use a randomized cross-over design to test the effects on core body temperature (esophageal) of combined oral contraceptive pills (estradiol-norethindrone, 4 weeks) compared with progestin only (norethindrone, 4 weeks) [107]. Eight women were first studied in their natural follicular and luteal phases, and there was a 4-week washout period between hormone regimes. During 20 min of rest in a thermoneutral environment, the esophageal temperature was higher when women were taking the progestin-only pill compared with their follicular phase. In addition, core body temperature was higher when taking progestin-only compared with the combined oral contraceptive. These findings show that progestin effectively increased core body temperature; however, when estrogen was administered with progestin, the effect was reversed [107]. The findings conflict with the above-mentioned studies examining core body temperature in women taking chronic combined oral contraceptives and also with early studies that showed the dominant thermoregulatory effect of progesterone over estrogen. Possibly, differences between study results can be explained by differences in the progestin used or ratio of progestin to estradiol. Norethindrone is less selective to the progesterone receptor than some other progestins and a higher dose may be required to exert a dominant thermogenic effect in the presence of estradiol (which would work to lower core body temperature). Alternatively, differences in the duration of use of oral contraceptives between this study (taken for 1 month) and others (convenience samples of women already taking oral contraceptives, generally for at least 3 months) could explain the discrepancies in results.
Changes in metabolic rate across the menstrual cycle
It has been suggested that the increase in core body temperature in the luteal phase can be explained, in part, by enhanced heat production associated with increased basal metabolic rate. The majority [29,108–113] but not all [114,115] studies find an increase in energy expenditure or basal metabolic rate of about 5% to 9% in the luteal phase. For example, in a study of 24-h energy expenditure using direct and indirect calorimetry, when conditions were relatively standardized, Webb [113],found an increase, on average, of 8.8% (with a standard deviation of 5.5%) in daily energy expenditure in 10 women in the luteal phase relative to the follicular phase. For eight of the women, the increase ranged between 8% and 16%, however, two other women showed a decrease of 2%. Some researchers have suggested that the increase in basal metabolic rate could be secondary (a Q10 effect) to the raised body temperature [29]; however, a rise in body temperature of 0.3–0.7°C would induce a small increase in metabolic heat production that may be within the range of measurement error [110]. Administration of a progestin-only contraceptive (depot-medroxyprogesterone acetate) increased resting metabolic rate by, on average, 9% (with 9 of 13 women showing an increase) in association with an increase in body temperature (measurement not defined) 3 weeks later, which is when maximum concentration is expected [116]. Two recent studies examined the effects on energy expenditure of sex hormone suppression with GnRH agonist therapy in women [109,117]. In the first study, a significant increase in resting energy expenditure in the mid-luteal phase was found compared with the early follicular phase, and a reduction in resting energy expenditure in response to GnRH agonist therapy (which suppressed progesterone and estrogen) [109]. The second study also found that with hormone suppression, resting energy expenditure was reduced (by 3.7%) and this reduction was prevented by estrogen therapy [117]. Together, these results support an association between sex steroid hormones and energy expenditure. Both progesterone and estrogen may contribute to this effect in the luteal phase.
Regulation of body temperature during exercise, heat and cold exposure, and fever across the menstrual cycle
If core body temperature is regulated at a higher operating point during the luteal phase, one would expect to find shifts in the thresholds for heat- and cold-defense effector mechanisms across the menstrual cycle. Indeed, a landmark study by Hessemer and Bruck [29] showed that there was a concomitant and almost identical change of all autonomic thermoregulatory thresholds (shivering, sweating and cutaneous vasodilation) from the early follicular to mid-luteal phase, in women exposed to environmental heating and cooling. It also had been reported previously that there were similar shifts in thermal perception, with the hand temperature at which women felt pleasant higher in the luteal phase [118] and subjects less sensitive to cold sensation in the luteal phase [119]. Further studies evaluating the autonomic and behavioral responses of women to heat and cold, as well as during exercise in hot environments, have supported the view that body temperature is defended at a higher level during the luteal phase.
Cold exposure
Core body temperature remained constantly higher in the luteal phase compared to in the follicular phase when women were exposed to a rapid decrease in air temperature (from 32°C to 12°C) that elicited shivering and an increase in metabolic rate (Figure 4; 29). The threshold core body temperature for heat production by shivering was 0.5°C higher in the luteal compared to the follicular phase. Although Hessemer and Bruck [29] did not examine cutaneous vasoconstriction, it has been shown that reflex thermoregulatory control of vasoconstriction also is shifted to a higher core body temperature, at least by exogenous female steroid hormones in oral contraceptives [105].
Figure 4.
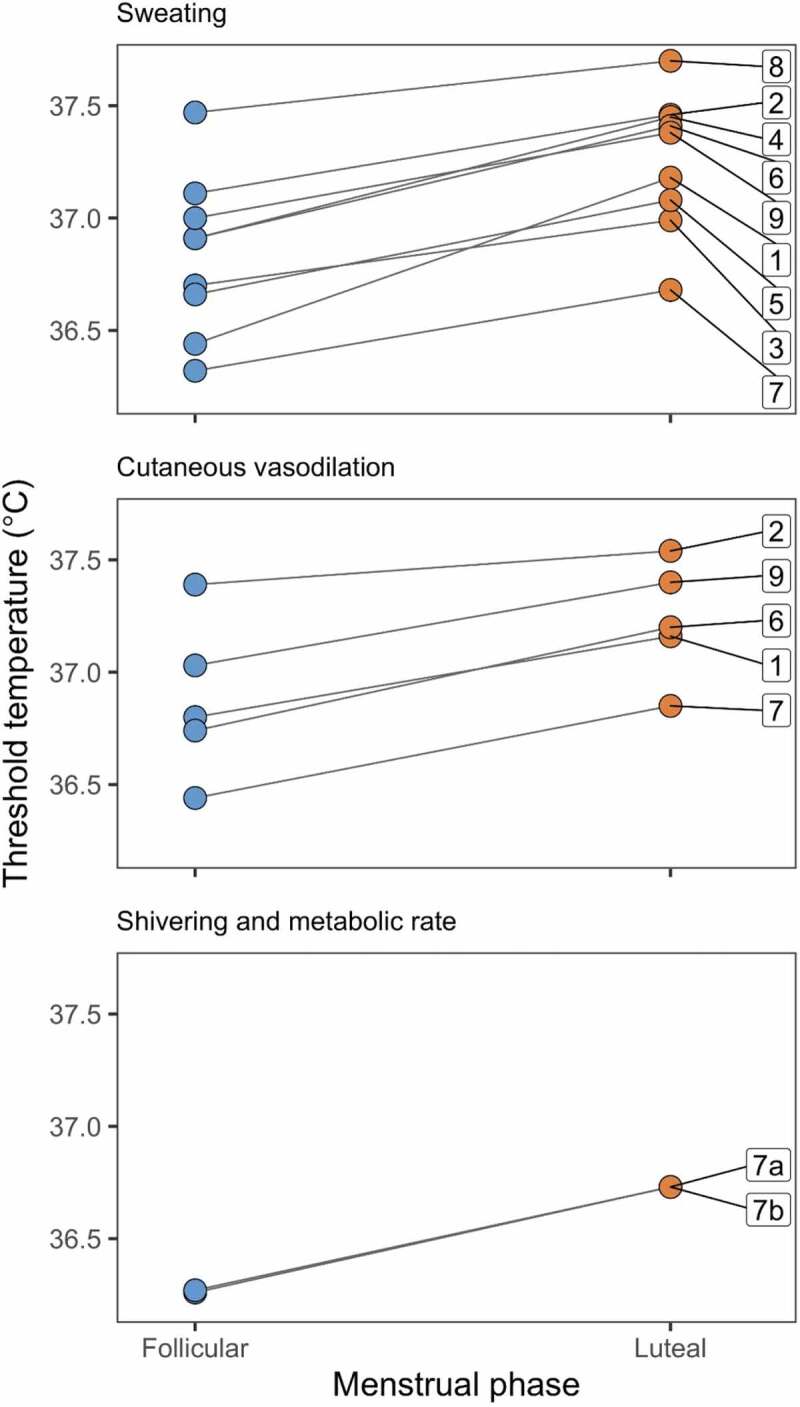
The threshold core body temperature for effector responses in the follicular and luteal phase of the menstrual cycle. Top panel shows the threshold for sweating, middle panel shows the threshold for cutaneous vasodilation, and the lower panel shows the thresholds for shivering (a) and increasing metabolic rate (b). Interventions are (1) during exercise in the early morning [126], (2) during exercise in the late afternoon [126], (3) during passive heat exposure [125], (4) during exercise in the heat [123], (5) during exercise [123], (6) during exercise [30], (7) during passive heat (upper and middle panels) or cold (lower panel) exposure [29], (8) during exercise [138], and (9) during exercise [139]. Core body temperatures are esophageal, rectal, or calculated mean body temperature.
In addition to physiological responses, there also is a shift in the onset of behavioral responses to cold across the menstrual cycle. Women exposed to decreasing air temperature (from 30°C to 15°C) dressed quicker and put on thicker clothes when in the luteal phase than when in the follicular phase [120]. They also reported that they felt cooler in the last 30 min of exposure to the decreasing air temperature in the luteal phase. In contrast, Matsuda-Nakamura and colleagues [121] found that thermal perception did not differ between the luteal and follicular phases when young health women were exposed to a mildly cool environment. Skin temperature and peripheral blood flow also decreased similarly during exposure to the cooler air in both phases of the menstrual cycle.
Heat exposure
During heat exposure, the higher core body temperature in the luteal phase is coupled with a higher core temperature threshold for heat loss effector function, including cutaneous vasodilation and sweating (Figure 4; 29,122-126). Despite the change in the threshold for sweating, it has been reported that the sweating rate and pattern of sweating at various cutaneous sites do not differ across the menstrual cycle [122,125]. In contrast, Lee and colleagues [127] found that women had a higher sweating rate and skin blood flow in the luteal phase when exposed to heat. Female reproductive hormones affect the peripheral mechanism of vasodilation, with differences in cutaneous vasodilation across the menstrual cycle, at least in some regions of the body [125]. Differences in sweating and blood flow responses between studies likely are attributed to different experimental protocols, and differences between subjects (for example, resulting from hydration or training – see below). Nevertheless, despite this variability, in all studies the difference in core body temperature between the luteal and follicular phases is maintained during exposure to heat [121,125].
Exercise
The higher body temperature during the luteal phase, including during exercise [128,129,130-132,140], has led to the suggestion that women are at a disadvantage when performing exercise during their luteal phase, especially in hot environments [131,133,134,140,141]. In recreationally active females, endurance time to fatigue in hot, humid conditions [140]) and exercise tolerance time in a hot, dry environment [132] were lower in the luteal phase compared to the follicular phase. In contrast, in well-trained women exercising in the heat, exercise performance did not differ across the menstrual cycle [135] and high-intensity intermittent running in the heat was not influenced by the phase of the menstrual cycle [136].
In thermoneutral conditions, exercise appears to have little effect on the core body temperature difference that exists between the luteal and follicular phases, regardless of exercise intensity [137]. Pivarnik and colleagues [131] reported a slightly greater increase in rectal temperature in women exercising at 22°C in the luteal compared to the follicular phase, and a perception by the women that the exercise was harder in the luteal phase [131]. In contrast, another study found that rectal temperature increased at a slightly greater rate in the follicular compared to the luteal phase in women exercising at 24°C, which the authors attributed to a greater gain for sweating in the luteal phase [138]. In subjects taking oral contraceptives in the same study, thermoregulatory responses to exercise were more uniform across the menstrual cycle with no changes in the dynamics of the sweating response [138].
During exercise in a hot and dry environment, body temperature also appears to increase at the same rate in both phases of the menstrual cycle, such that the core temperature difference between the luteal and follicular phases at rest is maintained during stead-state exercise [123]. Cumulative heat storage at different exercise intensities in a hot, dry environment was the same in both phases of the menstrual cycle, and there was no difference in whole body heat loss over the menstrual cycle [32]. Where fatigue is induced by hyperthermia during exercise [142], one, therefore, would expect women to reach a limiting hyperthermic core body temperature sooner in the luteal compared to the follicular phase.
Hydration and training state, however, may influence core body temperature responses across the menstrual cycle when exercising at high intensity or in severe heat. In women that were well-hydrated during exercise in a warm and humid environment, Garcia and colleagues [142] found that the sweating rate was higher during the luteal phase compared to their follicular phase. As a result, core body temperature did not rise excessively during exercise in the heat in the luteal phase, nor did their heart rate or perceived rating of exhaustion. Well-trained athletes also are likely to experience less variation in exercise performance across the menstrual cycle, partly because physical training improves heat loss responses in the mid-luteal phase [143]. In well-trained endurance athletes exercising in moderate heat during the mid-luteal phase, the body temperature threshold for heat loss responses was lower and the sensitivity of heat loss responses was higher compared to those of untrained athletes [139]. Well-trained women also have smaller changes in core body temperature over the menstrual cycle, associated with reduced ovarian hormone concentrations and fluctuation over the cycle [143]. In well-trained athletes taking oral contraceptives chronically, exercise performance in the heat did not differ over the menstrual cycle, despite the endogenous changes in core body temperature over the cycle still being present [144]. Chronic oral contraceptive use, however, attenuates the sweating response, which may impair performance if athletes are unable to pace themselves appropriately [144].
Fever
During a fever, core body temperature continues to be regulated but is actively defended at a higher level [9]. Fever results when cytokines, such as interleukin-1β (IL-1 β), interleukin-6 (IL-6) and tumor necrosis factor-α (TNF-α), released from leukocytes and other cells, induce the synthesis of cyclooxygenase-2, which results in increased prostaglandin synthesis. Prostaglandin E2 acts on the hypothalamus to alter the set point for body temperature regulation, leading to an increase in heat production, the conservation of heat, and ultimately fever [145]. Although mediators of the fever response in females and males do not differ, there is evidence, particularly from animals, that female reproductive hormones modulate the immune response and may affect the magnitude of the febrile response. Randomly-cycling female rats show a blunted febrile response compared to males when injected with the pyrogen lipopolysaccharide (LPS), possibly as a result of a reduced response of cytokine mediators of fever, including TNF-α and MIP-1α [146]. Treatment of ovariectomized female rats with estrogen and progesterone reduced blood levels of the cytokine IL-1β (but not IL-6) and the febrile response to LPS, compared to control ovariectomized female rats [147]. In human peripheral mononuclear cells, estrogen, and progesterone exert an inhibitory effect on IL-1 β mRNA synthesis and IL-1β release but not IL-6 secretion [148].
As far as we are aware, there are no data comparing patterns of fevers in women across phases of the menstrual cycle. Given the view that fever has an upper limit [149], one would expect to see a smaller increment in core body temperature in febrile women in the luteal phase compared to the follicular phase, possibly as a result of changes in cytokine responsiveness, such that core body temperature is similar at the peak of the fever in both phases.
Rare episodes of high and recurrent fever-like responses in the luteal phase have been reported. Rutanen and colleagues [150] reported a case where the patient had recurrent fevers associated with high progesterone during the luteal phase of the menstrual cycle, and upregulated cytokine levels across the entire menstrual cycle. In contrast, high and recurrent fevers in three other patients were not associated with any changes in inflammatory cytokines across the menstrual cycle, leading the authors to speculate that other mechanisms are responsible, possibly associated with a surge in LH affecting the central nervous system and hypothalamic thermoregulatory center [151–153].
Mechanisms of action of progesterone and estrogen on thermoregulation
Central actions of progesterone
The thermogenic effect of progesterone could be mediated centrally (to increase heat production and/or reduce heat loss), and could be a direct effect or an indirect effect achieved through another mediator. There are several lines of evidence supporting a direct effect of progesterone on the preoptic area of the hypothalamus to inhibit heat loss mechanisms (i.e., raise the set point at which behavioral and autonomic thermoeffector pathways are activated) and/or increase heat production. First, as described above, in association with the rise in core body temperature in the luteal phase, there is an increase in the threshold temperatures for sweating onset, cutaneous vasodilation, thermal comfort, and cold sensations (Figure 4), which likely reflects an orchestrated thermoregulatory response from the brain. Second, intravenous injection of progesterone in rabbits inhibits the firing rate of warm-sensitive neurons and stimulates cold-sensitive neurons in the POA and anterior hypothalamus, with a short latency (6–20 min) implying a non-genomic action [154]. Inhibition of warm-sensitive neurons would inhibit heat loss responses and increase heat production, thus raising body temperature. Third, Marrone and colleagues [101] showed in three animals that administration of progesterone directly into the POA increased colonic temperature by 0.5°C; four rats who received cholesterol administration as a control showed no consistent change in colonic temperature. Fourth, in vitro studies using slice preparations, which isolate the actions of progesterone on thermosensitive neurons in the POA from other progesterone-sensitive areas, showed that a greater proportion of warm-sensitive neurons (61%) than thermally insensitive neurons (15%) in the POA were inhibited by progesterone [155] (Table 2). Of the only four cold-sensitive neurons studied, three were inhibited by progesterone and one was activated; this sample is too small to draw any conclusions about the actions of progesterone on cold-sensitive neurons. Neurons responded, on average, at 8.3 ± 6.0 min, and recovered after 30–40 min, again indicating that the inhibitory effect of progesterone is rapid, and unlikely to be genomic. A second study by these same authors considered the priming effect of estrogen [156] and found that, similar to their earlier findings, progesterone alone tended to inhibit warm-sensitive neurons (Table 2). This effect persisted even when a progesterone receptor antagonist was applied, suggesting that it is not mediated via progesterone receptors. The authors suggest that progesterone’s inhibitory effect might be mediated by a direct action on GABAergic receptors [156].
Table 2.
Effects of progesterone and estrogen on neurons in the preoptic area. Progesterone data from Tsai et al. [155]. POA tissue slices from male and ovariectomized female rats were perfused with medium containing progesterone (3 ng.ml-1 and 30 ng.ml-1, which are within physiological ranges). There were no apparent sex or dose differences in responses so data were pooled. Estradiol data are from Silva and Boulant [16]. POA tissue slices were perfused in medium containing 17β-estradiol (30 pg.ml-1, which is within the physiological range). Progesterone 72 h after estradiol data are from Tsai et al. [156]. Ovariectomized rats were injected with 17β-estradiol benzoate (20 µg in 0.1 ml oil, subcutaneous) for 3 consecutive days before sacrifice. POA slices were then perfused with progesterone. In all cases, neurons that were excited or inhibited are shown; the remaining neurons showed no change to the hormones. See text for discussion.
| Progesterone |
Estradiol |
Progesterone 72 h after estradiol |
||||
|---|---|---|---|---|---|---|
| Reference | 154 | 16 | 155 | |||
| Effect | Excited | Inhibited | Excited | Inhibited | Excited | Inhibited |
| Warm-sensitive neurons | 5/31 (16%] | 19/31 (61%)* | 7/27 (26%) | 1/27 (4%) | 16/42 (38%) | 4/42 (10%) |
| Temperature- insensitive neurons | 3/26 (12%) | 4/26 (15%) | 2/22 (9%) | 1/22 (5%) | 1/21 (5%) | 5/21 (24%) |
| Cold-sensitive neurons | 1/4 (25%) | 3/4 (75%) | 0/3 (0%) | 0/3 [0%) | - | - |
In contrast, in preparations taken from ovariectomized rats treated with estrogen 72 h previously, progesterone excited warm-sensitive neurons [156]. This excitatory effect was not evident in slices from animals not pretreated with estrogen or in slices from animals treated with estrogen only 12 h prior, and the effect was blocked when a progesterone receptor antagonist was applied together with progesterone. These results elegantly show that the excitatory effect of progesterone on warm-sensitive neurons in the POA is likely mediated by progesterone receptors induced by estrogen [156]; induction of progesterone receptors in the preoptic area takes 24–30 h [157]. Taken together, these data support a rapid inhibitory effect of progesterone (not via progesterone receptors) on heat-loss and/or heat-production mechanisms in the POA. Following priming by estrogen, however, progesterone excites warm-sensitive neurons (via progesterone receptors), which would increase heat loss and reduce heat production. Both these mechanisms may coexist [156]. Putting these findings in the context of studies in women in the luteal phase, in whom both progesterone and estrogen are present, it seems possible that the ratio of progesterone to estrogen is critical in determining whether the inhibitory or excitatory effect of progesterone on warm-sensitive neurons in the POA is dominant. Indeed, correlational data by Cagnacci and colleagues [67] shows that a high ratio of progesterone to estrogen and not progesterone alone is correlated with higher core body temperature.
The cellular mechanisms and microcircuitry that progesterone acts on to induce its thermogenic effects are incompletely understood. Investigators have suggested norepinephrine [158] and cytokines [159] as potential secondary mediators. Pro-inflammatory cytokines such as IL-1 and TNF mediate fever after an infection, principally through stimulation of prostaglandin synthesis, resulting in an increased set point [160]. Progesterone has been shown to stimulate macrophages to produce IL-1 [161] and some researchers [but not all, e.g. 162] have shown that IL-1 activity [159] is increased after ovulation when progesterone is high, providing some support for the hypothesis that the rise in core body temperature in the luteal phase is similar to a fever [159]. Others have reported increased prostaglandin plasma concentrations in the luteal phase [163]. However, studies that have used a variety of antipyretics to inhibit cyclooxygenase, and therefore prostaglandin production, have shown no effect on core body temperature and thermoregulatory responses in the luteal phase. Two studies found no effect of prostaglandin synthesis inhibitors (acetaminophen or lysine acetylsalicylate) on the luteal phase-related upward shift in 24-h body core temperature rhythms or the blunted nocturnal amplitude of the core body temperature rhythm [65,68]. There also was no effect of acetaminophen on 24-h core body temperatures in the follicular phase [65]. Administration of salicylates together with progesterone across multiple days in men had inconsistent effects on basal body temperature [98]. Charkoudian and Johnson [105] administered ibuprofen to a combined group of women taking oral contraceptives (studied in the high progestin/estradiol phase, and at the end of the placebo phase) and women with natural menstrual cycles (luteal phase and follicular phase). Ibuprofen had no effect on the upward shift in short-term resting core body temperature, cutaneous vascular conductance and sweating responses in the high hormone phase (luteal phase/active pill). Brooks-Asplund et al. [164] found only minor effects of an antipyretic (aspirin) on oral body temperature and cytokine levels in postmenopausal women taking hormone therapy (estrogen or combined estrogen/progesterone), and no effect of aspirin on rectal temperature in the estrogen/progesterone group. The authors concluded that the thermoregulatory mechanism of steroid hormones is probably distinct from a classic infection-induced fever [164]. It remains to be determined whether progesterone could act via a prostaglandin-independent pathway involving factors like corticotropin-releasing factor and endothelins [165], to raise body temperature. Finally, as described above, there is also evidence that progesterone may act, at least in part, via inhibition of melatonin secretion from the pineal gland, reducing its hypothermic effect at night [66]. It has been suggested that melatonin’s hypothermic effect is centrally mediated via a reduction in the threshold for active vasodilation [166] and possibly also a shift in the vasoconstrictor system control to a lower internal temperature [167]. Of note, an effect of progesterone via thyroid hormone has been discounted [168]. Given that the field of the neuroscience of thermoregulation is still relatively young and the multifaceted neural network controlling thermoeffectors is still not completely understood [4], it is likely that an appreciation of how hormones like progesterone affect this network will be advanced in future work.
Central actions of estrogen
Unopposed estrogen has a core body temperature-lowering effect. There is evidence that similar to progesterone, estrogen could exert its effect by directly acting on thermosensitive neurons in the POA of the hypothalamus. Application of estradiol to isolated preoptic tissue slices from male rats causes an increase in the firing rate of warm-sensitive neurons, facilitating heat loss and reducing heat production [16] (Table 2). Estradiol had no effect on the cold-sensitive neurons tested. There is growing evidence from Rance and colleagues [169] showing that estradiol can also affect central thermoregulatory pathways via actions on a group of estrogen-sensitive arcuate neurons that co-express kisspeptin, neurokinin B, and dynorphin (KNDy neurons) involved in the regulation of GnRH secretion. There is considerable interest in this group of neurons because they are thought to be involved in triggering hot flashes in peri- and post-menopausal women: KNDy neurons are hypertrophied post-menopause after estrogen withdrawal and ablation of these neurons reduces cutaneous vasodilatation and partially blocks the effects of estrogen on thermoregulation [169]. Work examining the effects of estrogen withdrawal is informative about the thermoregulatory pathway through which estrogen acts. Recent findings in a mouse model suggest that estrogen-sensitive KNDy neurons modulate heat dissipation effectors indirectly via a subpopulation of glutamatergic neurokinin 3 receptor-expressing neurons in the median preoptic nucleus in the hypothalamus [170]. However, the ablation of neurokinin 3 receptor-expressing neurons did not prevent the thermoregulatory effects of estradiol, indicating that they are not essential for estrogen’s thermoregulatory effect [170]. Possibly, estrogen acts via this secondary pathway in addition to direct actions in the POA.
Actions of estrogen and progesterone at other sites
Progesterone and estrogen could influence thermoregulation via peripheral effects on the cutaneous vasculature in addition to their central action. Estrogen and progesterone influence local cutaneous blood flow, although mechanisms are complex and still not completely understood [3]. Estrogen has critical multiple effects on the vascular system, which are reviewed elsewhere [171]. Estrogen promotes vasodilation in part by stimulating nitric oxide (NO) and prostacyclin synthesis, and inhibits vasoconstriction by decreasing the production of vasoconstrictors like cyclooxygenase-derived products, angiotensin II, and endothelin-1 [171]. Estrogen induces upregulation of NO production in the endothelium via multiple mechanisms, including genomic (stimulation of NO synthase gene expression) and non-genomic activation of second messengers like Ca2+ cAMP [171]. The peripheral effect of progesterone is less clear although it seems to promote vasoconstriction by enhancing α-adrenergic responsiveness, mediated by cyclooxygenase [172].
Finally, limited work has shown that progesterone may increase heat production via its effects on mitochondria [116]. Resting metabolic rate is determined at the cellular level by processes related to mitochondrial activity [116]. A mitochondrial membrane progesterone receptor has been identified [173], potentially enabling a direct effect of progesterone, and progesterone also indirectly induces mitochondrial biogenesis [174].
In summary, progesterone and estrogen have the potential to act in multiple, complex, and interacting ways both centrally and in the periphery to modulate thermoregulation. The action of these hormones on the vasomotor state in the periphery likely also feeds back to the hypothalamus to further modulate core body temperature. It is critical to consider the dual and balanced actions of estrogen and progesterone, which are both present and active in the luteal phase. As emphasized by Charkoudian and Stachenfeld (2014) [3] in their seminal review, estrogen can modulate progesterone-related increases in body temperature through its actions in the POA and through peripheral vasodilation effects, meaning that the balance of these hormones is likely critical for thermoregulatory control.
Using consumer technology to monitor menstrual cycle changes in daily temperature
For decades, women have relied on measuring changes in their daily awakening temperature, typically taken with an oral thermometer once a day, to track ovulatory cycles [64]. de Mouzon and colleagues [55] investigated the reliability of basal core body temperature (morning rectal temperature) for determining the timing of ovulation. Women who were admitted to hospital for in vitro fertilization or sterility evaluation measured their temperature across the periovulatory period (72 h before until 72 h after ovulation) and four blood samples were taken each day. They concluded that basal core body temperature is an unreliable indicator of ovulation for three main reasons: (1) large variation in the interval between the core body temperature nadir or rise in temperature and ovulation; (2) the need for a 48-h window to confirm a basal core body temperature criterion because of a large number of false nadirs and temperature rises; (3) difficulty in interpreting basal core body temperature charts [55]. However, they suggest that basal body temperature may be of use in confirming ovulation has occurred after the fact. Despite these and other limitations [reviewed in 64], basal body temperature measurement is simple and cheap [175] and, therefore, remains a popular way of retrospectively tracking ovulatory cycles.
With advances in technology and machine learning algorithms, personalized self-monitoring consumer devices have been developed to track changes in daily activities, including body temperature across 24 h, over many days, weeks, and months. There is growing interest in applying these devices to monitor ovulatory cycles in women, with the primary aim of helping women with infertility. Table 3 describes wearables that have been marketed for the measurement of body temperature changes across the menstrual cycle, aimed at collecting multiple cycles of data to which algorithms can be applied to predict the optimal window of fertility in future cycles, potentially useful for women trying to fall pregnant or to avoid pregnancy. Devices have been developed to track daily changes in core body temperature (intra-vaginal), temperature of the ear canal or under the arm, while others rely on skin temperature of the wrist or finger, given its convenience and low invasiveness. As described above and reviewed elsewhere [176], skin temperature is influenced by many confounding factors, including environmental temperature, sensor design, and site of measurement. Controlled laboratory studies also show conflicting findings regarding the existence of a post-ovulatory rise in skin temperature [36]. For example, as discussed above, under controlled conditions, there was no difference in distal skin temperature between the follicular versus the luteal phase across the circadian cycle, and the rhythm of distal skin temperature was out of phase with that of core body temperature [83]. While the circadian rhythm of proximal skin temperature (e.g. thigh, abdomen) tracks that of core body temperature more closely, there is a lack of data evaluating how menstrual cycle phase impacts skin temperature measures depending on skin surface location and other factors. Skin temperature, therefore, cannot be used as a surrogate for core body temperature changes across the menstrual cycle.
Table 3.
Summary of studies conducted by internal company investigators or independent investigators on the performance of commercial wearable devices or apps that track changes in body temperature across the menstrual cycle.
| Wearable | Reference | Site of temperature measurement | Methods | Results | Comment |
|---|---|---|---|---|---|
| BodyMedia FIT | 175 | Band placed on upper arm, continuously measures skin T | Compared pre-wake T (device) with basal oral T (digital thermometer) over 17 days in 15 women | - Poor agreement between device and thermometer; wide range of Ts with device (29.7–36.7°C). - Poor agreement in detecting ovulation |
BodyMedia was acquired by Jawbone; products were discontinued in 2016 |
| Duofertility | 179 | - Under-arm sensor patch continuously measures nocturnal skin T & movement. - Proprietary algorithms identify date of ovulation |
Pilot study compared device with ultrasound monitoring for accuracy of detecting ovulation in 8 infertile women [18 cycles]. | Device detected ovulation in all cycles. | - Independent study. - High sensitivity in this small sample. - There were no annovulatory cycles recorded in this group, therefore, specificity in detecting ovulatory cycles could not be determined. - Sensor relies on skin T, which is not always a good reflection of core body T. |
| Ava bracelet | 177 | Wrist skin T, continuously measured across the night | Used device to track nocturnal T changes across MCs in 136 women (437 cycles], aged 20–40 y, with regular MCs, confirmed as ovulatory (LH surge). | - Showed an upward shift in wrist skin T in 82% of cycles, with higher average skin T in LP than early FP. - In only 41% of cycles, a T nadir (lowest T in a given cycle) was detected in a 5-day period pre-ovulation. |
- Study done by Ava team and collaborators. - Even when considering potential confounders [e.g. age, exercise, evening meal), effect of menstrual cycle phase remained. - Prolonged measurement of nocturnal skin T may be more robust than a single T measurement, however, skin T has limitations due to environmental T influences. |
| Ava bracelet | 178 | Wrist skin T, continuously measured across the night | Evaluated effectiveness of a fertility algorithm using multiple sensors in device (skin T, HR, HR variability, breathing rate] to estimate the ‘fertile window’ (encompasses ovulation). Data from 708 cycles in ~193 women. | - Skin T, HR and respiratory rate increased in LP. - Algorithm showed good performance (90% of cycles) in detecting 6-day fertile window, with specificity of 0.93 & sensitivity of 0.81. |
- Study done by Ava team and collaborators. - Suggests that multi-sensor data may be more sensitive than a T sensor alone in detecting ovulatory cycles, and possibly predicting a fertility period. |
| Ouraring | 180 | Placed on the finger, measures skin temperature, HR, and HR variability continuously | - Study compared skin T continuously measured with device and oral waking T. Also evaluated algorithms using skin T to predict menses and ovulation. - Data used from 22 women (21–49 y] across 120 to 150 days. Ovulation measured from LH surge. |
- Skin T with device was 0.30°C higher in LP vs FP, and correlated with waking oral T. - Sensitivity of the algorithm in detecting menses was 81.4% [window of ±3 days). - Sensitivity of the algorithm in detecting ovulation was 83.3% within a 6-day fertile window. |
- Some investigators are employed by Oura Health. - Shows a difference in skin T between menstrual cycle phases, and good sensitivity in detecting menses and ovulation. - Oura ring temperature sensors were not calibrated before measurements so absolute values could not be used. - Sensor relies on skin T, which has limitations due to environmental T influences. |
| Natural Cycles app | 181 | Web/mobile app. Algorithm uses predictive mathematical models applied to data entered daily by user: basal body T measured with external device; LH surge measured with external device; self-report info (e.g. age, cycle length, BMI, OC use) | - Retrospective study performed on 1501 cycles from 317 women aged 18 to 39 years. - A combination of information (waking oral T, ovulation test results in a subset, date of menses) was used to predict fertility window |
- Mean cycle length was 28.8 ± 5 days. - Algorithm estimated that day of ovulation was 1.9 ± 1.4 days after LH surge based on T data alone. |
- Study done by company founders. - Shows accuracy in assessing the fertile window. - Requires a high level of user compliance to measure basal body temperature (oral) every morning over multiple cycles to ensure the predictive models are accurate, and relies on user-entered information, subject to human error. |
| Natural Cycles app | 39 | Web/mobile app. | - Description of menstrual cycle data from 612,613 ovulatory cycles in 124,648 users worldwide. - Age range: 18–45 y BMI: 15–50 kg.m−2; not using hormonal contraception within past 12 months |
- FP length: 16.9 days (95% CI: 10–30), LP length: 12.4 days (95% CI: 7–17). - Cycle length ↓ by 0.18 days (95% CI: 0.17–0.18) and FP length ↓ by 0.19 days (95% CI: 0.19–0.20) per year from 25 to 45 years. - Variation of cycle length was 14% higher in women with a BMI >35 relative to BMI of 18.5–25. |
- Study funded and conducted by Natural Cycles Nordic AB. - Results are not fully representative since only cycles with ovulation detected were included in the study (ovulation was not detected in 48% of cycles, of which most did not have sufficient body T measurements to enable detection). - Same limitations as above. |
| Tempdrop | 33 | Placed under the arm; measures environmental and skin T, and activity continuously across the night | No data available | ||
| YONO | 182 | Sensor placed in the ear canal, measures tympanic T every 5 minutes across the night. | - Publication describes development of an adaptive statistical learning algorithm to predict the point of thermal shift using historical body T data. - Thermal shifts are used to forecast day of ovulation. - Data are from 125 cycles, from 34 users (22–42 y], data collection periods range from 28 to 222 days. |
- Using 64 cycles with sufficient T data, ovulation [LH surge] (± 3 days) was detected with 92.3% sensitivity using T sensor. - Historical data was used to predict ovulation in 39 cycles from 22 users. Rate of correct prediction using T sensor data was 76.92%, which improved further with 2 historical cycles. |
- Data collected by YONO labs. - Importantly, sensor was calibrated in a water bath and had low measurement error (±0.05° C). - Relies on user wearing the sensor; there were missing data as a result of users forgetting to wear sensor, or not taking it when traveling. - Relies on tympanic T, which can be contaminated by environmental T. |
| OvuSense fertility monitor [conference abstract) |
48 | Intra-vaginal T sensor that continuously measures core body T across the night. | - Conference abstract details comparison of the accuracy of vaginal T-based fertile period prediction (device) with that of LH surge, combination of LH/ultrasound folliculometry, or morning oral T. - 81 cycles from 21 women were analyzed. |
- Device-predicted probability of conception per fertile day curve closely matched that of ultrasound/LH combination. - Device prediction of fertile period was superior to oral T. |
- Some authors are paid consultants of Fertility Focus Ltd. - Uses a true core body T measurement site and tracks temperature continuously across the night. - Other data shows user acceptability [183], however, validity data are only briefly presented in abstract form. - More invasive T measurement than other body sites. |
| Ovularing | 184 | Intra-vaginal T sensor that measures core body T. Algorithms are applied to predict fertile window retrospectively and prospectively. |
- Study of 470 cycles in 158 women using Ovularing. - Hormonal assessments of LH, follicle-stimulating hormone, estradiol and progesterone, and vaginal ultrasound were performed in a sub-set across days 9–36 of the cycle, although unclear how these data were used. |
− 83.4% of the cycles were biphasic. – Ovulation day was determined 96.5% of the time in retrospective cycles. - A 7-day window of fertility could be predicted in 88.5% of prospective cycles, following measurement of 3 ovulatory cycles. |
- Unclear what was the gold-standard against which detection of ovulation from device was compared. - Uses a true core body T measurement site and tracks vaginal T continuously during use. - More invasive T measurement than other body sites. |
; BMI, body mass index; FP, follicular phase; HR, heart rate; LH, luteinizing hormone; LP, luteal phase; MC, menstrual cycle; OC, oral contraceptives; T, temperature
Nevertheless, there are several commercial wearables that incorporate skin temperature sensors and sophisticated algorithms to noninvasively and conveniently track and record body temperature as well as other physiological signals every few seconds or minutes. These devices often focus on the nighttime period, which produces more data and may be more reliable than a short period after waking. As outlined in Table 3, several of these devices have been investigated in preliminary studies, mostly by the company, and have shown promising results for the detection of the fertile window (encompassing ovulation) based on tracking an upward shift in temperature after ovulation. One study that investigated the possibility of detecting a decrease in skin temperature (measured at the wrist) before ovulation revealed that a pre-ovulatory temperature nadir was detected in only 41% of cycles, suggesting that prospective determination of ovulation with this method was not robust [177]. Some devices have gone beyond relying only on temperature and have integrated other signals, like heart rate and derived heart rate variability. Applying a machine learning algorithm to data from multiple sensors (contained in a wrist bracelet) revealed good performance in detecting the 6-day fertile window, showing the promise of multi-sensor data being applied to not only retrospectively identify ovulatory cycles but potentially to predict the period when fertility is highest [178].
While these wearables show promise in rejuvenating the usefulness of body temperature tracking to monitor ovulatory cycles if combined with other signals, and possibly to predict the ovulatory window, further independent evaluation of their validity against current gold standards, and across different groups of women, is required by the research community.
Conclusion
The rise in core body temperature during the luteal phase is a cardinal signal of an ovulatory menstrual cycle that is well recognized and easy to measure. Along with knowledge about the rise in core body temperature in the luteal phase, our knowledge has grown to reveal that thermoregulatory responses are also altered, with a shift to a higher threshold for effector responses, including shivering, sweating, and cutaneous vasodilation. We now understand that there is intricate circuitry linking the thermoregulatory and reproductive systems, with estrogen and progesterone interacting in complex ways to alter thermoregulation. The microcircuitry of how progesterone and estrogen interact in the brain to modify central thermoregulation as well as their effects in the periphery are not well understood, and further work is needed. Ultimately, the rise in core body temperature may serve a purpose for the potential of an ensuing pregnancy, although why an elevated temperature may be beneficial is not understood. Many questions remain about the anomalies in the literature: why do some women who have ovulatory cycles with a rise in progesterone not show a rise in core body temperature, for example? Could it simply be because most of these studies were undertaken using a single basal body temperature measurement in the morning or are some women less sensitive to the thermogenic effect of progesterone? It would be helpful to follow up with 24-h temperature measurement of core body temperature to confirm that an increase in temperature and blunted amplitude of the 24-h body temperature rhythm are really not evident, in addition to documenting ovulation and progesterone levels. Also, why, on rare occasions, do women show a magnified fever-like response when progesterone is increased as usual in the luteal phase? Are they more sensitive to progesterone, or is estrogen less effective, leading to an imbalance in central thermoregulatory control? Why in the baboon, with a true menstrual cycle, does the increase in core body temperature persist even after menstruation when progesterone is no longer present? While it is assumed that the thermoregulatory system is thoroughly understood, the interactions between it and the reproductive system are complex and remain to be fully uncovered.
Our understanding of female biology, including changes that occur across the menstrual cycle, is compromised by the paucity of data from females. It has been recognized across many disciplines that there is a greater need for research on females, and a need to report sex as a biological variable [185]. Male bias is evident in most fields of biological research, and particularly so in the field of neuroscience, where males outnumber females at a ratio of 5.5 to 1 [186]. For many years, the default model in human studies has been a 70 kg male [187] and most researchers avoid using female animals in research [188], primarily because of hormonal changes across the estrus cycle [187]. Bale and Eppetson [185] outline how researchers can focus on or control for particular hormonal states in animals and humans, and argue that females cannot be excluded from research simply because of an assumption that they have greater variability in responses. Indeed, proper consideration of the menstrual cycle may reduce noise in experiments and aid researchers in detecting differences between treatments or groups, including in measurements of body temperature.
Acknowledgments
We thank the Department of Science and Innovation and National Research Foundation for supporting Felicia Siboza in their internship programme from 2019 to 2020.
Biographies
Fiona C. Baker (on the left) is Senior Program Director of the Human Sleep Research Laboratory at SRI International, a nonprofit research organization in California, USA. She is also an Honorary Professorial Research Fellow in the School of Physiology at the University of the Witwatersrand, South Africa, where she received her PhD. Her research focuses largely on sleep and related physiology in women across the lifespan. She also studies maturational changes in sleep and brain structure/function in adolescents, considering sex differences and relationships between neuromaturation and sleep health with variations in adolescent behaviors.

Felicia Siboza is a DSI/NRF intern based at the Brain Function Research Group (BFRG) in the School of Physiology for the 2019/2020 period and holds a BSc in Human Genetics and B(Med)Sci (Hons) in Physiology. She is involved in research support of members of the BFRG with data collecting, extracting and capturing.

Andrea Fuller (top photo, on the right) is a professor in the School of Physiology at the University of the Witwatersrand, South Africa. Since 2007, she has directed the Brain Function Research Group, which carries out research in the fields of sleep, fever, pain, thermal and wildlife conservation physiology. Her primary research focus is in the areas of thermal and conservation physiology, with an emphasis on understanding the physiological plasticity available to mammals to cope with climate change.
Funding Statement
Fiona Baker is supported by National Institutes of Health [RF1AG061355]. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. Felicia Siboza is supported by the Department of Science and Innovation and National Research Foundation of South Africa internship programme.
Abbreviations
- FSH
follicle stimulating hormone
- GnRH
gonadotrophin-releasing hormone
- IL1
interleukin-1
- LH
luteinizing hormone
- LPS
lipopolysaccaharide
- NO
nitric oxide
- POA
preoptic area
- SCN
suprachiasmatic nucleus
- TNF
tumor necrosis factor
Disclosure statement
No conflicts of interest, financial or otherwise, in relation to this work, are declared by the authors.
Data Availability
There are no data associated with this review article.
References
- [1].Marshall J. A field trial of the basal-body-temperature method of regulating births. Lancet. 1968;2:8–10. [DOI] [PubMed] [Google Scholar]
- [2].Charkoudian N, Stachenfeld N.. Sex hormone effects on autonomic mechanisms of thermoregulation in humans. Auton Neurosci. 2016;196:75–80. [DOI] [PubMed] [Google Scholar]
- [3].Charkoudian N, Stachenfeld NS.. Reproductive hormone influences on thermoregulation in women. Compr Physiol. 2014;4:793–804. [DOI] [PubMed] [Google Scholar]
- [4].Morrison SF, Nakamura K. Central mechanisms for thermoregulation. Annu Rev Physiol. 2019;81:285–308. [DOI] [PubMed] [Google Scholar]
- [5].Romanovsky AA. The thermoregulation system and how it works. Handb Clin Neurol. 2018;156:3–43. [DOI] [PubMed] [Google Scholar]
- [6].Tan CL, Knight ZA. Regulation of body temperature by the nervous system. Neuron. 2018;98:31–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Tansey EA, Johnson CD. Recent advances in thermoregulation. Adv Physiol Educ. 2015;39:139–148. [DOI] [PubMed] [Google Scholar]
- [8].Mitchell D, Snelling EP, Hetem RS, et al. Revisiting concepts of thermal physiology: predicting responses of mammals to climate change. J Anim Ecol. 2018;87:956–973. [DOI] [PubMed] [Google Scholar]
- [9].Childs C. Body temperature and clinical thermometry. Handb Clin Neurol. 2018;157:467–482. [DOI] [PubMed] [Google Scholar]
- [10].Kräuchi K, Deboer T. The interrelationship between sleep regulation and thermoregulation. Front Biosci. 2010;15:604–625. [DOI] [PubMed] [Google Scholar]
- [11].Hetem RS, Maloney SK, Fuller A, et al. Heterothermy in large mammals: inevitable or implemented? Biol Rev. 2016;91:187–205. [DOI] [PubMed] [Google Scholar]
- [12].Flouris AD. Functional architecture of behavioural thermoregulation. Eur J Appl Physiol. 2011;111:1–8. [DOI] [PubMed] [Google Scholar]
- [13].Romanovsky AA. Skin temperature: its role in thermoregulation. Acta Physiol. 2014;210:498–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Wong BJ, Hollowed CG. Current concepts of active vasodilation in human skin. Temperature. 2017;4:41–59. doi: 10.1080/23328940.2016.1200203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Boulant JA, Dean JB. Temperature receptors in the central nervous system. Annu Rev Physiol. 1986;48:639–654. [DOI] [PubMed] [Google Scholar]
- [16].Silva NL, Boulant JA. Effects of testosterone, estradiol, and temperature on neurons in preoptic tissue slices. Am J Physiol. 1986;250:R625–632. [DOI] [PubMed] [Google Scholar]
- [17].Spencer RL, Chun LE, Hartsock MJ, et al. Glucocorticoid hormones are both a major circadian signal and major stress signal: how this shared signal contributes to a dynamic relationship between the circadian and stress systems. Front Neuroendocrinol. 2018;49:52–71. [DOI] [PubMed] [Google Scholar]
- [18].Keefe DL, Turek FW. Circadian time keeping processes in mammalian reproduction. Oxford RevReprod Biol. 1985;7:346. [PubMed] [Google Scholar]
- [19].Klerman EB. Clinical aspects of human circadian rhythms. J Biol Rhythms. 2005;20:375–386. [DOI] [PubMed] [Google Scholar]
- [20].Czeisler CA, Kronauer RE, Allan JS, et al. Bright light induction of strong (type 0) resetting of the human circadian pacemaker. Science. 1989;244:1328–1333. [DOI] [PubMed] [Google Scholar]
- [21].Moore-Ede MC, Sulzman FM, Fuller CA. The clocks that time us: physiology of the circadian timing system. Cambridge, Massachusetts: Harvard University Press; 1982. [Google Scholar]
- [22].Kräuchi K, Wirz-Justice A. Circadian rhythm of heat production, heart rate, and skin and core temperature under unmasking conditions in men. Am J Physiol. 1994;267:R819–29. [DOI] [PubMed] [Google Scholar]
- [23].Maloney SK, Goh G, Fuller A, et al. Amplitude of the circadian rhythm of temperature in homeotherms. CAB Rev. 2019;14:1–30. [Google Scholar]
- [24].Zitting KM, Vujovic N, Yuan RK, et al. Human resting energy expenditure varies with circadian phase. Curr Biol. 2018;28:3685–3690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Nyakudya TT, Fuller A, Meyer LC, et al. Body temperature and physical activity correlates of the menstrual cycle in chacma baboons (Papio hamadryas ursinus). Am J Primatol. 2012;74:1143–1153. [DOI] [PubMed] [Google Scholar]
- [26].Taylor NA, Tipton MJ, Kenny GP. Considerations for the measurement of core, skin and mean body temperatures. J Therm Biol. 2014;46:72–101. [DOI] [PubMed] [Google Scholar]
- [27].Brengelmann GL, Shiraki K, Yousef MK. Man in stressful environments: thermal and work physiology. Springfield, Ill: Charles C Thomas Publisher; 1987. [Google Scholar]
- [28].Mitchell D, Laburn HP. Pathophysiology of temperature regulation. Physiologist. 1985;28:507–517. [PubMed] [Google Scholar]
- [29].Hessemer V, Bruck K. Influence of menstrual cycle on shivering, skin blood flow, and sweating responses measured at night. J Appl Physiol. 1985a;59:1902–1910. [DOI] [PubMed] [Google Scholar]
- [30].Hessemer V, Bruck K. Influence of menstrual cycle on thermoregulatory, metabolic, and heart rate responses to exercise at night. J Appl Physiol. 1985b;59:1911–1917. [DOI] [PubMed] [Google Scholar]
- [31].Stephenson LA, Kolka MA. Esophageal temperature threshold for sweating decreases before ovulation in premenopausal women. J Appl Physiol. 1999;86:22–28. [DOI] [PubMed] [Google Scholar]
- [32].Notley SR, Dervis S, Poirier MP, et al. Menstrual cycle phase does not modulate whole body heat loss during exercise in hot, dry conditions. J Appl Physiol. 2019;126:286–293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Tempdrop , 2019, Get the accurate temp data you need for the fertility apps you love, viewed 3rd November, 2019. https://www.temp-drop.com/.
- [34].Abrams RM, Royston JP. Some properties of rectum and vagina as sites for basal body temperature measurement. Fertil Steril. 1981;35:313–316. [DOI] [PubMed] [Google Scholar]
- [35].Coyne MD, Kesick CM, Doherty TJ, et al. Circadian rhythm changes in core temperature over the menstrual cycle: method for noninvasive monitoring. Am J Physiol Regul Integr Comp Physiol. 2000;279:R1316–1320. [DOI] [PubMed] [Google Scholar]
- [36].Marsh SA, Jenkins DG. Physiological responses to the menstrual cycle. Sports Med. 2002;32:601–614. [DOI] [PubMed] [Google Scholar]
- [37].Rousseau ME. Women’s midlife health reframing menopause. J Nurse Midwifery. 1998;43:208–223. [DOI] [PubMed] [Google Scholar]
- [38].Cole LA, Ladner DG, Byrn FW. The normal variabilities of the menstrual cycle. Fertil Steril. 2009;91:522–527. [DOI] [PubMed] [Google Scholar]
- [39].Bull JR, Rowland SP, Scherwitzl EB, et al. Real-world menstrual cycle characteristics of more than 600,000 menstrual cycles. Npj Digital Med. 2019;2:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Faust L, Bradley D, Landau E, et al. Findings from a mobile application–based cohort are consistent with established knowledge of the menstrual cycle, fertile window, and conception. Fertil Steril. 2019;112:450–457. [DOI] [PubMed] [Google Scholar]
- [41].Baird DT, Baker TG, McNatty KP, et al. Relationship between the secretion of the corpus luteum and the length of the follicular phase of the ovarian cycle. Reproduction. 1975;45:611–619. [DOI] [PubMed] [Google Scholar]
- [42].Messinis IE, Messini CI, Dafopoulos K. Novel aspects of the endocrinology of the menstrual cycle. Reprod Biomed Online. 2014;28:714–722. [DOI] [PubMed] [Google Scholar]
- [43].Su HW, Yi YC, Wei TY, et al. Detection of ovulation, a review of currently available methods. Bioeng Transl Med. 2017;2:238–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Skinner DC, Caraty A, Allingham R. Unmasking the progesterone receptor in the preoptic area and hypothalamus of the ewe: no colocalization with gonadotropin-releasing neurons. Endocrinology. 2001;142:573–579. [DOI] [PubMed] [Google Scholar]
- [45].Skorupskaite K, George JT, Veldhuis JD, et al. Interactions between neurokinin B and kisspeptin in mediating estrogen feedback in healthy women. J Clin Endocrinol Metab. 2016;101:4628–4636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Buijs RM, Ruiz MAG, Hernández RM, et al. The suprachiasmatic nucleus; a responsive clock regulating homeostasis by daily changing the setpoints of physiological parameters. Auton Neurosci. 2019;218:43–50. [DOI] [PubMed] [Google Scholar]
- [47].Padilla SL, Perez JG, Ben-Hamo M, et al. Kisspeptin neurons in the arcuate nucleus of the hypothalamus orchestrate circadian rhythms and metabolism. Curr Biol. 2019;29:592–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Papaioannou S, Al Wattar B, Milnes R, et al. Quality index assessment of vaginal temperature based fertility prediction and comparison with luteinising hormone testing, ultrasound folliculometry and other home cycle monitors. Fertil Steril. 2013;100:S326–S327. [Google Scholar]
- [49].Metcalf MG. Incidence of ovulation from the menarche to the menopause: observations of 622 New Zealand women. N Z Med J. 1983;96:645–648. [PubMed] [Google Scholar]
- [50].Mordecai KL, Rubin LH, Maki PM. Effects of menstrual cycle phase and oral contraceptive use on verbal memory. Horm Behav. 2008;54:286–293. [DOI] [PubMed] [Google Scholar]
- [51].Sims ST, Heather AK. Myths and methodologies: reducing scientific design ambiguity in studies comparing sexes and/or menstrual cycle phases. Exp Physiol. 2018;103:1309–1317. [DOI] [PubMed] [Google Scholar]
- [52].Gogos A, Wu YC, Williams AS, et al. The effects of ethinylestradiol and progestins (“the pill”) on cognitive function in pre-menopausal women. Neurochem Res. 2014;39:2288–2300. [DOI] [PubMed] [Google Scholar]
- [53].Fotherby K. Interactions of contraceptive steroids with binding proteins and the clinical implications. Ann N Y Acad Sci. 1988;538:313–320. [DOI] [PubMed] [Google Scholar]
- [54].Cedars MI. Triphasic oral contraceptives: review and comparison of various regimens. Fertil Steril. 2002;77:1–14. [DOI] [PubMed] [Google Scholar]
- [55].de Mouzon J, Testart J, Lefevre B, et al. Time relationships between basal body temperature and ovulation or plasma progestins. Fertil Steril. 1984;41:254–259. [DOI] [PubMed] [Google Scholar]
- [56].Halbrecht I. Ovarian function and body temperature. Lancet. 1945;246:668–669. [DOI] [PubMed] [Google Scholar]
- [57].Marshall J. Thermal changes in the normal menstrual cycle. Br Med J. 1963;1:102–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Moghissi KS. Accuracy of basal body temperature for ovulation detection. Fertil Steril. 1976;27:1415–1421. [PubMed] [Google Scholar]
- [59].Zuspan KJ, Zuspan FP. Thermogenic alterations in the woman. II. Basal body, afternoon, and bedtime temperatures. Am J Obstet Gynecol. 1974;120:441–445. [DOI] [PubMed] [Google Scholar]
- [60].Israel SL, Schneller O. The thermogenic property of progesterone. Obstet Gynecol Surv. 1950;5:53–64. [Google Scholar]
- [61].Forman RG, Chapman MC, Steptoe PC. The effect of endogenous progesterone on basal body temperature in stimulated ovarian cycles. Hum Reprod. 1987;2:631–634. [DOI] [PubMed] [Google Scholar]
- [62].Davis ME, Fugo NW. The cause of physiologic basal temperature changes in women. J Clin Endocrinol. 1948;8:550–563. [DOI] [PubMed] [Google Scholar]
- [63].Barton M, Wiesner BP. Thermogenic effect of progesterone. Lancet. 1945;246:671–672. [DOI] [PubMed] [Google Scholar]
- [64].Barron ML, Fehring RJ. Basal body temperature assessment: is it useful to couples seeking pregnancy? MCN: Am J Maternal/Child Nurs. 2005;30:290–296. [DOI] [PubMed] [Google Scholar]
- [65].Baker FC, Driver HS, Paiker J, et al. Acetaminophen does not affect 24-h body temperature or sleep in the luteal phase of the menstrual cycle. J Appl Physiol. 2002;92:1684–1691. [DOI] [PubMed] [Google Scholar]
- [66].Cagnacci A, Soldani R, Laughlin GA, et al. Modification of circadian body temperature rhythm during the luteal menstrual phase: role of melatonin. J Appl Physiol. 1996;80:25–29. [DOI] [PubMed] [Google Scholar]
- [67].Cagnacci A, Volpe A, Paoletti AM, et al. Regulation of the 24-hour rhythm of body temperature in menstrual cycles with spontaneous and gonadotropin-induced ovulation. Fertil Steril. 1997;68:421–425. [DOI] [PubMed] [Google Scholar]
- [68].Cagnacci A, Arangino S, Tuveri F, et al. Regulation of the 24h body temperature rhythm of women in luteal phase: role of gonadal steroids and prostaglandins. Chronobiol Int. 2002;19:721–730. [DOI] [PubMed] [Google Scholar]
- [69].Kattapong KR, Fogg LF, Eastman CI. Effect of sex, menstrual cycle phase, and oral contraceptive use on circadian temperature rhythms. Chronobiol Int. 1995;12:257–266. [Google Scholar]
- [70].Lee KA. Circadian temperature rhythms in relation to menstrual cycle phase. J Biol Rhythms. 1988;3:255–263. [Google Scholar]
- [71].Parry BL, LeVeau B, Mostofi N, et al. Temperature circadian rhythms during the menstrual cycle and sleep deprivation in premenstrual dysphoric disorder and normal comparison subjects. J Biol Rhythms. 1997;12:34–46. [DOI] [PubMed] [Google Scholar]
- [72].Severino SK, Wagner DR, Moline ML, et al. High nocturnal body temperature in premenstrual syndrome and late luteal phase dysphoric disorder. Am J Psychiatry. 1991;148:1329–1335. [DOI] [PubMed] [Google Scholar]
- [73].Shibui K, Uchiyama M, Okawa M, et al. Diurnal fluctuation of sleep propensity and hormonal secretion across the menstrual cycle. Biol Psychiatry. 2000;48:1062–1068. [DOI] [PubMed] [Google Scholar]
- [74].Shechter A, Varin F, Boivin DB. Circadian variation of sleep during the follicular and luteal phases of the menstrual cycle. Sleep. 2010;33:647–656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Vidafar P, Gooley JJ, Burns AC, et al. Increased vulnerability to attentional failure during acute sleep deprivation in women depends on menstrual phase. Sleep. 2018;41:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Grant LK, Gooley JJ, St Hilaire MA, et al. Menstrual phase-dependent differences in neurobehavioral performance: the role of temperature and the progesterone/estradiol ratio. Sleep. 2019;54:972–977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Cagnacci A, Elliott JA, Yen SS. Melatonin: A major regulator of the circadian rhythm of core temperature in humans. J Clin Endocrinol Metab. 1992;75:447–452. [DOI] [PubMed] [Google Scholar]
- [78].Wright KP, Badia P. Effects of menstrual cycle phase and oral contraceptives on alertness, cognitive performance, and circadian rhythms during sleep deprivation. Behav Brain Res. 1999;103:185–194. [DOI] [PubMed] [Google Scholar]
- [79].Baker FC, Waner JI, Vieira EF, et al. Sleep and 24 hour body temperatures: a comparison in young men, naturally cycling women and women taking hormonal contraceptives. J Physiol. 2001b;530:565–574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Baker FC, Mitchell D, Driver HS. Oral contraceptives alter sleep and raise body temperature in young women. Pflugers Archiv Eur J Physiol. 2001a;442:729–737. [DOI] [PubMed] [Google Scholar]
- [81].Czeisler CA, Wright JKP. Influence of light on circadian rhythmicity in humans. In: Turek FW, Zee PC, editors. Regulation of sleep and circadian rhythms. New York: Marcel Dekker; 1999. p. 149–180. [Google Scholar]
- [82].Kräuchi K1, Konieczka K, Roescheisen-Weich C, et al. Diurnal and menstrual cycles in body temperature are regulated differently: a 28-day ambulatory study in healthy women with thermal discomfort of cold extremities and controls. Chronobiol Int. 2014;31:102–113. [DOI] [PubMed] [Google Scholar]
- [83].Shechter A, Boudreau P, Varin F, et al. Predominance of distal skin temperature changes at sleep onset across menstrual and circadian phases. J Biol Rhythms. 2011;26:260–270. [DOI] [PubMed] [Google Scholar]
- [84].Driver HS, Werth E, Dijk DJ, et al. The menstrual cycle effects on sleep. Sleep Med Clin. 2008;3:1–11. [Google Scholar]
- [85].Gao B, Franken P, Tobler I, et al. Effect of elevated ambient temperature on sleep, EEG spectra, and brain temperature in the rat. Am J Physiol. 1995;268:R1365–1373. [DOI] [PubMed] [Google Scholar]
- [86].Baker FC, Driver HS. Circadian rhythms, sleep, and the menstrual cycle. Sleep Med. 2007;8:613–622. [DOI] [PubMed] [Google Scholar]
- [87].de Zambotti M, Willoughby AR, Sassoon SA, et al. Menstrual cycle-related variation in physiological sleep in women in the early menopausal transition. J Clin Endocrinol Metab. 2015;100:2918–2926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Driver HS, Dijk DJ, Werth E, et al. Sleep and the sleep electroencephalogram across the menstrual cycle in young healthy women. J Clin Endocrinol Metab. 1996;81:728–735. [DOI] [PubMed] [Google Scholar]
- [89].Deboer T. Brain temperature dependent changes in the electroencephalogram power spectrum of humans and animals. J Sleep Res. 1998;7:254–262. [DOI] [PubMed] [Google Scholar]
- [90].Dijk DJ, Czeisler CA. Contribution of the circadian pacemaker and the sleep homeostat to sleep propensity, sleep structure, electroencephalographic slow waves, and sleep spindle activity in humans. J Neurosci;1995:15:3526–3538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].Baker FC, Selsick H, Driver HS, et al. Different nocturnal body temperatures and sleep with forced‐air warming in men and in women taking hormonal contraceptives. J Sleep Res. 1998;7:175–181. [DOI] [PubMed] [Google Scholar]
- [92].Sagot JC, Amoros C, Candas V, et al. Sweating responses and body temperatures during nocturnal sleep in humans. Am J Physiol. 1987;252:R462–470. [DOI] [PubMed] [Google Scholar]
- [93].Mong JA, Baker FC, Mahoney MM, et al. Sleep, rhythms, and the endocrine brain: influence of sex and gonadal hormones. J Neurosci. 2011;31:16107–16116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].de Zambotti M, Nicholas CL, Colrain IM, et al. Autonomic regulation across phases of the menstrual cycle and sleep stages in women with premenstrual syndrome and healthy controls. Psychoneuroendocrinology. 2013;38:2618–2627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [95].Carter JR, Lawrence JE, Klein JC. Menstrual cycle alters sympathetic neural responses to orthostatic stress in young, eumenorrheic women. Am J Physiol Endocrinol Metab. 2009;297:E85–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [96].Middlekauff HR, Park J, Gornbein JA. Lack of effect of ovarian cycle and oral contraceptives on baroreceptor and nonbaroreceptor control of sympathetic nerve activity in healthy women. Am J Physiol Heart Circ Physiol. 2012;302:H2560–H2566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [97].Minson CT, Halliwill JR, Young TM, et al. Influence of the menstrual cycle on sympathetic activity, baroreflex sensitivity, and vascular transduction in young women. Circulation. 2000;101:862–868. [DOI] [PubMed] [Google Scholar]
- [98].Rothchild I, Barnes AC. The effects of dosage, and of estrogen, androgen or salicylate administration on the degree of body temperature elevation induced by progesterone. Endocrinology. 1952;50:485–496. [DOI] [PubMed] [Google Scholar]
- [99].Buxton CL, Atkinson WB. Hormonal factors involved in the regulation of basal body temperature during the menstrual cycle and pregnancy. J Clin Endocrinol. 1948;8:544–549. [DOI] [PubMed] [Google Scholar]
- [100].Freeman ME, Crissman JK Jr, Louw GN, et al. Thermogenic action of progesterone in the rat. Endocrinology. 1970;86:717–720. [DOI] [PubMed] [Google Scholar]
- [101].Marrone BL, Gentry RT, Wade GN. Gonadal hormones and body temperature in rats: effects of estrous cycles, castration and steroid replacement. Physiol Behav. 1976;17:419–425. [DOI] [PubMed] [Google Scholar]
- [102].Nieburgs H, Greenblatt R. The role of the endocrine glands in body temperature regulation. J Clin Endocrinol Metab. 1948;8:622. [PubMed] [Google Scholar]
- [103].Brooks EM, Morgan AL, Pierzga JM, et al. Chronic hormone replacement therapy alters thermoregulatory and vasomotor function in postmenopausal women. J Appl Physiol. 1997;83:477–484. [DOI] [PubMed] [Google Scholar]
- [104].Tankersley CG, Nicholas WC, Deaver DR, et al. Estrogen replacement in middle-aged women: thermoregulatory responses to exercise in the heat. J Appl Physiol. 1992;73:1238–1245. [DOI] [PubMed] [Google Scholar]
- [105].Charkoudian N, Johnson JM. Altered reflex control of cutaneous circulation by female sex steroids is independent of prostaglandins. Am J Physiol Heart Circ Physiol. 1999;276:H1634–1640. [DOI] [PubMed] [Google Scholar]
- [106].Rogers SM, Baker MA. Thermoregulation during exercise in women who are taking oral contraceptives. Eur J Appl Physiol Occup Physiol. 1996;75:34–38. [DOI] [PubMed] [Google Scholar]
- [107].Stachenfeld NS, Silva C, Keefe DL. Estrogen modifies the temperature effects of progesterone. J Appl Physiol. 2000;88:1643–1649. [DOI] [PubMed] [Google Scholar]
- [108].Bisdee JT, James WPT, Shaw MA. Changes in energy expenditure during the menstrual cycle. Br J Nutr. 1989;61:187–199. [DOI] [PubMed] [Google Scholar]
- [109].Day DS, Gozansky WS, Van Pelt RE, et al. Sex hormone suppression reduces resting energy expenditure and β-adrenergic support of resting energy expenditure. J Clin Endocrinol Metab. 2005;90:3312–3317. [DOI] [PubMed] [Google Scholar]
- [110].Frascarolo P, Schutz Y, Jequier E. Decreased thermal conductance during the luteal phase of the menstrual cycle in women. J Appl Physiol. 1990;69:2029–2033. [DOI] [PubMed] [Google Scholar]
- [111].Meijer GA, Westerterp KR, Saris WH, et al. Sleeping metabolic rate in relation to body composition and the menstrual cycle. Am J Clin Nutr. 1992;55:637–640. [DOI] [PubMed] [Google Scholar]
- [112].Solomon SJ, Kurzer MS, Calloway DH. Menstrual cycle and basal metabolic rate in women. Am J Clin Nutr. 1982;36:611–616. [DOI] [PubMed] [Google Scholar]
- [113].Webb P. 24-hour energy expenditure and the menstrual cycle. Am J Clin Nutr. 1986;44:614–619. [DOI] [PubMed] [Google Scholar]
- [114].Bittel J, Henane R. Comparison of thermal exchanges in men and women under neutral and hot conditions. J Physiol. 1975;250:475–489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [115].Piers LS, Diggavi SN, Thangam S, et al. Changes in energy expenditure, anthropometry, and energy intake during the course of pregnancy and lactation in well-nourished Indian women. Am J Clin Nutr. 1995;61:501–513. [DOI] [PubMed] [Google Scholar]
- [116].Steward RG, Bateman LA, Slentz C, et al. The impact of short-term depot-medroxyprogesterone acetate treatment on resting metabolic rate. Contraception. 2016;93:317–322. [DOI] [PubMed] [Google Scholar]
- [117].Melanson EL, Gavin KM, Shea KL, et al. Regulation of energy expenditure by estradiol in premenopausal women. J Appl Physiol. 2015;119:975–981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [118].Cunningham DJ, Cabanac M. Evidence form behavioral thermoregulatory responses of a shift in setpoint temperature related to the menstrual cycle. J de Physiologie. 1971;63:236–238. [PubMed] [Google Scholar]
- [119].Kenshalo DR. Changes in the cool threshold associated with phases of the menstrual cycle. J Appl Physiol. 1966;21:1031–1039. [DOI] [PubMed] [Google Scholar]
- [120].Kim HE, Hiromi T. Effects of the menstrual cycle on dressing behavior in the cold. Physiol Behav. 1995;58:699–703. [DOI] [PubMed] [Google Scholar]
- [121].Matsuda-Nakamura M, Yasuhara S, Nagashima K. Effect of menstrual cycle on thermal perception and autonomic thermoregulatory responses during mild cold exposure. J Physiol Sci. 2015;65:339–347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [122].Frascarolo P, Schutz Y, Jequier E. Influence of the menstrual cycle on the sweating response measured by direct calorimetry in women exposed to warm environmental conditions. Eur J Appl Physiol Occup Physiol. 1992;64:449–454. [DOI] [PubMed] [Google Scholar]
- [123].Kolka MA, Stephenson LA. Control of sweating during the human menstrual cycle. Eur J Appl Physiol Occup Physiol. 1989;58:890–895. [DOI] [PubMed] [Google Scholar]
- [124].Hayashi K, Kawashima T, Suzuki Y. Effect of menstrual cycle phase on the ventilatory response to rising body temperature during exercise. J Appl Physiol. 2012;113:237–245. [DOI] [PubMed] [Google Scholar]
- [125].Inoue Y, Tanaka Y, Omori K, et al. Sex-and menstrual cycle-related differences in sweating and cutaneous blood flow in response to passive heat exposure. Eur J Appl Physiol. 2005;94:323–332. [DOI] [PubMed] [Google Scholar]
- [126].Stephenson LA, Kolka MA. Menstrual cycle phase and time of day alter reference signal controlling arm blood flow and sweating. Am J Physiol. 1985;249:R186–191. [DOI] [PubMed] [Google Scholar]
- [127].Lee H, Petrofsky J, Shah N, et al. Higher sweating rate and skin blood flow during the luteal phase of the menstrual cycle. Tohoku J Exp Med. 2014;234:117–122. [DOI] [PubMed] [Google Scholar]
- [128].Avellini BA, Kamon E, Krajewski JT. Physiological responses of physically fit men and women to acclimation to humid heat. J Appl Physiol Respir Environ Exerc Physiol. 1980;49:254–261. [DOI] [PubMed] [Google Scholar]
- [129].Carpenter AJ, Nunneley SA. Endogenous hormones subtly alter women’s response to heat stress. J Appl Physiol. 1988;65:2313–2317. [DOI] [PubMed] [Google Scholar]
- [130].Kolka MA, Stephenson LA. Resetting the thermoregulatory set‐point by endogenous estradiol or progesterone in women. Ann N Y Acad Sci. 1997;813:204–206. [DOI] [PubMed] [Google Scholar]
- [131].Pivarnik JM, Marichal CJ, Spillman T, et al. Menstrual cycle phase affects temperature regulation during endurance exercise. J Appl Physiol. 1992;72:543–548. [DOI] [PubMed] [Google Scholar]
- [132].Tenaglia SA, McLellan TM, Klentrou PP. Influence of menstrual cycle and oral contraceptives on tolerance to uncompensable heat stress. Eur J Appl Physiol Occup Physiol. 1999;7(2):76–83. [DOI] [PubMed] [Google Scholar]
- [133].Charkoudian N, Joyner MJ. Physiologic considerations for exercise performance in women. Clin Chest Med. 2004;25:247–255. [DOI] [PubMed] [Google Scholar]
- [134].Stephenson LA, Kolka MA. Thermoregulation in women. Exerc Sport Sci Rev. 1993;21:231–262. [PubMed] [Google Scholar]
- [135].Lei TH, Stannard SR, Perry BG, et al. Influence of menstrual phase and arid vs. humid heat stress on autonomic and behavioural thermoregulation during exercise in trained but unacclimated women. J Physiol. 2017;595:2823–2837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [136].Sunderland C, Nevill M. Effect of the menstrual cycle on performance of intermittent, high-intensity shuttle running in a hot environment. Eur J Appl Physiol. 2003;88:345–352. [DOI] [PubMed] [Google Scholar]
- [137].Fukuoka Y, Kaneko Y, Takita C, et al. The effects of exercise intensity on thermoregulatory responses to exercise in women. Physiol Behav. 2002;76(4–5):567–574. [DOI] [PubMed] [Google Scholar]
- [138].Grucza R, Pekkarinen H, Titov EK, et al. Influence of the menstrual cycle and oral contraceptives on thermoregulatory responses to exercise in young women. Eur J Appl Physiol Occup Physiol. 1993;67:279–285. [DOI] [PubMed] [Google Scholar]
- [139].Kuwahara T, Inoue Y, Abe M, et al. Effects of menstrual cycle and physical training on heat loss responses during dynamic exercise at moderate intensity in a temperate environment. Am J Physiol Regul Integr Comp Physiol. 2005b;288:R1347–1353. [DOI] [PubMed] [Google Scholar]
- [140].Janse de Jonge XA, Thompson MW, Chuter VH, Silk LN, Thom JM. Exercise performance over the menstrual cycle in temperate and hot, humid conditions.Med Sci Sports Exerc . 2012;44:2190-2198. [DOI] [PubMed] [Google Scholar]
- [141].Janse de Jonge XA. Effects of the menstrual cycle on exercise performance.Sports Med . 2003;33:833-851. [DOI] [PubMed] [Google Scholar]
- [142].Garcia AMC, Lacerda M, Fonseca I, et al. Effect of menstrual cycle on sweating. Braz J Med Biol Res. 2006;39:1255–1261. [DOI] [PubMed] [Google Scholar]
- [143].Kuwahara T, Inoue Y, Taniguchi M, et al. Effects of physical training on heat loss responses of young women to passive heating in relation to menstrual cycle. Eur J Appl Physiol. 2005a;94:376–385. [DOI] [PubMed] [Google Scholar]
- [144].Lei TH, Cotter JD, Schlader ZJ, et al. On exercise thermoregulation in females: interaction of endogenous and exogenous ovarian hormones. J Physiol. 2019;597:71–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [145].Roth J. Fever: mediators and mechanisms. In: Cavaillon J-M, Singer M, editors. Inflammation from molecular and cellular mechanisms to the clinic. Germany: Wiley-VCH, Weinheim; 2018. p. 861–889. [Google Scholar]
- [146].Brito HO, Radulski DR, Wilhelms DB, et al. Female sex hormones influence the febrile response induced by lipopolysaccharide, cytokines and prostaglandins but not by interleukin‐1β in rats. J Neuroendocrinol. 2016;28:1–10. [DOI] [PubMed] [Google Scholar]
- [147].Mouihate A, Pittman QJ. Neuroimmune response to endogenous and exogenous pyrogens is differently modulated by sex steroids. Endocrinology. 2003;144:2454–2460. [DOI] [PubMed] [Google Scholar]
- [148].Li ZG, Danis VA, Brooks PM. Effect of gonadal steroids on the production of il-1 and il-6 by blood mononuclear cells in vitro. Clin Exp Rheumatol. 1993;11:157––162.. [PubMed] [Google Scholar]
- [149].Mackowiak PA, Boulant JA. Fever’s glass ceiling. Clinl Infect Dis. 1996;22:525–536. [DOI] [PubMed] [Google Scholar]
- [150].Rutanen EM, Teppo AM, Stenman UH, et al. Recurrent fever associated with progesterone action and persistently elevated serum levels of immunoreactive tumor necrosis factor-alpha and interleukin-6. J Clin Endocrinol Metab. 1993;76:1594–1598. [DOI] [PubMed] [Google Scholar]
- [151].Nakamura Y, Hino K. A case of ovulatory cycle-dependent symptoms in woman with previous interferon β therapy. Endocr J. 2005;52:377–381. [DOI] [PubMed] [Google Scholar]
- [152].Yamasaki H, Oki T, Iwamoto I, et al. Fourteen-year-old girl with recurrent luteal-phase-dependent episodes of high fever. J Obstetrics Gynaecol Res. 2011;37:1166–1168. [DOI] [PubMed] [Google Scholar]
- [153].Yang OO, Currier JS. Reimann’s “habitual hyperthermia” responding to hormone therapy. Open Forum Infect Dis. 2016;3:ofw127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [154].Nakayama T, Suzuki M, Ishizuka N. Action of progesterone on preoptic thermosensitive neurones. Nature. 1975;258:80. [DOI] [PubMed] [Google Scholar]
- [155].Tsai CL, Matsumura K, Nakayama T. Effects of progesterone on thermosensitive neurons in preoptic slice preparations. Neurosci Lett. 1988;86:56–60. [DOI] [PubMed] [Google Scholar]
- [156].Tsai CL, Kanosue K, Matsumura K. Effects of estradiol treatment on responses of rat preoptic warm sensitive neurons to progesterone in vitro. Neurosci Lett. 1992;136:23–26. [DOI] [PubMed] [Google Scholar]
- [157].Barraclough CA, Camp P, Weiland N, et al. Stimulatory versus inhibitory effects of progesterone on estrogen-induced phasic lh and prolactin secretion correlated with estrogen nuclear and progestin cytosol receptor concentrations in brain and pituitary gland. Neuroendocrinology. 1986;42:6–14. [DOI] [PubMed] [Google Scholar]
- [158].Zuspan FP, Rao P. Thermogenic alterations in the woman. I. Interaction of amines, ovulation, and basal body temperature. Am J Obstet Gynecol. 1974;118:671–678. [DOI] [PubMed] [Google Scholar]
- [159].Cannon JG, Dinarello CA. Increased plasma interleukin-1 activity in women after ovulation. Science. 1985;227:1247–1249. [DOI] [PubMed] [Google Scholar]
- [160].Netea MG, Kullberg BJ, Van der Meer JWM. Circulating cytokines as mediators of fever. Clinl Infect Dis. 2000;5:S178–S184. [DOI] [PubMed] [Google Scholar]
- [161].Flynn A. Stimulation of interleukin-1 production from placental monocytes. Lymphokine Res. 1984;3:1–5. [PubMed] [Google Scholar]
- [162].Gonzalez RR, Blanchard LA, Allison WF. Cytokine interaction with the menstrual cycle during cold stress and exercise. Ann N Y Acad Sci. 1998;856:261–265. [DOI] [PubMed] [Google Scholar]
- [163].Fridén BE, Wallin A, Brännström M. Phase-dependent influence of nonsteroidogenic cells on steroidogenesis and prostaglandin production by the human corpus luteum. Fertil Sterility. 2000;73:359–365. [DOI] [PubMed] [Google Scholar]
- [164].Brooks-Asplund EM, Cannon JG, Kenney WL. Influence of hormone replacement therapy and aspirin on temperature regulation in postmenopausal women. Am J Physiol. 2000;279:839–848. [DOI] [PubMed] [Google Scholar]
- [165].Zampronio AR, Soares DM, Souza GE. Central mediators involved in the febrile response: effects of antipyretic drugs. Temperature. 2015;2:506–521. doi: 10.1080/23328940.2015.1102802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [166].Aoki K, Stephens DP, Zhao K, et al. Modification of cutaneous vasodilator response to heat stress by daytime exogenous melatonin administration. Am J Physiol. 2006;291:R619–24. [DOI] [PubMed] [Google Scholar]
- [167].Aoki K, Zhao K, Yamazaki F, et al. Exogenous melatonin administration modifies cutaneous vasoconstrictor response to whole body skin cooling in humans. J Pineal Res. 2008;44:141‐148. [DOI] [PubMed] [Google Scholar]
- [168].Rothchild I, Rapport RL. The thermogenic effect of progesterone and its relation to thyroid function. Endocrinology. 1952;50:580–583. [DOI] [PubMed] [Google Scholar]
- [169].Rance NE, Dacks PA, Mittelman-Smith MA, et al. Modulation of body temperature and LH secretion by hypothalamic KNDy (kisspeptin, neurokinin B and dynorphin) neurons: a novel hypothesis on the mechanism of hot flushes. Front Neuroendocrinol. 2013;34:211–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [170].Krajewski-Hall SJ, Miranda Dos Santos F, McMullen NT, et al. Glutamatergic Neurokinin 3 receptor neurons in the median preoptic nucleus modulate heat-defense pathways in female mice. Endocrinology. 2019;160:803–816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [171].Tostes RC, Nigro D, Fortes ZB, et al. Effects of estrogen on the vascular system. Braz J Med Biol Res. 2003;36:1143–1158. [DOI] [PubMed] [Google Scholar]
- [172].Wenner MM, Taylor HS, Stachenfeld NS. Progesterone enhances adrenergic control of skin blood flow in women with high but not low orthostatic tolerance. Journal of Physiology. 2011;589:975–986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [173].Saner KJ, Welter BH, Zhang F, et al. Cloning and expression of a novel, truncated, progesterone receptor. Mol Cell Endocrinol. 2003;200:155–163. [DOI] [PubMed] [Google Scholar]
- [174].Rodríguez-Cuenca S, Monjo M, Gianotti M, et al. Expression of mitochondrial biogenesis-signaling factors in brown adipocytes is influenced specifically by 17β-estradiol, testosterone, and progesterone. Am J Physiol Endocrinol Metab. 2007;292:E340–E346. [DOI] [PubMed] [Google Scholar]
- [175].Wark JD, Henningham L, Gorelik A, et al. Basal temperature measurement using a multi-sensor armband in Australian young women: a comparative observational study. JMIR Mhealth Uhealth. 2015;3:e94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [176].MacRae BA, Annaheim S, Spengler CM, et al. Skin temperature measurement using contact thermometry: a systematic review of setup variables and their effects on measured values. Front Physiol. 2018;9:29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [177].Shilaih M, Goodale BM, Falco L, et al. Modern fertility awareness methods: wrist wearables capture the changes in temperature associated with the menstrual cycle. Biosci Rep. 2018;38:BSR20171279–BSR20171279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [178].Goodale BM, Shilaih M, Falco L, et al. Wearable sensors reveal menses-driven changes in physiology and enable prediction of the fertile window: observational study. J Med Internet Res. 2019;21:e13404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [179].Rollason JC, Outtrim JG, Mathur RS. A pilot study comparing the DuoFertility®monitor with ultrasound in infertile women. Int J Women’s Health. 2014;6:657–662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [180].Maijala A, Kinnunen H, Koskimäki H, et al. Nocturnal finger skin temperature in menstrual cycle tracking: ambulatory pilot study using a wearable Oura ring. BMC Women’s Health. 2019;19:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [181].Berglund Scherwitzl E, Lindén Hirschberg A, Scherwitzl R. Identification and prediction of the fertile window using naturalCycles. Eur J Contraception Reprod Health Care. 2015;20:403–408. [DOI] [PubMed] [Google Scholar]
- [182].Luo L, She X, Cao J, et al. Detection and prediction of ovulation from body temperature measured by an in-ear wearable thermometer. IEEE Trans Biomed Eng. 2019. Epub ahead of print. DOI: 10.1109/TBME.2019.2916823 [DOI] [PubMed] [Google Scholar]
- [183].Papaioannou S, Aslam M, Al Wattar BH, et al. User’s acceptability of OvuSense: A novel vaginal temperature sensor for prediction of the fertile period. J Obstetrics Gynaecol. 2013;33:705–709. [DOI] [PubMed] [Google Scholar]
- [184].Regidor PA, Kaczmarczyk M, Schiweck E, et al. Identification and prediction of the fertile window with a new web-based medical device using a vaginal biosensor for measuring the circadian and circamensual core body temperature. Gynecological Endocrinol. 2018;34:256–260. [DOI] [PubMed] [Google Scholar]
- [185].Bale TL, Epperson CN. Sex as a biological variable: who, what, when, why, and how. Neuropsychopharmacology. 2017;42:386–396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [186].Beery AK, Zucker I. Sex bias in neuroscience and biomedical research. Neurosci Biobehav Rev. 2011;35:565–572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [187].Clayton JA. Studying both sexes: A guiding principle for biomedicine. Faseb J. 2016;30:519–524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [188].Zucker I, Beery AK. Males still dominate animal studies. Nature. 2010;465:690. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- Tempdrop , 2019, Get the accurate temp data you need for the fertility apps you love, viewed 3rd November, 2019. https://www.temp-drop.com/.
Data Availability Statement
There are no data associated with this review article.


