Abstract
Objectives
Examine possible pooling strategies designed to expand SARS-CoV-2 serological testing capacity.
Methods
Negative pools were assessed to determine optimal optical density (OD) cutoffs, followed by spiking weak or strong positive samples to assess initial assay performance. Samples were then randomly subjected to pool and individual testing approaches.
Results
Single positive specimens consistently converted pools of 5, 10, or 20 into positive outcomes. However, weaker IgG-positive samples failed to similarly convert pools of 50 to a positive result. In contrast, a stronger individual positive sample converted all pools tested into positive outcomes. Finally, examination of 150 samples configured into pools of 5, 10, 20 or 50 accurately predicted the presence of positive or negative specimens within each pool.
Conclusions
These results suggest that pooling strategies may allow expansion of serological testing capacity. While limitations exist, such strategies may aid in large-scale epidemiological screening or identification of optimal convalescent plasma donors.
Keywords: COVID-19, Serology, Pooling, Infectious disease
1. Introduction
The ongoing COVID-19 pandemic has presented a variety of challenges in the development of large-scale testing procedures capable of meeting evolving testing needs [1], [2]. Early testing relied heavily on SARS-CoV-2 viral RNA detection [3], [4], [5], which along with recent advances in viral antigen testing [6], continues to be the mainstay of COVID-19 diagnosis. However, individuals with asymptomatic SARS-CoV-2 infection often fail to immediately obtain SARS-CoV-2 testing, making it difficult to use negative nucleic acid test (NAT) results alone when seeking to effectively determine disease prevalence [7]. Furthermore, while a history of a positive NAT test initially served as a screening tool to identify possible convalescent plasma (CP) donors [8], current strategies require donor assessment for anti-SARS-CoV-2 antibody levels when procuring optimal CP units [9], [10]. While serological approaches certainly have limitations [11], anti-SARS-CoV-2 antibody-based testing may therefore provide a complementary surveillance tool when evaluating COVID-19 infection and aid in the identification of optimal CP donors [12], [13]. However, implementation of high throughput serological testing approaches can pose significant challenges [14].
The blood donor industry has faced the need of high-volume testing for acute and chronic infection for decades as a blood safety measure to reduce the probability of transfusion-transmitted infection [15]. In an effort to enhance testing capacity, while also creating flexibility based on the prevalence of disease and overall testing demand, sample pooling has been used for decades to expand testing volume capacity upon an existing testing infrastructure [16], [17]. However, this approach has nearly exclusively relied on NAT testing. Given the need to rapidly meet evolving serological testing demands, we reasoned that a similar strategy may prove useful when seeking to likewise rapidly expand anti-SARS-CoV-2 antibody detection capacity. To test the overall feasibility of this approach, we examined test performance of single-dilution and pooled serological samples. Our results suggest that optimal pooling approaches may be achievable depending on the population being tested and the overall antibody level being examined.
2. Methods
Pooling strategies were adapted from a recently Federal Drug Agency-Emergency Use Authorization (FDA EUA)-approved serological test that utilizes recombinant receptor binding domain (RBD) of SARS-CoV-2 as the target antigen. Single samples were diluted 1:50 in dilution buffer (0.2% Tween 20, 1% bovine serum albumin in phosphate buffered saline). For detection, horse radish peroxidase-conjugated anti-human IgG (Jackson ImmunoResearch, Catalog # 109-035-088) was used. Optical density (OD) values collected from pools replicated at least three times were used to derive cutoffs calculated as the sum of the mean OD value and the standard deviation multiplied by three (x̄ + 3*SD) for each pooling strategy. A spiked “weak sample”, (OD just above the single-dilution OD cutoff of 0.2) or a “strong” sample (OD > 1.5) were initially used to assess whether individual samples could convert corresponding pools to a positive test outcome. One hundred and fifty samples were partially randomly distributed into pools of 5, 10, 20 or 50 in order to achieve pools that would allow assessment of completely negative or possibly positive pool results based on previous testing outcomes, followed by pool testing and individual sample analysis. All samples were collected from residual specimens from convalescent plasma donors or healthcare workers under Emory Institutional Review Board approval.
3. Results and discussion
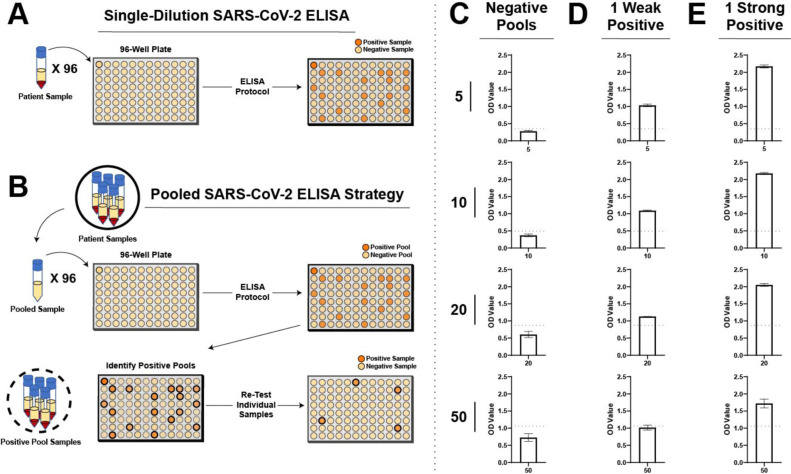
Adapting the single-dilution SARS-CoV-2 IgG ELISA protocol (Fig. 1 A), we explored pooling strategies designed to rapidly expand testing capabilities on existing instrumentation (Fig. 1B). As pooling requires additional sample material to be included in lieu of diluent in order to achieve the same dilution level for each individual sample, we first examined the possibility that negative pool OD values may change and therefore require unique cutoffs for each distinct pool. To this end, pools were generated using serologically negative individuals to produce pools reflective of 5, 10, 20 or 50 COVID-19 negative individuals. Using this approach, we observed a rise in total background OD values with increased pool size (Fig. 1C).
Fig. 1.
Pool size and individual sample positivity dictates the ability of pooling strategies to identify individual positive samples. A. Schematic representation of workflow for a single-dilution ELISA for anti-SARS-CoV-2 IgG antibodies. B. Schematic representation for a pooled ELISA strategy. C. Optical density (OD) values for negative pools of 5, 10, 20, or 50 are presented graphically. D. Pools spiked with a “weak” positive sample (individual OD value just greater than single assay cut off of 0.2) were examined for total OD results. E. Pools spiked with a “strong” positive sample (individual OD value > 1.5) were examined for total OD results. Error bars represent mean ± SD. Positive cutoff values were calculated as the mean + 3*SD. Experiments for all data presented were repeated at least 3 times.
Given the higher OD values with each increase in pooled samples, and thus serum volume, we next sought to determine whether a single positive specimen is capable of converting an otherwise negative pool into a positive result. Setting the OD cutoff value 3 standard deviations above the mean for each individual pool, we next spiked a single “weak” positive sample, defined as a sample ranging just above the OD cut off of 0.2 in a single-dilution assay, into each respective pool. Using this approach, a single weak positive sample introduced into pools of 5, 10 or 20 converted the entire pool into a positive result (Fig. 1D). In contrast, while the same sample increased the overall OD value when added to a pool of 50, the resulting strength of this reaction failed to overcome the threshold required for a positive signal (Fig. 1D). These results suggest that in pools of 20 or less, a single weakly positive sample may convert a pool to a positive result. However, when pools of 50 are employed, weak positive samples may be insufficient to overcome background OD values to produce a positive outcome.
As some serological testing procedures, such as those used for CP, may ultimately benefit from having positive results primarily triggered when only samples from individuals with high antibody levels are present, it remained possible that pools of 50 may still be useful in this setting. To test whether a single positive sample with higher antibody levels (OD > 1.5 in a single-dilution assay) alters pooling test results, we next used an individual CP donor sample with higher antibody levels. Similar to the outcome observed following use of a single weak positive sample in pools of 5, 10 or 20, the “stronger” sample converted the overall pool to a positive result, albeit at a much higher overall OD value than the weak sample (Fig. 1E). However, in contrast to the weaker sample, the single sample with higher anti-SARS-CoV-2 antibody levels readily converted the pool of 50 to a positive outcome (Fig. 1E). These results suggest that while sample matrix effects likely increase the background OD values as pool size increases, single positive samples can convert an entire pool to a positive outcome depending on the pool size and the strength of the positive individual sample present.
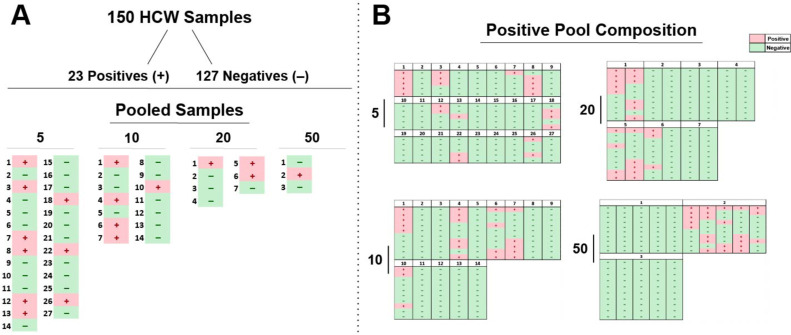
Having examined spiked positive samples among known negatives, we next sought to examine the serological pooling test performance using previously collected samples from 150 non-hospitalized individuals. While certainly not identical to randomly collected specimens in the general population, we reasoned that such an approach could provide some insight into the potential outcome of serological pool testing strategies. Each of the specimens were tested in pools of 5 (n = 27), 10 (n = 14), 20 (n = 7), and 50 (n = 3) (Fig. 2 A) and then individually. Thirty-three percent (9/27) of 5-sample pools, 36% (5/14) of 10-sample pools, 43% (3/7) of 20-sample pools, and 33% (1/3) of 50-sample pools yielded OD values greater than the precalculated cutoffs (Fig. 2A). To determine whether pools examined in this fashion accurately detected the presence of positive samples within each pool set, we compared pool testing results with the outcome of individually tested specimens (Fig. 2B). Individual sample testing outcomes accurately matched pooling results, with pools that produced a negative result consisting entirely of negative individual samples, while positive pooling results occurred when one or more positive individual samples were present (Fig. 2B).
Fig. 2.
Pooling strategies can accurately predict individual negative and positive sample content. A. Serological samples were randomized and tested for anti-SARS-CoV-2 IgG antibodies in pools of 5, 10, 20, or 50. Alpha values are presented for each pool, as determined by OD cutoff values for each respective pooling strategy. B. Pooled serology samples results are shown by composition of the individual patient sample results obtained by individual testing outcomes following pooled testing. Positive serological pools and samples (+) are presented in red whereas negative pools and samples (−) are shown in green.
As testing infrastructures can require significant investments from clinical laboratories, the rapid expansion of testing capacity can be challenging to achieve. The results of the present study suggest that pooling may represent one strategy to expand serological testing capacity. However, as with any clinical test, serological pooling certainly has limitations [11]. Serological tests in general are distinct from NAT tests in that they can suffer from relative lack of specificity due to a variety of factors [11], [18]. As a result, general limitations in serological testing results still apply with pooling approaches and may even be exacerbated by the accumulation of numerous individual low negative results as evidenced by higher OD values with increased pool size [19]. Furthermore, numerous positive results may produce a cumulative outcome that mimics a single or even multiple high positive results, suggesting that high pool positive results could reflect multiple outcomes. In addition, whether single positive specimens routinely and reliably convert pooled samples into positive results remains unknown. However, distinct thresholds and more limited pooling strategies may be used to enhance the efficacy of this approach; even pools of 5, where positive pool outcomes were most convincing, could in theory increase initial testing capacity 5-fold. As individual sample testing will always follow positive pool results, the different testing purposes and patient populations being examined can be used to dictate appropriate pooling numbers and thresholds to account for some assay limitations. Changes in pool volume can also occur in real time depending on alterations in testing goals and the overall seropositive prevalence [7]. Thus, while limitations certainly exist, the results of this study suggest that serum pooling strategies may be useful when seeking to rapidly expand serological testing capacity. Additional studies will be needed to determine the relative ability of such a strategy to be operative in distinct populations and with other testing platforms [20].
Disclosure of interest
The authors declare that they have no competing interest.
Acknowledgements
We thank Melanie Sherman for early technical assistance.
References
- 1.Cheng M.P., Papenburg J., Desjardins M., et al. Diagnostic testing for severe acute respiratory syndrome-related coronavirus 2: a narrative review. Ann Intern Med. 2020;172:726–734. doi: 10.7326/M20-1301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chan J.F., Yuan S., Kok K.H., et al. A familial cluster of pneumonia associated with the 2019 novel coronavirus indicating person-to-person transmission: a study of a family cluster. Lancet. 2020;395:514–523. doi: 10.1016/S0140-6736(20)30154-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Corman V.M., Landt O., Kaiser M., et al. Detection of 2019 novel coronavirus (2019-nCoV) by real-time RT-PCR. Euro Surveill. 2020;25:2000045. doi: 10.2807/1560-7917.ES.2020.25.3.2000045. [Article in press] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chu D.K.W., Pan Y., Cheng S.M.S., et al. Molecular diagnosis of a novel coronavirus (2019-nCoV) causing an outbreak of pneumonia. Clin Chem. 2020;66:549–555. doi: 10.1093/clinchem/hvaa029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Park G.S., Ku K., Baek S.H., et al. Development of reverse transcription loop-mediated isothermal amplification assays targeting severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) J Mol Diagn. 2020;22:729–735. doi: 10.1016/j.jmoldx.2020.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mak G.C., Cheng P.K., Lau S.S., et al. Evaluation of rapid antigen test for detection of SARS-CoV-2 virus. J Clin Virol. 2020;129 doi: 10.1016/j.jcv.2020.104500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mallapaty S. Antibody tests suggest that coronavirus infections vastly exceed official counts. Nature. 2020 doi: 10.1038/d41586-020-01095-0. [Article in press] [DOI] [PubMed] [Google Scholar]
- 8.Zhang B., Liu S., Tan T., et al. Treatment with convalescent plasma for critically Ill patients with severe acute respiratory syndrome coronavirus 2 infection. Chest. 2020;158:e9–e13. doi: 10.1016/j.chest.2020.03.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.FDA . 2020. Investigational COVID-19 Convalescent Plasma Guidance for Industry. [Accessed on 24th September 2020. Available from: https://www.fda.gov/vaccines-blood-biologics/investigational-new-drug-ind-or-device-exemption-ide-process-cber/recommendations-investigational-covid-19-convalescent-plasma] [Google Scholar]
- 10.Fang B., Meng Q.H. The laboratory's role in combating COVID-19. Crit Rev Clin Lab Sci. 2020;57:400–414. doi: 10.1080/10408363.2020.1776675. [DOI] [PubMed] [Google Scholar]
- 11.Stowell S., Guarner J. Role of serology in the COVID-19 pandemic. Clin Infect Dis. 2020 doi: 10.1093/cid/ciaa510. [Article in press] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Salazar E., Kuchipudi S.V., Christensen P.A., et al. Convalescent plasma anti-SARS-CoV-2 spike protein ectodomain and receptor binding domain IgG correlate with virus neutralization. J Clin Invest. 2020 doi: 10.1172/JCI141206. [Article in press] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tre-Hardy M., Blairon L., Wilmet A., et al. The role of serology for COVID-19 control: population, kinetics and test performance do matter. J Infect. 2020;81:e91–e92. doi: 10.1016/j.jinf.2020.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lippi G., Plebani M., Graber M.L. Building a bridge to safe diagnosis in health care. The role of the clinical laboratory. Clin Chem Lab Med. 2016;54:1–3. doi: 10.1515/cclm-2015-1135. [DOI] [PubMed] [Google Scholar]
- 15.Roth W.K., Weber M., Seifried E. Feasibility and efficacy of routine PCR screening of blood donations for hepatitis C virus, hepatitis B virus, and HIV-1 in a blood-bank setting. Lancet. 1999;353:359–363. doi: 10.1016/S0140-6736(98)06318-1. [DOI] [PubMed] [Google Scholar]
- 16.McMahon E.J., Fang C., Layug L., Sandler S.G. Pooling blood donor samples to reduce the cost of HIV-1 antibody testing. Vox Sang. 1995;68:215–219. doi: 10.1111/j.1423-0410.1995.tb02575.x. [DOI] [PubMed] [Google Scholar]
- 17.van Zyl G.U., Preiser W., Potschka S., Lundershausen A.T., Haubrich R., Smith D. Pooling strategies to reduce the cost of HIV-1 RNA load monitoring in a resource-limited setting. Clin Infect Dis. 2011;52:264–270. doi: 10.1093/cid/ciq084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Haaheim H., Vorland L., Gutteberg T.J. Laboratory diagnosis of respiratory diseases: PCR versus serology. Nucleosides Nucleotides Nucleic Acids. 2001;20:1255–1258. doi: 10.1081/NCN-100002530. [DOI] [PubMed] [Google Scholar]
- 19.Whitman J.D., Townsend R.L., Bern C., Stramer S.L. Evaluation of matrix effects and prolonged storage on Trypanosoma cruzi serology in blood donor specimens. Transfusion. 2020;60:1149–1153. doi: 10.1111/trf.15736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Loeffelholz M.J. Evaluation of high throughput serological tests for SARS-CoV-2. J Clin Microbiol. 2020 doi: 10.1128/JCM.02179-20. [Article in press] [DOI] [PMC free article] [PubMed] [Google Scholar]