Abstract
Background
Reduced blood or cerebrospinal fluid levels of allopregnanolone are involved in menstrual cycle-linked CNS disorders, such as catamenial epilepsy. This condition, like menstrually-related migraine, is characterized by severe, treatment-resistant attacks. We explored whether there were differences in allopregnanolone, progesterone and testosterone serum levels between women with menstrually-related migraine (MM, n = 30) or postmenopausal migraine without aura who had suffered from menstrually-related migraine during their fertile age (PM, n = 30) and non-headache control women in fertile age (FAC, n = 30) or post-menopause (PC, n = 30).
Methods
Participants were women with migraine afferent to a headache centre; controls were female patients’ acquaintances. Serum samples obtained were analyzed by HPLC-ESI-MS/MS.
Results
In menstrually-related migraine and postmenopausal migraine groups, allopregnanolone levels were lower than in the respective control groups (fertile age and post-menopause) (p < 0.001, one-way analysis of variance followed by Tukey-Kramer post-hoc comparison test) while progesterone and testosterone levels were similar. By grouping together patients with migraine, allopregnanolone levels were inversely correlated with the number of years and days of migraine/3 months (p ≤ 0.005, linear regression analysis).
Conclusion
Decreased GABAergic inhibition, due to low allopregnanolone serum levels, could contribute to menstrually-related migraine and persistence of migraine after menopause. For the management of these disorders, a rise in the GABAergic transmission by increasing inhibitory neurosteroids might represent a novel strategy.
Keywords: Menstrually-related migraine, menopause, headache, allopregnanolone, progesterone, testosterone, serum level, neurosteroid
Introduction
The cyclical fluctuations of steroid hormones in women affect susceptibility to various neurological disorders (1). The central nervous system (CNS), in turn, can metabolize and synthesize steroid hormones, collectively referred to as neurosteroids, which influence neuronal homeostasis by modulating the balance between neuroexcitation and neuroinhibition (2,3). The circulating steroid hormones, for their lipophilicity, penetrate the blood-brain barrier (BBB) and serve as precursors for the synthesis of neurosteroids. Among these, allopregnanolone (3α,5α-tetrahydroprogesterone), a derivative of pregnenolone and progesterone, shows neuroprotective, sedative, anesthetic, anxiolytic and antiepileptic properties. These effects depend mostly on allopregnanolone’s ability to act as a positive allosteric modulator of the GABA(A) receptor complex and therefore on its inhibitory effect on neuronal excitability (4,5). In addition, allopregnanolone has proved to exert analgesic effects and the capability to prevent or reverse maladaptive changes and painful behaviours that occur after nervous system damage in various experimental neuropathic conditions, including chemotherapy-evoked neuropathic pain in rats (6).
The downregulation of neurosteroid biosynthesis has been considered to be a possible contributor to the development of menstrual cycle-linked CNS disorders (7). Actually, reduced levels of allopregnanolone in the peripheral blood or cerebrospinal fluid were found to be associated with premenstrual dysphoric disorder, premenstrual syndrome, and catamenial epilepsy (8,9). Indeed, the ratio of allopregnanolone to progesterone decreases across the menstrual cycle, from the follicular to the luteal phase (10). Catamenial epilepsy is characterized by seizure exacerbation during particular phases of women’s menstrual cycles and refractoriness to specific treatments such as benzodiazepines and valproic acid (11). Nevertheless, in animal models of this condition, progesterone reduces seizure susceptibility, partly through its conversion to allopregnanolone, which enhances GABA function and thereby inhibits neuronal excitability (8).
Another neurological condition, linked to hormonal fluctuations, disabling, and difficult to treat, is menstrual migraine. This subtype of migraine is characterized by menstrual attacks occurring from 2 days before to 3 days after the beginning of the menstrual flow. Menstrual attacks are longer, more disabling, resistant to the treatment, and tend to recur more than non-menstrual attacks (12). Moreover, these attacks are associated with an increased risk of chronification; in fact, many women who suffer from chronic migraine and analgesic overuse initially suffered from menstrually-related migraine (13). The main trigger of menstrual attacks is believed to be the decrease in estrogen concentration after being exposed to high levels for several days (14). However, the mechanisms that mediate this effect, which appears only in some women with migraine but not in all, are unknown. After menopause, the prevalence of migraine falls by about one-half, at least partly as a result of hormonal stability (15). Nevertheless, in many women migraine does not end after menopause. In these cases, migraine seems associated with higher levels of mood disorders, disabilities and menopausal symptoms (16). The mechanisms of persisting migraine after menopause and hormonal changes in this condition have also been poorly studied (17).
We hypothesized a role of allopregnanolone, as in catamenial epilepsy, also in menstrually-related and postmenopausal migraine. Therefore the objectives of our pilot study were to evaluate the serum levels of allopregnanolone, progesterone and testosterone in women suffering from menstrually-related migraine and in women who had suffered from it during their fertile age, then continued to suffer from migraine also after menopause, and to compare them with serum levels of these hormones in non-headache women, both in fertile age and in post-menopause, as controls. Another objective was to explore whether, overall, in migraine women, there was a relationship between serum concentrations of allopregnanolone and the severity of migraine.
Methods
Study groups
This pilot, cross-sectional, study involved fertile and menopausal women (Table 1). Fertile women (aged 18 to 45 years) comprised 30 women diagnosed with menstrually-related migraine without aura (MM group) according to the diagnostic criteria of the International Classification of Headache Disorders, 3rd edition (ICHD-3, appendix A1.1.2) (18) and 30 non-headache age-matched women as control (FAC, fertile age control group). Menopausal women (aged no more than 65 years and in spontaneous menopause for at least 1 year) comprised 30 women suffering from migraine without aura (PM, postmenopausal migraine group) who had suffered from menstrually-related migraine during their fertile age according to the diagnostic criteria of the ICHD-3 (18) and 30 non-headache, age-matched women as control (PC, postmenopausal control group). Only subjects with absence of major medical or psychiatric comorbidities, normal liver and kidney functions were included in the study; women of FAC and PC groups were required to present no more than 3 days of tension-type headache per month and no diagnosis of any other type of primary or secondary headache, according to ICHD-3 criteria (18). Women taking hormonal therapy (contraceptive or post-menopause therapy), migraine prophylaxis, drugs that could modify allopregnanolone levels and unable to understand the study purpose were excluded. The women with migraine were enrolled from consecutive patients attending, for the first time, at the Headache Centre of the University Hospital of Modena; non-headache women were patients’ acquaintances. All women provided their written consent to participation in the study, which was conducted in accordance with the ethical principles of the Helsinki Declaration, last edition (2013) and approved by the Ethics Committee of Area Vasta Emilia Nord (Italy) (prot. 0013510/18). The subjects were enrolled from July 2018 to May 2019.
Table 1.
Demographics and migraine characteristics (
MM: menstrually-related migraine group; FAC: fertile age control group; PM: postmenopausal migraine group; PC: postmenopausal control group).
| Variable |
Fertile age (%) |
Postmenopause (%) |
||
|---|---|---|---|---|
| MM (n = 30) | FAC (n = 30) | PM (n = 30) | PC (n = 30) | |
| Mean age ± SD | 33.5 ± 7.1 | 30.9 ± 7.9 | 56.6 ± 4.5# | 56.1 ± 4.5# |
| Age range (years) | 19 ÷ 45 | 18 ÷ 44 | 50 ÷ 65 | 50 ÷ 63 |
| BMI (kg/m2) (mean ± SD) | 23.0 ± 3.3 | 23.5 ± 4.2 | 24.1 ± 4.5 | 23.1 ± 2.4 |
| Menstrual cycle lengths (mean days ± SD) | 27.8 ± 1.1 | 28.4 ± 1.0 | 0 | 0 |
| Range of menstrual cycle length (days) | 26 ÷ 30 | 26 ÷ 31 | 0 | 0 |
| Married (%) | 21 (70) | 9 (30)§ | 24 (80) | 26 (87) |
| Employed (%) | 24 (80) | 17 (57) | 22 (73) | 26 (87) |
| Degree (%) | 5 (17) | 21 (70)¥ | 11 (37)$ | 6 (20) |
| Smokers (%) | 5 (17) | 6 (20) | 5 (17) | 4 (13) |
| Alcohol consumption (%) | 16 (53) | 21 (70) | 13 (43) | 21 (70) |
| Coffee consumption (%) | 21 (70) | 17 (57) | 16 (53) | 19 (63) |
| Years of migraine (mean ± SD) | 17.4 ± 8.9* | 0 | 33.5 ± 11.9 | 0 |
| Migraine days/3 months (mean ± SD) | 25.5 ± 25.3 | 0 | 35.1 ± 24.5 | 0 |
#p < 0.001 vs. MM and FAC groups, one-way analysis of variance followed by Tukey-Kramer post-hoc comparison test.
§p = 0.001 vs. MM, PM and PC groups.
¥p < 0.001 vs. MM, PM and PC groups.
$p = 0.002, vs. MM and PC groups, chi-squared test for the homogeneity of odds.
*p < 0.001 vs. PM, one-way analysis of variance followed by the Tukey-Kramer post-hoc comparison test.
Procedures
A sample of venous blood was collected in the morning at fast from each woman enrolled in the study for the quantitative determination of allopregnanolone, progesterone and testosterone. The samples (10 mL) were allowed to clot at room temperature for 1 h and then centrifuged at 2000 × g for 10 min at +4°C to collect sera. In fertile women (MM and FAC groups), blood samples were taken between the 7th and 10th day of the menstrual cycle and, in women with migraine (MM and PM groups), during the interictal period, at least 2 days after the last migraine attack. At the medical examination, a form was compiled for each subject to collect personal data, life habits, clinical history and, for MM and PM groups, also the history and characteristics of migraine.
Sample processing and LC-MS/MS analysis
Calibrators in human albumin and serum samples from the subjects were purified to remove proteins and phospholipids, evaporated to dryness and derivatized prior to LC-MS/MS. Chromatographic analyses were performed on a Kinetex XB-C18 column under gradient elution and the target compounds were detected by multiple reaction monitoring (MRM) in positive ion electrospray mode. Three selected MS/MS transitions were monitored for each analyte and deuterated allopregnanolone (internal standard) to achieve unambiguous identification. Serum levels of allopregnanolone, progesterone and testosterone were determined via the calibration curve calculated by plotting concentration against the ratio of analyte area to that of the internal standard. Details concerning chemicals, sample processing and LC-MS/MS quantitative analyses are provided in the Supplementary material.
Data and statistical analysis
All collected data, made anonymous, were entered into a specific database. Descriptive analysis of all variables was conducted. We compared the demographic and headache characteristics in the studied groups; subsequently, we assessed the serum levels of allopregnanolone, progesterone and testosterone in each migraine group (MM and PM) compared to those in the correspondent control group (FAC and PC) and the levels in the MM group compared to those in the PM group. Furthermore, we investigated whether there was a relationship between serum concentrations of allopregnanolone and the severity of the migraine assessed as years of migraine and number of migraine days in the last 3 months, considering MM and PM groups individually and also all women with migraine together (MM and PM groups, n = 60).
Statistical analysis was carried out by StataIC 13 software. The continuous variables normally distributed were expressed as mean ± standard deviation (SD) and the dichotomous variables as counts and percentages. The comparison between means was done by one-way analysis of variance followed by the Tukey-Kramer post-hoc comparison test, while the chi-squared test for the homogeneity of odds was used for binary variables. The magnitude of the mean differences in allopregnanolone levels (effect size) was determined with Cohen’s d. Linear regression analysis was conducted to assess the relationship between serum level of allopregnanolone (independent variable) and migraine seriousness (dependent variable). The magnitude of the dependence between the above-mentioned variables was explored using the R2 value. Differences were considered significant if the p-value (two-tailed) was lower than 0.05.
Results
Analyses of allopregnanolone, progesterone and testosterone (Figure 1) in fertile women showed that allopregnanolone levels were significantly lower in the women with migraine of the MM group (mean value ± SD: 52 ± 18 pg/mL) than in the FAC control group (mean value ± SD: 78 ± 36 pg/mL, p < 0.001), while progesterone levels were similar between MM group (mean value ± SD: 135 ± 47 pg/mL) and FAC group (mean value ± SD: 162 ± 120 pg/mL, p = 0.30); also testosterone levels showed no difference between MM group (mean value ± SD: 298 ± 116 pg/mL) and the FAC group (mean value ± SD: 327 ± 124 pg/mL, p = 0.43, one-way analysis of variance followed by Tukey-Kramer post-hoc comparison test). Cohen’s d value for allopregnanolone serum levels between MM and FAC was −1.05 [−1.69 ÷ −0.4]. Analyses of serum samples in the post-menopause groups showed allopregnanolone levels significantly lower in the PM group (mean value ± SD: 25 ± 13 pg/mL) compared to the respective PC control group (mean value ± SD: 80 ± 17 pg/mL, p < 0.001); conversely, progesterone levels were similar between the PM group (mean value ± SD: 88 ± 24 pg/mL) and PC group (mean value ± SD: 106 ± 83 pg/mL, p = 0.94) and also testosterone levels did not differ in the PM group (mean value ± SD: 209 ± 88 pg/mL) compared to the PC group (mean value ± SD: 201 ± 56 pg/mL, p > 0.99, one-way analysis of variance followed by Tukey-Kramer post-hoc comparison test). Cohen’s d value for allopregnanolone serum levels between PM and PC was −3.71 [−4.69 ÷ −2.72]. Postmenopausal women with migraine (PM) presented serum levels significantly lower than those in fertile age (MM) for all the target analytes: Allopregnanolone (p < 0.001), progesterone (p = 0.027) and testosterone (p < 0.001, one-way analysis of variance followed by Tukey-Kramer post-hoc comparison test). Cohen’s d value for allopregnanolone serum levels between PM and MM was −1.68 [−2.27 ÷ −1.09].
Figure 1.
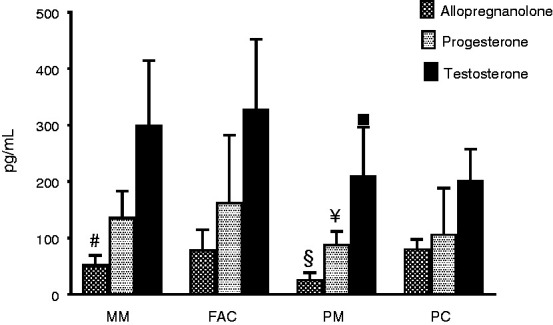
Serum levels (mean ± SD) of allopregnanolone, progesterone and testosterone in fertile age (MM and FAC groups) and postmenopausal (PM and PC groups) women.
#p < 0.001 vs. FAC group; §p < 0.001 vs. PC and MM groups; ¥p = 0.027 vs. MM group; ▪p < 0.001 vs. MM group, one-way analysis of variance followed by the Tukey-Kramer post-hoc comparison test.
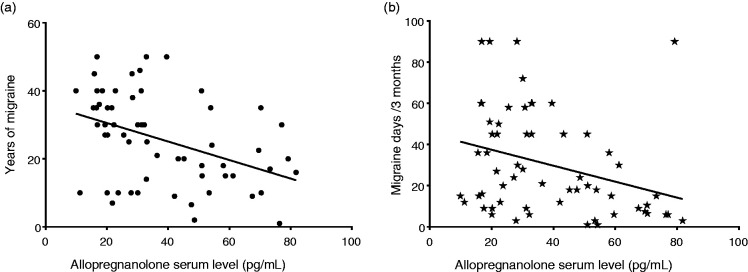
Considering MM, serum levels of allopregnanolone presented a negative correlation with years of migraine (R2 = 0.13, p = 0.069) and a statistically significant negative correlation with migraine days in the last 3 months (R2 = 0.18, p = 0.019; linear regression analysis). Serum levels of allopregnanolone in the PM group presented a negative correlation, although not statistically significant, with both years of migraine (R2 = 0.002, p = 0.81) and migraine days in the last 3 months (R2 = 0.04, p = 0.33; linear regression analysis). Indeed, considering both migraine groups together (MM plus PM, n = 60) (Figure 2), serum levels of allopregnanolone presented a statistically significant negative correlation with years of migraine (R2 = 0.19, p = 0.001) and with migraine days in the last three months (R2 = 0.13, p = 0.005; linear regression analysis).
Figure 2.
Correlation between allopregnanolone serum level and (a) years of migraine (p = 0.001) and (b) migraine days/3 months (p = 0.005, linear regression analysis) in migraine groups (MM plus PM, n = 60).
Discussion
Migraine and epilepsy are episodic disorders with distinct characteristics but sharing cortical hyperexcitability, probably due to an imbalance between excitatory (glutamate) and inhibitory (GABA) factors (19,20). Low levels of allopregnanolone have been hypothesized underlying catamenial epilepsy, a menstrual cycle-related seizure, refractory to treatment with specific anticonvulsant drugs (11). Perhaps either menstrually-related migraine or catamenial epilepsy might be driven by dysfunctions in the same steroid regulation mechanisms, particularly low allopregnanolone levels. Actually, we found that women with migraine (Figure 1, MM and PM groups) had significantly lower allopregnanolone serum levels compared to those measured in their respective controls FAC and PC groups (p < 0.001, one-way analysis of variance followed by Tukey-Kramer post-hoc comparison test). Moreover, according to Sawilowsky (21), the magnitude of the difference of allopregnanolone levels was large between MM and FAC and huge between PM and PC, even if negative. However, to confirm the role of low allopregnanolone levels in menstrually-related migraine, comparative studies are needed that take also into account women suffering from migraine without menstrual attacks.
Recent studies suggest that allopregnanolone has a specific neuroprotective action in the central and peripheral nervous system and a beneficial effect in neurodegenerative disorders (3,22) in which an inflammatory activation of the microglia and astrocytes is present. Neuroinflammation is a physiological response to infection or injury but if it becomes chronic, it induces neurodegeneration (23). Neuroinflammation plays an important role also in migraine, in inducing central sensitization and chronicity of the disorder (24). Really, in experimental models of migraine, allopregnanolone is capable of extinguishing the neurogenic inflammation. In particular, in trigeminal pain, allopregnanolone blocks neurogenic inflammation, probably with mechanisms mediated by the GABA(A) receptor (25). In another model of migraine (neurogenic edema in the rat meninges) allopregnanolone reduces plasma extravasation induced by electrical stimulation of the trigeminal ganglion and substance P (26). In addition, allopregnanolone regulates neuroinflammatory responses also by inhibiting TLR4-dependent pro-inflammatory signalling (27,28). In the context of migraine, impaired neuronal homeostasis for inadequate levels of allopregnanolone could result in a lower neuroprotective, analgesic and anti-inflammatory action. It was unlikely that the reduced allopregnanolone levels in the studied women with migraine were the consequence of ovarian insufficiency, since the serum concentrations of progesterone and testosterone showed no differences compared to control females. The adrenal cortex contributes largely to the circulating levels of allopregnanolone, either directly or through the synthesis of a precursor, peripherally converted to allopregnanolone. This neurosteroid is also synthesized in the nervous tissue by specific enzymes, crosses the BBB, and its synthesis is influenced by neuroinflammation (29). Low concentrations of allopregnanolone have been found in other conditions characterized, like migraine, by neuroinflammation such as Parkinson’s and Alzheimer’s disease and multiple sclerosis (3). Thus, we assumed that significantly lower allopregnanolone levels in patients with migraine (both MM and PM groups) than in non-headache control women were possibly associated with migraine. Indeed, non-headache women (FAC and PC groups) showed no difference in allopregnanolone levels, despite one group consisting of young women in fertile age (Table 1) and the other of postmenopausal women. Therefore, in migraine women, reduced levels of allopregnanolone could contribute to the persistence of migraine even after menopause, when instead, due to the complete cessation of the monthly cycling of estrogen, migraine generally improves and its prevalence decreases by about half (30). This was further supported by the large magnitude of effect, evaluated by Cohen’s d (21), of the lower levels of allopregnanolone found in the PM group compared with the MM one.
In fertile age women, the timing of blood sampling is crucial for the interpretation of allopregnanolone levels. In fact, the levels of this neurosteroid increase physiologically in the late luteal phase while after menopause its fluctuations are limited (10,29). Recently, allopregnanolone serum levels have been found to be significantly increased in patients with episodic and chronic migraine compared to healthy controls. Since this study (31) does not specify the phase of the sampling, and not even how many women were of fertile or menopausal age and the number of males (apart from those with cluster headache), the comparison with our results is unfeasible.
In preclinical experimental models, allopregnanolone exhibits pronounced analgesic properties, but few studies focused on the relationship between pain and allopregnanolone in clinical populations (32). Two studies in US Army male veterans show that allopregnanolone levels are inversely related to pain (muscle soreness, chest pain, low back pain and total pain, including headache), suggesting that allopregnanolone may act as a potent endogenous analgesic and that supplementation with exogenous allopregnanolone could have therapeutic potential (33,34). Notably, grouping all women with migraine together (MM plus PM) (Figure 2), we found that serum concentrations of allopregnanolone were significantly and inversely correlated with both the history of migraine (years) (p = 0.001) and number of migraine days in the last three months (p = 0.005, linear regression analysis), even if the effect size of these correlations was mild to moderate according to Cohen (35). These results supported the possibility of an anti-migraine action of allopregnanolone, not only an analgesic one.
We did not find (Figure 1) differences in testosterone and progesterone levels between MM and the corresponding non-headache control group (FAC). Postmenopausal migraine women (PM group) had significantly lower levels of these hormones than women in fertile age, but there were no differences compared to postmenopausal, non-headache control women (PC group). These results were in agreement with the observation that testosterone, in women who do not take estrogens, does not intervene in migraine (36).
Our study has limitations. The sample was of small size (Table 1), being a pilot study. However, it was conducted rigorously and there are no significant differences among groups regarding lifestyle. In women of fertile age, analyte determinations were performed in the follicular phase when allopregnanolone levels are physiologically lower than in the luteal phase (29). Therefore, the differences found between women with migraine and the corresponding age-matched non-headache controls were relevant. However, measuring the levels of allopregnanolone in late luteal phase in women with and without menstrually-related migraine might have provided a more relevant insight on the mechanism of menstrual attack. Blood sampling was planned during the late follicular phase in order to avoid menstrual attack and compare allopregnanolone levels between women of fertile and postmenopausal age, since in this phase the levels should be similar and allopregnanolone levels were not found to vary with age in women (29).
Conclusion
Women suffering from migraine presented low serum levels of allopregnanolone, a neurosteroid that modulates GABAergic inhibition (9). In this context, the increased cortical excitability found in migraine may not be sufficiently offset by the allopregnanolone-mediated enhancement of the GABAergic transmission. Consequently, the reduced GABAergic inhibition could inadequately protect women with migraine against inflammatory and algogenic stimuli. In particular, it could contribute to menstrually-related migraine and persistence of migraine even after menopause. Moreover, serum levels of allopregnanolone, in our study carried out in the clinic, were inversely related to the years and frequency of migraine, also indicating an anti-migraine and not only analgesic action of this neurosteroid.
Our findings, obtained in a pilot study, if confirmed in larger case series, could provide the background for novel therapies aimed at raising the GABAergic transmission by drugs increasing the biosynthetic pathway of inhibitory neurosteroids or by the use of synthetic allopregnanolone analogues for the treatment of both menstrually-related and postmenopausal migraine.
Clinical implications
Women suffering from migraine, either menstrually-related or postmenopausal, had lower allopregnanolone serum levels than non-headache control women.
In these women with migraine, allopregnanolone serum levels were inversely related to the years and frequency of migraine.
Drugs increasing the biosynthetic pathway of inhibitory neurosteroids or synthetic allopregnanolone analogues could represent a possible strategy for the management of menstrually-related and postmenopausal migraine.
Supplemental Material
Supplemental material, sj-pdf-1-cep-10.1177_0333102420937742 for Serum levels of allopregnanolone, progesterone and testosterone in menstrually-related and postmenopausal migraine: A cross-sectional study by Cecilia Rustichelli, Elisa Bellei, Stefania Bergamini, Emanuela Monari, Carlo Baraldi, Flavia Lo Castro, Aldo Tomasi and Anna Ferrari in Cephalalgia
Acknowledgements
The authors thank the “Centro Interdipartimentale Grandi Strumenti” (CIGS) of the University of Modena and Reggio Emilia for technical assistance. LC-MS/MS instruments at CIGS were granted by the Foundation “Fondazione Cassa di Risparmio di Modena – FCRM.”
Authorship
All authors participated in study design, performed research, data analysis, and drafting of manuscript content. All authors reviewed and approved the manuscript.
Declaration of conflicting interests
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Data accessibility
The datasets generated and analysed during these studies are not publicly available. All relevant data are included in this published article and in the Supplementary material.
Ethics or Institutional Review Board approval
All women participants in this study gave their written consent. The research was conducted in accordance with the ethical principles of the Helsinki Declaration, last edition (2013) and approved by the Ethics Committee of Area Vasta Emilia Nord (AVEN) (Italy) (prot. 0013510/18).
Funding
The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was carried out with research funds from the University of Modena and Reggio Emilia (Italy) attributed to Dr Anna Ferrari (FAR 2016).
ORCID iD
Carlo Baraldi https://orcid.org/0000-0001-8432-1888
References
- 1.Wu X, Gangisetty O, Carver CM, et al. Estrous cycle regulation of extrasynaptic δ-containing GABA(A) receptor-mediated tonic inhibition and limbic epileptogenesis. J Pharmacol Exp Ther 2013; 346: 146–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Finocchi C, Ferrari M. Female reproductive steroids and neuronal excitability. Neurol Sci 2011; 32: S31–S35. [DOI] [PubMed] [Google Scholar]
- 3.Mendell AL, MacLusky NJ. Neurosteroid metabolites of gonadal steroid hormones in neuroprotection: Implications for sex differences in neurodegenerative disease. Front Mol Neurosci 2018; 11: 359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Reddy DS. Neurosteroids: Endogenous role in the human brain and therapeutic potentials. Prog Brain Res 2010; 186: 113–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chuang S-H, Reddy DS. 3β-methyl-neurosteroid analogs are preferential positive allosteric modulators and direct activators of extrasynaptic δ-subunit γ-aminobutyric acid type A receptors in the hippocampus dentate gyrus subfield. J Pharmacol Exp Ther 2018; 365: 583–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Coronel MF, Labombarda F, González SL. Neuroactive steroids, nociception and neuropathic pain: A flashback to go forward. Steroids 2016; 110: 77–87. [DOI] [PubMed] [Google Scholar]
- 7.Bernardi F, Pluchino N, Begliuomini S, et al. Disadaptive disorders in women: Allopregnanolone, a sensitive steroid. Gynecol Endocrinol 2004; 19: 344–353. [DOI] [PubMed] [Google Scholar]
- 8.Reddy DS. Role of neurosteroids in catamenial epilepsy. Epilepsy Res 2004; 62: 99–118. [DOI] [PubMed] [Google Scholar]
- 9.Schüle C, Nothdurfter C, Rupprecht R. The role of allopregnanolone in depression and anxiety. Prog Neurobiol 2014; 113: 79–87. [DOI] [PubMed] [Google Scholar]
- 10.Kimball A, Dichtel LE, Nyer MB, et al. The allopregnanolone to progesterone ratio across the menstrual cycle and in menopause. Psychoneuroendocrinology 2020; 112: 104512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Reddy DS, Rogawski MA. Enhanced anticonvulsant activity of ganaxolone after neurosteroid withdrawal in a rat model of catamenial epilepsy. J Pharmacol Exp Ther 2000; 294: 909–915. [PubMed] [Google Scholar]
- 12.MacGregor EA, Victor TW, Hu X, et al. Characteristics of menstrual vs nonmenstrual migraine: A post hoc, within-woman analysis of the usual-care phase of a nonrandomized menstrual migraine clinical trial. Headache 2010; 50: 528–538. [DOI] [PubMed] [Google Scholar]
- 13.Calhoun A, Ford S. Elimination of menstrual-related migraine beneficially impacts chronification and medication overuse. Headache 2008; 48: 1186–1193. [DOI] [PubMed] [Google Scholar]
- 14.Somerville BW. Estrogen-withdrawal migraine. I. Duration of exposure required and attempted prophylaxis by premenstrual estrogen administration. Neurology 1975; 25: 239–244. [DOI] [PubMed] [Google Scholar]
- 15.Freeman EW, Sammel MD, Lin H, et al. Symptoms in the menopausal transition: Hormone and behavioral correlates. Obstet Gynecol 2008; 111: 127–136. [DOI] [PubMed] [Google Scholar]
- 16.Carturan P, Scorcine C, Fragoso YD. Migraine in the post-menopausal period is associated with higher levels of mood disorders, disability, and more menopausal symptoms. Arq Neuropsiquiatr 2016; 74: 999–1002. [DOI] [PubMed] [Google Scholar]
- 17.Delaruelle Z, Ivanova TA, Khan S, et al. Male and female sex hormones in primary headaches. J Headache Pain 2018; 19: 117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Headache Classification Committee of the International Headache Society (IHS). The International Classification of Headache Disorders, 3rd edition. Cephalalgia 2018; 38: 1–211. [DOI] [PubMed] [Google Scholar]
- 19.Mantegazza M, Cestèle S. Pathophysiological mechanisms of migraine and epilepsy: Similarities and differences. Neurosci Lett 2018; 667: 92–102. [DOI] [PubMed] [Google Scholar]
- 20.Liao J, Tian X, Wang H, et al. Epilepsy and migraine – are they comorbidity? Genes Dis 2018; 5: 112–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sawilowsky S. New effect size rules of thumb. J Mod Appl Stat Met 2009; 8: 467–474. [Google Scholar]
- 22.Kalakh S, Mouihate A. Enhanced remyelination during late pregnancy: Involvement of the GABAergic system. Sci Rep 2019; 9: 7728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Noorbakhsh F, Baker GB, Power C. Allopregnanolone and neuroinflammation: A focus on multiple sclerosis. Front Cell Neurosci 2014; 8: 134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Edvinsson L, Haanes KA, Warfvinge K. Does inflammation have a role in migraine? Nat Rev Neurol 2019; 15: 483–490. [DOI] [PubMed] [Google Scholar]
- 25.Cutrer FM, Moskowitz MA. Wolff Award 1996. The actions of valproate and neurosteroids in a model of trigeminal pain. Headache 1996; 36: 579–585. [DOI] [PubMed] [Google Scholar]
- 26.Limmroth V, Lee WS, Moskowitz MA. GABAA-receptor-mediated effects of progesterone, its ring-A-reduced metabolites and synthetic neuroactive steroids on neurogenic oedema in the rat meninges. Br J Pharmacol 1996; 117: 99–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yilmaz C, Karali K, Fodelianaki G, et al. Neurosteroids as regulators of neuroinflammation. Front Neuroendocrinol 2019; 55: 100788. [DOI] [PubMed] [Google Scholar]
- 28.Balan I, Beattie MC, O’Buckley TK, et al. Endogenous neurosteroid (3α,5α)3-Hydroxypregnan-20-one inhibits toll-like-4 receptor activation and pro-inflammatory signaling in macrophages and brain. Sci Rep 2019; 9: 1220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Melcangi RC, Panzica GC. Allopregnanolone: State of the art. Prog Neurobiol 2014; 113: 1–5. [DOI] [PubMed] [Google Scholar]
- 30.MacGregor EA. Migraine, menopause and hormone replacement therapy. Post Reprod Health 2018; 24: 11–18. [DOI] [PubMed] [Google Scholar]
- 31.Koverech A, Cicione C, Lionetto L, et al. Migraine and cluster headache show impaired neurosteroids patterns. J Headache Pain 2019; 20: 61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Joksimovic SL, Covey DF, Jevtovic-Todorovic V, et al. Neurosteroids in pain management: A new perspective on an old player. Front Pharmacol 2018; 9: 1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kilts JD, Tupler LA, Keefe FJ, et al. Neurosteroids and self-reported pain in veterans who served in the U.S. Military after September 11, 2001. Pain Med 2010; 11: 1469–1476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Naylor JC, Kilts JD, Szabo ST, et al. Allopregnanolone levels are inversely associated with self-reported pain symptoms in U.S. Iraq and Afghanistan-era veterans: Implications for biomarkers and therapeutics. Pain Med 2016; 17: 25–32. [DOI] [PubMed] [Google Scholar]
- 35.Cohen J. A power primer. Psych Bull 1992; 112: 155–159. [DOI] [PubMed] [Google Scholar]
- 36.Mattsson P. Serum levels of androgens and migraine in postmenopausal women. Clin Sci 2002; 103: 487–491. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-pdf-1-cep-10.1177_0333102420937742 for Serum levels of allopregnanolone, progesterone and testosterone in menstrually-related and postmenopausal migraine: A cross-sectional study by Cecilia Rustichelli, Elisa Bellei, Stefania Bergamini, Emanuela Monari, Carlo Baraldi, Flavia Lo Castro, Aldo Tomasi and Anna Ferrari in Cephalalgia
Data Availability Statement
The datasets generated and analysed during these studies are not publicly available. All relevant data are included in this published article and in the Supplementary material.