Abstract
Although anxiety disorders represent a major societal problem demanding new therapeutic targets, these efforts have languished in the absence of a mechanistic understanding of this subjective emotional state. While it is impossible to know with certainty the subjective experience of a rodent, rodent models hold promise in dissecting well-conserved limbic circuits. The application of modern approaches in neuroscience has already begun to unmask the neural circuit intricacies underlying anxiety by allowing direct examination of hypotheses drawn from existing psychological concepts. This information points toward an updated conceptual model for what neural circuit perturbations could give rise to pathological anxiety and thereby provides a roadmap for future therapeutic development.
In health, the neural systems that underpin emotion dynamically integrate internal states and external stimuli to enable the rapid selection of situationally appropriate behaviors. The function of highly reciprocal limbic circuits is to integrate the barrage of signals received by an individual, including motivational drives1, environmental context and learned associations based on past events, and to consolidate them into a singular gestalt experience that is emotional in quality and directs the behavioral response.
The idea that emotions invigorate behaviors that aid survival has a long history. In The Expression of the Emotions in Man and Animals, Darwin conjectured that emotions evolved as a result of natural selection2, and, consistent with this evolutionary provenance, emotional behaviors can be observed across species. Whether animals experience emotions as humans do is a matter of controversy3,4; however, as Anderson and Adolphs argue in their 2014 review, “primitive emotion states are expressed by externally observable behaviors” across species, allowing the objective study of these states and the neural systems that drive them in model organisms5.
Here we review the state of our field and postulate that anxiety disorders arise from disruptions in the highly interconnected circuits normally serving to process the stream of stimuli detected by our brains from the outside world. We propose that information processing in distributed, interlinked nodes results in the assignment of emotional value to environmental stimuli, operationalized here as “interpretation,” as well as the weighing of potential threats against previously learned associations and competing motivational needs, referred to here as “evaluation.” Moreover, we suggest that computations in corticolimbic circuits resulting in the interpretation of environmental threat subsequently drive an observable anxiety-like response. Finally, we posit that perturbations anywhere in these circuits disrupt balance in the entire system, resulting in a fundamental misinterpretation of neutral sensory information as threatening and leading to the inappropriate emotional—and thereby behavioral—responses seen in anxiety disorders, as well as other psychiatric disease states.
Anxiety disorders: too much of a normal thing
Anxiety represents a state of high arousal and negative valence6 and results in enhanced vigilance in the absence of an immediate threat7. Occasional anxiety is a normal aspect of the emotional repertoire and aids survival by increasing awareness and enabling rapid responses to possible hazards. Anxiety is characterized by subjective experiences such as tension and worried thoughts, as well as physiological changes including sweating, dizziness and increases in blood pressure and heart rate. This emotional state can be triggered by stimuli that do not pose immediate danger, or can be internally generated. By contrast, the related emotional state of fear occurs acutely in response to a real or perceived imminent threat and dissipates on removal of the eliciting stimulus7.
Although healthy individuals experience sporadic bouts of anxiety, anxiety that is persistent, disruptive or disproportionate to actual danger can be debilitating and is considered pathological. The Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition divides pathological anxiety into three main categories of disorders, including obsessive-compulsive and related disorders, trauma- and stressor-related disorders, and anxiety disorders8. The precipitating stimuli differ among these diagnoses; however, in all cases, the somatic, cognitive and behavioral manifestations of anxiety interfere with normal functioning and lead to substantial economic and personal burdens9-11.
The prevalence (see Box 1) of anxiety disorders is estimated to be 18% among adults12, with a lifetime prevalence of more than 28% (ref. 13); however, a considerable portion of pathologically anxious individuals do not receive adequate treatment14. Despite the pervasiveness of anxiety disorders, relatively few therapeutic targets have been identified for the treatment of anxiety (for review of pharmacotherapeutic strategies for the treatment of anxiety, see refs. 15-17). Since the 1950s, new molecular entities approved by the US Food and Drug Administration (FDA) with primary indications for anxiety have been limited to benzodiazepines and the atypical anxiolytics buspirone, meprobamate and hydroxyzine pamoate18. More recently, the label indications for antidepressants such as certain tricyclic antidepressants, selective serotonin reuptake inhibitors (SSRIs) and serotonin norepinephrine reuptake inhibitors (SNRIs) have been extended by the FDA to include anxiety19. Despite these additions, the therapeutic arsenal for anxiety disorders remains inadequate; the available drugs do not successfully relieve anxiety in all patients, and unwanted side effects reduce compliance among those for whom they do. By comparison, development of new classes of drugs for the treatment of hypertension has remained steady over the same time period18 (Fig. 1). To improve treatment strategies for anxiety, a thorough understanding of the neural circuits governing this emotional state in health and disorder is needed20.
Box 1. Definitions.
In human populations
Prevalence.
The proportion of a population with a condition.
Lifetime prevalence.
The proportion of a population that will experience the condition at some point during their lives.
In animal models of disease
Face validity.
The extent to which observable phenotypes in the animal reproduce the human condition; to satisfy face validity, the anxiety response of the animal should be behaviorally and physiologically similar to the anxiety response in humans.
Predictive validity.
The extent to which pharmacological agents that reduce anxiety in model organisms also reduce anxiety in humans; to satisfy predictive validity, anxiety in animals should be sensitive to clinically effective anxiolytics.
Construct validity.
The extent to which the underlying causes of the human condition and phenotypes in the animal are equivalent; to satisfy construct validity, an animal model of anxiety should result from the same neurobiological processes as anxiety in humans.
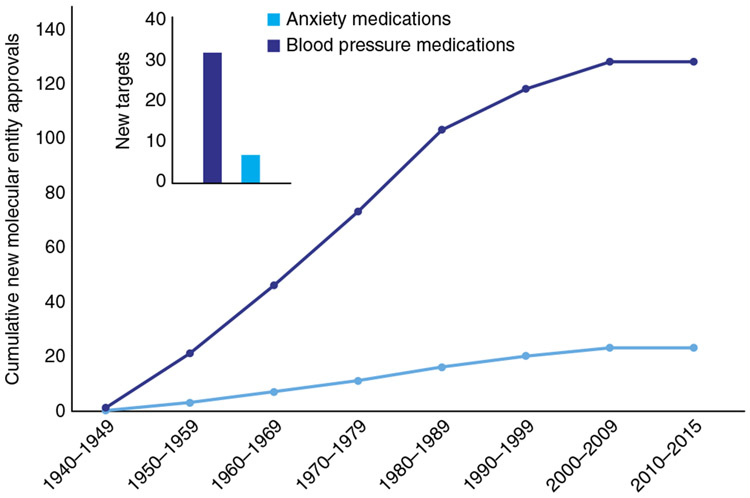
Figure 1.
Few new pharmacotherapies for the treatment of anxiety have been developed since the 1940s. The cumulative FDA approvals of medications with an indication for anxiety (blue line) are compared to those for medications with an indication for hypertension (dark blue line), a more thoroughly understood condition. In addition to the comparatively slow rate of overall approvals of anxiolytics, a lesser number of mechanistically distinct targets have been identified for the treatment of anxiety than hypertension during the past 75 years (inset). The relative paucity of pharmacological strategies for the treatment of anxiety disorders and the imperfect efficacy of these drugs betrays a need for a more thorough understanding of the neural substrates of anxiety.
Measuring anxiety-related behaviors in rodents
For a truly mechanistic understanding of the neural underpinnings of anxiety, the ability to manipulate specific circuit components is required, demanding the use of next-generation technologies in model organisms21. To provide insights about the pathophysiology of anxiety disorders, measurements of anxiety-like behaviors in animals must meet the essential criterion of validity (see Box 1) (refs. 22,23). Despite the inherent challenges of using model organisms to study the neural substrates of anxiety disorders, a diverse array of strategies for assessing externally observable anxiety-related phenotypes in animals has been developed5.
Rodents are well suited to this purpose and have historically been used in basic research and drug development to model fear learning, stress and anxiety. In this review, we focus on ‘state’ anxiety, defined as a behavioral state of enhanced arousal and vigilance in response to uncertain situations24. This classification refers to anxiety evoked by potentially hazardous situations25, rather than the pathological ‘trait’ anxiety of the clinical literature, wherein anxiety is a persistent, nonadaptive attribute that leads individuals to overestimate potential dangers in conditions of ambiguity24. This contrast notwithstanding, the neural systems implicated in anxiety by rodent studies are consistent with those identified in human patients26-34, underscoring the utility and translatability of these rodent studies. Tests of fear and anxiety in rodents have been reviewed extensively elsewhere22,23,25; we offer here a brief description of commonly used strategies for measuring anxiety-like behaviors in mice (Fig. 2).
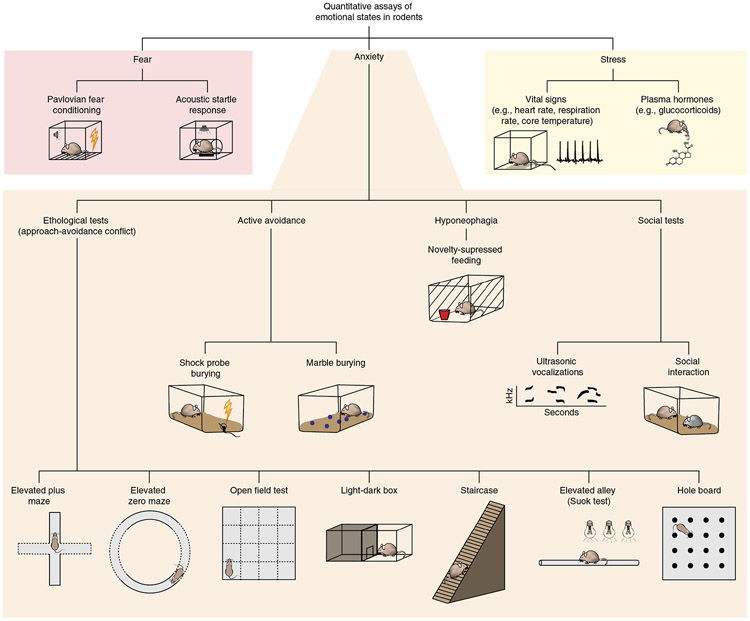
Figure 2.
Validated tests to assay anxiety and related emotional states in mice. State anxiety in mice is measured behaviorally through a variety of tests, highlighted in orange. Exemplars of fear and physiological stress assays are highlighted in red and yellow, respectively.
Most anxiety assays adapted for mice are ethologically based. These tasks capitalize on the innate, conflicting drives of rodents to explore novel spaces and to avoid open, exposed and brightly illuminated areas where they might be more vulnerable to environmental threats. Generally in these approach-avoidance conflict tasks, mice with an anxious phenotype tend to spend more time in enclosed or ‘safe’ zones of the behavioral apparatus compared to controls. In the elevated plus maze (EPM), anxious mice avoid the open arms of the maze in favor of the walled arms35. The ambiguous center of the EPM is eliminated in the elevated zero maze; however, the task resembles the EPM otherwise in that anxious mice prefer the walled quadrants to the open ones36. In the open field test (OFT), anxious animals remain along the edges of the enclosure37, whereas in the light-dark box, anxiety-related behavior is measured as a propensity to remain in the darkened portion of the chamber38. Other, less commonly used ethological tests include the staircase task39, in which anxious mice tend to rear more and climb fewer steps, the elevated alley40, in which mice must traverse a partially illuminated rod, and the hole board41, in which animals can forage in holes along the floor of the otherwise exposed apparatus.
These approach-avoidance assays are appealing for a number of reasons. They have a high degree of face validity in that most anxiety disorders include a component of avoidance of a potentially hazardous stimulus. Furthermore, anxiety-like behaviors in these tasks are reduced by anxiolytic drugs, particularly benzodiazepines42. Finally, limited training is required for animals to perform them, rendering this group of anxiety assays high throughput. An important caveat of these tasks, however, is that they are sensitive to overall changes in locomotion, so the use of control tests for motor deficits is necessary when employing these measures to assess anxiety-like behavior.
A further confound of exploration-based tasks is that they cannot distinguish a reduced anxiety phenotype from increased novelty-seeking, exploration or impulsive approach behavior23. Some alternative tasks for assessing anxiety-related behaviors in mice avoid this drawback; for instance, in active-avoidance tasks, mice direct their energies toward minimizing threatening stimuli. Rodents will bury an electrified probe after having received shocks from the probe43 and will even spontaneously bury marbles introduced as a novelty into their home cages44. This burying behavior is quantifiable and sensitive to anxiolytics45,46. Hyponeophagia (novelty-suppressed feeding) has also been used to assess anxiety-like behavior in rodents; in a novel environment, hungry mice exhibit an increased latency to feed that is sensitive to benzodiazepines and SSRIs47,48.
Anxiety phenotypes are also assessed using measures based on rodent social interactions. Rodents produce and detect ultrasonic vocalizations as a means of communication, and the frequency of these vocalizations varies relative to the apparent emotional state of the animal49. When removed from their mothers, pups emit ultrasonic vocalizations in the frequency range associated with fear and anxiety-like responses, and such stress-induced vocalizations are reduced by anxiolytics50. In the social interaction task, social contacts between unfamiliar individuals in a brightly illuminated arena are quantified; anxiolytics have been found to increase social interaction in this task51.
Complementing the behavioral assays, physiological measures of vital signs and circulating levels of stress hormones provide an additional indicator of anxiety-like phenotypes in mice. Although these types of measures are more commonly collected to quantify stress, their use in the assessment of anxiety in rodents has its basis in the strong somatic components of clinical anxiety8. Moreover, a thorough understanding of the neural circuits underlying anxiety must include a consideration of its somatic symptoms, as changes in heart rate, respiration and glucocorticoid levels require the activation of distinct pathways from those that drive anxiety-like behaviors31,52. Similarly, protocols traditionally used to study fear, such as auditory fear conditioning—in which auditory cues (conditioned stimuli, CS) predict delivery of foot shocks (unconditioned stimuli, US)—and the innate acoustic startle response to unexpected bursts of high decibel noise, are often used to interrogate anxiety circuits and to draw inferences about how fear learning may be disrupted in anxiety disorders53.
Recent technological developments have been instrumental in advancing our understanding of the neural substrates of anxiety measured in these behavioral assays. For example, the use of optogenetics to manipulate behaviors in these tasks has been central in establishing causal relationships between the activity of neural circuits and anxiety. Such nascent strategies have catapulted preclinical neuroscience research into a new era of circuit-level investigations. We focus here on insights gained as a result of this circuit-level approach, which is rapidly becoming the mainstay of the current era of neuroscience research.
Neural pathways of fear and anxiety
For more than a century after the original discovery that temporal lobe structures govern emotional behaviors54, our understanding of the neural substrates of anxiety was largely restricted to insights gleaned through lesion and inactivation studies. While this important early work led to the identification of key loci controlling anxiety, notably including the amygdala, the bed nucleus of the stria terminalis (BNST), the ventral hippocampus (vHPC) and the prefrontal cortex (PFC), progress in dissecting the contributions of regional microcircuits to this emotional state was limited. Moreover, the impact of changes in individual structures on activity in distal projection targets, or how such changes may functionally result in anxiety, remained elusive. Innovative approaches in animal research, especially targeted manipulations of neurons based on projection target or genetic identity via optogenetics, have opened these questions for causal testing and accelerated the pace of discovery. The resulting paradigm shift in anxiety research has expanded the focus to broader circuit-level interactions in emotional processing, such that it is now clear that anxiety requires the recruitment of a distributed array of interlinked neural circuits. Here we discuss the contributions of individual nodes in the context of larger circuit relationships that have been unveiled as a result of recent technological advances and reconcile region-specific studies of anxiety into a broader circuit-level network.
Interpreting threats on the macrocircuit level.
To assess a situation as threatening and render an anxiety-like response, an individual must first detect environmental stimuli through the sensory systems and then identify them as aversive or potentially dangerous. Coordinated activity in the amygdala, BNST, vHPC and PFC enables such interpretation of threat in the environment. These structures are highly interconnected, with multiple reciprocal projections facilitating macrocircuit-level interactions, which subsequently initiate vigilance and defensive behaviors through the recruitment of brainstem and hypothalamic nuclei. In this macrocircuit, sensory information regarding potential threats flows along circular loops in which it is transmitted both forward (from the amygdala onward to the BNST, vHPC and medial PFC (mPFC), and consequently to downstream effector nuclei) and backward (from the mPFC and vHPC back to the amygdala and BNST). Threats are detected and interpreted as worthy of enhanced vigilance in the forward direction, and this initial interpretation is evaluated in the backward direction.
A major determinant of whether environmental stimuli are interpreted as threatening occurs in the amygdala, wherein sensory stimuli are imbued with emotional value. As the main input nucleus in the amygdala, the basolateral amygdala (BLA, containing the lateral, basal and basomedial sections) receives excitatory afferents regarding sensory stimuli from the thalamus and sensory cortices55. Processing of this sensory information in the BLA results in the formation of associations between neutral predictive stimuli and outcomes of positive or negative valence56 via Hebbian mechanisms57-59. In this way, cues predicting threat are themselves recognized as threatening60 and, likewise, those predicting reward themselves become rewarding61,62.
The emotional valence of these cues then determines whether canonical fear or reward pathways are recruited downstream of the BLA63; in fear- or anxiety-provoking circumstances, projections from the BLA targeting the central amygdala (CeA, containing the lateral (CeL) and medial (CeM) subdivisions)53,64 and the BNST31,65 are activated. The activity of BLA neurons is not exclusively shaped by sensory input, however; principal neurons and interneurons in the BLA have been shown to receive monosynaptic input from mPFC and vHPC, and send reciprocal projections to both of these regions66-68. Activity in these pathways can both directly invigorate the anxiety response (for example, through projections of the vHPC to the lateral septum and hypothalamic nuclei; see below), as well as influence the likelihood of a threat appraisal (for example, through fear-memory retrieval via mPFC projections to the amygdala; see below and ref. 69).
Microcircuits and interactions among nodes.
Within this larger macrocircuit, individual nodes process information via local pathways. The advent of optogenetics is allowing in-depth interrogation of these microcircuits to enhance our understanding of the mechanisms whereby genetically or projection target-defined populations of cells shape processing within and among the amygdala, BNST, vHPC and mPFC. These modern technologies have enabled detailed elaboration upon the canonical amygdala microcircuit mediating fear learning. Briefly, activation of lateral amygdala neurons induces unconditioned freezing70. These cells develop robust responses to shock-predictive cues, corresponding to freezing behavior when the cues are presented71. The presence of an unambiguous threat such as foot shock results in rapid fear responses by engaging microcircuits in the CeA. In this circuit, glutamatergic signals from the BLA are conducted onward to the CeL, where they impinge on two populations of mutually inhibitory GABAergic neurons, the protein kinase C-δ (PKCδ)-negative CeLON cells and the PKCδ-positive CeLOFF cells72,75. Inhibition of the latter population by the former results in the disinhibition of CeM output neurons, consequently driving freezing behavior and changes in heart rate via CeM projections to the periaqueductal gray and dorsal vagal complex, respectively52 (Fig. 3). Consistent with the apparent role of PKCδ-positive neurons in gating fear responses, direct activation of these cells produces an anxiolytic effect in the EPM, OFT and light-dark box tests74. Activation of somatostatin (SOM)-positive CeL neurons by the lateral amygdala75 and paraventricular nucleus of the thalamus76,77 has also been shown to drive fear learning through an overall increase in inhibition in the CeL. Furthermore, a population of cells in the BLA projecting directly to the CeM shows evidence of potentiation following fear conditioning, and photoinhibition of these neurons inhibits the acquisition of the fear association63. For more detailed consideration of the microcircuits driving fear learning, see refs. 53,78.
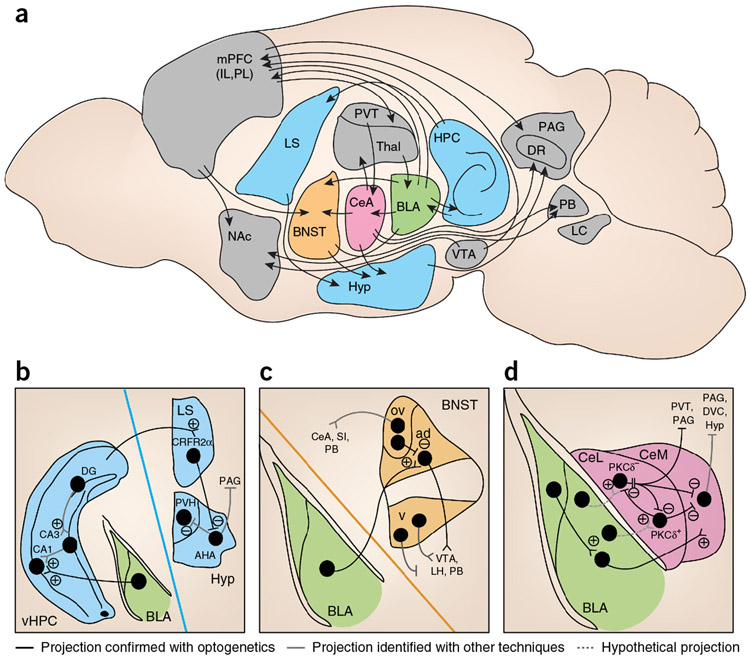
Figure 3.
Neural circuits implicated in anxiety-related behaviors in the rodent brain. Recent work using optogenetics, behavioral neuroscience and electrophysiology has begun to establish causal relationships between anxiety behaviors and activity in specific neural circuits. (a) A sagittal view of rodent brain including distal circuits implicated in anxiety-related behaviors. (b) Septohippocampal microcircuitry linked to anxiety. (c,d) Extended amygdala microcircuits involved in anxiety-related behaviors, including BLA projections to the BNST (c) and BLA projections to CeA (d). ad, anterodorsal nucleus of the BNST; AHA, anterior hypothalamic area; BLA, basolateral amygdala; BNST, bed nucleus of the stria terminalis; CeA, central amygdala; CeL, lateral subdivision of the central amygdala; CeM, centromedial subdivision of the amygdala; CRFR2α, type 2 corticotropin releasing factor receptor; DR, dorsal raphe nucleus; DVC, dorsal vagal complex; HPC, hippocampus; Hyp, hypothalamus; IL, infralimbic division of the mPFC; LC, locus coeruleus; LH, lateral hypothalamus; LS, lateral septum; mPFC, medial prefrontal cortex; NAc, nucleus accumbens; ov, oval nucleus of the BNST; PAG, periaqueductal gray; PB, parabrachial nucleus; PL, prelimbic division of the mPFC; PVH, paraventricular nucleus of the hypothalamus; PVT, paraventricular thalamus; SI substantia innominata; Thal, thalamus; v, ventral nucleus of the BNST; vHPC, ventral hippocampus; VTA, ventral tegmental area.
In the absence of immediate threat, anxiety-like behaviors can arise from recruitment of these same amygdala pathways. For instance, tonic activity in a subset of BLA neurons has been shown to track with anxiety-like behavior in the EPM and OFT79. Moreover, photoactivation of BLA somata increases measures of innate anxiety33, as well as learned inhibitory avoidance80. Targeted photostimulation of BLA inputs to CeL, by contrast, is anxiolytic, presumably via enhanced inhibitory influence over CeM projection neurons55. This work demonstrates the importance of projection-defined interrogation of these circuits, as individual projection neurons in the same region can enact opposing behavioral effects depending on their efferent targets.
Initiation of fear and sustained anxiety responses requires the recruitment of the BNST7, which occurs partly as a result of direct innervation by BLA afferents, as well as through dense glutamatergic input from the hippocampus81,82, mPFC, entorhinal cortex and insular cortex55,83. Some redundancy of amygdala and BNST function ensures that fear learning remains intact in the face of localized damage or dysfunction. For example, in the absence of a functional BLA, the BNST acts as a compensatory site in the acquisition of fear memories, although this BLA-independent fear learning requires more training84. Moreover, individual subregions of the BNST have been shown to differentially regulate separable features of the anxiety phenotype31,85 (Fig. 3). BLA inputs to the anterodorsal BNST promote behavioral and physiological anxiolysis; local inhibition of anterodorsal BNST by the oval nucleus of the BNST is anxiogenic31. These effects on the innate anxiety state are mediated via projections from the anterodorsal BNST to the ventral tegmental area (VTA), lateral hypothalamus and parabrachial nucleus, which independently regulate subjective preference, risk avoidance in the EPM and OFT, and respiration rate, respectively31. BLA inputs to the ventrolateral BNST promote freezing during uncontrollable stress and subsequent reduction in social interaction, both of which responses are reduced by inhibition of ventrolateral BNST86. The ventral BNST also regulates anxiety through its innervation of the VTA; activity in glutamatergic ventral BNST neurons projecting to the VTA produces anxiety-like behaviors, whereas GABAergic neurons in the ventral BNST exert anxiolysis via a parallel pathway85.
Reciprocal interactions between the BLA and the vHPC have also been shown to regulate fear learning and anxiety-like behaviors. The hippocampus is a highly structured brain region with an elaborated internal microcircuit that has been reviewed in detail elsewhere87-89. BLA inputs to the vHPC promote anxiety-like behavior; for example, BLA projections to the hippocampal formation through the entorhinal cortex are necessary for the acquisition of contextual fear memories, as photoinhibition of these terminals during learning blocks freezing during context reexposure90. Furthermore, glutamatergic terminals of BLA fibers onto pyramidal neurons in the CA1 region of the vHPC bidirectionally control innate anxiety-related behaviors in the EPM and OFT66, such that increased activity in this projection enhances anxiety-like behavior and inactivation of this pathway reduces anxiety-like behavior27,66. These targeted investigations of BLA input to vHPC suggest that increased activation of cells in this region produce anxiety; however, direct photostimulation of ventral dentate gyrus granule cells has been in fact found to suppress anxiety-like behaviors in the EPM and OFT91. Although these findings appear to conflict with those obtained through manipulation of BLA inputs to the vHPC, they may potentially be reconciled, as the low-frequency stimulation of ventral dentate gyrus used by Kheirbek et al.91 has been previously demonstrated to result in feedforward inhibition of CA3 pyramidal cells92.
The effects of vHPC activation on anxiety-related behaviors occur partially through its connections to the lateral septum93, which in turn is reciprocally connected to the hypothalamus94. Ipsilateral projections from vHPC to lateral septum convey an anxiety signal; bilateral blockade of activity in either structure using muscimol increases open-arm exploration in the EPM, as does disconnection of the two using pharmacological inactivation of vHPC in one hemisphere and concurrent inactivation of the contralateral lateral septum95. Corticotropin releasing factor receptor (CRFR) activation in the lateral septum increases anxiety-related phenotypes96 by way of activation of CRFR type 2–expressing GABAergic projection neurons residing there97. Activation of these neurons results in inhibition of cells in the anterior hypothalamic area, which themselves inhibit the paraventricular nucleus of the hypothalamus and periaqueductal gray. The resultant disinhibition of these regions promotes neuroendocrine and behavioral aspects of the persistent anxiety state97, which redouble the complementary actions of CeA and BNST activation.
In addition to producing anxiety-like behaviors via projections to the lateral septum, the vHPC supports the interpretation of a given situation as threatening through its reciprocal connection with the BLA, as well as its efferent projections to the mPFC. The vHPC serves as an important source of synchrony between the amygdala and mPFC during threatening situations (discussed in detail below). Furthermore, the vHPC specifically targets fear-encoding neurons in the basal amygdala (which themselves project to the mPFC), and the activation of these cells is necessary for fear renewal after extinction67. Neighboring cells in the basal amygdala that are innervated by PFC afferents fire in response to conditioned stimuli that have been extinguished, and they project reciprocally to the PFC67.
Evaluation of threat: regulation of interpretation.
Naturalistic environments are often characterized by ambiguity, and the absolute absence of potential danger is rarely assured. In psychology, it has been proposed that there is an evaluative system that guides subsequent modulation of emotion in a regulatory fashion98. To prevent unchecked activation of proanxiety circuits, an extra layer of processing is required to evaluate whether interpretations of environmental threats are accurate and weighted appropriately given internal (for example, homeostatic, hormonal) or external (for example, support of the conspecific group, extent of perceived danger) conditions. Such an evaluative system could provide permissive or restrictive feedback to interpretation circuits to promote or suppress the expression of anxiety-like behaviors.
An integral source of evaluation for threat interpretations is the mPFC, which regulates subcortical responses to threatening stimuli. As a neocortical structure, the mPFC is organized into six layers in humans (I–VI; layer IV is absent in rodents) containing excitatory pyramidal neurons and a diverse array of inhibitory interneurons99. The mPFC can be parsed into distinct subregions on the basis of their cytoarchitectures, which are consistent with divergent functions among these regions100. The primary subdivisions of the mPFC in rodents include the prelimbic and infralimbic cortices. These receive inputs from midline thalamic nuclei, the BLA and the hippocampus, and send reciprocal projections to BLA, as well as efferent projections to the striatum101.
Reciprocal connections between the mPFC and the amygdala have been extensively studied in the learned fear response, as well as in anxiety disorders in both humans102,103 and rodents, which we explore in depth below. The evaluative function of the PFC is demonstrated in this exemplar circuit, as PFC interactions with the BLA differ depending on the degree of environmental threat. Correlated activity between the dorsal anterior cingulate region of the PFC and BLA underlies acquisition of aversive memories, and maintenance of such cross-regional correlations promotes resistance to extinction of fear memories104. During auditory fear conditioning, activity in basal amygdala neurons projecting to the prelimbic cortex is increased105, and prelimbic cortex responses to shock-predicting cues are increased after learning106. This increase in prelimbic cortex activation is likely due in part to phasic inhibition of local parvalbumin-positive interneurons, which is necessary for cue-induced freezing behavior107. By contrast, extinction of the learned fear association increases activity in basal amygdala neurons targeting the infralimbic cortex105. Fittingly, increased activity in the infralimbic cortex is associated with fear extinction108 (but see also refs. 109-111), and electrical stimulation of infralimbic cortex reduces freezing to the CS even in animals that are resistant to extinction training108,112. In naive mice, these two subdivisions of mPFC exert similar levels of excitation and feedforward inhibition on BLA principal neurons. Following auditory fear conditioning, however, excitatory responses evoked by prelimbic cortical inputs in the BLA are increased as a result of enhanced AMPA-type glutamate receptor function113. Furthermore, prelimbic cortex activation of amygdalar subregions is necessary for retrieval of fear memories. Direct activation of the BLA by prelimbic cortical inputs mediates freezing responses to CS presentations within 6 h of auditory fear conditioning; however, fear memory retrieval more than 24 h after conditioning necessitates prelimbic cortex recruitment of inputs from the paraventricular nucleus of the thalamus to CeL114. Taken together, these findings indicate that prelimbic cortical inputs to the amygdala facilitate responses to threatening stimuli, whereas infralimbic cortex inputs to the amygdala suppress them. For a more in-depth consideration of how interactions between the amygdala and mPFC influence fear learning, readers are directed to ref. 115.
The distinct pathways engaged by the mPFC in conditions of high and low threat represent a mechanism whereby this region may dynamically permit or prevent anxiogenic activity in interpretation circuits. In addition, the mPFC has been demonstrated to exert powerful feedforward inhibition over the baseline activity of BLA principal neurons in vivo116 (but see ref. 117) and also to suppress responses of lateral amygdala to conditioned aversive stimuli118. Such inhibitory control enables the mPFC to trump threat interpretations in conditions of acceptably low risk in favor of engaging in appetitive behaviors. Moreover, the mPFC is ideally poised to orchestrate shifts between anxiety-like and reward-motivated behaviors, as it sends projections both to the amygdala and nucleus accumbens (NAc). mPFC efferents to the latter affect information processing in striatal projection neurons119, and stimulation of this pathway is reinforcing120.
Habituation can also suppress threat interpretations. As opposed to extinction, in which responses to a learned CS are diminished over time as a result of multiple unpaired presentations, habituation occurs when evoked behavioral responses are diminished after repeated presentations of a neutral or unconditioned stimulus121. This process is especially relevant to anxiolysis in the case of stimuli that are ambiguously threatening, such as police sirens in a busy neighborhood; although these stimuli may portend danger initially, over multiple exposures they no longer evoke an anxiety response. The neural mechanisms of habituation have long been suggested to involve reduced transmission in stimulus–response pathways after repeated presentation of the stimulus122.
Energy homeostasis and motivation signals also shape the degree to which threat interpretations govern behavioral responses. Given sufficient motivation to seek food, to drink or to mate, an animal will engage in these appetitive behaviors despite the presence of potential dangers. The comparison of apparent risks or costs to possible reward is a computation performed by circuits supporting motivated behavior, critically including the NAc and the mesocorti-colimbic dopamine system (reviewed in depth elsewhere123-125). In cases in which expected benefits outweigh perceived costs, the impact of threat interpretations on behavior is diminished. Hypothalamic drives to maintain homeostasis (that is, to seek and consume food or water126,127) can also overwhelm threat avoidance and anxiety-like behaviors. Stimulation of lateral hypothalamic terminals in the VTA, for example, promotes sucrose seeking, even when mice must cross an electrified floor to obtain the sucrose reward128. Thus, subcortical motivational systems contribute to the evaluation of threat interpretations by shifting the balance toward appetitive behaviors and away from anxiety-like behaviors.
Synchrony between interpretation and evaluation circuits.
The combined actions of distributed neural circuits emerging from the amygdala, BNST, vHPC and mPFC result in the interpretation and evaluation of the emotional value of environmental stimuli. If such stimuli are identified as threatening on the basis of this assessment, anxiety-like behaviors may result. However, coordinated processing of potentially threatening stimuli must occur to elicit a well-defined behavioral response. Synchronization of local field potentials (LFPs) provides a mechanism whereby the timing of activity in these distributed regions could be orchestrated.
Interactions among the “emotional triad” (a term coined by Joshua Gordon)—BLA, mPFC and vHPC—are shaped by dynamic changes in the degree of synchrony in the LFP oscillations of these regions. Theta activity (4–12 Hz) in the vHPC is associated with anxiety-like behavior in rodents129. This rhythm is thought to be driven by brainstem nuclei via projections through the medial septum130, and it is universally reduced by anxiolytic drugs131. This hippocampal theta rhythm has been shown to entrain single-unit activity in both pyramidal cells and interneurons of the PFC, as well as PFC gamma-band activity (40–120 Hz)132. Synchronization of PFC activity to the hippocampal theta rhythm has been observed in anxiety assays; in the threatening zones of the EPM and OFT (open arms and center, respectively), theta activity has been observed in the mPFC, which is entrained to vHPC theta129. Theta rhythm synchrony between hippocampus and amygdala may underlie anxiety behaviors as well, as cross-correlation of activity in the theta frequency in lateral amygdala and CA1 increase in response to the CS following fear conditioning133. Following extinction of fear conditioning, spontaneous freezing to the extinguished CS, representing an inappropriate assessment of threat, is associated with synchronized theta rhythms among infralimbic cortex, CA1 and lateral amygdala134. Moreover, there is a greater degree of theta coherence between these regions in serotonin receptor 1A knockout mice, which serve as a genetic mouse model of trait anxiety, than in wild-type controls129. Disruption of the vHPC theta rhythm using pharmacological blockade of gap junctions reduces anxiety-like behaviors in the EPM and OFT, and disconnection of LFP coherence by unilateral administration of gap junction blockers in contralateral vHPC and mPFC reduces theta activity in both regions, as well as innate anxiety-like behaviors in ethological tasks135. vHPC-originating theta rhythms potentially enable the mPFC to construct representations of aversive features in the environment, as mPFC neurons with strong task-related responses are strongly coupled to the vHPC theta rhythm136. Together these data suggest that the vHPC theta rhythm conveys an anxiogenic signal to the mPFC and BLA.
Theta rhythms originating in the mPFC, however, provide a safety signal to BLA neurons. These theta rhythms originate in the mPFC as a result of phasic inhibition of parvalbumin interneurons residing there, which mediates local theta-rhythm phase resetting107. Following successful extinction of fear learning, infralimbic cortex spike firing has been observed to lead the theta rhythm in hippocampus and lateral amygdala during presentations of the CS134. Conversely, artificial theta synchronization of hippocampus and lateral amygdala during extinction recall (such that PFC firing no longer leads these rhythms) increases freezing to the CS137. BLA field potentials and spike activity are entrained to the mPFC theta rhythm during periods of perceived safety (for example, when mice enter the relative safety of the periphery of the open field32). This synchronization of BLA oscillations to mPFC during safety also extends to other LFP frequency bands in the BLA, including theta-coupled gamma, particularly in the fast gamma (70–120 Hz) range138. Uncoupling of PFC theta oscillations from the hippocampal theta could in part be mediated by reciprocal inputs from the BLA to the PFC, which heterosynaptically suppress the influence of hippocampal inputs there139. As judged from the opposing contributions of prelimbic and infralimbic cortices to fear learning and anxiety, the PFC safety signal likely originates in the infralimbic cortex. Thus, oscillatory activity among vHPC, mPFC and BLA appears to reflect the internal anxiety state. The dominant source of theta activity may dynamically shift between the vHPC and mPFC in periods of relative threat and security to aid in the interpretation and evaluation of environmental events and support circuit-level activity promoting defensive or exploratory behaviors.
Perspective on the neural circuitry of anxiety
We emphasize that limbic circuits are loops, rather than one-way streams of information flow. Anxiety arises from activity in these circuits when ambiguous environmental stimuli are interpreted as threatening. For this interpretation to occur, an organism must first detect that the stimuli exist through its sensory systems. Once potential threats are detected, the highly interconnected circuits described here ‘interpret’ the meaning of those stimuli and determine whether they portend danger. This interpretation is in part dictated by the individual’s previous experience and includes the assignment of valence to the stimulus via BLA circuits56. Following the interpretation of an ambiguous event, additional circuits including the PFC and nuclei at the intersection of the limbic system and motor effectors140 must evaluate whether the external events reflect expectations and whether they meet or contradict the animal’s needs in order to engage the appropriate behavioral response. Consequently, that response is initiated through downstream motor pathways, brainstem nuclei in control of autonomic responses, and the neuroendocrine system. Activation of these effectors results in the observable responses that we identify as anxiety or lack thereof.
On the basis of this model (Fig. 4), we conceive of anxiety as occurring between the stimulus and response, at the level of internal processing. Two individuals may equivalently detect a particular stimulus, but different interpretation of that stimulus may result in the selection of opposing behavioral responses. For example, a sudden, loud noise might be interpreted as fireworks by one person and as a gunshot by another, leading the first to curiously look to the sky and the second to duck and cover. The selected response also depends on the evaluation of potential threat compared against actual threat (as determined by previous learning), as well as the individual’s drive state. For instance, if an animal is thirsty, it will risk visiting the watering hole despite interpreting the setting as threatening. In fact, this approach-avoidance conflict resulting from the incongruence between the animal’s needs and the interpretation of threat promotes vigilance and apprehension, which serve a protective function for the animal and produce behaviors we can measure as anxiety (see above). Although we cannot measure the internal state or emotional experience of an animal, we can determine which circuits govern the interpretation and evaluation of environmental stimuli and result in the selection of such anxiety-like behaviors.
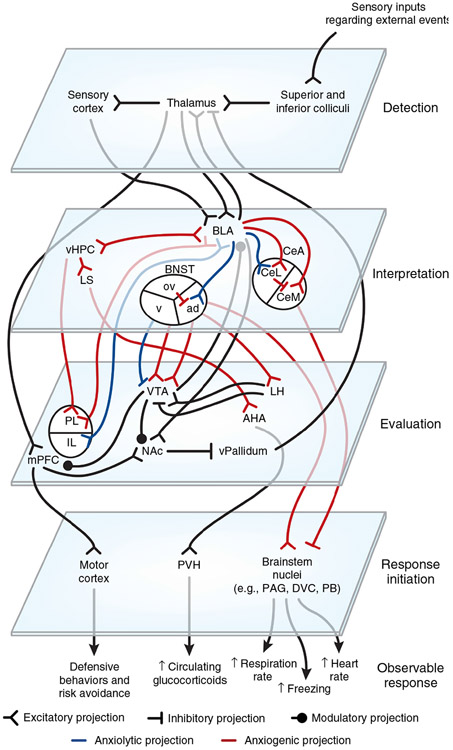
Figure 4.
Circuit organization in anxiety: a problem with interpretation. We propose a four-step model wherein external events are detected, interpreted, evaluated, and responded to by succeeding levels of highly interconnected neural circuits. Whether events are interpreted as threatening or nonthreatening depends on the balance between opposing circuits among the amygdala, vHPC, mPFC and BNST. In anxiety, balance is shifted toward projections interpreting events as threatening. Red, anxiogenic pathway; blue, anxiolytic pathway. ad, anterodorsal nucleus of the BNST; AHA, anterior hypothalamic area; BLA, basolateral amygdala; BNST, bed nucleus of the stria terminalis; CeA, central amygdala; CeL, lateral subdivision of the central amygdala; CeM, centromedial subdivision of the amygdala; DVC, dorsal vagal complex; IL, infralimbic division of the mPFC; LH, lateral hypothalamus; LS, lateral septum; mPFC, medial prefrontal cortex; NAc, nucleus accumbens; ov, oval nucleus of the BNST; PAG, periaqueductal gray; PB, parabrachial nucleus; PL, prelimbic division of the mPFC; PVH, paraventricular nucleus of the hypothalamus; v, ventral BNST; vHPC, ventral hippocampus; vPallidum, ventral pallidum; VTA, ventral tegmental area.
Whether or not events are interpreted as threatening depends on the balance between circuits supporting exploratory versus defensive behaviors. An important mechanism that may allow one system to over-come the other is the recruitment of projection-defined populations of neurons in the BLA. Positive and negative valence are oppositely encoded by BLA neurons projecting to canonical reward and fear systems (NAc and CeM, respectively63), and specific activation of CeM-projecting BLA neurons might bias the interpretation system toward a threat appraisal. The relative strengths of these competing circuits may also contribute to interpretations of danger in the environment; for instance, repeated exposure to stressors or threatening stimuli may cause specific potentiation of circuits promoting anxiety-related behavior, such that, in ambiguous situations, anxiety circuits prevail. Natural variations in the expression of certain genes may also influence the function of the interpretation system. For instance, the release of monoamines such as serotonin at different nuclei in the corticolimbic network affects anxiety (that is, innate anxiety-like behaviors in the EPM are associated with decreased levels of serotonin in the mPFC, amygdala and hippocampus141), and polymorphisms in the serotonin transporter gene have been found to influence trait anxiety in rodents and humans142,143. These findings are particularly interesting in light of the efficacy of SSRIs and SNRIs in the treatment of anxiety.
Here we reframe corticolimbic circuitry from the perspective of anxiety. The model that we propose builds on existing models, within a framework of four steps: detection, interpretation, evaluation and response initiation. Both bottom-up and top-down processes determine which external events will be detected144; top-down suppression of attention to ambiguous stimuli may reduce the anxiety response. Furthermore, the valence detection function of BLA projection neurons56 fits within the interpretation circuits described here, helping to determine whether a given stimulus is threatening or appetitive. Actor-critic models145-147 operate within the evaluation system: dopamine inputs to the striatum push the animal toward obtaining maximum reward, a process that must take into account possible dangers. These dangers are recognized by interpretation circuits and fed into the actor-critic interplay occurring within evaluation circuits, subsequently determining what course of action must be taken. This action is taken as a result of increased activity in the basal ganglia, which serve as the limbic-motor interface140 and facilitate the initiation of motor responses following upstream processing in the interpretation system.
To select appropriate behavioral responses in a given environmental context, an animal must weigh potential rewards against potential risks. To use a balance metaphor, interpretation circuits may determine the relative weights of various environmental stimuli on either end of the balance while evaluation circuits govern the location of the fulcrum that influences the balance point.
Circuit-based intervention: the future of anxiety therapy
In cases of pathological anxiety, excessive apprehension occurs in response to minimally threatening stimuli or even in the absence of provocation, implying dysfunction at the level of interpretation. Bias of the system toward rendering a threat interpretation in the absence of probable danger may result from undue Hebbian plasticity in limbic pathways (particularly in cued anxiety disorders, such as social anxiety disorder and specific phobias) or may be the outcome of a disruption to homeostatic mechanisms that normally maintain balance between circuits supporting defensive versus exploratory behaviors (particularly in persistent anxiety, such as generalized anxiety disorder).
Because disruption to any one part of a highly interconnected system results in changes to the whole, effective solutions require interventions that consider the dynamics of the entire system. Likewise, future therapies for anxiety disorders must take a circuit-level approach. We have previously extolled neural circuit reprogramming, in which the brain’s own plasticity mechanisms are directed toward resolving the symptoms of psychiatric illnesses, as a promising future therapeutic strategy20. Targeted plasticity, perhaps via transcranial magnetic stimulation or focal ultrasound, directed at certain nodes in the reciprocal loops described in this review could elicit positive downstream changes, ameliorating undesirable anxiety. For example, potentiation of inputs from infralimbic cortex to the BLA could enhance ‘safety’ signals, and depotentiation of BLA inputs to the CeM could reduce the expression of learned fear associations. There is reason to believe that neural circuit reprogramming is a viable future strategy for the treatment of anxiety disorders; already it has been shown that fear memories can be inactivated and reactivated via induction of LTP and LTD in amygdalar microcircuits148. Moreover, the best intervention so far available for the treatment of anxiety disorders is cognitive-behavioral therapy (CBT)149, which aims to replace maladaptive interpretations of events with more helpful ones, a process that almost certainly occurs through plastic changes in the interpretation circuits described here.
Before this innovative strategy can become reality, however, a comprehensive understanding of the dynamic interactions occurring within the distributed networks underlying anxiety is needed. Although this prospect seems daunting, inroads are rapidly being made that advance the state of knowledge toward this goal. Optogenetic studies of individual pathways within circuits subserving interpretation and evaluation of external stimuli provide valuable insights into how individual components of the system function. Work toward mapping the neural connectome could potentially identify new pathways among genetically defined populations of cells that will supply avenues for future discovery150.
One important clue as to how these highly interconnected pathways might interact and impact one another’s activity may be derived from investigating the comorbidity among psychiatric disorders. Although comorbidities are systematically eliminated in pharmaceutical drug development, the overlapping constellations of symptoms might shed light on which aspects of the neural substrates of these symptoms covary. For instance, substance abuse and addiction are often coincident with anxiety disorders; however, the symptoms of anxiety or depression in an addicted person may not be observable until the addictive substance is withdrawn. Studying an individual with comorbid disorders in both states might unveil how the interconnected loops shift dominance during different emotional and behavioral states, and provide new targets for future treatment strategies.
Treating complex psychiatric disorders that arise from disruption to complex, highly interconnected neural systems requires a broad, circuit-level approach. A paradigm shift in how we consider the neural substrates underpinning anxiety—such that individual nodes are not interrogated in a vacuum, independent of the larger circuits they comprise—promises to transform how anxiety disorders are treated.
ACKNOWLEDGMENTS
We thank M. Kinch and M. Kheirbek for correspondence, C. Vander Weele and R. Wichmann for reading and comments on our text, and the entire Tye laboratory for discussion. K.M.T. is a New York Stem Cell Foundation – Robertson Investigator, and this work was supported by funding from the JPB Foundation, PIIF, PNDRF, JFDP, Whitehall Foundation, Klingenstein Foundation, McKnight Foundation, NARSAD Young Investigator Award, Alfred P Sloan Foundation, New York Stem Cell Foundation, Whitehead Career Development Chair, US National Institutes of Health (NIH) R01-MH102441-01 (National Institute of Mental Health) and NIH Director’s New Innovator Award DP2-DK-102256-01 (National Institute of Diabetes and Digestive and Kidney Diseases). G.G.C. is supported by the JFDP Postdoctoral Fellowship from the JPB Foundation.
Footnotes
COMPETING FINANCIAL INTERESTS
The authors declare no competing financial interests.
References
- 1.Hull CL Principles of Behavior: An Introduction to Behavior Theory (Appleton-Century-Crofts, 1943). [Google Scholar]
- 2.Darwin C The Expression of the Emotions in Man and Animals (Impression anastalitique Culture et Civilisation, 1872). [Google Scholar]
- 3.LeDoux J Rethinking the emotional brain. Neuron 73, 653–676 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Panksepp J Affective consciousness: core emotional feelings in animals and humans. Conscious. Cogn 14, 30–80 (2005). [DOI] [PubMed] [Google Scholar]
- 5.Anderson DJ & Adolphs R A framework for studying emotions across species. Cell 157, 187–200 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Russell JA A circumplex model of affect. J. Pers. Soc. Psychol 39, 1161–1178 (1980). [Google Scholar]
- 7.Davis M, Walker DL, Miles L & Grillon C Phasic vs sustained fear in rats and humans: role of the extended amygdala in fear vs anxiety. Neuropsychopharmacology 35, 105–135 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders 5th edn., http://dsm.psychiatryonline.org/doi/book/10.1176/appi.books.9780890425596 (American Psychiatric Association, 2013). [Google Scholar]
- 9.Bereza BG, Machado M & Einarson TR Systematic review and quality assessment of economic evaluations and quality-of-life studies related to generalized anxiety disorder. Clin. Ther 31, 1279–1308 (2009). [DOI] [PubMed] [Google Scholar]
- 10.Mondin TC et al. Anxiety disorders in young people: a population-based study. Rev. Bras. Psiquiatr 35, 347–352 (2013). [DOI] [PubMed] [Google Scholar]
- 11.Pagotto LF et al. The impact of posttraumatic symptoms and comorbid mental disorders on the health-related quality of life in treatment-seeking PTSD patients. Compr. Psychiatry 58, 68–73 (2015). [DOI] [PubMed] [Google Scholar]
- 12.Kessler RC, Chiu WT, Dernier O, Merikangas KR & Walters EE Prevalence, severity, and comorbidity of 12-month DSM-IV disorders in the National Comorbidity Survey Replication. Arch. Gen. Psychiatry 62, 617–627 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kessler RC et al. Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the National Comorbidity Survey Replication. Arch. Gen. Psychiatry 62, 593–602 (2005). [DOI] [PubMed] [Google Scholar]
- 14.Lecrubier Y Widespread underrecognition and undertreatment of anxiety and mood disorders: results from 3 European studies. J. Clin. Psychiatry 68 (suppl. 2): 36–41 (2007). [PubMed] [Google Scholar]
- 15.Berlin HA, Hamilton H & Hollander E Experimental therapeutics for refractory obsessive-compulsive disorder: translational approaches and new somatic developments. Mt. Sinai J. Med 75, 174–203 (2008). [DOI] [PubMed] [Google Scholar]
- 16.Davidson JR et al. A psychopharmacological treatment algorithm for generalised anxiety disorder (GAD). J. Psychopharmacol 24, 3–26 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hoffman EJ & Mathew SJ Anxiety disorders: a comprehensive review of pharmacotherapies. Mt. Sinai J. Med 75, 248–262 (2008). [DOI] [PubMed] [Google Scholar]
- 18.Kinch MS, Haynesworth A, Kinch SL & Hoyer D An overview of FDA-approved new molecular entities: 1827–2013. Drug Discov. Today 19, 1033–1039 (2014). [DOI] [PubMed] [Google Scholar]
- 19.U.S. Food and Drug Administration. Drugs@FDA: FDA Approved Drug Products http://www.accessdata.fda.gov/scripts/cder/drugsatfda/index.cfm.
- 20.Tye KM Neural circuit reprogramming: a new paradigm for treating neuropsychiatric disease? Neuron 83, 1259–1261 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Allsop SA, Vander Weele CM, Wichmann R & Tye KM Optogenetic insights on the relationship between anxiety-related behaviors and social deficits. Front. Behav. Neurosci 8, 241 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Belzung C & Griebel G Measuring normal and pathological anxiety-like behavior in mice: a review. Behav. Brain Res 125, 141–149 (2001). [DOI] [PubMed] [Google Scholar]
- 23.Cryan JF & Holmes A The ascent of mouse: advances in modelling human depression and anxiety. Nat. Rev. Drug Discov 4, 775–790 (2005). [DOI] [PubMed] [Google Scholar]
- 24.Syivers P, Lilienfeld SO & LaPrairie JL Differences between trait fear and trait anxiety: implications for psychopathology. Clin. Psychol. Rev 31, 122–137 (2011). [DOI] [PubMed] [Google Scholar]
- 25.Lister RG Ethologically-based animal models of anxiety disorders. Pharmacol. Ther 46, 321–340 (1990). [DOI] [PubMed] [Google Scholar]
- 26.Adhikari A Distributed circuits underlying anxiety. Front. Behav. Neurosci 8, 112 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Degroot A & Treit D Anxiety is functionally segregated within the septohippocampal system. Brain Res. 1001, 60–71 (2004). [DOI] [PubMed] [Google Scholar]
- 28.Eden AS et al. Emotion regulation and trait anxiety are predicted by the microstructure of fibers between amygdala and prefrontal cortex. J. Neurosci 35, 6020–6027 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Etkin A, Prater KE, Schatzberg AF, Menon V & Greicius MD Disrupted amygdalar subregion functional connectivity and evidence of a compensatory network in generalized anxiety disorder. Arch. Gen. Psychiatry 66, 1361–1372 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Irle E et al. Reduced amygdalar and hippocampal size in adults with generalized social phobia. J. Psychiatry Neurosci 35, 126–131 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kim S-Y et al. Diverging neural pathways assemble a behavioural state from separable features in anxiety. Nature 496, 219–223 (2013).First study to provide causal evidence that separate circuits control the behavioral and physiological features of anxiety.
- 32.Likhtik E, Stujenske JM, Topiwala MA, Harris AZ & Gordon JA Prefrontal entrainment of amygdala activity signals safety in learned fear and innate anxiety. Nat. Neurosci 17, 106–113 (2014).Identifies a macrocircuit-level mechanism to discern safety from threat, whereby BLA firing is tuned to mPFC oscillatory activity.
- 33.Tye KM et al. Amygdala circuitry mediating reversible and bidirectional control of anxiety. Nature 471, 358–362 (2011).Original optogenetic dissection of the control of anxiety by separate aspects of the BLA microcircuit and first study to employ projection-specific optogenetic manipulations of behavior.
- 34.Yassa MA, Hazlett RL, Stark CEL & Hoehn-Saric R Functional MRI of the amygdala and bed nucleus of the stria terminalis during conditions of uncertainty in generalized anxiety disorder. J. Psychiatr. Res 46, 1045–1052 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pellow S, Chopin P, File SE & Briley M Validation of open:closed arm entries in an elevated plus-maze as a measure of anxiety in the rat. J. Neurosci. Methods 14, 149–167 (1985). [DOI] [PubMed] [Google Scholar]
- 36.Shepherd JK, Grewal SS, Fletcher A, Bill DJ. & Dourish CT Behavioural and pharmacological characterisation of the elevated ‘zero-maze’ as an animal model of anxiety. Psychopharmacology (Berl.) 116, 56–64 (1994). [DOI] [PubMed] [Google Scholar]
- 37.Hall C & Ballachey EL A study of the rat’s behavior in a field. A contribution to method in comparative psychology. Univ. Calif. Publ. Psychol 6, 1–12 (1932). [Google Scholar]
- 38.Ambrogi Lorenzini C, Bucherelli C & Giachetti A Passive and active avoidance behavior in the light-dark box test. Physiol. Behav 32, 687–689 (1984). [DOI] [PubMed] [Google Scholar]
- 39.Simiand J, Keane PE & Morre M The staircase test in mice: a simple and efficient procedure for primary screening of anxiolytic agents. Psychopharmacology (Berl.) 84, 48–53 (1984). [DOI] [PubMed] [Google Scholar]
- 40.Kalueff AV et al. The regular and light-dark Suok tests of anxiety and sensorimotor integration: utility for behavioral characterization in laboratory rodents. Nat. Protoc 3, 129–136 (2008). [DOI] [PubMed] [Google Scholar]
- 41.File SE & Wardill AG The reliability of the hole-board apparatus. Psychopharmacologia 44, 47–51 (1975). [DOI] [PubMed] [Google Scholar]
- 42.Borsini F, Podhorna J & Marazziti D Do animal models of anxiety predict anxiolytic-like effects of antidepressants? Psychopharmacology (Berl.) 163, 121–141 (2002). [DOI] [PubMed] [Google Scholar]
- 43.Degroot A & Nomikos GG Genetic deletion and pharmacological blockade of CB1 receptors modulates anxiety in the shock-probe burying test. Eur. J. Neurosci 20, 1059–1064 (2004). [DOI] [PubMed] [Google Scholar]
- 44.Njung’e K & Handley SL Evaluation of marble-burying behavior as a model of anxiety. Pharmacol. Biochem. Behav 38, 63–67 (1991). [DOI] [PubMed] [Google Scholar]
- 45.Nicolas LB, Kolb Y & Prinssen EPM A combined marble burying-locomotor activity test in mice: a practical screening test with sensitivity to different classes of anxiolytics and antidepressants. Eur. J. Pharmacol 547, 106–115 (2006). [DOI] [PubMed] [Google Scholar]
- 46.Treit D, Pinel JPJ & Fibiger HC Conditioned defensive burying: A new paradigm for the study of anxiolytic agents. Pharmacol. Biochem. Behav 15, 619–626 (1981). [DOI] [PubMed] [Google Scholar]
- 47.Dulawa SC, Holick KA, Gundersen B & Hen R Effects of chronic fluoxetine in animal models of anxiety and depression. Neuropsychopharmacology 29, 1321–1330 (2004). [DOI] [PubMed] [Google Scholar]
- 48.Merali Z, Levac C & Anisman H, Validation of a simple, ethologically relevant paradigm for assessing anxiety in mice. Biol. Psychiatry 54, 552–565 (2003). [DOI] [PubMed] [Google Scholar]
- 49.Sánchez C Stress-induced vocalisation in adult animals. A valid model of anxiety? Eur. J. Pharmacol. 463, 133–143 (2003). [DOI] [PubMed] [Google Scholar]
- 50.Gardner CR Distress vocalization in rat pups. A simple screening method for anxiolytic drugs. J. Pharmacol. Methods 14, 181–187 (1985). [DOI] [PubMed] [Google Scholar]
- 51.File SE Animal models for predicting clinical efficacy of anxiolytic drugs: social behaviour. Neuropsychobiology 13, 55–62 (1985). [DOI] [PubMed] [Google Scholar]
- 52.Viviani D et al. Oxytocin selectively gates fear responses through distinct outputs from the central amygdala. Science 333, 104–107 (2011).Identification of discrete CeA efferent pathways separately regulating behavioral and physiological fear responses.
- 53.Duvarci S & Pare D Amygdala microcircuits controlling learned fear. Neuron 82, 966–980 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Brown S & Schäfer E An investigation into the functions of the occipital and temporal lobes of the monkey’s brain. Philos. Trans. R. Soc. Lond. B 179, 303–327 (1888). [Google Scholar]
- 55.McDonald AJ Cortical pathways to the mammalian amygdala. Prog. Neurobiol 55, 257–332 (1998). [DOI] [PubMed] [Google Scholar]
- 56.Janak PH & Tye KM, From circuits to behaviour in the amygdala. Nature 517, 284–292 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Maren S & Quirk GJ Neuronal signalling of fear memory. Nat. Rev. Neurosci 5, 844–852 (2004). [DOI] [PubMed] [Google Scholar]
- 58.McKernan MG & Shinnick-Gallagher P Fear conditioning induces a lasting potentiation of synaptic currents in vitro. Nature 390, 607–611 (1997). [DOI] [PubMed] [Google Scholar]
- 59.Rogan MT, Stäubli UV & LeDoux JE Fear conditioning induces associative long-term potentiation in the amygdala. Nature 390, 604–607 (1997). [DOI] [PubMed] [Google Scholar]
- 60.LeDoux JE Emotion circuits in the brain. Annu. Rev. Neurosci 23, 155–184 (2000). [DOI] [PubMed] [Google Scholar]
- 61.Tye KM & Janak PH Amygdala neurons differentially encode motivation and reinforcement. J. Neurosci 27, 3937–3945 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Tye KM, Stuber GD, de Ridder B, Bonci A & Janak PH Rapid strengthening of thalamo-amygdala synapses mediates cue-reward learning. Nature 453, 1253–1257 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Namburi P et al. A circuit mechanism for differentiating positive and negative associations. Nature 520, 675–678 (2015).First evidence of largely non-overlapping, projection target-defined populations of neurons in the BLA oppositely encoding valence in fear and reward learning.
- 64.Tye KM & Deisseroth K Optogenetic investigation of neural circuits underlying brain disease in animal models. Nat. Rev. Neurosci 13, 251–266 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Fox AS, Oler JA, Tromp DPM, Fudge JL & Kalin NH Extending the amygdala in theories of threat processing. Trends Neurosci. 38, 319–329 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Felix-Ortiz AC et al. BLA to vHPC inputs modulate anxiety-related behaviors. Neuron 79, 658–664 (2013).Provides the first direct evidence of bidirectional control of anxiety via the BLA projection to vHPC.
- 67.Herry C et al. Switching on and off fear by distinct neuronal circuits. Nature 454, 600–606 (2008). [DOI] [PubMed] [Google Scholar]
- 68.Hübner C, Bosch D, Gall A, Lüthi A & Ehrlich I Ex vivo dissection of optogenetically activated mPFC and hippocampal inputs to neurons in the basolateral amygdala: implications for fear and emotional memory. Front. Behav. Neurosci 8, 64 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Herry C & Johansen JP Encoding of fear learning and memory in distributed neuronal circuits. Nat. Neurosci 17, 1644–1654 (2014). [DOI] [PubMed] [Google Scholar]
- 70.Johansen JP et al. Optical activation of lateral amygdala pyramidal cells instructs associative fear learning. Proc. Natl. Acad. Sci. USA 107, 12692–12697 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Quirk GJ, Repa C & LeDoux JE Fear conditioning enhances short-latency auditory responses of lateral amygdala neurons: parallel recordings in the freely behaving rat. Neuron 15, 1029–1039 (1995). [DOI] [PubMed] [Google Scholar]
- 72.Ciocchi S et al. Encoding of conditioned fear in central amygdala inhibitory circuits. Nature 468, 277–282 (2010)Optogenetic interrogation of amygdala microcircuits controlling learned fear on the basis of neural response profiles (see also ref. 73).
- 73.Haubensak W et al. Genetic dissection of an amygdala microcircuit that gates conditioned fear. Nature 468, 270–276 (2010)Optogenetic dissection of the control of learned fear by genetically defined populations of neurons in the amygdala microcircuit (see also ref. 72).
- 74.Cai H, Haubensak W, Anthony TE & Anderson DJ Central amygdala PKC-δ+ neurons mediate the influence of multiple anorexigenic signals. Nat. Neurosci 17, 1240–1248 (2014).Presents the first causal evidence that a population of CeL neurons mediate inhibition of feeding, and also demonstrates that activity in these cells is not anxiogenic.
- 75.Li H et al. Experience-dependent modification of a central amygdala fear circuit. Nat. Neurosci 16, 332–339 (2013).Presents the first evidence of fear conditioning–induced plasticity in the CeL.
- 76.Penzo MA, Robert V & Li B Fear conditioning potentiates synaptic transmission onto long-range projection neurons in the lateral subdivision of central amygdala. J. Neurosci 34, 2432–2437 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Penzo MA et al. The paraventricular thalamus controls a central amygdala fear circuit. Nature 519, 455–459 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Tovote P, Fadok JP & Lüthi A Neuronal circuits for fear and anxiety. Nat. Rev. Neurosci 16, 317–331 (2015). [DOI] [PubMed] [Google Scholar]
- 79.Wang DV et al. Neurons in the amygdala with response-selectivity for anxiety in two ethologically based tests. PLoS ONE 6, e18739 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Huff ML, Miller RL, Deisseroth K, Moorman DE & LaLumiere RT Posttraining optogenetic manipulations of basolateral amygdala activity modulate consolidation of inhibitory avoidance memory in rats. Proc. Natl. Acad. Sci. USA 110, 3597–3602 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Cullinan WE, Herman JP & Watson SJ Ventral subicular interaction with the hypothalamic paraventricular nucleus: evidence for a relay in the bed nucleus of the stria terminalis. J. Comp. Neurol 332, 1–20 (1993). [DOI] [PubMed] [Google Scholar]
- 82.Dong HW, Petrovich GD & Swanson LW Topography of projections from amygdala to bed nuclei of the stria terminalis. Brain Res. Brain Res. Rev 38, 192–246 (2001). [DOI] [PubMed] [Google Scholar]
- 83.Stamatakis AM et al. Amygdala and bed nucleus of the stria terminalis circuitry: implications for addiction-related behaviors. Neuropharmacology 76 (part B): 320–328 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Poulos AM, Ponnusamy R, Dong H-W & Fanselow MS Compensation in the neural circuitry of fear conditioning awakens learning circuits in the bed nuclei of the stria terminalis. Proc. Natl. Acad. Sci. USA 107, 14881–14886 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Jennings JH et al. Distinct extended amygdala circuits for divergent motivational states. Nature 496, 224–228 (2013)Presents causal evidence of functionally opposed, neurochemically defined populations of projection neurons within a single efferent pathway.
- 86.Christianson JP et al. Safety signals mitigate the consequences of uncontrollable stress via a circuit involving the sensory insular cortex and bed nucleus of the stria terminalis. Biol. Psychiatry 70, 458–464 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.van Strien NM, Cappaert NLM & Witter MP The anatomy of memory: an interactive overview of the parahippocampal-hippocampal network. Nat. Rev. Neurosci 10, 272–282 (2009). [DOI] [PubMed] [Google Scholar]
- 88.Witter MP, Canto CB, Couey JJ, Koganezawa N & O’Reilly KC Architecture of spatial circuits in the hippocampal region. Phil. Trans. R. Soc. Lond. B 369, 20120515 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Amaral D & Lavenex P in The Hippocampus Book (eds. Anderson P, Morris R, Amaral D, Bliss T & O’Keefe J) 37–114 (Oxford Univ. Press, 2007). [Google Scholar]
- 90.Sparta DR et al. Inhibition of projections from the basolateral amygdala to the entorhinal cortex disrupts the acquisition of contextual fear. Front. Behav. Neurosci. 8, 129 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Kheirbek MA et al. Differential control of learning and anxiety along the dorsoventral axis of the dentate gyrus. Neuron 77, 955–968 (2013).Using optogenetics, clarifies the specific influence of dorsal and ventral dentate gyrus to exploration and encoding of fear association versus innate anxiety, respectively.
- 92.Mori M, Abegg MH, Gähwiler BH & Gerber U A frequency-dependent switch from inhibition to excitation in a hippocampal unitary circuit. Nature 431, 453–456 (2004). [DOI] [PubMed] [Google Scholar]
- 93.Risold PY & Swanson LW Structural evidence for functional domains in the rat hippocampus. Science 272, 1484–1486 (1996). [DOI] [PubMed] [Google Scholar]
- 94.Risold PY & Swanson LW Connections of the rat lateral septal complex. Brain Res. Brain Res. Rev 24, 115–195 (1997). [DOI] [PubMed] [Google Scholar]
- 95.Trent NL & Menard JL The ventral hippocampus and the lateral septum work in tandem to regulate rats’ open-arm exploration in the elevated plus-maze. Physiol. Behav 101, 141–152 (2010). [DOI] [PubMed] [Google Scholar]
- 96.Henry B, Vale W & Markou A The effect of lateral septum corticotropin-releasing factor receptor 2 activation on anxiety is modulated by stress. J. Neurosci 26, 9142–9152 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Anthony TE et al. Control of stress-induced persistent anxiety by an extra-amygdala septohypothalamic circuit. Cell 156, 522–536 (2014)First optogenetic interrogation of the septohippocampal system in anxiety, which has not been otherwise evaluated using modern tools.
- 98.Gross JJ The emerging field of emotion regulation: an integrative review. Rev. Gen. Psychol 2, 271–299 (1998). [Google Scholar]
- 99.Markram H et al. Interneurons of the neocortical inhibitory system. Nat. Rev Neurosci 5, 793–807 (2004). [DOI] [PubMed] [Google Scholar]
- 100.Van De Werd HJJM & Uylings HBM Comparison of (stereotactic) parcellations in mouse prefrontal cortex. Brain Struct. Funct 219, 433–459 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Groenewegen HJ, Wright CI & Uylings HB The anatomical relationships of the prefrontal cortex with limbic structures and the basal ganglia. J. Psychopharmacol 11, 99–106 (1997). [DOI] [PubMed] [Google Scholar]
- 102.Kim MJ et al. The structural and functional connectivity of the amygdala: from normal emotion to pathological anxiety. Behav Brain Res. 223, 403–410 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Ochsner KN, Bunge SA, Gross JJ & Gabrieli JDE Rethinking feelings: an fMRI study of the cognitive regulation of emotion. J. Cogn. Neurosci 14, 1215–1229 (2002). [DOI] [PubMed] [Google Scholar]
- 104.Livneh U & Paz R Amygdala-prefrontal synchronization underlies resistance to extinction of aversive memories. Neuron 75, 133–142 (2012). [DOI] [PubMed] [Google Scholar]
- 105.Senn V et al. Long-range connectivity defines behavioral specificity of amygdala neurons. Neuron 81, 428–437 (2014). [DOI] [PubMed] [Google Scholar]
- 106.Burgos-Robles A, Vidal-Gonzalez I & Quirk GJ Sustained conditioned responses in prelimbic prefrontal neurons are correlated with fear expression and extinction failure. J. Neurosci 29, 8474–8482 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Courtin J et al. Prefrontal parvalbumin interneurons shape neuronal activity to drive fear expression. Nature 505, 92–96 (2014)Original causal demonstration that mPFC microcircuit dynamics locally produce the mPFC theta rhythm to control the timing of fear behaviors.
- 108.Milad MR & Quirk GJ Neurons in medial prefrontal cortex signal memory for fear extinction. Nature 420, 70–74 (2002). [DOI] [PubMed] [Google Scholar]
- 109.Baeg EH et al. Fast spiking and regular spiking neural correlates of fear conditioning in the medial prefrontal cortex of the rat. Cereb. Cortex 11, 441–451 (2001). [DOI] [PubMed] [Google Scholar]
- 110.Chang C, Berke JD & Maren S Single-unit activity in the medial prefrontal cortex during immediate and delayed extinction of fear in rats. PLoS ONE 5, e11971 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Holmes A et al. Chronic alcohol remodels prefrontal neurons and disrupts NMDAR-mediated fear extinction encoding. Nat. Neurosci 15, 1359–1361 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Maroun M, Kavushansky A, Holmes A, Wellman C & Motanis H Enhanced extinction of aversive memories by high-frequency stimulation of the rat infralimbic cortex. PLoS ONE 7, e35853 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Arruda-Carvalho M & Clem RL Pathway-selective adjustment of prefrontal-amygdala transmission during fear encoding. J. Neurosci 34, 15601–15609 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Do-Monte FH, Quiñones-Laracuente K & Quirk GJ A temporal shift in the circuits mediating retrieval of fear memory. Nature 519, 460–463 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Likhtik E & Paz R Amygdala-prefrontal interactions in (mal)adaptive learning. Trends Neurosci. 38, 158–166 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Grace AA & Rosenkranz JA Regulation of conditioned responses of basolateral amygdala neurons. Physiol. Behav 77, 489–493 (2002). [DOI] [PubMed] [Google Scholar]
- 117.Likhtik E, Pelletier JG, Paz R & Paré D Prefrontal control of the amygdala. J. Neurosci 25, 7429–7437 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Rosenkranz JA, Moore H & Grace AA The prefrontal cortex regulates lateral amygdala neuronal plasticity and responses to previously conditioned stimuli. J. Neurosci 23, 11054–11064 (2003).Early demonstration, using technically challenging in vivo intracellular recordings, that mPFC can regulate affective processing via inhibition of amygdala activity.
- 119.Calhoon GG & O’Donnell P Closing the gate in the limbic striatum: prefrontal suppression of hippocampal and thalamic inputs. Neuron 78, 181–190 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Britt JP et al. Synaptic and behavioral profile of multiple glutamatergic inputs to the nucleus accumbens. Neuron 76, 790–803 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Mackintosh NJ Neurobiology, psychology and habituation. Behav Res. Ther 25, 81–97 (1987). [DOI] [PubMed] [Google Scholar]
- 122.Byrne JH, Castellucci VF, Carew TJ. & Kandel ER Stimulus-response relations and stability of mechanoreceptor and motor neurons mediating defensive gill-withdrawal reflex in Aplysia. J. Neurophysiol 41, 402–417 (1978). [DOI] [PubMed] [Google Scholar]
- 123.Balleine BW Neural bases of food-seeking: affect, arousal and reward in corticostriatolimbic circuits. Physiol. Behav 86, 717–730 (2005). [DOI] [PubMed] [Google Scholar]
- 124.Orsini CA, Moorman DE, Young JW, Setlow B & Floresco SB Neural mechanisms regulating different forms of risk-related decision-making: insights from animal models. Neurosci. Biobehav. Rev doi: 10.1016/j.neubiorev.2015.04.009 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Phillips PEM, Walton ME & Jhou TC Calculating utility: preclinical evidence for cost-benefit analysis by mesolimbic dopamine. Psychopharmacology (Berl.) 191, 483–495 (2007). [DOI] [PubMed] [Google Scholar]
- 126.Opland DM, Leinninger GM & Myers MG Modulation of the mesolimbic dopamine system by leptin. Brain Res. 1350, 65–70 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Sternson SM Hypothalamic survival circuits: blueprints for purposive behaviors. Neuron 77, 810–824 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Nieh EH et al. Decoding neural circuits that control compulsive sucrose seeking. Cell 160, 528–541 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Adhikari A, Topiwala MA & Gordon JA Synchronized activity between the ventral hippocampus and the medial prefrontal cortex during anxiety. Neuron 65, 257–269 (2010).Early demonstration that vHPC (but not dorsal HPC) theta rhythm synchrony with mPFC increases during anxiety.
- 130.Vertes RP & Kocsis B Brainstem-diencephalo-septohippocampal systems controlling the theta rhythm of the hippocampus. Neuroscience 81, 893–926 (1997). [DOI] [PubMed] [Google Scholar]
- 131.McNaughton N, Kocsis B & Hajós M Elicited hippocampal theta rhythm: a screen for anxiolytic and procognitive drugs through changes in hippocampal function? Behav. Pharmacol 18, 329–346 (2007). [DOI] [PubMed] [Google Scholar]
- 132.Sirota A et al. Entrainment of neocortical neurons and gamma oscillations by the hippocampal theta rhythm. Neuron 60, 683–697 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Seidenbecher T, Laxmi TR, Stork O & Pape H-C Amygdalar and hippocampal theta rhythm synchronization during fear memory retrieval. Science 301, 846–850 (2003). [DOI] [PubMed] [Google Scholar]
- 134.Lesting J et al. Directional theta coherence in prefrontal cortical to amygdalo-hippocampal pathways signals fear extinction. PLoS ONE 8, e77707 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Schoenfeld TJ. et al. Gap junctions in the ventral hippocampal-medial prefrontal pathway are involved in anxiety regulation. J. Neurosci. 34, 15679–15688 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Adhikari A, Topiwala MA & Gordon JA Single units in the medial prefrontal cortex with anxiety-related firing patterns are preferentially influenced by ventral hippocampal activity. Neuron 71, 898–910 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Lesting J et al. Patterns of coupled theta activity in amygdala-hippocampal-prefrontal cortical circuits during fear extinction. PLoS ONE 6, e21714 (2011).Initial investigation of the macrocircuit dynamics underlying fear learning, characterized by coordinated oscillatory activity among the amygdala, hippocampus and PFC.
- 138.Stujenske JM, Likhtik E, Topiwala MA & Gordon JA Fear and safety engage competing patterns of theta-gamma coupling in the basolateral amygdala. Neuron 83, 919–933 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Tejeda HA & O’Donnell P Amygdala inputs to the prefrontal cortex elicit heterosynaptic suppression of hippocampal inputs. J. Neurosci 34, 14365–14374 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Mogenson GJ, Jones DL & Yim CY From motivation to action: functional interface between the limbic system and the motor system. Prog. Neurobiol 14, 69–97 (1980). [DOI] [PubMed] [Google Scholar]
- 141.Carvalho MC, Albrechet-Souza L, Masson S & Brandão ML Changes in the biogenic amine content of the prefrontal cortex, amygdala, dorsal hippocampus, and nucleus accumbens of rats submitted to single and repeated sessions of the elevated plus-maze test. Braz. J. Med. Biol. Res 38, 1857–1866 (2005). [DOI] [PubMed] [Google Scholar]
- 142.Collier DA et al. A novel functional polymorphism within the promoter of the serotonin transporter gene: possible role in susceptibility to affective disorders. Mol. Psychiatry 1, 453–460 (1996). [PubMed] [Google Scholar]
- 143.Lesch K-P et al. Association of anxiety-related traits with a polymorphism in the serotonin transporter gene regulatory region. Science 274, 1527–1531 (1996). [DOI] [PubMed] [Google Scholar]
- 144.Corbetta M & Shulman GL Control of goal-directed and stimulus-driven attention in the brain. Nat. Rev. Neurosci 3, 201–215 (2002). [DOI] [PubMed] [Google Scholar]
- 145.Houk JC, Adams JL & Barto AG in Models of Information Processing in the Basal Ganglia (eds. Houk JC, Davis JL & Beiser DG) 249–270 (MIT Press, 1995). [Google Scholar]
- 146.Joel D, Niv Y & Ruppin E Actor-critic models of the basal ganglia: new anatomical and computational perspectives. Neural Netw. 15, 535–547 (2002). [DOI] [PubMed] [Google Scholar]
- 147.Sugrue LP, Corrado GS & Newsome WT Choosing the greater of two goods: neural currencies for valuation and decision making. Nat. Rev. Neurosci. 6, 363–375 (2005). [DOI] [PubMed] [Google Scholar]
- 148.Nabavi S et al. Engineering a memory with LTD and LTP. Nature 511, 348–352 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Butler AC, Chapman JE, Forman EM & Beck AT The empirical status of cognitive-behavioral therapy: a review of meta-analyses. Clin. Psychol. Rev 26, 17–31 (2006). [DOI] [PubMed] [Google Scholar]
- 150.Sporns O, Tononi G & Kötter R The human connectome: a structural description of the human brain. PLoS Comput. Biol 1, e42 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]