Abstract
Flow-mediated slowing (FMS), defined as the minimum pulse wave velocity (PWVmin) during reactive hyperemia, is potentially a simple, user-objective test for examining endothelial function. The purpose of the current study was to determine the effects of a known endothelial dysfunction protocol on arm PWV and PWVmin. Complete data were successfully collected in 22 out of 23 healthy adults (23.8 years [SD 4.1], 16 F, 22.8 kg/m2 [SD 2.8]). Local endothelial dysfunction was induced by increasing retrograde shear stress in the upper arm, through inflation of a distal (forearm) tourniquet to 75 mmHg, for 30 min. Pre- and post-endothelial dysfunction, PWV was measured followed by simultaneous assessment of PWVmin and flow-mediated dilation (FMD). PWV was measured between the upper arm and wrist using an oscillometric device, and brachial FMD using ultrasound. FMD (%) and PWVmin (m/s) were calculated as the maximum increase in diameter and minimum PWV during reactive hyperemia, respectively. Endothelial dysfunction resulted in a large effect size (ES) decrease in FMD (∆ = −3.10%; 95% CI: –4.15, –2.05; ES = −1.3), and a moderate increase in PWV (∆ = 0.38 m/s; 95% CI: 0.07, 0.69; ES = 0.5) and PWVmin (∆ = 0.16 m/s; 95% CI: 0.05, 0.28; ES = 0.6). There was a large intra-individual (pre- vs post-endothelial dysfunction) association between FMD and PWVmin (r = −0.61; 95% CI: –0.82, –0.24). In conclusion, acute change in PWV and PWVmin are at least partially driven by changes in endothelial function.
Keywords: arterial stiffness, endothelial dysfunction, flow-mediated dilation, measurement validity, retrograde shear stress
Introduction
Vascular homeostasis, and therefore cardiovascular disease risk, is partly governed by the vascular endothelium.1,2 The gold-standard noninvasive approach for assessing endothelial function is the ultrasound-based flow-mediated dilation (FMD) test.3,4 The FMD test measures an artery’s vasodilatory response to increased shear stress, where increased shear stress is induced by reactive hyperemia following 5 min of downstream ischemia.3,4 While the FMD test is highly valid, it is technically challenging, operator-dependent, and requires high levels of operator training.3,4 A simpler, user-objective test would be of high value to both epidemiological and physiological investigations. One potential test is the novel flow-mediated slowing (FMS) technique.
FMS may be defined as the minimum pulse wave velocity (PWVmin) during reactive hyperemia, with a lower minimum thought to represent greater endothelial function.5–9 PWV, which is the speed at which the forward pressure wave travels from a proximal to distal arterial segment, is considered the ‘gold-standard’ measure of arterial stiffness.10,11 However, the assessment of PWV during reactive hyperemia can also be used to reflect endothelial function.12–16 According to the Moens–Korteweg equation, PWV is directly proportional to the arterial wall width and Young’s modulus, and inversely proportional to blood viscosity and vessel diameter.17 Vessel diameter increases during FMD, due to endothelium-dependent vasodilation.3,18 The endothelium-dependent increase in diameter would be expected to result in a reciprocal decline in PWV. However, while FMD and PWV are the most widely reported noninvasive assessments of endothelial function and arterial stiffness, respectively, FMS has garnered little attention.5–9 Moreover, none of the known studies have investigated whether FMS is able to track acute changes in endothelial function.
Endothelial function naturally fluctuates throughout the day and is enhanced or impaired by external and internal factors which alter the local hemodynamic environment.2,19–23 For example, while unidirectional shear stress enhances endothelial function,19 oscillatory shear stress can impair endothelial function.19–22 A model that we20 and others21,22 have used to increase retrograde, and subsequently oscillatory shear stress, and acutely impair endothelial function, is the inflation of a tourniquet to a low-moderate pressure (50–75 mmHg) distal to the arterial segment of interest for 20-30 min. The down-stream resistance, as the result of tourniquet inflation, increases retrograde and oscillatory shear stress. In turn, the oscillatory shear stress leads to diminished FMD (endothelial function).21,22 This model can be used to confirm whether PWV and FMS are able to similarly track acute impairments in endothelial function. Therefore, the objective of the current study was to determine the effects of acute local endothelial dysfunction, induced by increasing retrograde shear stress for 30 min in the upper arm, on arm PWV and PWVmin. It was hypothesized that acute local endothelial dysfunction would increase PWV and PWVmin.
Methods
This study is reported in accordance with CONSORT (Consolidated Standards of Reporting Trials) guidelines.24 Ethical approval was obtained from the University of North Carolina at Chapel Hill Institutional Review Board, and all participants provided written informed consent prior to participating in the study.
Participants
Twenty-three young (18–35 years), healthy individuals were recruited from the host institution, a large state university. A healthy population sample was recruited to mitigate the risk of age or disease-related influences on the primary outcomes. Participants were excluded if they were pregnant, reported any known cardio-metabolic disorders, were taking medications known to affect cardiovascular function, or reported cigarette smoking.
Experimental design
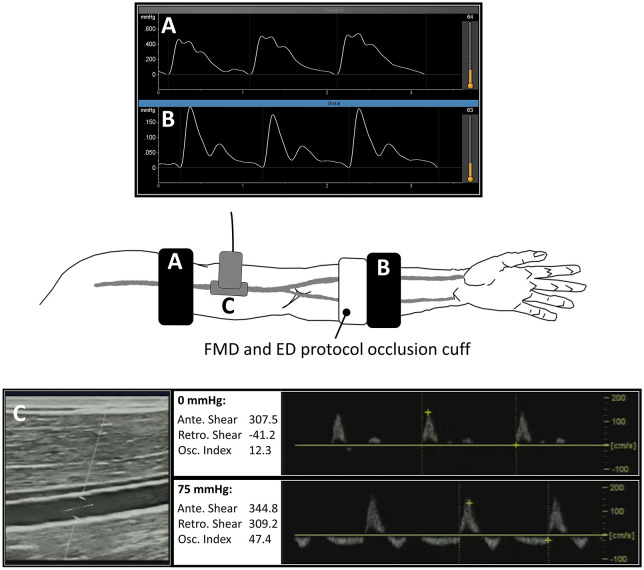
This was a quasi-experimental, pretest–post-test design in which FMD and PWVmin were assessed pre- and post-acute local endothelial dysfunction. Following a familiarization visit, all measurements were collected on a single occasion in an environmentally controlled laboratory (average: 22°C, 50% humidity, 748 mmHg). Participants arrived between 0600 and 1000, having fasted for 12 h, consuming only water, and having refrained from supplement intake that morning. Additionally, participants were asked to avoid strenuous physical activity and alcohol for 24 h prior to experimentation. Following anthropometric assessments, participants were asked to rest quietly and supine for 20 min with both arms resting at right angles to the body and at heart level.25 During the 20-min rest period a blood pressure cuff attached to the SphygmoCor XCEL (AtCor Medical, Sydney, Australia) device was fitted to the right upper arm to permit collection of control hemodynamic assessments. Primary outcome apparatus, including ultrasound (LOGIQ P6; GE Healthcare, Wauwatosa, WI, USA) for FMD and Vicorder (Arterial Stiffness Model; SMT Medical, Wuerzburg, Germany) for PWV/PWVmin, were positioned on the left arm (Figure 1). The quality of ultrasound and Vicorder pressure waveform signals were adjudicated, and adjustments were made if the signals were not optimal.
Figure 1.
Experimental set-up.
To measure PWV at baseline, and then the minimum PWV during reactive hyperemia (FMS), arterial pressure waveforms were simultaneously captured at proximal (A) and distal (B) sites using an oscillometric device. Duplex Doppler ultrasound was used to measure brachial artery FMD, and to capture pulsed Doppler waveforms (C) at baseline (0 mmHg) and during the ED protocol (75 mmHg).
Ante, antegrade; ED, endothelial dysfunction; FMD, flow-mediated dilation; FMS, flow-mediated slowing; Osc, oscillatory; PWV, pulse wave velocity; Retro, retrograde.
Following a 20-min rest, the baseline brachial diameter (Dbase) and PWV were recorded. Subsequently, the maximum diameter (Dmax) and PWVmin response to reactive hyperemia were simultaneously recorded, where reactive hyperemia was induced by inflating a pneumatic tourniquet (SC10; Hokanson, Bellevue, WA, USA) around the mid-forearm to 250 mmHg for 5 min. Following a 3-min recovery, endothelial dysfunction was induced by inflating the mid-forearm tourniquet to a pressure of 75 mmHg (Figure 1). This moderate pressure does not prevent arterial inflow, but does induce arterial wave reflections with resultant retrograde blood flow.20,21,26,27 Following 30 min of increased retrograde shear stress, the tourniquet was slowly (over 30 s) deflated, and then all assessments were immediately repeated as described above.
Experimental measures
Ultrasound: Flow-mediated dilation and shear rate
A 11-2 MHz linear array probe (LOGIQ P6; GE Healthcare) was used to record brachial artery brightness-mode images and pulsed Doppler waveforms for the measurement of FMD and shear rate, respectively.3 Using a custom-built probe-holding device, the ultrasound probe was fixed on the brachial artery 5–10 cm proximal to the antecubital fossa. The insonation angle was kept constant between 45° and 60° and the sample volume included most of the vessel. For the entire FMD procedure, video recordings were captured at 30 Hz using an external video capture system (AV.io HD Frame Grabber; Epiphan Video, Palo Alto, CA, USA). During the period of increased retrograde shear stress, one 30-s recording was captured to confirm increased retrograde shear rate. The captured videos were analyzed offline using specialized image analysis software (FMD Studio; Quipu srl, Pisa, Italy). The second-by-second diameter, antegrade velocity and retrograde velocity data were smoothed (3-s bins) and processed using custom-written Visual Basic code.20,28 FMD (%) was calculated as (Dmax – Dbase)/Dbase * 100%, where Dbase is the mean diameter averaged over 2 min preceding the cuff inflation period, and Dmax is the peak diameter response to reactive hyperemia (Figure 2). To estimate the FMD stimulus, shear rate area-under-the-curve (AUC) up to 40 s (Shear AUC) after cuff release was calculated.28 Shear rate (s−1) was calculated as 4 * mean velocity/diameter, and oscillatory index as retrograde shear rate/(antegrade shear rate + retrograde shear) * 100.29
Figure 2.
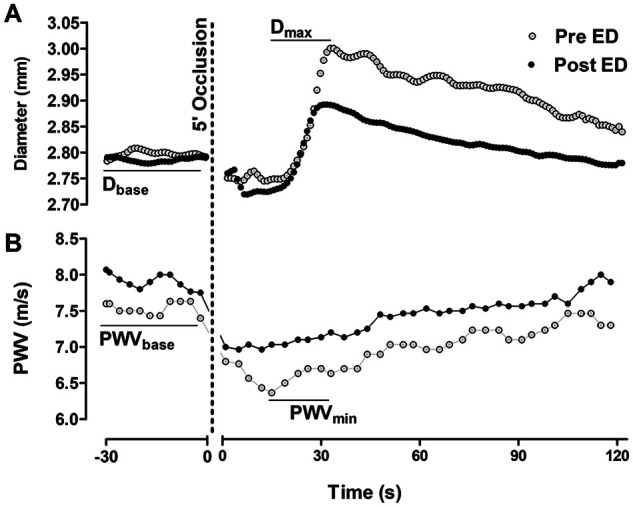
Example traces of FMD (A) and FMS (B) data recorded before and after the ED protocol.
(A) Diameters were recorded at 30 Hz for 2 min at baseline, during the 5 min of occlusion, and for 3 min following occlusion release. The diameters were analyzed offline using specialized image analysis software. FMD was calculated as (Dmax – Dbase)/Dbase * 100. (B) PWV was automatically recorded every 3.5 s by the Vicorder device. For PWVbase, PWV was recorded continuously for 2 min and then averaged. For PWVmin, the minimum PWV following occlusion release was automatically detected and then manually confirmed.
Dbase, baseline brachial diameter; Dmax, maximum brachial diameter during reactive hyperemia r; ED, endothelial dysfunction; FMD, flow-mediated dilation; FMS, flow-mediated slowing; PWV, pulse wave velocity; PWVbase, PWV at baseline; PWVmin, minimum PWV during reactive hyperemia.
Pulse wave velocity and flow-mediated slowing
Simultaneous to FMD, the Vicorder device was used to measure PWV and PWVmin. PWV (m/s) was calculated by dividing arterial path length by the pulse transit time between a proximal (upper arm) and distal (wrist) arterial segment. Specifically, path length was directly measured, using a custom device to bypass body contours, between the mid-point of the two cuffs. To measure pulse transit time, the cuffs were simultaneously inflated to a low (~50 mmHg) pressure, and a proprietary algorithm was used to automatically calculate the time between the foot of the proximal pressure waveform to the foot of the distal pressure waveform. As part of the FMS test, PWV was recorded continuously for 2 min prior to inflation of the forearm tourniquet used to induce reactive hyperemia (Figure 2). This 2-min recording was averaged to derive PWV. Subsequently, PWVmin was captured by continuously recording PWV during reactive hyperemia, and averaging PWV over 3.5-s cycles. PWVmin was also expressed as the change relative to baseline (PWVmin%), and the integrated 30 (PWV30), 60 (PWV60), 90 (PWV90), and 120-s (PWV120) response relative to baseline.
Peripheral and central hemodynamics
The SphygmoCor XCEL (AtCor Medical) was used to measure peripheral and central BP as well as arterial wave reflection. An appropriately sized cuff was placed around the upper portion of the right arm. Each measurement cycle lasts ~60 s. This includes inflating the cuff to measure brachial systolic (SBP) and diastolic (DBP) blood pressure, and then re-inflating 5 s later to 10 mmHg below DBP to acquire a volumetric displacement signal for 10 s.30 The brachial waveforms are calibrated using the cuff-measured SBP and DBP, and mean arterial pressure (MAP) is derived by integrating the AUC. A corresponding aortic pressure waveform is generated using a validated proprietary transfer function and calibrated using DBP and MAP.30 The aortic waveform is used to derive central SBP (cSBP), aortic backward pressure (Pb), and aortic forward pressure (Pf). The Pf and Pb wave pressures were determined by assuming a triangular flow wave.31 This method creates a triangular-shaped flow wave by matching the start, peak, and end of the flow wave to the timings of the foot, inflection point, and incisura of the aortic pressure wave, respectively.
Sample size
Sample size calculations were made using GPower 3.1. A prior study reported that the endothelial dysfunction protocol used in the current study decreased FMD% from 7.2% (SD: 3.0) to 4.2% (SD: 3.4), with an effect size (ES) of 1.0.21 Using a more conservative effect size of 0.5, a Type I error of 5% and Type II error of 20%, 21 subjects were required. This number was inflated to 23 to account for the potential for missing data.
Statistical analysis
Statistical analyses were performed using Jamovi (2019, Version 1.0.1) and the rmcorr (repeated measures correlation) package for R (R Foundation for Statistical Computing, Vienna, Austria).32 Only participants who had complete data for the primary outcomes were included in the analyses. The significance level was set a priori for all statistical procedures at α = 0.05. Raw data are presented as mean (SD) and mixed model data are presented as mean (95% CI). The corresponding author (LS) had full access to the data in the study and was responsible for the integrity of the data set and the data analysis.
The effect of endothelial dysfunction on PWV, PWVmin, and FMD were tested using paired Student’s t-tests. Additionally, linear mixed models (random intercept, fixed slope) were used to adjust FMD for within-subject changes in shear AUC, and to adjust PWV and PWVmin for within-subject changes in MAP. Linear mixed models were also used to assess the effect of condition (baseline, during cuff inflation (endothelial dysfunction protocol), post-cuff inflation) on hemodynamic data. When the condition effect was significant, during cuff inflation and post-cuff inflation were compared to baseline, with Bonferroni adjustments. Intra-individual (within-subject) associations between FMD with PWV and PWVmin were analyzed using rmcorr. The rmcorr statistical technique determines the overall within-individual relationship among paired measures assessed on two or more occasions.32
For paired t-tests and linear mixed models, ES were calculated as Cohen’s d where ⩽ 0.2 was defined as trivial, 0.2–0.3 as small, 0.4–0.8 as moderate, and ⩾ 0.8 as large. For the mixed models, Cohen’s d was calculated as the effect of condition (β) from linear mixed models divided by the baseline SD. For rmcorr, r value estimates of 0.1, 0.3, and 0.5 were defined as small, medium, and large, respectively.33
Results
Participants
Complete data were collected on 22 of the 23 participants (23.8 years [SD 4.1], 73% F, 22.8 kg/m2 [SD 2.8]). One participant (20 years, F, 20.8 kg/m2) was excluded from data analysis due to inadequate image quality (i.e. the ultrasound image analysis software inadequately traced the vessel walls). The PWV data for this participant were similar to the group mean (PWV 8.6 vs 8.5 m/s and PWVmin 7.1 vs 6.9 m/s, respectively).
Hemodynamic data
Hemodynamic/control data are presented in Table 1. Across conditions (baseline, during cuff inflation, post-cuff inflation), the brachial diameter did not significantly change (p = 0.720). There was a significant change in the shear rate parameters, including antegrade shear rate (p < 0.001), retrograde shear rate (p < 0.001), and oscillatory index (p < 0.001). Compared to baseline, each of the shear rate parameters significantly increased during partial occlusion (p < 0.001 for all measures) but were not different to baseline post-occlusion (p = 1.000 for all measures).
Table 1.
Hemodynamic measures (n = 22).
| Baseline |
Cuff |
Post |
P
Overall
|
P
Cuff
|
P
Post
|
||||
|---|---|---|---|---|---|---|---|---|---|
| Mean | (SD) | Mean | (SD) | Mean | (SD) | ||||
| Diameter (mm) | 3.01 | (0.41) | 3.03 | (0.45) | 3.01 | (0.44) | 0.720 | ||
| Ante shear | 186.3 | (54.6) | 305 | (81.1) | 175 | (51.0) | < 0.001 | < 0.001 | 0.508 |
| Retro shear | −37.4 | (27.4) | −201 | (90.3) | −38.3 | (30.3) | < 0.001 | < 0.001 | 0.952 |
| Osc index | 17.6 | (13.4) | 38.4 | (10.4) | 18.7 | (13.3) | < 0.001 | < 0.001 | 0.749 |
| MAP (mmHg) | 78.3 | (8.90) | 79.5 | (7.68) | 82.3 | (9.16) | < 0.001 | 0.226 | < 0.001 |
| DBP (mmHg) | 66.1 | (7.02) | 68.0 | (7.47) | 70.1 | (7.48) | < 0.001 | 0.022 | < 0.001 |
| SBP (mmHg) | 112.3 | (10.4) | 112.9 | (9.8) | 115.7 | (9.4) | 0.008 | 0.602 | 0.004 |
| cSBP (mmHg) | 98.7 | (9.4) | 97.9 | (8.6) | 100.3 | (9.4) | 0.142 | ||
| Pb (mmHg) | 11.8 | (2.38) | 11.3 | (2.07) | 11.5 | (1.87) | 0.401 | ||
| Pf (mmHg) | 25.3 | (4.16) | 24.9 | (3.98) | 25.4 | (4.41) | 0.736 | ||
Ante, antegrade; cSBP, central systolic blood pressure; DBP, diastolic blood pressure; MAP, mean arterial pressure; Osc, oscillatory; Pb, aortic backward pressure; Pf, aortic forward pressure; Retro, retrograde; SBP, systolic blood pressure.
There was a significant change in MAP (p < 0.001), DBP (p < 0.001), and SBP (p < 0.001). However, there was a non-significant change in central hemodynamic parameters, including cSBP (p = 0.142), Pb (p = 0.401), and Pf (p = 0.736).
Flow-Mediated Dilation (FMD)
Example data from one subject are shown in Figure 2, and the primary study outcomes are reported in Table 2. Following the 30-min endothelial dysfunction protocol, there was a large significant decrease (∆ = −3.10; 95% CI: –4.15, –2.05; ES = −1.3) in FMD. There was a moderate decrease in shear AUC (∆ = −4124; 95% CI: –6923, –1326; ES = −0.7). After adjusting FMD for the shear AUC stimulus, the decrease in FMD remained large (ES = −2.4, p < 0.001).
Table 2.
Primary outcomes pre- and post-acute endothelial dysfunction (n = 22).
| Baseline |
Post |
p | ES | |||
|---|---|---|---|---|---|---|
| Mean | (SD) | Mean | (SD) | |||
| Shear AUC | 30,426 | (9080) | 26,302 | (7647) | 0.006 | −0.7 |
| FMD (%) | 6.17 | (2.00) | 3.07 | (2.40) | < 0.001 |
−1.3 |
| PWV (m/s) | 8.47 | (1.17) | 8.85 | (1.17) | 0.020 | 0.5 |
| PWVmin (m/s) | 6.87 | (0.67) | 7.04 | (0.63) | 0.009 | 0.6 |
| PWVmin (%) | 18.3 | (6.51) | 19.6 | (5.62) | 0.396 | 0.2 |
| PWV30 (%) | 15.9 | (6.42) | 15.9 | (6.05) | 0.995 | 0.0 |
| PWV60 (%) | 13.9 | (6.39) | 13.1 | (6.01) | 0.590 | −0.2 |
| PWV90 (%) | 12.3 | (6.42) | 11.2 | (5.87) | 0.474 | −0.2 |
| PWV120 (%) | 11.2 | (6.47) | 9.89 | (5.74) | 0.388 | −0.2 |
AUC, area-under-the-curve; ES, effect size (Cohen’s d); FMD, flow-mediated dilation; PWV, pulse wave velocity; PWVmin, minimum pulse wave velocity; PWV30–120, PWV integrated to 30–120 s during reactive hyperemia relative to baseline.
Pulse Wave Velocity (PWV)
Following the 30-min endothelial dysfunction protocol, there was a moderate significant increase (∆ = 0.38; 95% CI: 0.07, 0.69; ES = 0.5) in PWV. After adjusting for MAP, the effect remained moderate (ES = 0.5, p < 0.022). As reported in Figure 3A, there was a moderate but inconclusive intra-individual association between FMD and PWV (r = −0.3; 95% CI: –0.67, 0.12; p = 0.121).
Figure 3.
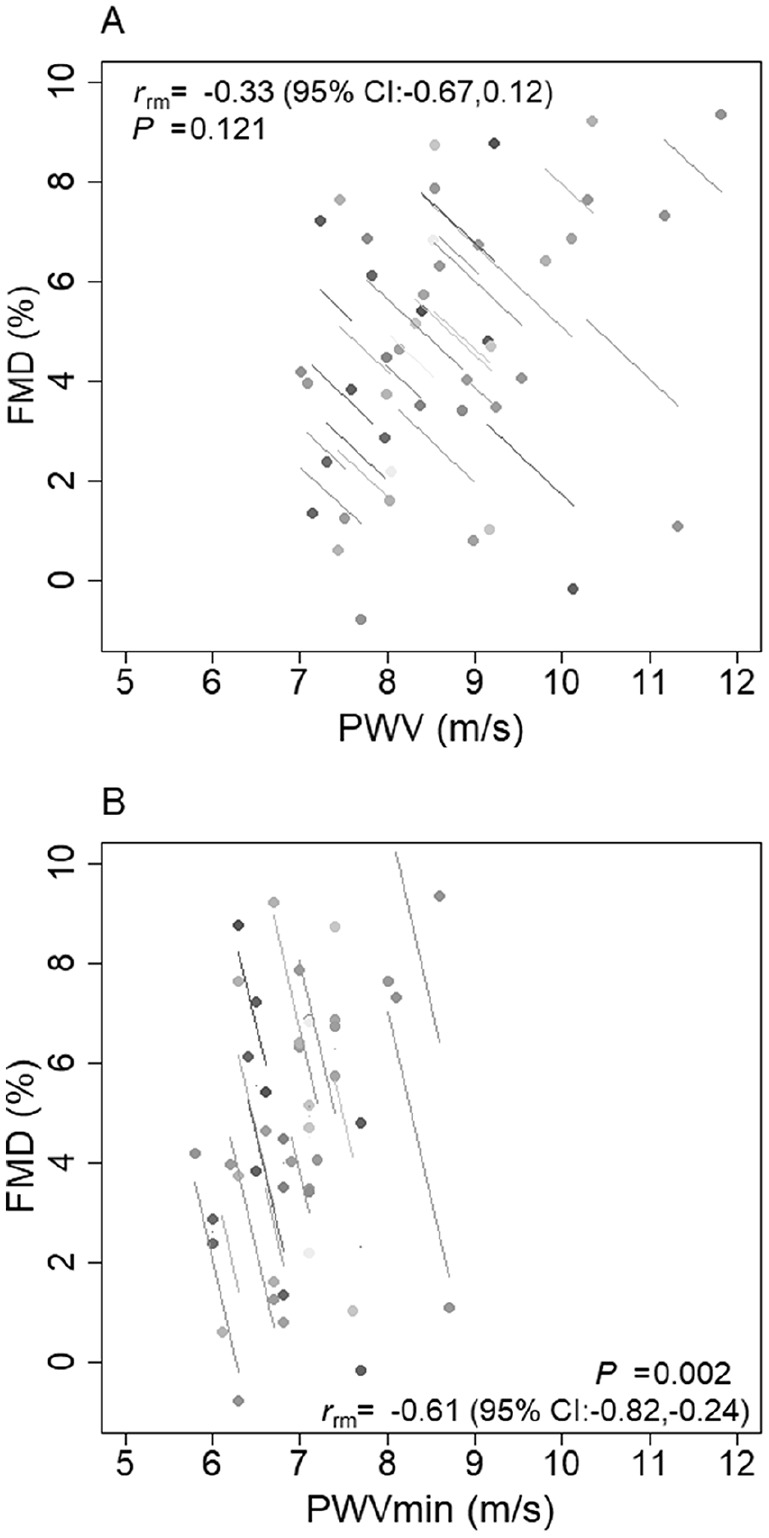
Intra-individual (pre- vs post-endothelial dysfunction) associations between FMD with PWV (A) and PWVmin (B) (n = 22 repeated measures).
FMD, flow-mediated dilation; PWV, pulse wave velocity; PWVmin, minimum pulse wave velocity.
Flow-Mediated Slowing (FMS)
Following the 30-min endothelial dysfunction protocol, there was a moderate significant increase (∆ = 0.16; 95% CI: 0.05, 0.28; ES = 0.6) in PWVmin. After adjusting for MAP, the effect remained moderate (ES = 0.6, p < 0.009). There was a large intra-individual association between FMD and PWVmin (r = −0.61; 95% CI: –0.82, –0.24; p = 0.002) (Figure 3B). The endothelial dysfunction protocol had an inconclusive effect on the remainder of the FMS parameters (Table 2), with non-significant intra-individual associations with FMD (p = 0.295–0.991).
Discussion
The objective of the current study was to determine the effects of acute endothelial dysfunction on PWV and PWVmin. Acute endothelial dysfunction, localized to the arm, resulted in a moderate and significant increase in arm PWV (∆ = 0.38 m/s) and PWVmin (∆ = 0.16 m/s). These findings suggest that acute changes in PWV and PWVmin are at least partly driven by endothelial function.
Comparison to literature: PWV
In support of our hypothesis, the endothelial dysfunction protocol increased PWV by 0.38 m/s. We did not measure molecules associated with endothelial function in this study and can only speculate with respect to mechanisms. That being said, the increase in PWV following a known endothelial dysfunction protocol is in line with a number of studies reporting that infusion of a nitric oxide (NO) synthesis inhibitor, NG-monomethyl-l-arginine (l-NMMA) or NG-nitro-l-arginine methyl ester (l-NAME), decreases compliance and increases PWV.12–15 Conversely, infusion of glyceryl trinitrate (nitroglycerin), which is metabolized to NO within the vascular wall, increases arterial compliance and decreases PWV.12–14,16 While NO is not the only molecule regulating endothelial function,18 these previous findings do support a direct association between endothelial function and arterial mechanical function. The current study extends these previous findings by demonstrating that non-specific local endothelial dysfunction increases arterial stiffness.
In the current study, we did see a 5.0 mmHg increase in MAP. MAP is a known determinant of PWV11 and could have driven the PWV response. Stewart et al.16 infused l-NMMA into a small (n = 8) sample of men and found that carotid–femoral PWV increased by 0.7 m/s. The authors argued that this response may have been driven by the 7.8 mmHg increase in MAP. In support, infusion of norepinephrine and dobutamine resulted in similar increases in PWV and MAP to that observed with l-NMMA infusion. However, association is not causation and the authors did not manipulate NO while controlling for MAP, nor did they statistically control for changes in MAP. The authors did test the association between MAP and PWV using ordinary least squares regression, but they used aggregate data and included the four conditions in the same model. This would have violated the assumption of statistical independence of errors, and could have resulted in an erroneous conclusion.32 The findings of Stewart et al.16 are contrary to Wilkinson et al.,13 who used an ovine hind-limb model and reported that glyceryl trinitrate decreased PWV while l-NMMA increased PWV, and that these changes occurred independent of MAP. In the current study, we did statistically adjust for within-subject changes in MAP, and there remained a moderate ES increase in PWV. The intra-individual association between FMD and PWV was inconclusive, but we were not statistically powered to assess this association and the lack of association may have been driven by the restricted range of change values.34
Comparison to literature: FMS
We found that the endothelial dysfunction protocol had an inconclusive effect on each of the PWVmin% calculations (response relative to baseline). However, there was a moderate significant increase (∆ = 0.16 m/s) in the raw FMS signal (i.e. PWVmin). As such, while a limited number of studies have reported that PWVmin% is reduced in those with cardiovascular complications,5–7 and that PWVmin% is associated with cardiovascular risk factors,9 these findings may not be attributable to endothelial function. Alternatively, the lack of effect of endothelial dysfunction on PWVmin% in the current study may be attributable to a shifting numerator. Baseline PWV did increase by 0.38 m/s, which may have masked any change in the denominator, PWVmin. The PWVmin did increase during reactive hyperemia following endothelial dysfunction, which is consistent with the reduced peak arterial diameter and the assumption made by the Moens–Korteweg equation.17 As such, at least for studies investigating acute change in endothelial function, the raw PWVmin signal may be superior to PWVmin%. The use of PWVmin is supported by the large intra-individual association between FMD and PWV (r = −0.61).
Limitations and strengths
This study had several considerations, which were considered when designing the study. First, a healthy population sample was recruited to mitigate the risk of age or disease-related influences on the primary outcomes. While our sample population did permit optimal signal-to-noise-ratio, future studies with older and clinical populations are now required to better generalize the findings. Second, we were not sufficiently powered to stratify our sample by sex. Further investigation is required to ascertain whether endothelial dysfunction similarly affects PWV and PWVmin in men and women. Third, the study was powered to estimate the effect of the endothelial dysfunction protocol on PWVmin, not to determine associations between PWV and PWVmin with FMD. As such, the association data should not be directly used to adjudicate the hypothesis. Lastly, FMD was assessed simultaneously to PWVmin, meaning the upper arm and wrist cuffs were inflated to 50 mmHg throughout the FMD test. Pilot testing indicated that 50 mmHg was the minimum inflation pressure for permitting quality pressure waveforms. We did consider conducting FMD and FMS measurements on separate arms, or on separate days, but both alternative approaches would have introduced error variance. Rather, we completed both tests simultaneously, under the assumption that partial occlusion would have similarly affected both tests. Nonetheless, we cannot discount that partial occlusion did introduce some noise, particularly to the FMD data.
There were several strengths of the current study. First, we used the standard FMD test to confirm endothelial dysfunction. Second, we non-specifically induced local endothelial dysfunction. While previous studies have demonstrated an association between PWV and endothelial function by targeting NO bioavailability,12–16 it should be acknowledged that NO is not the only molecule regulating endothelial function.18
Implications
Findings from this study indicate that PWVmin and PWV are viable options for measuring acute changes in endothelial function. These options are attractive alternatives to existing methods, including FMD, because they are user-objective, automated, and likely more precise (reliable) than FMD. For example, one study compared the reliability of brachial FMD to brachial–radial PWVmin% in a group of 22 healthy participants (28–65 years old), reporting a coefficient of variation (CV) of 7.3% for PWVmin% and 26.6% for FMD.7 The same study reported a CV of 3.3% for PWV, and, while PWVmin was not reported, the CV for the absolute change in PWV (PWVmin – baseline PWV) was 8.2%. The reported reliability of FMD and PWV are in accordance with the available literature.35–37 To confirm viability, additional investigation is warranted to: (i) estimate the precision of PWVmin; (ii) confirm whether PWV and PWVmin decrease to a greater extent following a perturbation known to improve endothelial function; (iii) confirm whether an increase or decrease in PWV and PWVmin corresponds with changes in the balance agonist and antagonistic molecules known to regulate endothelial function (e.g. NO and endothelin-1); (iv) use a technique which does not require partial occlusion to measure PWV (e.g. tonometry of photoplethysmography) to determine whether partial occlusion during reactive hyperemia modifies the PWVmin response; and (v) compare PWVmin and FMD in terms of the ability to discriminate between those with and without known cardiovascular disease.
Conclusions
The objective of the current study was to determine the effects of acute local endothelial dysfunction, induced by increasing retrograde shear stress in the arm for 30 min, on arm PWV and PWVmin. Endothelial dysfunction resulted in a moderate significant increase in PWV and PWVmin. These findings indicate that PWV and PWVmin are viable, user-objective, and automated tools for monitoring acute changes in endothelial function.
Acknowledgments
The authors would like to thank Arnd Warmuth, SMT Medical, for providing technical insights.
Footnotes
Declaration of conflicting interests: The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Lee Stoner  https://orcid.org/0000-0002-0682-2270
https://orcid.org/0000-0002-0682-2270
References
- 1. Jia G, Aroor AR, Jia C, et al. Endothelial cell senescence in aging-related vascular dysfunction. Biochim Biophys Acta Mol Basis Dis 2019; 1865: 1802–1809. [DOI] [PubMed] [Google Scholar]
- 2. Lee D-Y, Chiu J-J. Atherosclerosis and flow: Roles of epigenetic modulation in vascular endothelium. J Biomed Sci 2019; 26: 56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Stoner L, Sabatier MJ. Use of ultrasound for non-invasive assessment of flow-mediated dilation. J Atheroscler Thromb 2012; 19: 407–421. [DOI] [PubMed] [Google Scholar]
- 4. Bots ML, Westerink J, Rabelink TJ, et al. Assessment of flow-mediated vasodilatation (FMD) of the brachial artery: Effects of technical aspects of the FMD measurement on the FMD response. Eur Heart J 2005; 26: 363–368. [DOI] [PubMed] [Google Scholar]
- 5. Naka KK, Tweddel AC, Doshi SN, et al. Flow-mediated changes in pulse wave velocity: A new clinical measure of endothelial function. Eur Heart J 2006; 27: 302–309. [DOI] [PubMed] [Google Scholar]
- 6. Rusak EJ, Bellido CA, Iavicoli OR, et al. Assessment of endothelial function by means of flow-mediated changes using pulse wave velocity. J Clin Hypertens 2010; 12: 495–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ellins EA, New KJ, Datta DBN, et al. Validation of a new method for non-invasive assessment of vasomotor function. Eur J Prev Cardiol 2016; 23: 577–583. [DOI] [PubMed] [Google Scholar]
- 8. Pereira T, Almeida A, Conde J. Flow-mediated slowing as a methodological alternative to the conventional echo-tracking flow-mediated dilation technique for the evaluation of endothelial function: A preliminary report. Mayo Clin Proc Innov Qual Outcomes 2018; 2: 199–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Cauwenberghs N, Wei F-F, Kuznetsova T, et al. Flow-mediated slowing of brachial-radial pulse wave velocity: Methodological aspects and clinical determinants. Artery Res 2018; 21: 29–37. [Google Scholar]
- 10. Townsend RR, Wilkinson IB, Schiffrin EL, et al. Recommendations for improving and standardizing vascular research on arterial stiffness: A scientific statement from the American Heart Association. Hypertension 2015; 66: 698–722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kucharska-Newton AM, Stoner L, Meyer ML. Determinants of vascular age: An epidemiological perspective. Clin Chem 2019; 65: 108–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kinlay S, Creager MA, Fukumoto M, et al. Endothelium-derived nitric oxide regulates arterial elasticity in human arteries in vivo. Hypertension 2001; 38: 1049–1053. [DOI] [PubMed] [Google Scholar]
- 13. Wilkinson IB, Qasem A, McEniery CM, et al. Nitric oxide regulates local arterial distensibility in vivo. Circulation 2002; 105: 213–217. [DOI] [PubMed] [Google Scholar]
- 14. McVeigh GE, Allen PB, Morgan DR, et al. Nitric oxide modulation of blood vessel tone identified by arterial waveform analysis. Clin Sci (Lond) 2001; 100: 387–393. [PubMed] [Google Scholar]
- 15. Bank AJ, Kaiser DR. Smooth muscle relaxation: Effects on arterial compliance, distensibility, elastic modulus, and pulse wave velocity. Hypertension 1998; 32: 356–359. [DOI] [PubMed] [Google Scholar]
- 16. Stewart AD, Millasseau SC, Kearney MT, et al. Effects of inhibition of basal nitric oxide synthesis on carotid-femoral pulse wave velocity and augmentation index in humans. Hypertension 2003; 42: 915–918. [DOI] [PubMed] [Google Scholar]
- 17. Caro CG, Pedley TJ, Schroter RC, et al. The mechanics of the circulation. 2nd ed. Cambridge: Cambridge University Press, 2012. [Google Scholar]
- 18. Stoner L, Erickson ML, Young JM, et al. There’s more to flow-mediated dilation than nitric oxide. J Atheroscler Thromb 2012; 19: 589–600. [DOI] [PubMed] [Google Scholar]
- 19. Harrison DG, Widder J, Grumbach I, et al. Endothelial mechanotransduction, nitric oxide and vascular inflammation. J Intern Med 2006; 259: 351–363. [DOI] [PubMed] [Google Scholar]
- 20. Stoner L, McCully KK. Velocity acceleration as a determinant of flow-mediated dilation. Ultrasound Med Biol 2012; 38: 580–592. [DOI] [PubMed] [Google Scholar]
- 21. Thijssen DHJ, Dawson EA, Tinken TM, et al. Retrograde flow and shear rate acutely impair endothelial function in humans. Hypertension 2009; 53: 986–992. [DOI] [PubMed] [Google Scholar]
- 22. Tremblay JC, Grewal AS, Pyke KE. Examining the acute effects of retrograde versus low mean shear rate on flow-mediated dilation. J Appl Physiol (1985) 2019; 126: 1335–1342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Green DJ, Hopman MTE, Padilla J, et al. Vascular adaptation to exercise in humans: Role of hemodynamic stimuli. Physiol Rev 2017; 97: 495–528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Schulz KF, Altaian DG, Moher D. CONSORT 2010 Statement: Updated guidelines for reporting parallel group randomized trials. BMJ 2010; 340: c332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Stoner L, Lambrick DM, Faulkner J, et al. Guidelines for the use of pulse wave analysis in adults and children. J Atheroscler Thromb 2013; 20: 404–406. [DOI] [PubMed] [Google Scholar]
- 26. Carter SA. Effect of age, cardiovascular disease, and vasomotor changes on transmission of arterial pressure waves through the lower extremities. Angiology 1978; 29: 601–606. [DOI] [PubMed] [Google Scholar]
- 27. Stoner L, Young JM, Fryer S, et al. The importance of velocity acceleration to flow-mediated dilation. Int J Vasc Med 2012; 2012: 589213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Stoner L, McCully KK. Peak and time-integrated shear rates independently predict flow-mediated dilation. J Clin Ultrasound 2012; 40: 341–351. [DOI] [PubMed] [Google Scholar]
- 29. Stoner L, Stone K, Zieff G, et al. The impact of upper-limb position on estimated central blood pressure waveforms. J Hum Hypertens 2019; 33: 444–453. [DOI] [PubMed] [Google Scholar]
- 30. Butlin M, Qasem A, Avolio AP. Estimation of central aortic pressure waveform features derived from the brachial cuff volume displacement waveform. Conf Proc IEEE Eng Med Biol Soc 2012; 2012: 2591–2594. [DOI] [PubMed] [Google Scholar]
- 31. Qasem A, Avolio A. Determination of aortic pulse wave velocity from waveform decomposition of the central aortic pressure pulse. Hypertension 2008; 51: 188–195. [DOI] [PubMed] [Google Scholar]
- 32. Bakdash JZ, Marusich LR. Repeated measures correlation. Front Psychol 2017; 8: 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Cohen J. Statistical power analysis for the behavioral sciences. 2nd ed. Hillsdale, NJ: Erlbaum, 1988, pp.75–108. [Google Scholar]
- 34. Bland JM, Altman DG. Correlation in restricted ranges of data. BMJ 2011; 342: d556. [DOI] [PubMed] [Google Scholar]
- 35. Hwang MH, Yoo JK, Kim HK, et al. Validity and reliability of aortic pulse wave velocity and augmentation index determined by the new cuff-based SphygmoCor Xcel. J Hum Hypertens 2014; 28: 475–481. [DOI] [PubMed] [Google Scholar]
- 36. Magda SL, Ciobanu AO, Florescu M, et al. Comparative reproducibility of the noninvasive ultrasound methods for the assessment of vascular function. Heart Vessels 2013; 28: 143–150. [DOI] [PubMed] [Google Scholar]
- 37. Ghiadoni L, Faita F, Salvetti M, et al. Assessment of flow-mediated dilation reproducibility: A nationwide multicenter study. J Hypertens 2012; 30: 1399–1405. [DOI] [PubMed] [Google Scholar]