Abstract
Background/objective:
Endurance exercise can improve memory function in persons with multiple sclerosis (pwMS), but the effects on hippocampal functioning are currently unknown. We investigated the effects of a running intervention on memory and hippocampal functional connectivity in pwMS.
Methods/results:
Memory and resting-state functional magnetic resonance imaging (fMRI) data were collected in a running intervention (n = 15) and waitlist group (n = 14). Visuospatial memory improvement was correlated to increased connectivity between the hippocampus and the default-mode network (DMN) in the intervention group only.
Conclusion:
As a result of endurance exercise, improvements in visuospatial memory may be mediated by a stronger functional embedding of the hippocampus in the DMN.
Keywords: Multiple sclerosis, cognitive rehabilitation, endurance exercise, functional connectivity, hippocampus, default-mode network
Introduction
Cognitive deficits occur in 43%–70% of the people with multiple sclerosis (pwMS), with memory being one of the most frequently impaired domains.1 A recent study showed that a 12-week community-located running training improved visuospatial memory in pwMS with mild disability.2 Animal work demonstrated exercise-induced increases in neurogenesis, angiogenesis, and trophic factor signaling in the hippocampus, a region crucial for memory function. In addition, studies in pwMS reported associations between cardiorespiratory fitness and hippocampal volume, thereby also suggesting a beneficial effect of endurance exercise on the hippocampus and memory function.3,4
In addition to the structural characteristics of the hippocampus, memory function is also thought to be governed by the functional connections between the hippocampus and memory-related networks, most importantly the default-mode network (DMN). Furthermore, better memory function in aging individuals is related to higher functional connectivity (FC) within the DMN and between the hippocampus and the DMN. In contrast, persons with Alzheimer’s disease show reduced FC in this network.5 Yet, the effect of running training (i.e. endurance exercise) on FC of the hippocampus with the DMN has not been studied before.
This study aimed to expand the previous finding of improved visuospatial memory after a 12-week running intervention by investigating changes in hippocampus–DMN resting-state FC in pwMS, being the first pilot study to specifically focus on the relationship between endurance exercise, visuospatial memory function, and hippocampal connectivity in pwMS.
Methods
Participants
A total of 29 pwMS, representing a subsample from a larger trial, were included in this work. For detailed inclusion criteria and procedures, see supplementary methods and the work by Feys et al.2 Participants were randomized into the intervention (n = 15) or the waiting-list control group (n = 14). The study was approved by the involved institutional review boards. Written informed consent was obtained from all participants prior to participation.
Experimental design and intervention
The intervention group completed a 12-week, community-located “start-to-run” program, in which participants followed a gradually increasing running training three times a week working toward a continuous 5-km run (see Figure S1 for training details). Before and after 12 weeks, participants’ walking capacity was assessed with the 6-minute walk test (6MWT), visuospatial and verbal memory were measured with the spatial recall test (SPART),6 and the selective reminding test (SRT).7 Magnetic resonance imaging (MRI) data were also collected.
Functional MRI data collection and analysis
MRI scanning (3 T) included a high-resolution three-dimensional (3D)-T1 weighted sequence, a 3D fluid-attenuated inversion recovery, and a resting-state functional magnetic resonance imaging (fMRI) scan.
FC values were computed as Pearson correlations of the individual region’s time series and then averaged over the bilateral hippocampus and the DMN. The DMN was defined as 38 cortical regions, spanning bilateral medial prefrontal areas, temporal and parietal regions, and posterior cingulate cortex. For detailed acquisition, preprocessing, and analysis, see supplementary material.
Statistics
Results on the SPART, SRT, and FC values were analyzed using 2 × 2 analyses of variance (ANOVAs) with group (intervention vs control) as between-subjects factor and time (pre vs post) as within-group factor. Delta scores (post minus pre; Δ) were calculated for the SRT, SPART, and for hippocampus–DMN FC. Pearson correlation between SPART and FC delta scores was calculated. For specificity, other cognitive test scores were post hoc correlated to the hippocampus–DMN FC. Statistical analyses were performed in SPSS 22.0 (Armonk, NY, USA).
Results
Demographics
Both groups did not differ on gender and disease duration; the intervention group was slightly younger and had a lower body mass index (BMI) (p < 0.05; Table 1).
Table 1.
Demographic and clinical measures.
| Intervention group (n = 15) | Control group (n = 14) | p value | |
|---|---|---|---|
| Age (years) | 38.1 (8.1) | 44.7 (7.5) | 0.028 |
| Gender (female/male) | 15/0 | 13/1 | 0.224 |
| Disease duration (years) | 9.9 (6.1) | 8.8 (5.8) | 0.641 |
| Body mass index | 23.7 (5.9) | 28.1 (3.3) | 0.026 |
| Handedness (right/left) | 13/2 | 13/1 | 0.584 |
| 6MWT pre (m) | 589.1 (56.2) | 577.2 (56.3) | 0.575 |
| 6MWT post (m) | 594.3 (49.7) | 578.6 (67.2) | 0.479 |
| SPART prea | 43.0 [41.0–48.0] | 44.5 [42.0–47.5] | 0.621 |
| SPART posta | 48.0 [44.0–53.0] | 43.5 [38.75–47.25] | 0.046 |
| SRT prea | 51.5 [43.75–54.0] | 51.5 [47.5–53.0] | –b |
| SRT posta | 50.0 [38.0–56.0] | 52.5 [42.75–59.25] | –b |
| NGMV (mL) | 810.3 (43.5) | 798.1 (21.4) | 0.344 |
| NHipV (mL) | 10.7 (7.5) | 10.8 (10.3) | 0.710 |
| Lesion volume (mL) | 6.0 [3.8–6.7] | 4.4 [3.7–6.2] | 0.505 |
6MWT = 6-minute walk test; SPART = spatial recall test; NGMV = normalized gray matter volume; NHipV = normalized bilateral hippocampus volume.
Values are mean (SD) unless otherwise specified. Values in bold significant at p<.05.
Median [IQR]; tested with Mann–Whitney U due to non-normal distribution.
No group comparisons performed due to absence of significant group*time interaction.
Visuospatial memory
The SPART showed a significant group*time interaction effect (F(1,27) = 5.82, p = 0.023, partial eta squared = 0.177). Post hoc t tests indicated that the intervention group improved significantly on visuospatial memory from pre to post (Δ score = 4.6(7.3), t(14) = −2.21, p = 0.045), whereas the control group did not (Δ score = −2.5(7.6); p > 0.05). These results became borderline significant after correction for age: F(1,26) = 2.99, p = 0.095, partial eta squared = 0.103. No group*time interaction was noted on the SRT.
FC
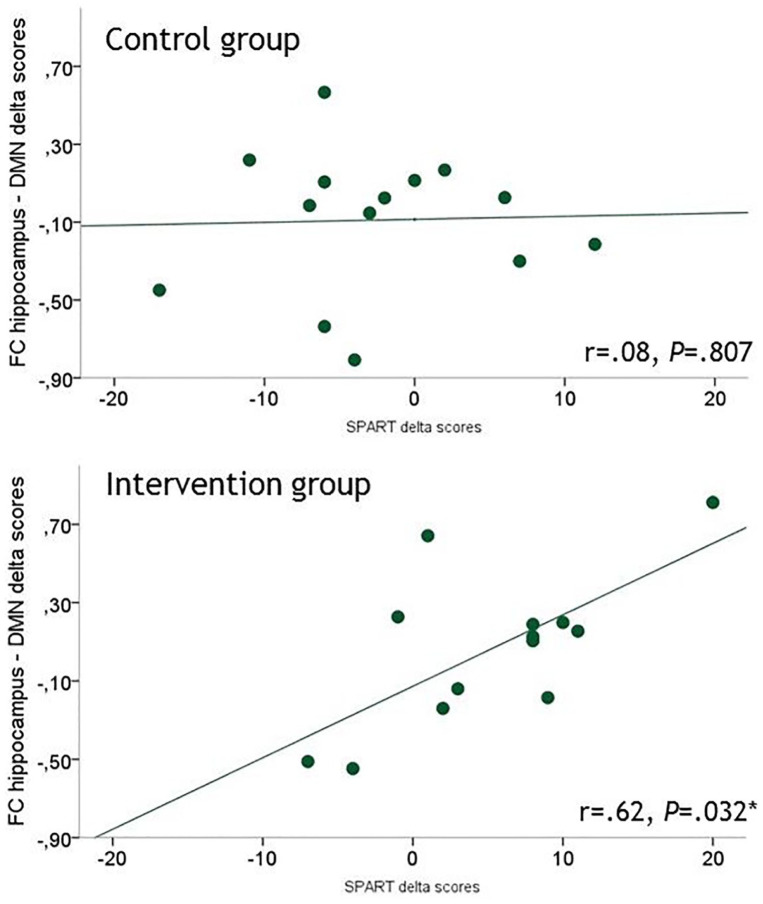
No group differences were observed in hippocampus–DMN FC at both time points. However, an association was found between the Δ-SPART and the Δ-hippocampus–DMN FC (r = 0.62, p = 0.032 corrected for age), indicating that an improvement on the SPART was related to an increase in FC of the hippocampus with the DMN. This association was only observed in the intervention group and not with the SRT or any of the other cognitive tests (Figure 1 and Table S2).
Figure 1.
Scatterplots of Δ-SPART versus Δ-hippocampus–DMN functional connectivity showing a relationship in the intervention group, but not in the control group. Relationships are partial correlations, corrected for age.
*Significant at p < 0.05.
Discussion
The improvement in visuospatial memory after a 12-week community-located running intervention correlated positively with increased hippocampus–DMN FC in mildly disabled pwMS. This could suggest that the effects of running on visuospatial memory are mediated by changes in the connectivity between the hippocampus and DMN.
The hippocampus is well known for its role in visuospatial memory and has extensive connections with the DMN, both structurally and functionally.5 Interestingly, increased hippocampal FC with DMN regions has previously been related to better memory function in older adults, emphasizing the importance of hippocampus–DMN FC in memory function.8
Physical exercise has been shown to improve memory function in MS, which has also been related to increased hippocampal and DMN FC in elderly adults.2,9,10 This could indicate that the functional network has reorganized, potentially due to changes in perfusion, trophic factor signaling or increased hippocampal neurogenesis.4,10 It is also possible that global functional integration is involved, in addition to the specific connectivity between the hippocampus and the DMN. Due to power, this hypothesis could not be examined here and may also explain the lack of a significant improvement on SPART and SRT scores after correcting for age. Alternatively, it might be that FC changes are more sensitive to exercise effects and that interventions of longer duration or higher intensity are needed to realize changes in general hippocampal memory function. Another limitation is the lack of Expanded Disability Status Scale (EDSS) scores and MS phenotypes, although the 6MWT scores and inclusion criteria assured that only mildly disabled pwMS were included.
In conclusion, we demonstrated that improvements in visuospatial memory function are correlated with the functional embedding of the hippocampus in the DMN after 12 weeks of community-located endurance exercise in mildly disabled pwMS. Next, one should investigate whether this stronger embedding of the hippocampus in the DMN persists and whether this might result in a more resilient network formation supportive of memory performance.
Supplemental Material
Supplemental material, MSJ863644_supplemental_material for A pilot study of the effects of running training on visuospatial memory in MS: A stronger functional embedding of the hippocampus in the default-mode network? by Marijn Huiskamp, Lousin Moumdjian, Paul van Asch, Veronica Popescu, Menno Michiel Schoonheim, Martijn D Steenwijk, Ellen Vanzeir, Bart van Wijmeersch, Jeroen JG Geurts, Peter Feys and Hanneke E Hulst in Multiple Sclerosis Journal
Acknowledgments
The non-for-profit organization Move to Sport (www.movetosport.be) initiated the study. The authors acknowledge Prof. Dr P. Parizel (UZA Antwerp) for facilitation of neuroimaging at UZA Wilrijk and Novartis and the MS Network Limburg for funding related operational costs.
Footnotes
Declaration of Conflicting Interests: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: J.J.G.G. has received study grants from Biogen Idec, Sanofi Genzyme, and Novartis Pharma and is Editor for Europe at Multiple Sclerosis Journal. P.F. is steering committee member of Neurocompass, participated in advisory board meetings of BIOGEN IDEC, and received teaching honoraria for EXCEMED and PARADIGMS. M.M.S. serves on the editorial board of Frontiers of Neurology, receives research support from the Dutch MS research Foundation (grant number 13-820), and has received compensation for consulting services or speaker honoraria from EXCEMED, Genzyme, and Biogen. H.E.H. receives research support from the Dutch MS Research Foundation (grant number 08-648) and serves as a consultant for Genzyme, Merck-Serono, Teva Pharmaceuticals, and Novartis.
Funding: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
ORCID iDs: Menno Michiel Schoonheim  https://orcid.org/0000-0002-2504-6959
https://orcid.org/0000-0002-2504-6959
Peter Feys  https://orcid.org/0000-0002-5680-5495
https://orcid.org/0000-0002-5680-5495
Supplemental material: Supplemental material for this article is available online.
Contributor Information
Marijn Huiskamp, Department of Anatomy & Neurosciences, MS Center Amsterdam, Amsterdam UMC, Vrije Universiteit Amsterdam, Amsterdam, The Netherlands.
Lousin Moumdjian, REVAL Rehabilitation Research Center, BIOMED, Faculty of Rehabilitation Sciences, Hasselt University, Hasselt, Belgium; Institute of Psychoacoustics and Electronic Music (IPEM), Faculty of Arts and Philosophy, Gent University, Gent, Belgium.
Paul van Asch, Fit up Physiotherapy Center, Kontich, Belgium.
Veronica Popescu, REVAL Rehabilitation Research Center, BIOMED, Faculty of Rehabilitation Sciences, Hasselt University, Hasselt, Belgium; Rehabilitation and MS Centre Overpelt, Overpelt, Belgium.
Menno Michiel Schoonheim, Department of Anatomy & Neurosciences, MS Center Amsterdam, Amsterdam UMC, Vrije Universiteit Amsterdam, Amsterdam, The Netherlands.
Martijn D Steenwijk, Department of Anatomy & Neurosciences, MS Center Amsterdam, Amsterdam UMC, Vrije Universiteit Amsterdam, Amsterdam, The Netherlands.
Ellen Vanzeir, REVAL Rehabilitation Research Center, BIOMED, Faculty of Rehabilitation Sciences, Hasselt University, Hasselt, Belgium.
Bart van Wijmeersch, REVAL Rehabilitation Research Center, BIOMED, Faculty of Rehabilitation Sciences, Hasselt University, Hasselt, Belgium; Rehabilitation and MS Centre Overpelt, Overpelt, Belgium.
Jeroen JG Geurts, Department of Anatomy & Neurosciences, MS Center Amsterdam, Amsterdam UMC, Vrije Universiteit Amsterdam, Amsterdam, The Netherlands.
Peter Feys, REVAL Rehabilitation Research Center, BIOMED, Faculty of Rehabilitation Sciences, Hasselt University, Hasselt, Belgium.
Hanneke E Hulst, Department of Anatomy & Neurosciences, MS Center Amsterdam, Amsterdam UMC, Vrije Universiteit Amsterdam, Amsterdam, The Netherlands.
References
- 1. Benedict RHB, Cookfair D, Gavett R, et al. Validity of the minimal assessment of cognitive function in multiple sclerosis (MACFIMS). J Int Neuropsychol Soc 2006; 12: 549–558. [DOI] [PubMed] [Google Scholar]
- 2. Feys P, Moumdjian L, Van Halewyck F, et al. Effects of an individual 12-week community-located “start-to-run” program on physical capacity, walking, fatigue, cognitive function, brain volumes, and structures in persons with multiple sclerosis. Mult Scler J 2019; 25: 92–103. [DOI] [PubMed] [Google Scholar]
- 3. Motl RW, Pilutti LA, Hubbard EA, et al. Cardiorespiratory fitness and its association with thalamic, hippocampal, and basal ganglia volumes in multiple sclerosis. Neuroimage Clin 2015; 7: 661–666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Rocca MA, Barkhof F, De Luca J, et al. The hippocampus in multiple sclerosis. Lancet Neurol 2018; 17(10): 918–926. [DOI] [PubMed] [Google Scholar]
- 5. Jeong W, Chung CK, Kim JS. Episodic memory in aspects of large-scale brain networks. Front Hum Neurosci 2015; 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Rao SM, Hammeke T, McQuillen MP, et al. Memory disturbance in chronic progressive multiple sclerosis. Arch Neurol 1984; 41(6): 625–631. [DOI] [PubMed] [Google Scholar]
- 7. Buschke H. Selective reminding for analysis of memory and learning. J Verbal Learning Verbal Behav 1973; 12: 543–550. [Google Scholar]
- 8. Wang L, Laviolette P, O’Keefe K, et al. Intrinsic connectivity between the hippocampus and posteromedial cortex predicts memory performance in cognitively intact older individuals. Neuroimage 2010; 51(2): 910–917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Zimmer P, Bloch W, Schenk A, et al. High-intensity interval exercise improves cognitive performance and reduces matrix metalloproteinases-2 serum levels in persons with multiple sclerosis: A randomized controlled trial. Mult Scler 2018; 24(12): 1635–1644. [DOI] [PubMed] [Google Scholar]
- 10. Voss MW, Erickson KI, Prakash RS, et al. Functional connectivity: A source of variance in the association between cardiorespiratory fitness and cognition. Neuropsychologia 2010; 48(5): 1394–1406. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, MSJ863644_supplemental_material for A pilot study of the effects of running training on visuospatial memory in MS: A stronger functional embedding of the hippocampus in the default-mode network? by Marijn Huiskamp, Lousin Moumdjian, Paul van Asch, Veronica Popescu, Menno Michiel Schoonheim, Martijn D Steenwijk, Ellen Vanzeir, Bart van Wijmeersch, Jeroen JG Geurts, Peter Feys and Hanneke E Hulst in Multiple Sclerosis Journal