Abstract
Integrins are transmembrane receptors that mediate cell-extracellular matrix and cell–cell interactions. The β1-integrin subunit is highly expressed by embryonic neural stem cells (NSCs) and is critical for NSC maintenance in the developing nervous system, but its role in the adult hippocampal niche remains unexplored. We show that β1-integrin expression in the adult mouse dentate gyrus (DG) is localized to radial NSCs and early progenitors, but is lost in more mature progeny. Although NSCs in the hippocampal subgranular zone (SGZ) normally only infrequently differentiate into astrocytes, deletion of β1-integrin significantly enhanced astrocyte differentiation. Ablation of β1-integrin also led to reduced neurogenesis as well as depletion of the radial NSC population. Activation of integrin-linked kinase (ILK) in cultured adult NSCs from β1-integrin knockout mice reduced astrocyte differentiation, suggesting that at least some of the inhibitory effects of β1-integrin on astrocytic differentiation are mediated through ILK. In addition, β1-integrin conditional knockout also resulted in extensive cellular disorganization of the SGZ as well as non-neurogenic regions of the DG. The effects of β1-integrin ablation on DG structure and astrogliogenesis show sex-specific differences, with the effects following a substantially slower time-course in males. β1-integrin thus plays a dual role in maintaining the adult hippocampal NSC population by supporting the structural integrity of the NSC niche and by inhibiting astrocytic lineage commitment.
Keywords: neural stem cells, integrins, astrocytes, neurogenesis, hippocampus
Introduction
Integrins are transmembrane receptors composed of an α and β subunit that allow cells to recognize and communicate with the ECM (Srichai and Zent, 2010). The largest integrin subfamily is the β1-integrin subfamily, and β1-integrin is highly expressed in mammalian NSCs (Campos et al., 2004; Flanagan et al., 2006; Hall et al., 2006; Lathia et al., 2007). Binding of β1-integrin to ECM molecules, including laminin, collagen, and tenascin-C, triggers conformational changes that activate β1-integrin (Srichai and Zent, 2010). Although β1-integrin has no intrinsic catalytic activity, signaling molecules, such as ILK and focal adhesion kinase (FAK), associate with its cytoplasmic tail and transduce downstream effects (Hannigan et al., 1996; Schlaepfer et al., 1999).
In the developing nervous system, β1-integrin is expressed by embryonic NSCs and regulates NSC maintenance via cross-talk with growth factor signaling pathways (Campos et al., 2004, 2006; Leone et al., 2005). β1-integrin also promotes NSC maintenance during development by regulating apical adhesion of NSCs (Graus-Porta et al., 2001; Loulier et al., 2009; Radakovits et al., 2009). In the adult subventricular zone (SVZ), β1-integrin also participates in NSC adhesion, and β1-integrin blockade alters cell proliferation and disrupts structural organization of the niche (Shen et al., 2008; Kazanis et al., 2010).
Although the anatomical structure of the adult SVZ niche, including the role of ECM components, is relatively well characterized (Mercier et al., 2002; Shen et al., 2008; Tavazoie et al., 2008), less is known about how interactions with the ECM may regulate neural stem and progenitor cell fate decisions in the adult hippocampus (Faissner and Reinhard, 2015; Kazanis and Ffrench-Constant, 2011). β1-integrin is required for formation of the radial glial scaffold in the developing hippocampus (Forster et al., 2002), suggesting that β1-integrin might also help maintain the structure and function of NSCs in the adult hippocampal SGZ.
In the adult DG, radially-oriented NSCs located in the SGZ give rise to a population of proliferative neural progenitor cells otherwise known as intermediate progenitor cells or transit-amplifying progenitors (TAPs) (Bonaguidi et al., 2011; Morrens et al., 2012). TAPs display a nonradial morphology and undergo multiple rounds of cell division before differentiating into neuroblasts (Bonaguidi et al., 2011; Encinas et al., 2011). NSCs in the adult SGZ in vivo differentiate predominantly into neurons (Bonaguidi et al., 2011; Encinas et al., 2011), but in culture, astrocytes are a frequent progeny (Bonaguidi et al., 2008). Thus NSCs must encounter signals in the SGZ in vivo that inhibit astrocytic lineage commitment. β1-integrin signaling inhibits astrocytic differentiation of spinal cord ependymal stem cells and perinatal SVZ NSCs (North et al., 2015; Pan et al., 2014), suggesting that it might be involved in limiting astrocytic lineage commitment in the adult SGZ.
Sex-dependent differences in integrin functions have been observed in the central nervous system (Brahmachari and Pahan, 2010; Recouvreux et al., 2013), and there are also sex-dependent differences in mechanisms regulating adult hippocampal neurogenesis (Galea et al., 2013; Hillerer et al., 2013; Lee et al., 2014). We therefore compared the effects of β1-integrin ablation on the adult hippocampal stem cell niche in male and female mice.
In this study, we find that β1-integrin expression in the adult DG is localized to radial NSCs where it plays a dual role in maintaining NSCs by supporting the structural integrity of the hippocampal stem cell niche and by inhibiting astrocytic differentiation. Further, we observe sex-specific differences in the time course of the effects of β1-integrin ablation on SGZ structure and NSC differentiation.
Materials and Methods
Generation of Transgenic Mice
β1-integrin floxed (β1flx/flx) mice (Jackson Laboratories) were bred to mice harboring a LoxP flanked stop sequence followed by Enhanced Yellow Fluorescent Protein (YFP) in the ROSA locus (ROSA:YFP; Jackson Laboratories #006148) to produce β1flx/flx; ROSA:YFP and β1+/+; ROSA:YFP mice. They were mated on a C57Bl/6 background such that β1flx/flx; ROSA:YFP and β1+/+; ROSA:YFP mice were littermates. 8 to 10-week-old mice were used for all viral injection experiments.
Viral Vector Production and Stereotaxic Virus Injections
Cre recombinase was cloned into the lentiviral backbone, pBOB-IRES2-mCherry (Bond et al., 2014), to generate pBOB-Cre-IRES2-mCherry (LV-Cre). A constitutively active form of ILK (caILK; Pan et al., 2014) was cloned into pBOB-IRES2-mCherry to generate pBOB-caILK-IRES2-mCherry (LV-caILK). Lentivirus (LV) was produced by transfection of HEK 293T cells (Bond et al., 2014) and was used at a titer of 108 colony forming units per milliliter. 8 to 10-week-old mice were stereotaxically injected as described at bregma coordinates posterior 2 mm, lateral 1.5mm, depth 1.9 mm (Bond et al., 2014). Virally infected brains were analyzed 14 or 28 days postinjection.
Adult NSC Cultures and Differentiation
Adult NSCs were isolated from 8 to 12 weeks old β1flx/flx; Rosa:YFP and β1+/+; Rosa:YFP mice by dissecting the hippocampus as described (Bonaguidi et al., 2008). Cells were dissociated and grown as neurospheres in serum-free neurosphere medium (NM) containing DMEM:F12 (Invitrogen) with N2, B27, penicillin/streptomycin/glutamine, and heparin (Sigma). NM was supplemented with 20 ng/mL epidermal growth factor (EGF, Millipore) and 10 ng/mL basic fibroblast growth factor (Millipore). Cells were incubated at 37°C in humidified 5% CO2. Neurospheres were passaged every 3–5 days. After the third passage, cultures were infected with adenovirus expressing Cre (Vector Biolabs) and either LV-caILK or control lentivirus (LV-mCherry). Seven days postinfection, cells were dissociated and plated for differentiation at 2 × 104 cells/cm2 on to glass coverslips coated with poly-d-lysine (Sigma) and laminin (Roche) in 24-well plates. Cells were allowed to differentiate for 7 days in NM supplemented with 0.2 ng/mL EGF. Differentiated cells were then fixed in 4% paraformaldehyde for 15 min at room temperature (RT) and immunostained.
Immunohistochemistry
Mice were sacrificed by CO2 inhalation and immediately trans-cardially perfused with cold Hank’s Balanced Salt Solution (HBSS, Corning). Brains were fixed for 2 h in 4% PFA, cryoprotected in 30% sucrose overnight, and embedded in OCT. Fixed brains were sectioned into 20 µm sections using a cryostat. Immunostaining was performed using standard techniques (Bond et al., 2014). (Table 1 for primary antibody dilutions). For β1-integrin staining, biotin-conjugated goat or donkey anti-rat (1:500) was the secondary antibody, and fluorophore-conjugated streptavidin (1:100, Invitrogen) was used to amplify signal. Immunostaining was performed on every sixth serial section of the hippocampus, and quantification was performed on four or more Z-stacks per brain (see below).
Table 1:
Antibodies
| Immunohistochemistry Antibodies: | ||||
|---|---|---|---|---|
| Antibody | Host species | Vendor | Dilution | Treatment |
| Aldh1 | Rabbit | Abcam | 1:500 | Antigen Retrieval |
| β1-integrin | Rat | Chemicon | 1:500 | |
| DCX | Goat | Santa Cruz | 1:500 | |
| GFAP | Rabbit | Dako | 1:1000 | |
| GFAP | Mouse IgG1 | Sigma | 1:1000 | |
| GFP | Rabbit | Invitrogen | 1:1000 | |
| GFP | Chicken | Abcam | 1:1000 | |
| mCherry | Rabbit | Clontech | 1:1000 | |
| MCM2 | Mouse IgG1 | BD Biosciences | 1:500 | Antigen Retrieval |
| Nestin | Mouse IgG1 | BD Biosciences | 1:500 | Antigen Retrieval |
| NeuN | Mouse IgG1 | Chemicon | 1:500 | |
| S100β | Rabbit | Dako | 1:1000 | |
| SOX2 | Goat | Santa Cruz | 1:500 | Antigen Retrieval |
| vWF | Rabbit | Abcam | 1:2000 | |
| Western Antibodies: | ||||
| Antibody | Host species | Vendor | Dilution | Treatment |
| β1-integrin | Rat | Chemicon | 1:2000 | Non-reducing |
| FAK | Rabbit | Cell Signaling | 1:1000 | |
| GAPDH | Mouse | Millipore | 1:5000 | |
| GFP | Chicken | Abcam | 1:5000 | |
| ILK | Rabbit | Millipore | 1:5000 | |
| pFAK | Rabbit | Cell Signaling | 1:1000 | |
| pILK | Rabbit | Abgent | 1:1000 | |
Confocal Imaging and Quantification
All imaging and quantification was done blinded to genotype and condition. Sections of similar rostral-caudal position in dorsal DG were imaged using a Leica SP5 Confocal Microscope. Sequential scanning of channels was performed to prevent false-positive colocalization. For each immunohistochemical marker, four or more Z-stacks of equivalent thickness were quantified for each brain. Z-stacks were 10–15 µm thick and the step size was 1 µm. The numbers of mice per genotype used for immunohistochemical quantification are specified in the results. In viral injection experiments, only sections with recombination or visible viral infection were considered for quantification. Unbiased, stereological cell counting was done using ImageJ software. For per volume quantifications, granule cell layer (GCL) volume was calculated and used as the denominator. The volume was calculated by manually tracing the GCL and measuring the selected surface area using ImageJ. The GCL area was then multiplied by the Z-stack thickness to determine the total GCL volume in that Z-stack. For in vitro differentiation experiments, cells were counted in 10 random fields per coverslip with a minimum of three coverslips per condition for each independent experiment.
Protein Extraction and Western Blotting
Protein was extracted from dissected DG (Hagihara et al., 2009) using M-PER protein extraction reagent and HALT protease and phosphatase inhibitor cocktail (Thermo Scientific). Protein samples were boiled in denaturing conditions for 20 min and 5 µg protein was loaded on to 4–20% polyacrylamide gels. Protein was transferred on to polyvinylidene difluoride membranes at 4°C for 2 h. Membranes then were blocked in tris-buffered saline with 0.05% Tween 20 and 5% nonfat dry milk or 5% BSA for 1 h at RT. Membranes were then incubated with primary antibody in blocking solution overnight at 4°C (Table 1 for antibody information). After washing, membranes were incubated with the appropriate horseradish peroxidase-conjugated secondary antibody (1:2000 in blocking solution; Santa Cruz) and developed using Pierce Enhanced Chemiluminescent Western Blotting Substrate (Thermo Scientific).
Statistical Analysis
Statistical analysis was performed with two-tailed, unpaired Student’s t test or with ANOVA (with Tukey post hoc test), as indicated in the figures and text.
Results
Cell Type-Specific Expression of β1-Integrin in the Adult DG
In the adult SVZ, β1-integrin is expressed by TAPs and activated NSCs, but not quiescent NSCs (Kazanis et al., 2010). In contrast, embryonic NSCs highly express β1-integrin (Campos et al., 2004; Flanagan et al., 2006; Hall et al., 2006; Lathia et al., 2007). We therefore sought to determine what stages of neural stem/progenitor cells express β1-integrin in the adult DG.
In addition to expression in blood vessels, β1-integrin was preferentially localized in DG to the SGZ where NSCs and TAPs reside (Fig. 1). β1-integrin was expressed in the cell bodies of most SRY(Sex Determining Region Y)-Box2 (SOX2) positive stem/progenitor cells in the SGZ, as well as in radial processes of NSCs which express both SOX2 and glial fibrillary acidic protein (GFAP) (Fig. 1A). Similarly, β1-integrin colocalized with Nestin+ GFAP+ radial processes of NSCs (Fig. 1B). In fact, most β1-integrin in the granule cell layer (GCL) outside of the SGZ localized to radial processes of NSCs (Fig. 1A,B, arrows). β1-integrin also colocalized with minichromosome maintenance complex component 2 (MCM2), a prereplication complex protein expressed beginning in G1 phase that is required for initiation of DNA replication and is therefore specific to dividing cells (Maslov et al., 2004) (Fig. 1C). In the DG, MCM2+ cells are predominantly TAPs (Bonaguidi et al., 2011). β1-integrin expression was less apparent in later stages of the stem cell lineage, and was absent in most doublecortin (DCX) positive neuroblasts (Fig. 1D). Most neuronal nuclei (NeuN) positive neurons, which reside outside of the SGZ, did not coexpress β1-integrin (Fig. 1E). Finally, outside of the GCL, β1-integrin expression was found in GFAP+ astrocytes in the hilus (Fig. 1A, arrowheads), and in blood vessels. In summary, β1-integrin was highly expressed in the cell bodies and radial processes of NSCs, in dividing TAPs, and in blood vessels in the adult mouse DG.
FIGURE 1:
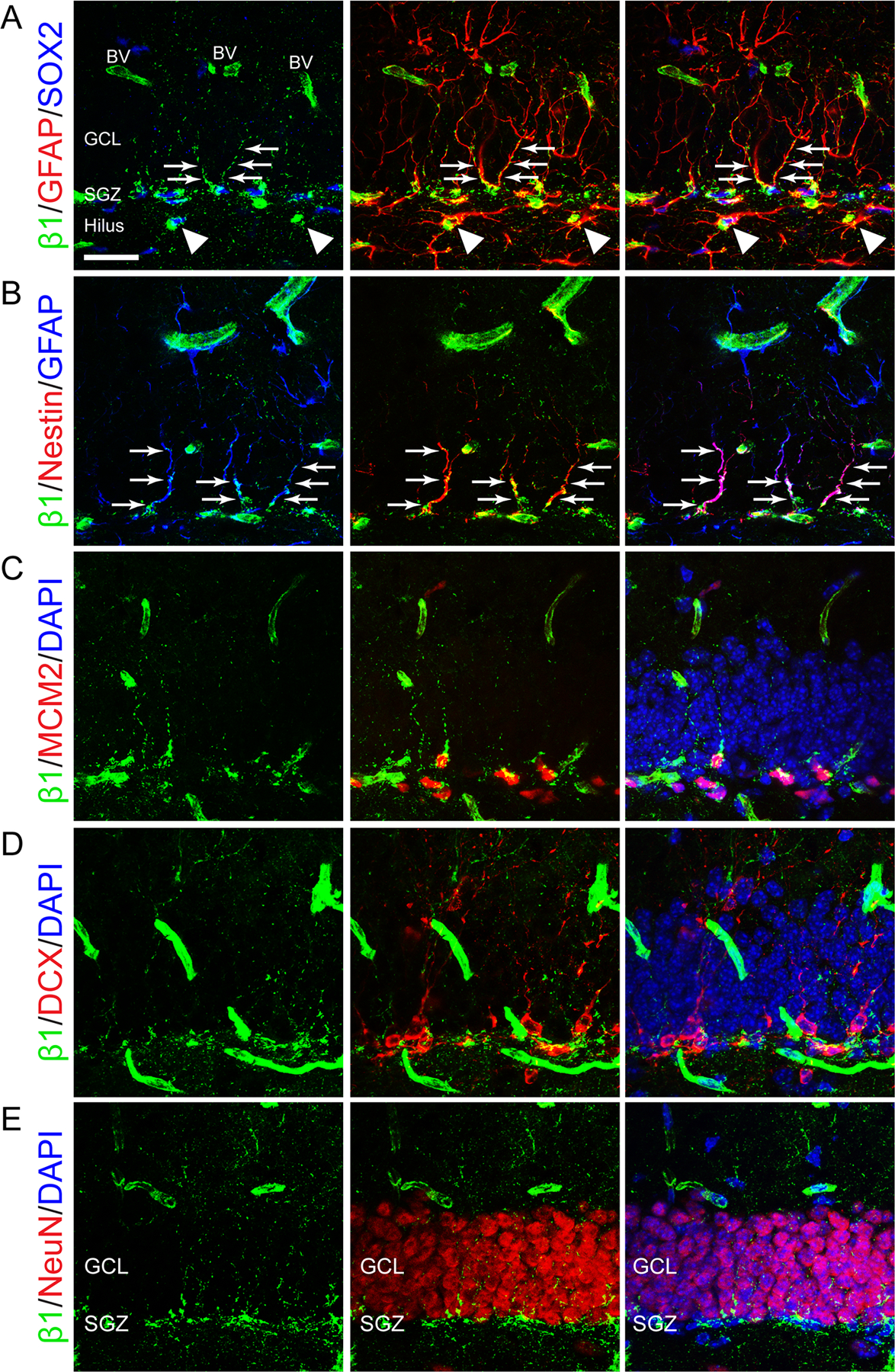
Cell type-specific expression of β1-integrin in the adult dentate gyrus. A: Immunostaining for β1-integrin (β1, green), SOX2 (blue), and GFAP (red) shows that β1-integrin localizes to the cell body of SOX2+ cells. β1-integrin also colocalizes with the radial processes of SOX2+GFAP+ stem cells in the SGZ (arrows). Outside of the GCL, β1-integrin is expressed by GFAP+ astrocytes in the hilus (arrowheads), and in blood vessels (BV). B: Immunostaining for β1-integrin (green), GFAP (blue), and Nestin (red) shows that β1-integrin expression localizes to radial processes of GFAP+Nestin+ stem cells in the SGZ (arrows). C: Immunostaining for β1-integrin (green), MCM2 (red), and DAPI (blue) shows β1-integrin expression in dividing MCM2+ progenitors. D: Immunostaining for β1-integrin (green), DCX (red), and DAPI (blue) shows that β1-integrin expression does not usually colocalize with DCX+ neuroblasts. E: Immunostaining for β1-integrin (green), NeuN (red), and DAPI (blue) shows that β1-integrin expression is localized to the SGZ and is not expressed in NeuN+ granule cell neurons. Scale bar 25 µm. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
β1-Integrin Is Critical for Structural Integrity of the DG
To define the role of β1-integrin in the adult hippocampal NSC niche, we examined Cre recombinase-mediated deletion of β1-integrin. β1-integrin floxed (β1flx/flx) mice were bred to a Cre-inducible reporter line (ROSA:YFP) to generate β1flx/flx; ROSA:YFP and β1+/+; ROSA:YFP mice. Because we observed that β1-integrin expression was localized to both radial NSCs, a largely quiescent cell population, as well as TAPs, a proliferative population, we targeted both dividing and nondividing cells using a lentivirus that expresses Cre recombinase (LV-Cre).
LV-Cre was stereotaxically injected into the DG of 8–10 week old β1flx/flx; ROSA:YFP (β1 KO) and β1+/+; ROSA:YFP (control) mice and tissue was analyzed 14 days post injection (D14; Fig. 2A). At this timepoint, we observed more efficient ablation of β1-integrin protein in female compared to male mice (see Fig. 7), and therefore focused our initial analyses on females. Recombination as indicated by YFP expression was equivalent in β1 KO and control mice (Fig. 2C). Cre successfully ablated β1-integrin in neurogenic lineages in the DG of β1 KO female mice as indicated by immunostaining (Fig. 2E). β1-integrin knockout significantly disrupted the structural integrity of the DG, including the molecular layer (ML), the GCL, and the hilus (Fig. 2B). Nuclear DAPI staining illustrated the organization of the DG in control mice: tightly packed dentate granule cells formed the GCL, while the ML and hilus had sparse cell densities (Fig. 2B). After β1-integrin ablation, cell density in the GCL, ML, and hilus became more uniform and the regions less distinct (Fig. 2B). The SGZ is a narrow layer of cells between the GCL and the hilus that is marked by the presence of stem/progenitor cells. In controls, the SGZ was well defined by SOX2+ stem/progenitor cells and dividing MCM2+ progenitors (Fig. 2D, dotted line). However, in β1 KO mice the distinct structure of the SGZ was lost. SOX2+ stem/progenitor cells were dispersed throughout the DG and some dividing progenitors (SOX2+ MCM2+; white arrows Fig. 2D) were located well outside the predicted SGZ region depicted by the dotted line. In addition, the number of MCM2+ cells in the DG increased after β1-integrin deletion (Control 38,996 ± 10991 n = 4, β1 KO 98,468 ± 19,655 n = 4, P < 0.05). This was largely attributable to a marked increase in MCM2+ cell numbers in the GCL (Control 11,398 ± 4,734 n = 4, β1 KO 54,780 ± 12,924 n = 4, P < 0.05), whereas there was no significant change in MCM2+ cells in the SGZ (Control 27,598 ± 7,175 n = 4, β1 KO 43,687 ± 7,390 n = 4, P > 0.05). This suggests that β1-integrin ablation causes progenitor cells to migrate out of the SGZ and proliferate. This increase in MCM2 immunoreactivity largely did not colocalize with YFP, a marker of β1 KO cells, suggesting that the proliferation phenotype is non-cell-autonomous and perhaps secondary to loss of β1-integrin function in NSCs.
FIGURE 2:
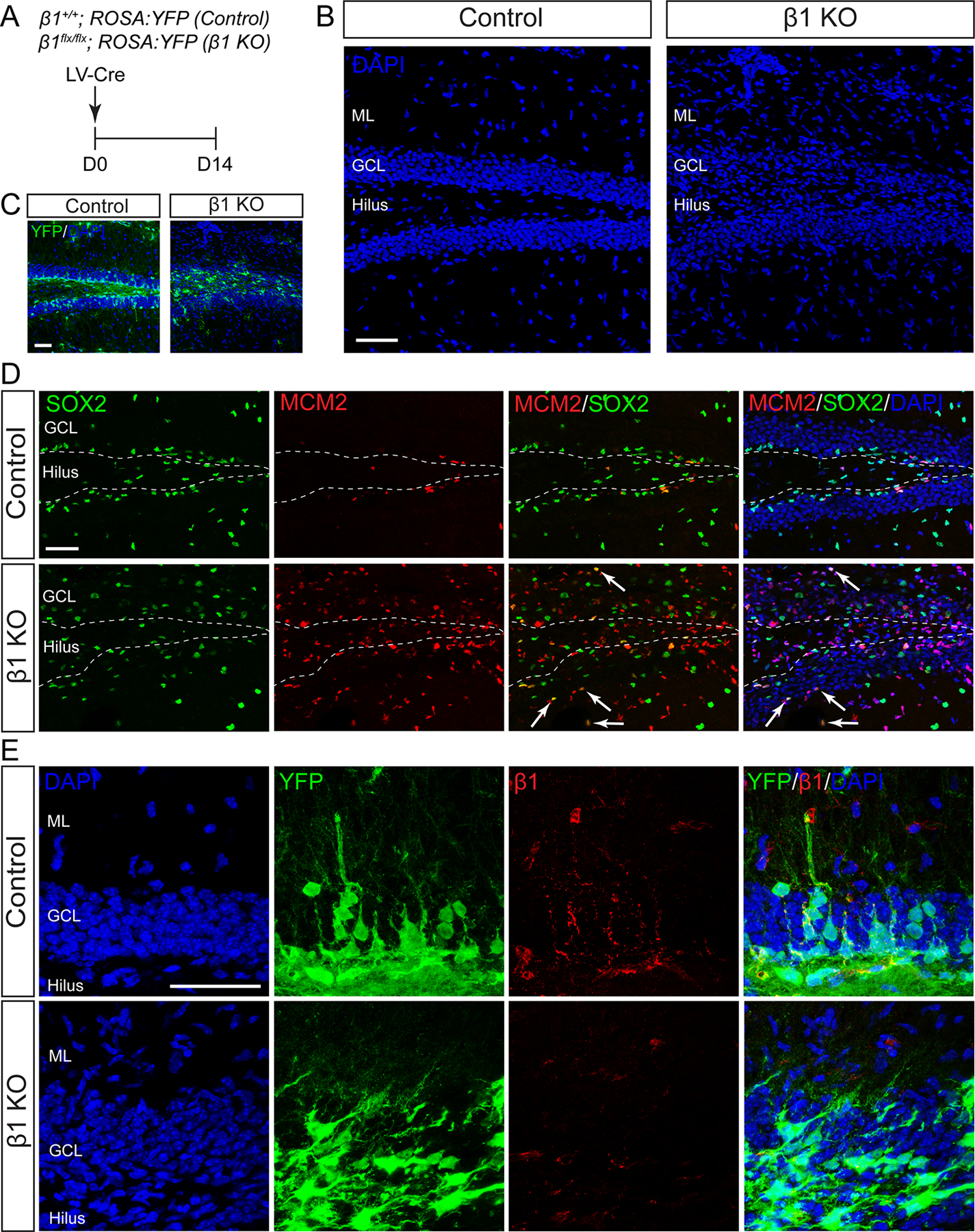
β1-integrin knockout disrupts the structural integrity of the dentate gyrus. A: Lentivirus expressing Cre (LV-Cre) was stereotaxically injected into the DG of adult female β1+/+; ROSA:YFP (Control) or β1flx/flx; ROSA:YFP (β1 KO) mice on day 0 (D0) and analyses were completed on D14. B: DAPI nuclear staining (blue) shows that β1-integrin knockout causes severe disorganization throughout the molecular layer (ML), granule cell layer (GCL), and hilus of the DG. C: Immunostaining for YFP (green) and DAPI (blue) of the same DGs shown in B shows equivalent recombination in control and β1 KO conditions. D: Immunostaining for SOX2 (green), MCM2 (red), and DAPI (blue). In control, dividing MCM2+ cells and SOX2+ stem/progenitor cells are mostly confined to the SGZ (dotted line). In β1-integrin knockouts, SOX2+MCM2+ dividing progenitors are found outside the SGZ (white arrows). β1-integrin knockout increases the number of MCM2+, particularly in the GCL. E: High magnification DAPI staining (blue) shows that β1-integrin knockout increases cell density in the ML and causes the GCL to lose its compact organization. Immunostaining for YFP (green) reveals that control recombined cells have neuronal morphology, while cells in β1-integrin KO mice have a multipolar morphology. Immunostaining for β1-integrin (red) shows that LV-Cre largely eliminates β1-integrin in the DG of β1 KO mice. Scale bars 50 µm. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
β1-integrin knockout also altered the morphology of the recombined cells (Fig. 2E). Recombined YFP+ cells in controls lined the SGZ, and those with neuronal morphology were present in the GCL (Fig. 2E). In contrast, YFP+ cells in β1 KO mice exhibited a multipolar morphology and were found throughout the GCL and the region where the GCL merged into the hilus (Fig. 2E). Together these data indicated that β1-integrin is critical for structural support in the DG, and that β1-integrin ablation altered the phenotype of cells generated in the DG.
β1-Integrin Expression Is Conserved in Endothelial Cells
In the DG, β1-integrin is expressed by vascular endothelial cells (ECs) as well as by NSCs and TAPs. Since progenitor cells in the SGZ reside in close proximity to vasculature (Palmer et al., 2000), we assessed whether ablation of β1-integrin in ECs following LV-Cre injection might contribute to the observed structural disorganization. Only a very small percentage of YFP+ cells expressed the EC marker von Willebrand factor (vWF) in either control or β1 KO mice (Control 1.27 ± 0.20% n = 4, β1 KO 2.16 ± 0.54% n = 3, P > 0.05), suggesting a minimal level of Cre recombination in ECs. Similarly, only a small percentage of vWF-expressing cells were YFP+ in either condition (Control 7.70 ± 0.87% n = 4, β1 KO 9.42 ± 1.71% n = 3, P > 0.05), demonstrating that the majority of ECs did not undergo Cre-mediated recombination. We further observed strong colocalization of β1-integrin with vWF in both control and β1 KO tissue (Fig. 3A, arrows), indicating that β1-integrin expression is preserved in ECs. By contrast, analysis of the neural elements of the DG demonstrated absence of β1-integrin expression in YFP+ recombined cells in β1 KO mice but preservation in nonrecombined cells (Fig. 3B).
FIGURE 3:

β1-integrin expression is preserved in endothelial cells. A: Colocalization of vWF (green) and β1-integrin (red) shows that expression of β1-integrin remains in endothelial cells (arrows) in the β1 KO DG. Exposure time for β1-integrin images was chosen to highlight β1-integrin expression in the endothelium-a longer exposure time would reveal that β1-integrin is also expressed in NSCs and progenitor cells in the SGZ (see Fig. 1). B: Immunostaining for YFP (green) and β-integrin (red) shows that in β1 KO tissue, recombined YFP+ cells do not express β1-integrin (arrowheads), but β1-integrin expression is preserved in YFP-negative cells. Scale bars: 50 µm. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
β1-Integrin Inhibits Astrocyte Differentiation
Since β1-integrin suppresses astrocytic differentiation in perinatal SVZ NSCs and adult spinal cord ependymal stem cells (Pan et al., 2014; North et al., 2015), we examined colocalization of YFP+ recombined cells with astrocyte-specific markers. GFAP is expressed by both astrocytes and radial NSCs in the DG. β1-integrin knockout increased the percentage of GFAP+ cells almost 3-fold (Fig. 4A,D; Control 23.7 ± 4.8% n = 4, β1 KO 60.3 ± 10.0% n = 4, P < 0.05). ALDH1L1 (aldehyde dehydrogenase 1 family, member L1), is a more broadly expressed astrocyte marker (Cahoy et al., 2008). As expected, very few YFP+ cells coimmunostained with ALDH1L1 in controls since lineage commitment in the adult DG is biased toward the neuronal fate (Bonaguidi et al., 2011; Encinas et al., 2011). In contrast, β1-integrin knockout more than tripled the percentage of ALDH1L1+ astrocytes (Fig. 4B,D; Control 8.6 ± 2.5% n = 4, β1 KO 30.1 ± 7.8% n = 5, P < 0.05). Finally, colocalization of YFP+ cells with S100β, a calcium binding protein expressed by mature astrocytes but not adult NSCs (Steiner et al., 2004; Raponi et al., 2007), showed that β1 ablation more than doubled the percentage of S100β+YFP+ astrocytes (Fig. 4C,D; Control 4.8 ± 1.6% n = 4, β1 KO 11.3 ± 1.7% n = 5, P < 0.05).
FIGURE 4:

β1-integrin knockout promotes astrocyte differentiation. Female control or β1 KO tissue was analyzed at 14 dpi. A: Immunostaining for YFP (green) and GFAP (red) shows that β1-integrin knockout increases colocalization of YFP+ recombined cells with the astrocyte marker GFAP. B: Immunostaining for YFP (green) and ALDH1L1 (red) shows that β1-integrin knockout increases colocalization of YFP+recombined cells with the astrocyte marker ALDH1L1. C: Immunostaining for YFP (green) and S100β (red) shows that β1-integrin knockout increases colocalization of YFP+ recombined cells with the astrocyte marker S100β. D: Quantification of GFAP+YFP+ cells, ALDH1L1+YFP+ cells, and S100β+YFP+ cells shows that β1-integrin knockout increases astrocyte differentiation. Scale bar 50 µm. Data are means ± SEM. Unpaired Student’s t test: *P < 0.05. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
To further address whether the increase in astrocytic differentiation was a cell-autonomous effect of β1-integrin ablation, we analyzed what proportion of ALDH1L1-expressing astrocytes were YFP+. β1-integrin knockout increased the total number of ALDH1L1+ astrocytes (Control 41,913 ± 6,054 n = 4, β1 KO 74,989 ± 11,438 n = 4, P < 0.05) as well as the number of ALDH1L1+ YFP+ astrocytes (Control 14,643 ± 3,297 n = 4, β1 KO 41,795 ± 6,670 n = 4, P < 0.05). The increase in ALDH1L1+ YFP+ astrocytes accounted for 82% of the increase in the total ALDH1L1+ population (difference between control and β1 KO populations: ALDH1L1+ YFP+=27,152; ALDH1L1+=33,076), suggesting that over 80% of newly-generated ALDH1L1 astrocytes were YFP+. Furthermore, there was no significant change in the number of ALDH1L1+ YFP-cells (Control 27,270 ± 2,880 n = 4, β1 KO 33,193 ± 5,126 n = 4, P > 0.05). This suggests that ablation of β1-integrin increases astrocytic differentiation in a cell-autonomous manner. Taken together, these data indicate that β1-integrin suppresses astrocytic differentiation in the adult hippocampal NSC niche.
β1-Integrin Is Required for Radial NSC Maintenance and Neurogenesis
We next examined the role of β1-integrin in NSC maintenance. β1-integrin knockout decreased the number of SOX2+ stem/progenitor cells in the SGZ (Fig. 5A,D; Control 77,665 ± 7,691 n = 4, β1 KO 59,725 ± 3,009 n = 5, P < 0.05) with dispersion of SOX2+ cells throughout the GCL. Further, β1-integrin knockout severely diminished the size of the GFAP+ SOX2+ radial NSC population (Fig. 5A,D; Control 11,222 ± 1,240 n = 4, β1 KO 2,219 ± 1,101 n = 5, P < 0.001). Notably, the decrease in GFAP+ SOX2+ radial glia in β1 KO mice accounted for over 50% of the decrease in the overall SOX2+ population (difference between control and β1 KO populations: radial GFAP+ SOX2+ = 9,003; SOX2+ = 17,940), suggesting that the NSC population was significantly affected. Quantification of radial Nestin+ cells provided additional evidence that β1-integrin knockout decreased the size of the radial NSC population (Fig. 5B,D; Control 22,953 ± 5,493 n = 3, β1 KO 2,069 ± 969 n = 4, P < 0.01). Notably, Nestin+ GFAP+ cells in β1 KO mice were localized throughout the DG and had a multipolar morphology typical of differentiated astrocytes whereas most Nestin+ GFAP+ cells in controls exhibited a radial morphology and were localized to the SGZ where NSCs reside (Fig. 5B). These data indicated that β1-integrin is critical for maintenance of the radial NSC population in the adult hippocampal niche.
FIGURE 5:
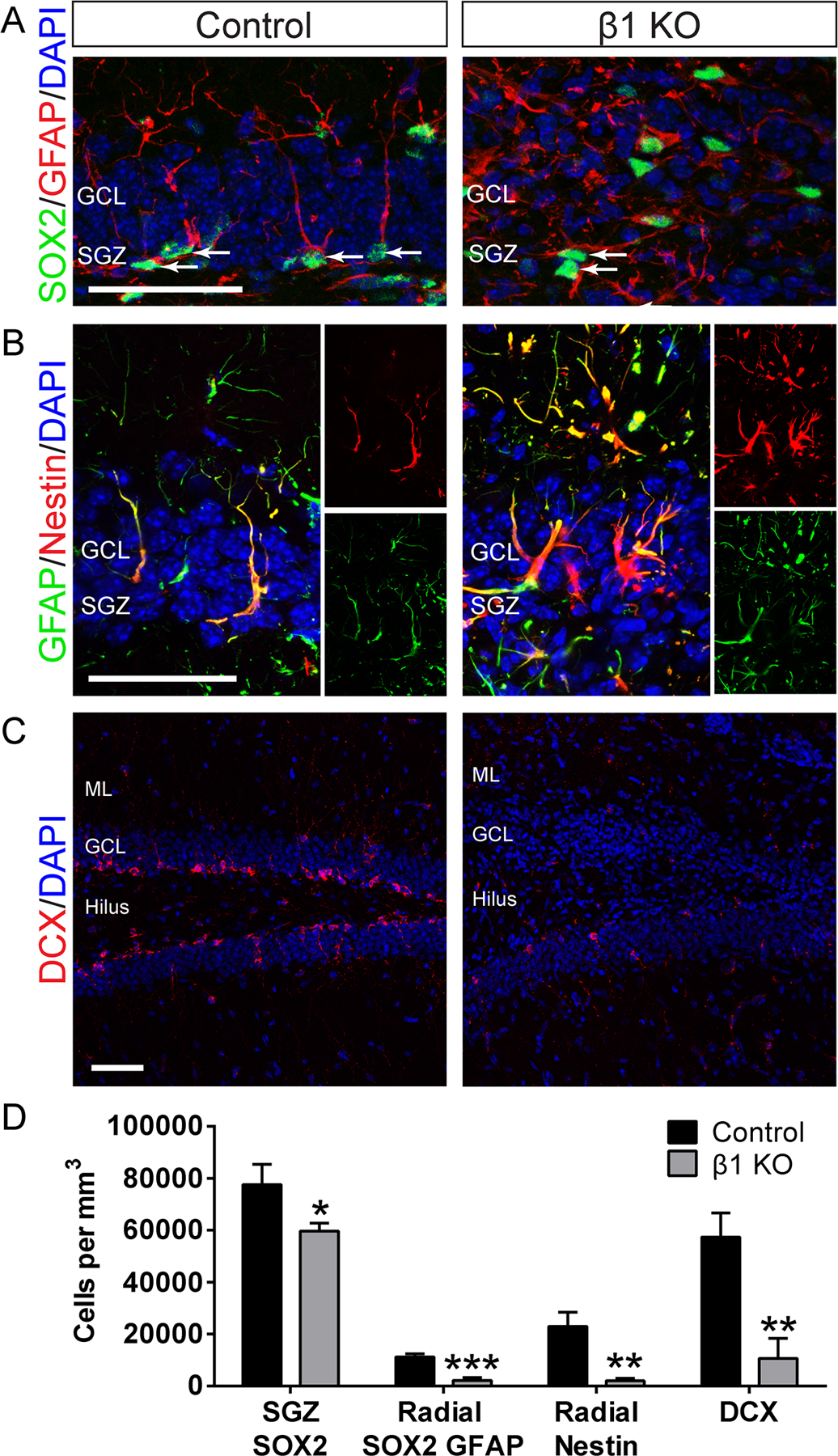
β1-integrin knockout decreases the number of radial NSCs and decreases neurogenesis. Female control or β1 KO tissue was analyzed at 14 dpi. A: Immunostaining for SOX2 (green) and GFAP (red) shows that SOX2+GFAP+ cells have a radial morphology in control and a multipolar morphology when β1-integrin is knocked out. SOX2+ cells are largely confined to the SGZ in control but are located throughout the GCL after β1-integrin ablation. Arrows denote SOX2+ cells in SGZ. B: Immunostaining for GFAP (green) and Nestin (red) shows that GFAP+Nestin+ cells have a radial morphology in control and a multipolar morphology when β1-integrin is knocked out. C: Immunostaining for DCX (red) shows that β1-integrin knockout significantly decreases the number of neuroblasts in the DG. D: Quantification of SOX2+ cells shows that there are fewer SOX2+ stem/progenitor cells in the SGZ after β1-integrin ablation. Quantification of radial SOX2+GFAP+ cells and radial Nestin+ cells shows significantly fewer radial NSCs in the SGZ after β1-integrin ablation. Quantification of DCX+ neuroblasts shows that β1-integrin ablation decreases neurogenesis. Scale bars 50 µm. Data are means ± SEM. Unpaired Student’s t test: *P < 0.05, **P < 0.01, ***P < 0.001. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
Because β1-integrin ablation decreased the radial NSC population and promoted astrocyte differentiation, we hypothesized that neurogenesis would be decreased. In fact, β1-integrin knockout severely reduced the number of DCX+ neuroblasts in the DG (Fig. 5C,D; Control 57,335 ± 9,345 n = 4, β1 KO 10,591 ± 7,811 n = 5, P < 0.01), indicating that β1-integrin is important for maintaining adult hippocampal neurogenesis.
β1-Integrin Signaling Regulates Astrocyte Differentiation of Adult NSCs through ILK
β1-integrin does not have intrinsic catalytic activity, but its cytoplasmic tail associates with signaling molecules, such as ILK and FAK, which mediate downstream effects. To determine whether ILK or FAK mediates β1-integrin function in the hippocampal niche, we first examined protein levels of these signaling molecules after LV-Cre-mediated β1-integrin ablation (protocol as in Fig. 2A). We observed a ~35% reduction in phosphorylated-ILK (pILK) and a ~55% reduction in total ILK in the β1-integrin KO DG (Fig. 6A; β1: Control 1 ± 0.130 n = 6, β1 KO 0.600 ± 0.057 n = 8, P < 0.01; pILK: Control 1 ± 0.044 n = 6, β1 KO 0.662 ± 0.074 n = 8, P < 0.01; ILK: Control 1 ± 0.133 n = 6, β1 KO 0.427 ± 0.046 n = 8, P < 0.001). In contrast, levels of both phosphorylated-FAK (pFAK) and total FAK remained unchanged (Fig. 6A; pFAK: Control 1 ± 0.215 n = 6, β1 KO 1.161 ± 0.231 n = 8, P > 0.05; FAK: Control 1 ± 0.134 n = 6, β1 KO 1.186 ± 0.117 n = 8, P > 0.05). This suggested that ILK may mediate some effects of β1-integrin in the DG.
FIGURE 6:
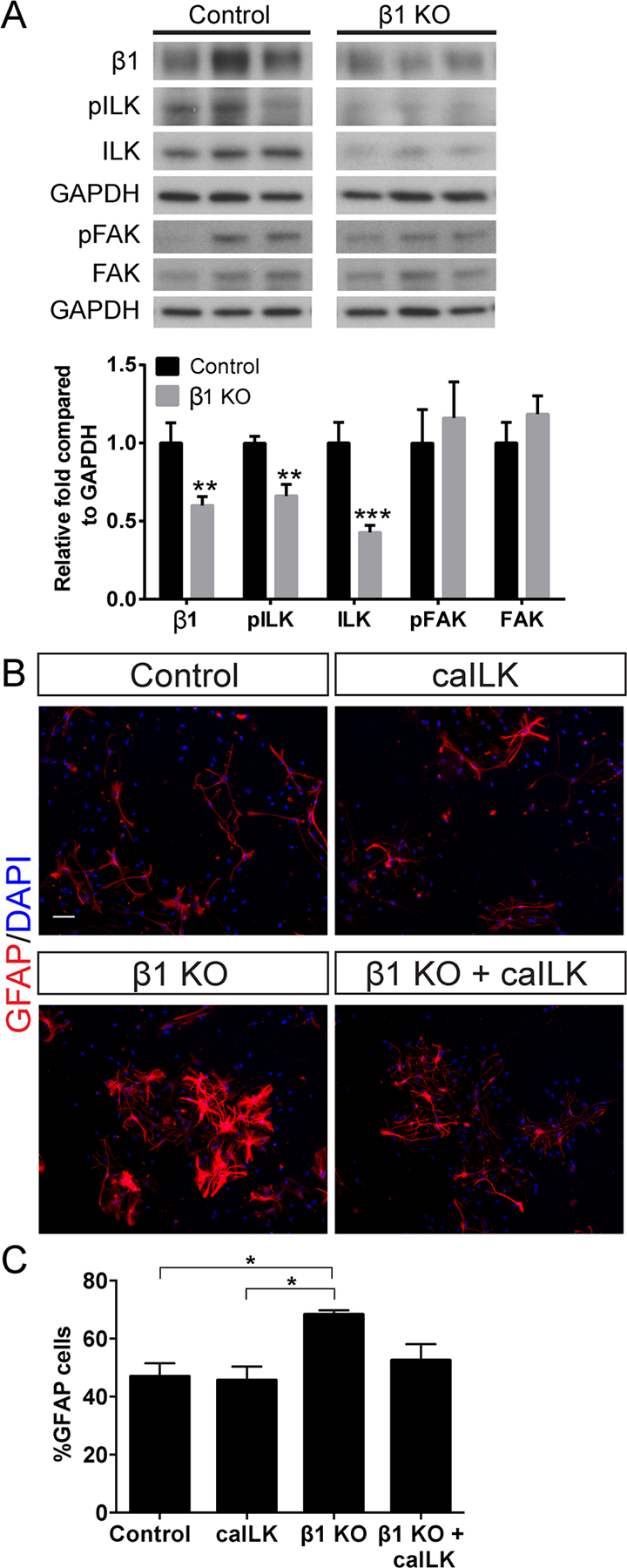
β1-integrin regulates astrocyte differentiation through ILK. A: Western blot of DG tissue after β1-integrin knockout as in Fig. 2A. β1-integrin ablation decreases levels of pILK and total ILK but does not change levels of pFAK or total FAK. GAPDH was the loading control and values are expressed as relative fold compared to control. B: Hippocampal NSC cultures from female β1+/+ ROSA:YFP or β1+/+; ROSA:YFP mice were infected with adenovirus expressing Cre (AV-Cre) to generate control and β1KO neurospheres. Lentivirus expressing constitutively active ILK (LV-caILK) was used to increase ILK signaling. Immunostaining for GFAP revealed that β1-integrin knockout increases astrocyte differentiation and that addition of caILK to β1 KO cultures rescues the increase in astrocyte differentiation. C: Quantification of GFAP+ cells shows that β1-integrin ablation increases the percentage of infected cells that differentiate into GFAP+ astrocytes compared to control, while addition of caILK to β1 KO cultures reduces astrocyte differentiation back towards control levels. Scale bar 50 µm. Data are means ± SEM. Unpaired Student’s t test: **P < 0.01, ***P < 0.001. One-way ANOVA with Tukey’s post hoc *P < 0.05. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
β1-integrin can provide both adhesive and signaling functions through its extracellular ligand-binding domain and its cytoplasmic domain, respectively. β1-integrin knockout eliminates both functions. We hypothesized that the structural disorganization after β1-integrin knockout was due to cell adhesion defects, but that the increased astrocytic differentiation reflected loss of intracellular β1-integrin signaling. However, it was also possible that NSCs lacking β1-integrin may have detached from the niche and thus become exposed to exogenous factors that promoted astrocyte differentiation. We therefore examined adult hippocampal NSCs cultured from adult β1flx/flx; ROSA:YFP and β1+/+; ROSA:YFP mice to test the hypothesis that β1-integrin regulates NSC maintenance and astrocyte differentiation through ILK. We infected neurospheres with adenovirus expressing Cre (AV-Cre) to generate β1 KO (β1flx/flx; ROSA:YFP) and control (β1+/+; ROSA:YFP) neurospheres and plated the cells for differentiation. β1-integrin knockout significantly increased astrocyte differentiation (Fig. 6B,C; Control 46.98 ± 4.50% n = 3, β1 KO 68.32 ± 1.42% n = 3, Tukey’s post hoc P < 0.05). We used a lentivirus expressing a constitutively active form of ILK (LV-caILK, Pan et al., 2014) to determine whether this rescued the effects of β1-integrin knockout on astrocyte differentiation. Expression of caILK did not significantly alter the percentage of GFAP+ cells in control cultures (Fig. 6B,C; Control 46.98 ± 4.50% n = 3, caILK 45.63 ± 4.76% n = 3, Tukey’s post hoc P > 0.05). However, caILK expression in β1 KO cultures reduced the percentage of GFAP+ cells to control levels (Fig. 6B,C; Control 46.98 ± 4.50% n = 3, β1 KO + caILK 52.53 ± 5.55% n = 3, Tukey’s post hoc P > 0.05). Thus β1-integrin signaling inhibits astrocyte differentiation, at least in part, through ILK.
Effects of β1-Integrin Ablation on the Hippocampal NSC Niche Follow a Slower Time Course in Males
We next turned our analysis to the male mice to examine sex-dependent differences in effects of β1-integrin ablation on the hippocampal niche. LV-Cre was injected into the DG of male β1flx/flx; ROSA:YFP (β1 KO) and β1+/+; ROSA:YFP (control) mice and tissue was analyzed at 14 or 28 days post injection (dpi). At 14 dpi in males, β1 KO had minimal effect on DG structure (Fig. 7A) in contrast to the marked DG disorganization in females at this time (Fig. 2B). Similarly, β1 KO in males at 14 dpi had no significant effect on either neurogenesis, assessed by the number of DCX+ neuroblasts (Fig. 7B,D; Control 38,594 ± 6,513 n = 5, β1 KO 29,824 ± 13,302 n = 4, P > 0.05) or gliogenesis, assessed by the percentage of GFAP+ YFP+ cells (Fig. 7C,E; Control 21.5 ± 3.8% n = 5, β1 KO 35.6 ± 8.9% n = 4, P > 0.05). In contrast, β1 KO in females significantly increased gliogenesis and reduced neurogenesis at 14 dpi (Figs. 4 and 5). We therefore analyzed male β1 KO mice at 28 dpi to test the hypothesis that effects of β1-integrin ablation on the hippocampal niche are temporally delayed in males. At 28 dpi, there was decreased density of cells in the GCL and dispersion of cells into the hilus in β1 KO mice (Fig. 7A). Neurogenesis was also markedly decreased at 28 dpi, as demonstrated by a greater than tenfold reduction in DCX+ neuroblasts in β1 KO males (Fig. 7B,D; Control 48,144 ± 8,885 n = 3, β1 KO 4,675 ± 169.3 n = 3, P < 0.01). Furthermore, analysis of astrocytic differentiation at 28 dpi revealed that β1 KO male mice had over a twofold increase in the percentage of GFAP+ YFP+ cells (Fig. 7C,E; Control 13.6 ± 2.4% n = 3, β1 KO 28.9 ± 3.7% n = 3, P < 0.05). We then performed immunoblotting to examine effects of β1 KO on levels of β1-integrin protein in the DG at 14 and 28 dpi (Fig. 7F). In females there was a significant reduction in β1-integrin expression by 14 dpi (Fig. 6A). By contrast, in males β1 KO did not reduce β1-integrin levels at 14 dpi (Fig. 7F; β1: Control 1 ± 0.100 n = 6, β1 KO 1.162 ± 0.055 n = 8, P > 0.05), but by 28 dpi there was a significant reduction in β1-integrin expression (Fig. 7F; Control 1 ± 0.085 n = 6, β1 KO 0.573 ± 0.051 n = 6, P < 0.01). We next asked whether there are sex-specific differences in ILK following β1-integrin ablation. Whereas both pILK and total ILK were reduced in β1 KO females at 14 dpi (Fig. 6A), there were no such changes in males at this time (Fig. 7F; pILK: Control 1 ± 0.164 n = 6, β1 KO 1.082 ± 0.071 n = 8, P > 0.05; ILK: Control 1 ± 0.056 n = 6, β1 KO 1.131 ± 0.141 n = 8, P > 0.05). However, by 28 dpi, there was a 42% reduction in pILK and a 27% reduction in total ILK in β1 KO male mice (Fig. 7F; pILK: Control 1 ± 0.130 n = 6, β1 KO 0.577 ± 0.119 n = 6, P < 0.05; ILK: Control 1 ± 0.095 n = 6, β1 KO 0.728 ± 0.087 n = 6, P = 0.060), suggesting that the effects of β1-integrin on intracellular signaling occur at a slower rate in males. Taken together, these results suggest that the effects of β1-integrin gene knockout on NSC maintenance and fate commitment in the DG follow a substantially slower time course in males compared to females because of increased persistence of β1-integrin protein in males.
FIGURE 7:

The effects of β1-integrin knockout on the NSC niche follow a slower time course in males. Male control or β1 KO tissue was analyzed at 14 and 28 dpi. A: DAPI staining (blue) shows that by 28 dpi β1-integrin knockout alters the structural integrity of the DG. B: Immunostaining for DCX (red) shows that by 28 dpi β1-integrin knockout significantly decreases the number of neuroblasts in the male DG. C: Immunostaining for YFP (green) and GFAP (red) shows that by 28 dpi β1-integrin knockout increases colocalization of YFP+ recombined cells with the astrocyte marker GFAP. D: Quantification of DCX+ neuroblasts shows that β1-integrin knockout does not alter neurogenesis at 14 dpi, but substantially reduces neurogenesis by 28 dpi. E: Quantification of GFAP+YFP+ cells shows that β1-integrin knockout does not significantly alter astrocyte differentiation at 14 dpi, but does increase astrocyte differentiation by 28 dpi. F. Western blots of male DG tissue show no change in β1-integrin expression at 14 dpi, but reduced expression at 28 dpi. β1-integrin knockout decreases levels of pILK and total ILK by 28 dpi. GAPDH was the loading control and values are expressed as relative fold compared to control. Scale bars 50 µm. Data are means ± SEM. Unpaired Student’s t test: *P < 0.05, **P < 0.01. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
Discussion
The distinct microenvironment in the neurogenic niche necessary to support and maintain NSCs consists of a complex mixture of morphogens, neurotransmitters, cytokines, and ECM molecules. Most prior studies have focused on secreted signaling molecules, and the contribution of ECM to adult hippocampal NSC niches has been less thoroughly explored. We investigated the role of β1-integrin, an ECM receptor subunit, in the adult hippocampal NSC niche. We demonstrate that β1-integrin maintains the NSC population by supporting the structural integrity of both the niche and the DG, and by inhibiting astrocytic differentiation of NSCs.
β1-integrin is enriched in both mouse and human embryonic NSCs (Campos et al., 2004; Flanagan et al., 2006; Hall et al., 2006; Lathia et al., 2007). In the adult DG, we found that β1-integrin expression is localized to the SGZ and is present in NSCs. β1-integrin expression decreased in later stages of lineage commitment and was seemingly absent in mature dentate granule cells. This suggested that β1-integrin might be important for NSC function in the SGZ. In the adult SVZ, β1-integrin is limited to ependymal cells and actively dividing NSCs, but is not expressed in quiescent NSCs (Kazanis et al., 2010). In the SGZ we observed β1-integrin expression in virtually all radial NSCs; because many NSCs are quiescent, our data thus suggest that the expression pattern of β1-integrin differs in the two adult niches. This discordance is likely due to the different cellular architectures of the SVZ and SGZ, and suggests that β1-integrin may have distinct functions in these niches.
Cre-mediated ablation of β1-integrin resulted in extensive cellular disorganization of the SGZ as well as nonneurogenic regions of the adult DG. Following β1-integrin ablation, we observed that β1-integrin was depleted from both NSCs and TAPs but maintained in endothelial cells, suggesting that the observed phenotype was due to loss of expression of β1-integrin in neural elements. One primary function of β1-integrin is adhesion to the ECM framework, and this structural role appears to be conserved across adult and embryonic NSC niches. β1-integrin plays a critical role in anchoring NSCs to the basement membrane in the developing neocortex, and loss of function causes NSC detachment, accompanied by layering defects (Graus-Porta et al., 2001; Loulier et al., 2009; Radakovits et al., 2009). In the adult SVZ, β1-integrin blocking antibodies cause NSC detachment and, at high concentrations, cause disorganization within the niche (Kazanis et al., 2010; Shen et al., 2008). Similarly, we observed that loss of β1-integrin in the DG caused disorganization within the SGZ and caused progenitors to detach and migrate to regions outside of the SGZ. We observed a significant increase in the number of proliferating MCM2-expressing cells in the GCL, consistent with studies in the SVZ that showed that blockade of β1-integrin causes ectopic migration and increased proliferation of progenitor cells (Kazanis et al., 2010; Shen et al., 2008). Surprisingly, we found that β1-integrin deletion also led to disorganization throughout the non-neurogenic regions of the DG. Our expression analysis suggested that dentate granule neurons express little, if any, β1-integrin, and Cre recombination in this region was low, which implies that the structural disorganization of the DG may be, in part, cell non-autonomous. This suggests that ECM components of the SGZ niche may play a larger role in supporting the overall structure of the DG, and that β1-integrin in NSCs regulates more than just cell adhesion.
Our findings indicate that β1-integrin helps to maintain radial NSCs in the SGZ and inhibits astrocytic differentiation of these cells. Ablation of β1-integrin from ependymal zone stem cells also increases astrogliogenesis following spinal cord injury (North et al., 2015), consistent with a role for β1-integrin in restricting astrocytic differentiation. Furthermore, genetic deletion of β1-integrin in astrocytes during development leads to a partial reactive gliosis phenotype in the early postnatal and adult central nervous system, characterized by upregulation of GFAP expression and astrocytic hypertrophy in the brain and spinal cord (Robel et al., 2009). Therefore, in addition to modulating astrocytic differentiation of NSCs, β1-integrin may also regulate the phenotype of astrocytic progeny. It is possible that the effects of β1-integrin ablation on NSC maintenance and differentiation are caused by detachment and migration of these cells from the SGZ, resulting in exposure to different exogenous signals. It is difficult to examine this issue in vivo because of the complex and undefined signaling milieu. However, the astrocytic differentiation phenotype remained when we deleted β1-integrin from NSCs in vitro, suggesting that β1-integrin restricts astrocyte differentiation of hippocampal NSCs via its effects on intracellular signaling pathways.
Two kinases that associate with the cytosolic tail of β1-integrin, ILK and FAK, mediate many effects of interactions with the ECM. Ablation of β1-integrin in the DG decreased ILK pathway components, but did not affect FAK. Further, overexpression of constitutively active ILK rescued the effects of β1-integrin knockout on astrocyte differentiation. Thus, the effects of β1-integrin on NSC maintenance and astrocyte differentiation are mediated, at least in part, through ILK. Several pathways known to act downstream of ILK have been implicated in regulating astrocyte differentiation. For example, ILK can direct the phosphorylation of intracellular targets, including protein kinase B/Akt (Troussard et al., 2003) and GSK3β (Naska et al., 2006), and overexpression of Akt in hippocampal neural progenitor cells inhibits astrogliogenesis (Peltier et al., 2007). Environmental enrichment increases levels of ILK, and knockdown of ILK in the DG prevents the stimulatory effects of environmental enrichment on hippocampal neurogenesis and cognition (Xu et al., 2015). This is consistent with our finding that ablation of β1-integrin reduces levels of hippocampal ILK and markedly inhibits neurogenesis. Inhibition of GSK3β rescues the effect of ILK knockdown on neurogenesis (Xu et al., 2015), suggesting that ILK-mediated inhibition of GSK3β may contribute to its effects on NSC maintenance and fate commitment. ILK can also modulate the BMP signaling pathway (Su et al., 2010; Tseng et al., 2010), which is a key regulator of astrocyte differentiation (Gross et al., 1996). In addition, ILK acts as a scaffolding protein, forming a complex with PINCH and parvin, which link integrins to the cytoskeleton and serve as a platform through which multiple signaling pathways are regulated (Legate et al., 2006). A recent study suggests that ILK regulates NSCs in the SVZ and SGZ through inhibition of PINCH1/2-dependent enhancement of c-Jun N-terminal protein kinase activity (Porcheri et al., 2014). Genetic ablation of ILK reportedly increased proliferation both in the adult neurogenic niches and in vitro and the effect was mediated by Ras suppressor unit-1, which modulates signaling pathways involved in cell cycle regulation (Porcheri et al., 2014). However, others have observed that knockdown of ILK in the DG reduces proliferation (Xu et al., 2015). We observed that deletion of β1-integrin enhanced the number of proliferating MCM2-expressing progenitors in areas outside of the SGZ. However, the increase in dividing MCM2+ cells was largely in nonrecombined cells, suggesting that the change in proliferation was a cell nonautonomous effect of β1-integrin ablation, possibly secondary to the structural disorganization and ectopic migration of progenitors in the DG.
Although constitutively active ILK rescued the increase in astrocyte differentiation after knockout of β1-integrin in vitro, the level of astrocytic differentiation observed was still higher than in the SGZ in vivo, suggesting that β1-integrin-mediated inhibition of astrocytic differentiation is not solely mediated through ILK. β1-integrin also regulates NSC signaling by interacting with growth factor receptors in the membrane (Campos et al., 2006; Leone et al., 2005). For example, β1-integrin modulates growth factor signaling by regulating the localization of growth factor receptors within lipid rafts (Baron et al., 2003, 2005). Inhibition of BMP signaling allows propagation of SGZ NSCs in vitro and inhibits astrocytic differentiation (Bonaguidi et al., 2008). Further, in perinatal SVZ NSCs, β1-integrin reduces movement of the BMP type 1b receptor subunit into lipid rafts thereby blocking astrocytic differentiation in response to BMP signaling (North et al., 2015). Given the high level of BMP signaling in the adult hippocampus (Bonaguidi et al., 2008; Bond et al., 2014) β1-integrin may inhibit astrocytic lineage commitment in hippocampal NSCs by similar mechanisms.
β1-integrin ablation produced divergent effects in males and females. In particular, the effects of β1-integrin ablation on DG structure and NSC differentiation were observed at 14 dpi in females, but were absent in males at this time. However, at 28 dpi males began to display DG structural abnormalities as well as increased astrocyte differentiation, concomitant with decreased neurogenesis. This demonstrates that the effects of β1-integrin ablation follow a slower time-course in males. We hypothesize that this finding results from a slower turn-over rate of β1-integrin in the male DG. Alternatively, different integrin subtypes could be differentially expressed in the DG of males and females, partially compensating for the loss of β1-integrin in males. Sex-dependent differences in integrin function have been observed previously, including differences in expression of β1-integrin on the surface of myelin basic protein-primed T cells (Brahmachari and Pahan, 2010) and differential responses of β8-integrin to dopaminergic manipulations in male and female pituitaries (Recouvreux et al., 2013). Sex-dependent differences in regulation of adult neurogenesis also have been observed. For instance, male and female rats have differential responses to chronic stress, with males displaying a decrease in NSC proliferation in the DG and females displaying a decrease in newborn neuron survival in the DG following stress (Hillerer et al., 2013). Furthermore, sex steroid hormones can regulate adult progenitor cell proliferation and neuronal survival in both males and females (Galea et al., 2013; Tatar et al., 2013). Treatment of female rats with estradiol increases progenitor cell proliferation in the DG, but the effect is dependent on dose and duration of treatment (Barha et al., 2009; Galea et al., 2013). In addition, progesterone can increase proliferation in the DG in both male and female rodents (Barha et al., 2011; Liu et al., 2010). In males, chronic androgen treatment increases newborn neuron survival, with no effect on progenitor proliferation (Spritzer and Galea, 2007). These results underscore the importance of examining sex differences when evaluating integrin functions and hippocampal neurogenesis.
In summary, this study highlights the dual role of β1-integrin in the adult hippocampus—both in maintaining the structural integrity of the NSC niche and the DG, and in modulating intracellular signaling pathways involved in lineage commitment.
Acknowledgment
Grant sponsor: NIH Grants; Grant number: R01NS020778, TL1R000108, T32MH067564, T32GM008152, and F31NS089154.
Abbreviations
- ALDH1L1
aldehyde dehydrogenase 1 family member L1
- AV
adenovirus
- β1
β1-integrin
- β1 KO
β1-integrin knockout
- BV
blood vessel
- caILK
constitutively active integrin-linked kinase
- DCX
doublecortin
- DG
dentate gyrus
- dpi
days post injection
- EC
endothelial cell
- ECM
extracellular matrix
- FAK
focal adhesion kinase
- GCL
granule cell layer
- GFAP
glial fibrillary acidic protein
- ILK
integrin-linked kinase
- LV
lentivirus
- MCM2
minichromosome maintenance complex component 2
- ML
molecular layer
- NeuN
neuronal nuclei
- NSC
neural stem cell
- pFAK
phosphorylated-focal adhesion kinase
- pILK
phosphorylated-integrin-linked kinase
- SGZ
subgranular zone
- SOX2
SRY(sex determining region Y)-Box2
- SVZ
subventricular zone
- TAP
transit-amplifying progenitor
- vWF
von Willebrand factor
- YFP
yellow fluorescent protein
Footnotes
Disclosure: The authors declare no conflict of interest.
References
- Barha CK, Ishrat T, Epp JR, Galea LA, Stein DG. 2011. Progesterone treatment normalizes the levels of cell proliferation and cell death in the dentate gyrus of the hippocampus after traumatic brain injury. Exp Neurol 231:72–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barha CK, Lieblich SE, Galea LA. 2009. Different forms of oestrogen rapidly upregulate cell proliferation in the dentate gyrus of adult female rats. J Neuroendocrinol 21:155–166. [DOI] [PubMed] [Google Scholar]
- Baron W, Colognato H, ffrench-Constant C. 2005. Integrin-growth factor interactions as regulators of oligodendroglial development and function. Glia 49:467–479. [DOI] [PubMed] [Google Scholar]
- Baron W, Decker L, Colognato H, ffrench-Constant C. 2003. Regulation of integrin growth factor interactions in oligodendrocytes by lipid raft microdo-mains. Curr Biol 13:151–155. [DOI] [PubMed] [Google Scholar]
- Bonaguidi MA, Peng CY, McGuire T, Falciglia G, Gobeske KT, Czeisler C, Kessler JA. 2008. Noggin expands neural stem cells in the adult hippocampus. J Neurosci 28:9194–9204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonaguidi MA, Wheeler MA, Shapiro JS, Stadel RP, Sun GJ, Ming GL, Song H. 2011. In vivo clonal analysis reveals self-renewing and multipotent adult neural stem cell characteristics. Cell 145:1142–1155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bond AM, Peng CY, Meyers EA, McGuire T, Ewaleifoh O, Kessler JA. 2014. BMP signaling regulates the tempo of adult hippocampal progenitor maturation at multiple stages of the lineage. Stem Cells 32:2201–2214. [DOI] [PubMed] [Google Scholar]
- Brahmachari S, Pahan K. 2010. Gender-specific expression of beta1 integrin of VLA-4 in myelin basic protein-primed T cells: implications for gender bias in multiple sclerosis. J Immunol 184:6103–6113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cahoy JD, Emery B, Kaushal A, Foo LC, Zamanian JL, Christopherson KS, Xing Y, Lubischer JL, Krieg PA, Krupenko SA, Thompson WJ, Barres BA. 2008. A transcriptome database for astrocytes, neurons, and oligodendrocytes: A new resource for understanding brain development and function. J Neurosci 28:264–278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campos LS, Decker L, Taylor V, Skarnes W. 2006. Notch, epidermal growth factor receptor, and beta1-integrin pathways are coordinated in neural stem cells. J Biol Chem 281:5300–5309. [DOI] [PubMed] [Google Scholar]
- Campos LS, Leone DP, Relvas JB, Brakebusch C, Fassler R, Suter U, ffrench-Constant C. 2004. Beta1 integrins activate a MAPK signalling pathway in neural stem cells that contributes to their maintenance. Development 131: 3433–3444. [DOI] [PubMed] [Google Scholar]
- Encinas JM, Michurina TV, Peunova N, Park JH, Tordo J, Peterson DA, Fishell G, Koulakov A, Enikolopov G. 2011. Division-coupled astrocytic differentiation and age-related depletion of neural stem cells in the adult hippocampus. Cell Stem Cell 8:566–579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faissner A, Reinhard J. 2015. The extracellular matrix compartment of neural stem and glial progenitor cells. Glia 63:1330–1349. [DOI] [PubMed] [Google Scholar]
- Flanagan LA, Rebaza LM, Derzic S, Schwartz PH, Monuki ES. 2006. Regulation of human neural precursor cells by laminin and integrins. J Neurosci Res 83:845–856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forster E, Tielsch A, Saum B, Weiss KH, Johanssen C, Graus-Porta D, Muller U, Frotscher M. 2002. Reelin, disabled 1, and beta 1 integrins are required for the formation of the radial glial scaffold in the hippocampus. Proc Natl Acad Sci USA 99:13178–13183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galea LA, Wainwright SR, Roes MM, Duarte-Guterman P, Chow C, Hamson DK. 2013. Sex, hormones and neurogenesis in the hippocampus: hormonal modulation of neurogenesis and potential functional implications. J Neuroendocrinol 25:1039–1061. [DOI] [PubMed] [Google Scholar]
- Graus-Porta D, Blaess S, Senften M, Littlewood-Evans A, Damsky C, Huang Z, Orban P, Klein R, Schittny JC, Muller U. 2001. Beta1-class integrins regulate the development of laminae and folia in the cerebral and cerebellar cortex. Neuron 31:367–379. [DOI] [PubMed] [Google Scholar]
- Gross RE, Mehler MF, Mabie PC, Zang Z, Santschi L, Kessler JA. 1996. Bone morphogenetic proteins promote astroglial lineage commitment by mammalian subventricular zone progenitor cells. Neuron 17:595–606. [DOI] [PubMed] [Google Scholar]
- Hagihara H, Toyama K, Yamasaki N, Miyakawa T. 2009. Dissection of hippocampal dentate gyrus from adult mouse. J Vis Exp. [DOI] [PMC free article] [PubMed]
- Hall PE, Lathia JD, Miller NG, Caldwell MA, ffrench-Constant C. 2006. Integrins are markers of human neural stem cells. Stem Cells 24:2078–2084. [DOI] [PubMed] [Google Scholar]
- Hannigan GE, Leung-Hagesteijn C, Fitz-Gibbon L, Coppolino MG, Radeva G, Filmus J, Bell JC, Dedhar S. 1996. Regulation of cell adhesion and anchorage-dependent growth by a new beta 1-integrin-linked protein kinase. Nature 379:91–96. [DOI] [PubMed] [Google Scholar]
- Hillerer KM, Neumann ID, Couillard-Despres S, Aigner L, Slattery DA. 2013. Sex-dependent regulation of hippocampal neurogenesis under basal and chronic stress conditions in rats. Hippocampus 23:476–487. [DOI] [PubMed] [Google Scholar]
- Kazanis I, ffrench-Constant C. 2011. Extracellular matrix and the neural stem cell niche. Dev Neurobiol 71:1006–1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kazanis I, Lathia JD, Vadakkan TJ, Raborn E, Wan R, Mughal MR, Eckley DM, Sasaki T, Patton B, Mattson MP, Hirschi KK, Dickinson ME, ffrench-Constant C. 2010. Quiescence and activation of stem and precursor cell populations in the subependymal zone of the mammalian brain are associated with distinct cellular and extracellular matrix signals. J Neurosci 30:9771–9781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lathia JD, Patton B, Eckley DM, Magnus T, Mughal MR, Sasaki T, Caldwell MA, Rao MS, Mattson MP, ffrench-Constant C. 2007. Patterns of laminins and integrins in the embryonic ventricular zone of the CNS. J Comp Neurol 505: 630–643. [DOI] [PubMed] [Google Scholar]
- Lee TT, Wainwright SR, Hill MN, Galea LA, Gorzalka BB. 2014. Sex, drugs, and adult neurogenesis: sex-dependent effects of escalating adolescent cannabinoid exposure on adult hippocampal neurogenesis, stress reactivity, and amphetamine sensitization. Hippocampus 24:280–292. [DOI] [PubMed] [Google Scholar]
- Legate KR, Montanez E, Kudlacek O, Fassler R. 2006. ILK, PINCH and parvin: the tIPP of integrin signalling. Nat Rev Mol Cell Biol 7:20–31. [DOI] [PubMed] [Google Scholar]
- Leone DP, Relvas JB, Campos LS, Hemmi S, Brakebusch C, Fassler R, Ffrench-Constant C, Suter U. 2005. Regulation of neural progenitor proliferation and survival by beta1 integrins. J Cell Sci 118:2589–2599. [DOI] [PubMed] [Google Scholar]
- Liu L, Zhao L, She H, Chen S, Wang JM, Wong C, McClure K, Sitruk-Ware R, Brinton RD. 2010. Clinically relevant progestins regulate neurogenic and neuroprotective responses in vitro and in vivo. Endocrinology 151:5782–5794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loulier K, Lathia JD, Marthiens V, Relucio J, Mughal MR, Tang SC, Coksaygan T, Hall PE, Chigurupati S, Patton B, Colognato H, Rao MS, Mattson MP, Haydar TF, ffrench-Constant C. 2009. beta1 integrin maintains integrity of the embryonic neocortical stem cell niche. PLoS Biol 7:e1000176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maslov AY, Barone TA, Plunkett RJ, Pruitt SC. 2004. Neural stem cell detection, characterization, and age-related changes in the subventricular zone of mice. J Neurosci 24:1726–1733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mercier F, Kitasako JT, Hatton GI. 2002. Anatomy of the brain neurogenic zones revisited: fractones and the fibroblast/macrophage network. J Comp Neurol 451:170–188. [DOI] [PubMed] [Google Scholar]
- Morrens J, Van Den Broeck W, Kempermann G. 2012. Glial cells in adult neurogenesis. Glia 60:159–174. [DOI] [PubMed] [Google Scholar]
- Naska S, Park KJ, Hannigan GE, Dedhar S, Miller FD, Kaplan DR. 2006. An essential role for the integrin-linked kinase-glycogen synthase kinase-3 beta pathway during dendrite initiation and growth. J Neurosci 26:13344–13356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- North HA, Pan L, McGuire TL, Brooker S, Kessler JA. 2015. beta1-Integrin alters ependymal stem cell BMP receptor localization and attenuates astrogliosis after spinal cord injury. J Neurosci 35:3725–3733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer TD, Willhoite AR, Gage FH. 2000. Vascular niche for adult hippocampal neurogenesis. J Comp Neurol 425:479–494. [DOI] [PubMed] [Google Scholar]
- Pan L, North HA, Sahni V, Jeong SJ, McGuire TL, Berns EJ, Stupp SI, Kessler JA. 2014. beta1-integrin and integrin linked kinase regulate astrocytic differentiation of neural stem cells. PLoS One 9:e104335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peltier J, O’Neill A, Schaffer DV. 2007. PI3K/Akt and CREB regulate adult neural hippocampal progenitor proliferation and differentiation. Dev Neurobiol 67:1348–1361. [DOI] [PubMed] [Google Scholar]
- Porcheri C, Suter U, Jessberger S. 2014. Dissecting integrin-dependent regulation of neural stem cell proliferation in the adult brain. J Neurosci 34: 5222–5232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radakovits R, Barros CS, Belvindrah R, Patton B, Muller U. 2009. Regulation of radial glial survival by signals from the meninges. J Neurosci 29:7694–7705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raponi E, Agenes F, Delphin C, Assard N, Baudier J, Legraverend C, Deloulme JC. 2007. S100B expression defines a state in which GFAP-expressing cells lose their neural stem cell potential and acquire a more mature developmental stage. Glia 55:165–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Recouvreux MV, Lapyckyj L, Camilletti MA, Guida MC, Ornstein A, Rifkin DB, Becu-Villalobos D, Diaz-Torga G. 2013. Sex differences in the pituitary transforming growth factor-beta1 system: Studies in a model of resistant prolactinomas. Endocrinology 154:4192–4205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robel S, Mori T, Zoubaa S, Schlegel J, Sirko S, Faissner A, Goebbels S, Dimou L, Gotz M. 2009. Conditional deletion of beta1-integrin in astroglia causes partial reactive gliosis. Glia 57:1630–1647. [DOI] [PubMed] [Google Scholar]
- Schlaepfer DD, Hauck CR, Sieg DJ. 1999. Signaling through focal adhesion kinase. Prog Biophys Mol Biol 71:435–478. [DOI] [PubMed] [Google Scholar]
- Shen Q, Wang Y, Kokovay E, Lin G, Chuang SM, Goderie SK, Roysam B, Temple S. 2008. Adult SVZ stem cells lie in a vascular niche: A quantitative analysis of niche cell–cell interactions. Cell Stem Cell 3:289–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spritzer MD, Galea LA. 2007. Testosterone and dihydrotestosterone, but not estradiol, enhance survival of new hippocampal neurons in adult male rats. Dev Neurobiol 67:1321–1333. [DOI] [PubMed] [Google Scholar]
- Srichai MB and Zent R 2010. Integrin structure and function In: Zent R, Pozzi A, editors. Cell-extracellular matrix interactions in cancer. New York: Springer; pp 19–41. [Google Scholar]
- Steiner B, Kronenberg G, Jessberger S, Brandt MD, Reuter K, Kempermann G. 2004. Differential regulation of gliogenesis in the context of adult hippocampal neurogenesis in mice. Glia 46:41–52. [DOI] [PubMed] [Google Scholar]
- Su JL, Chiou J, Tang CH, Zhao M, Tsai CH, Chen PS, Chang YW, Chien MH, Peng CY, Hsiao M, Kuo ML, Yen ML. 2010. CYR61 regulates BMP-2-dependent osteoblast differentiation through the {alpha}v{beta}3 integrin/integrin-linked kinase/ERK pathway. J Biol Chem 285:31325–31336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tatar C, Bessert D, Tse H, Skoff RP. 2013. Determinants of central nervous system adult neurogenesis are sex, hormones, mouse strain, age, and brain region. Glia 61:192–209. [DOI] [PubMed] [Google Scholar]
- Tavazoie M, Van der Veken L, Silva-Vargas V, Louissaint M, Colonna L, Zaidi B, Garcia-Verdugo JM, Doetsch F. 2008. A specialized vascular niche for adult neural stem cells. Cell Stem Cell 3:279–288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Troussard AA, Mawji NM, Ong C, Mui A, St -Arnaud R, Dedhar S. 2003. Conditional knock-out of integrin-linked kinase demonstrates an essential role in protein kinase B/Akt activation. J Biol Chem 278:22374–22378. [DOI] [PubMed] [Google Scholar]
- Tseng WP, Yang SN, Lai CH, Tang CH. 2010. Hypoxia induces BMP-2 expression via ILK, Akt, mTOR, and HIF-1 pathways in osteoblasts. J Cell Physiol 223:810–818. [DOI] [PubMed] [Google Scholar]
- Xu XF, Li T, Wang DD, Chen B, Wang Y, Chen ZY. 2015. Integrin-linked kinase is essential for environmental enrichment enhanced hippocampal neurogenesis and memory. Sci Rep 5:11456. [DOI] [PMC free article] [PubMed] [Google Scholar]


