Abstract
Thrombotic microangiopathy (TMA) is a condition characterized by thrombocytopenia and microangiopathic hemolytic anemia (MAHA) with varying degrees of organ damage in the setting of normal international normalized ratio and activated partial thromboplastin time. Complement has been implicated in the etiology of TMA, which are classified as primary TMA when genetic and acquired defects in complement proteins are the primary drivers of TMA (complement-mediated TMA or atypical hemolytic uremic syndrome, aHUS) or secondary TMA, when complement activation occurs in the context of other disease processes, such as infection, malignant hypertension, autoimmune disease, malignancy, transplantation, pregnancy, and drugs. It is important to recognize that this classification is not absolute because genetic variants in complement genes have been identified in patients with secondary TMA, and distinguishing complement/genetic-mediated TMA from secondary causes of TMA can be challenging and lead to potentially harmful delays in treatment. In this review, we focus on data supporting the involvement of complement in aHUS and in secondary forms of TMA associated with malignant hypertension, drugs, autoimmune diseases, pregnancy, and infections. In aHUS, genetic variants in complement genes are found in up to 60% of patients, whereas in the secondary forms, the finding of genetic defects is variable, ranging from almost 60% in TMA associated with malignant hypertension to less than 10% in drug-induced TMA. On the basis of these findings, a new approach to management of TMA is proposed.
Keywords: autoimmune disease, complement, drugs, HUS, hypertension, thrombotic microangiopathy
Challenges in the classification and diagnosis of TMA
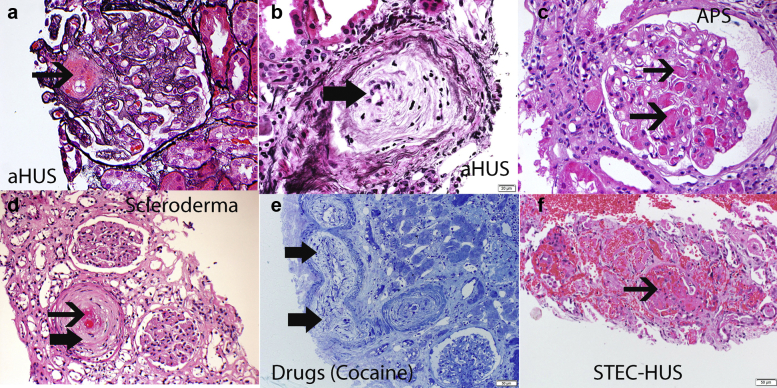
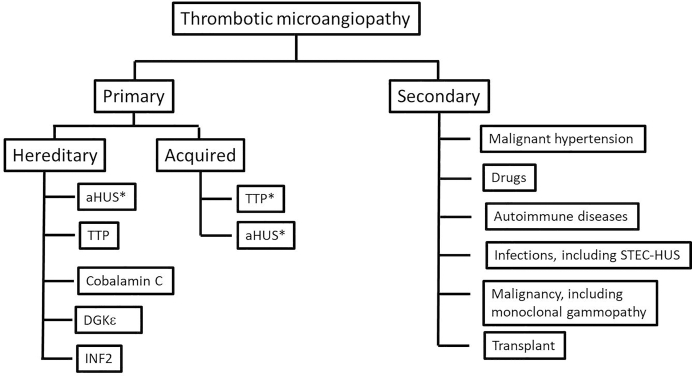
Thrombotic microangiopathy (TMA) is an overarching term used to describe any condition characterized by nonimmune thrombocytopenia and microangiopathic hemolytic anemia1 with varying degrees of organ damage in the setting of normal prothrombin time and activated partial thromboplastin time.2 Thrombocytopenia due to peripheral consumption should also be ruled out. Although microthrombi in tissue specimens, mainly kidney biopsies, is the hallmark of TMA (Figure 1), TMA is often inferred from the observation of thrombocytopenia and MAHA in the appropriate clinical setting. Determining the underlying etiology can be a major challenge but is important for directed therapy. One of the most useful classifications is that originally proposed by Nester and George,3 which was subsequently modified by others grouping TMA into primary (genetic and acquired) and secondary causes4,5 (Figure 2).
Figure 1.
Pathology of thrombotic microangiopathy (TMA). The pathology of TMA on light microscopy includes glomerular and vascular changes. Glomerular changes: thrombi in glomerular capillaries, mesangiolysis, and, in the chronic phase, thickened capillary walls with double contour formation. Vascular changes: thrombi, fragmented red cells, intimal swelling, and fibrous thickening with onion skinning. Representative findings of TMA in a case of aHUS (a,b), antiphospholipid syndrome (c), scleroderma (d), drug (cocaine) (e), and infection (Shiga toxin hemolytic uremic syndrome; STEC-HUS) (f). (f) STEC-HUS shows severe TMA with cortical necrosis. Thin black arrow points to thrombi in glomerular capillaries and arteries; thick black arrow points to myxoid change and onion-skinning of arterial walls. In general, the glomerular and vascular TMA findings are not specific for a particular etiology. (a,b: silver methenamine stain 40×; c, periodic acid–Schiff stain 40×; d, hematoxylin and eosin 20×; e, toluidine blue 10×; and f, hematoxylin and eosin 10×).
Figure 2.
Classification of thrombotic microangiopathy. aHUS, atypical hemolytic uremic syndrome; DGKe, diacylglycerol kinase epsilon; INF2, inverted formin 2; STEC-HUS, Shiga toxin hemolytic uremic syndrome; TTP, thrombotic thrombocytopenic purpura. ∗aHUS and TTP can be both hereditary and acquired; aHUS more likely to be hereditary, whereas TTP is more likely to be acquired.
Primary genetic causes of TMA include (i) deficiency of ADAMTS13 (a disintegrin and metalloprotease with thrombospondin type-1 repeats, 13th member), which is seen in congenital thrombotic thrombocytopenic purpura (TTP)6; (ii) complement-mediated hemolytic uremic syndrome (HUS), also known as aHUS, which is driven by abnormalities in complement genes; (iii) and a few rare diseases such as cobalamin C (cblC) deficiency, which is most commonly due to mutations in the MMACHC (methylmalonic aciduria cblC type with homocystinuria) gene, mutations of DGKE (diacylglycerol epsilon), and mutations in the INF2 (inverted formin 2) gene. Primary acquired causes of TMA include (i) TTP secondary to autoantibodies to ADAMTS13 (acquired TTP) and (ii) complement-mediated HUS secondary mainly to autoantibodies to factor H (FH).
Secondary TMA signifies a TMA occurring in the context of another disease process, such as infection, malignant hypertension, autoimmune disease, malignancy, transplantation, pregnancy, or drugs. It is important to recognize that this classification is not absolute because genetic variants in complement genes have been identified in patients with secondary TMA associated with pregnancy, transplantation, infections, systemic and glomerular diseases, and malignant hypertension, suggesting an overlap between primary and secondary TMA and illustrating the importance of genetic background in disease susceptibility.
Regardless of etiology, TMA is frequently associated with increased mortality or end-organ damage.7, 8, 9, 10, 11 Although TTP,12 Shiga toxin HUS,13, 14, 15 and Streptococcus pneumoniae–associated HUS16 have well-established diagnostic tests and treatment guidelines, distinguishing aHUS and other genetic types of TMA from secondary causes of TMA can be challenging and lead to potentially harmful delays in treatment. Cavero et al.17 have demonstrated that patients with secondary TMA respond to eculizumab as a temporary approach to complement blockade.18
In this article, we focus on data supporting the involvement of alternative complement pathway in secondary forms of TMA associated with malignant hypertension, drug exposure, autoimmune diseases, pregnancy, IgA nephropathy (IgAN), and infections. For an in-depth overview of specific analysis of complement pathways, we recommend the articles from Skattum et al.19 and Angioi et al.20
aHUS: The Prototype of Complement-Mediated TMA
aHUS is a disease characterized by dysregulation of the alternative pathway of complement on cell surfaces. Uncontrolled activity of the terminal complement pathway leads to the generation of massive amount of membrane attack complex with consequent damage to endothelial cells and the generation of fibrin thrombi in the microvasculature,21 culminating in platelet adhesion, mechanical intravascular destruction of erythrocytes, and tissue ischemia. There is no diagnostic test that conclusively confirms aHUS, and the diagnosis is considered to be one of exclusion.
Genetics
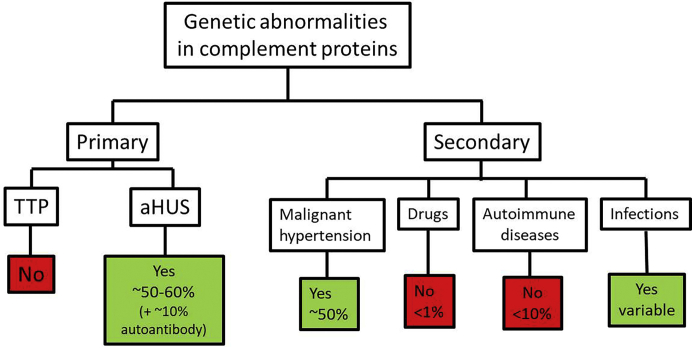
Although genetic data are useful in the diagnosis of aHUS, the absence of detectable mutations in complement proteins does not rule out aHUS because genetic abnormalities are only identified in about half of patients.7,8,22, 23, 24, 25, 26, 27 Genetic data can be broadly divided into inactivating mutations in genes that encode complement regulating proteins such as CFH, CFI, and MCP or a gain-of-function mutations in genes that encode complement activating proteins such as C3 and CFB (Figure 3). The penetrance of aHUS in families is complicated and has been estimated to be 50%.7 In light of incomplete penetrance, the current hypothesis is that the development of aHUS requires “2 hits” (combination of genetic background and a trigger). Approximately 50% of cases are triggered by infections.8,28 Pregnancy is another frequent trigger for women, and most will present in the postpartum period29 (discussed subsequently). CFH mutations can result in either aHUS or C3G. In aHUS, the mutations tend to be missense mutations involving the C-terminus of CFH with normal CFH regulatory activity in plasma (but limited capacity to protect cells at tissue level), whereas in C3G, the mutations tend to be at the N-terminus of CFH with decreased complement regulating activities of CFH in the plasma (fluid phase dysregulation).30
Figure 3.
Genetic abnormalities in complement genes in primary and secondary thrombotic microangiopathy. aHUS, atypical hemolytic uremic syndrome; TTP, thrombotic thrombocytopenic purpura.
Determination of pathogenicity of identified variants follows American College of Medical Genetics and Genomics guidelines.31 Accurate classification is paramount to clinical care and remains one of the main challenges today. As such, testing is best done in laboratories with specialized expertise in complement genetics.
With regard to acquired, disease FH autoantibodies are also associated with aHUS, typically in children who are homozygous deleted for the CFHR1 gene, a member of the CFH gene family. The frequency of this deletion allele varies across the globe from a high of more than 50% in Nigeria to very rare in South America and Japan. How deletion of CFHR1 leads to development of FH autoantibodies is complicated and may involve slight differences in structural conformation of FHR1 and FH as well as an individual’s susceptibility to the development of autoantibodies in general.32,33
Complement Evaluation
Complement pathway assessment can be a useful tool to aide in diagnosis of aHUS; however, similar to genetic analysis, normal results do not exclude a diagnosis of aHUS. Standard complement evaluation comprises quantitative and qualitative analyses. Reduction in complement proteins are not invariably seen with aHUS, but determining serum proteins levels can assist in the interpretation of genetic and functional data.34 In aHUS patients, there is preferential activation of the alternative complement pathway, and the expected serum complement profile consists of low serum C3 and normal C4, which reflects the preferential activation of the alternative complement pathway even though C3 levels are reduced in only 30% of patients. Low C3 with normal or decreased FB concentration, associated with normal C4, suggests alternative pathway–mediated complement activation. The concentrations of FH, factor D, and factor I can clarify the mechanism of C3 consumption. It is common that abnormal results may not all be found simultaneously, and in approximately 60% of aHUS patients, all complement protein levels are normal. This has given rise to an extensive debate on which tests are the most sensitive and specific for aHUS.35, 36, 37, 38
Finally, complement activation and C5b-9 (membrane attack complex) deposition on endothelial cell surface can also be determined in the laboratory by incubating serum/plasma using endothelial cell culture (HMEC-1).39,40 Complement serology may be useful, when available, for monitoring of C5 blockade in patients receiving complement directed therapy.
TMA and Secondary Causes
One of the challenges in TMA classification is whether patients with secondary causes also have a significant component of TMA from complement activation and whether these patients will benefit from anticomplement therapy. Here we discuss the available data on the role of complement in various secondary causes of TMA.
TMA and Malignant Hypertension
Patients with hypertensive emergency, defined as out-of-range elevation in blood pressure associated with failure of at least 3 organs,41 may present with features of MAHA.42,43 Scleroderma renal crisis should be ruled out (discussed subsequently). In a study of 97 patients with malignant hypertension, one-third had MAHA, of which 56% needed dialysis (as opposed to only 3% in the group without MAHA), and only 40% where able to stop dialysis after blood pressure control, suggesting additional mechanisms of injury.44 Indeed, it has recently been shown that complement abnormalities are present in a significant number of patients who develop TMA in the setting of malignant hypertension. In recent studies, variants in genes encoding regulatory proteins of the alternative complement pathway were identified in 6 of 9 patients.45,46 In another series of 26 patients with malignant hypertension and TMA,43 35% of the patients had variants in complement genes. In addition, soluble and glomerular deposits of C5b-9 have been identified in the patients with malignant hypertension who develop TMA.47 Thus, patients who had massive tissue deposition of C5b-9 more often progressed to end-stage renal disease (ESRD; 72% vs. 38%) than patients with minor deposition, irrespective of complement genetic status; the patients with significant complement activation also had more glomerular thrombi. In this same cohort, complement inhibition with eculizumab prevented ESRD in 5 of 6 patients with massive ex vivo complement activation after blood pressure lowering failed.
In another cohort of patients with aHUS, HELLP (hemolysis, elevated liver enzymes, low platelet count) syndrome, preeclampsia, and malignant hypertension, a modified endothelial cell assay for deposition of C5b-9 (using activated plasma instead of serum) was used to detect complement activation. Ex vivo C5b-9 deposition was increased in the active phase of patients with aHUS, as well as in 100% of patients with HELLP and 90% of patients with preeclampsia. In the subgroup of patients with malignant hypertension, C5b-9 deposition was similar to control levels, and there was partial TMA response after blood pressure control alone.40
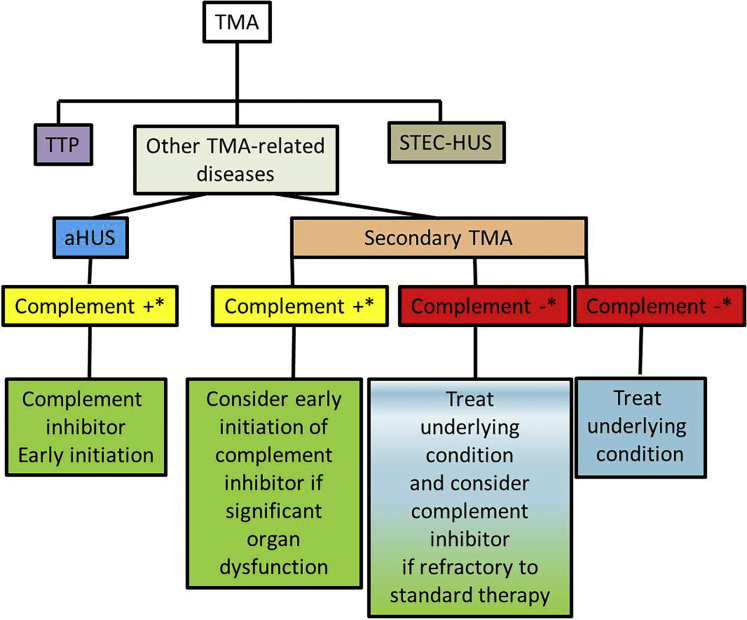
Given the severity of renal disease presenting with TMA in the setting of malignant hypertension, early detection of complement involvement may help identify a subset of patients that would benefit from complement inhibition especially when blood pressure lowering does not improve TMA.48 Eculizumab (Soliris, Alexion Pharma, New Haven, CT) has been used for a variable lengths in patients developing TMA in the setting of malignant hypertension49,50 with good response even when initiated 5.5 months after initial TMA presentation.10 The length of administration of complement blockers may be guided by TMA response and presence of a positive genetic test for complement genes (Figure 4).
Figure 4.
Approach to thrombotic microangiopathy (TMA) management according to evidence of complement involvement. ∗Evidence of complement activation, if available: (i) genetics: pathogenic/likely pathogenic variant or risk haplotype in alternative complement pathway genes; (ii) antibody: autoantibodies to complement factors (mainly anti–factor H and anti–factor B), (iii) functional assays: soluble C5b-9, tissue deposition of C5b-9, others; (iv) biopsy: staining for C4d, C5b-9. aHUS, atypical hemolytic uremic syndrome; STEC-HUS, Shiga toxin hemolytic uremic syndrome; TTP, thrombotic thrombocytopenic purpura.
In summary, on the basis of available data, approximately one-third of patients with malignant hypertension may present with TMA, of which complement abnormalities are found in 35% to 65%. These patients have worse outcome compared with patients without complement defects, and usually TMA does not subside with blood pressure control. Complement abnormalities should be suspected in patients with TMA in the setting of malignant hypertension.
Drug-Induced TMA
Thrombotic microangiopathy may be mediated by drugs and medicinal plants.51 Drug-induced TMA (DITMA) has 2 primary underlying mechanisms: (i) dose-related idiosyncrasies that are immune mediated and (ii) direct toxic effects dependent on the dose and time of drug administration. TMA resulting from anti–vascular endothelial growth factors such as bevacizumab has been demonstrated to involve injury to renal podocytes.52 Other examples of medications inducing TMA by toxic effects include cocaine, cyclosporine, docetaxel, everolimus, and interferon-alpha, interferon-beta, interferon polycarboxylate, pentostatin, sirolimus, sunitinib, tacrolimus, and vincristine.
Quinine is the hallmark of immune-mediated drug-related TMA, and purpura related to exposure to this antimalarial drug has been known for more than a century53; other examples of medications that can cause immune-mediated TMA include muromonab-CD3, penicillin, mitomycin, quetiapine, sulfisoxazole, and trielina. Some medications such as gemcitabine and oxaliplatin induce TMA via both immune-mediated and toxic effects.54
DITMA is suspected when there is a sudden onset of severe systemic symptoms, usually acute kidney injury with anuria, within days (usually <21 days in antibody-mediated TMA) or hours after exposure to the drug (in cases of direct toxicity), although some cases may occur long after drug exposure. There may be a history of malaise after previous exposure. Another diagnostic criterion is resolution or improvement of TMA when the suspected drug is stopped or dose reduced, although some degree of kidney injury may persist.55 The detection of antibody-dependent reaction to the drug supports the clinical diagnosis; however, a negative test does not exclude drug-induced TMA.53 Management of DITMA predominantly involves withholding the causative medication; often that alone is not enough to lead to clinical recovery, however, and in patients with escalating renal injury other therapies such as plasma exchange and eculizumab sometimes needs to be considered. As noted earlier, gemcitabine may have direct endothelial toxicity, with release of large amounts of von Willebrand factor multimers and concomitant activation of the coagulation cascade,56 although a few case reports of the benefit of steroids, plasma exchange, and rituximab57,58 in gemcitabine-related TMA also support the role of an immune mediated mechanism. Further highlighting the complexity of DITMA are case reports of gemcitabine-related TMA resolution with short-term eculizumab after no response to plasma exchange, raising the possibility of complement involvement in the pathogenesis of this entity in adults59, 60, 61, 62 and children.63 In a large registry study,64 120 patients with gemcitabine-related TMA were treated with plasma exchange/plasma infusion (42%), corticosteroids (15%) or eculizumab (5%) after complete drug withdrawal. Overall response was similar among all treatments, and no genetic complement abnormalities were found in the patients, which is consistent with the analysis of 110 patients with secondary TMA in which the genetic findings were similar to healthy individuals.65
Similarly, eculizumab was also used in a cohort of 29 patients with secondary TMA, of which 15 patients had DITMA and 14 patients had other causes (systemic diseases, pregnancy/postpartum, cancer-related, acute humoral rejection in a transplant recipient, and primary intestinal lymphangiectasia).17 All 15 DITMA patients were kidney transplant recipients and 14 patients were using the combination of tacrolimus with an mammalian target of rapamycin (mTOR) inhibitor. All but 1 patient withdrew the offending drug, and 12 patients also received plasma exchange. Eculizumab was started 4 to 53 days after TMA detection and no graft was lost. Genetic variants of complement genes were not detected in the 15 patients, which corroborates that the most probable cause of TMA was tacrolimus/mTOR use.
In summary, DITMA may be immune mediated or a consequence of direct toxicity of the offending drug to endothelial cells. Even though the majority of patients lack complement genetic variants, response of DITMA to eculizumab may provide indirect evidence of complement activation in some cases. Functional studies of complement activation in DITMA are lacking.
TMA and Autoimmune Diseases
A variety of autoimmune diseases that can be associated with TMA,66 both at the onset or during the course of the disease. In this review, we focus on systemic lupus erythematosus (SLE), antiphospholipid syndrome (APS), and scleroderma. The pathophysiology of TMA in autoimmune diseases is complex and multifactorial because both classical and alternative complement pathways may be involved in a somewhat paradoxical manner.67
TMA in patients with SLE may be renal limited or present with systemic features. It is important to rule out secondary TTP through determination of ADAMTS13 activity and antibody, which has therapeutic implications. Elevated anti-DNA antibody titers and hypocomplementemia are not predictive of renal TMA in lupus nephritis.66 TMA is a rare manifestation (3%–9%) in SLE and results in poor renal outcome irrespective of the presence antiphospholipid antibodies.68 In a study of 148 biopsies of lupus nephritis, 36 cases (24%) presented with TMA, of which 80% were isolated and 20% were associated with other conditions (TTP, HUS, malignant hypertension, and scleroderma); the patients with TMA had worse kidney outcome, especially if C4d staining was positive and serum FH levels were low.69 The complement system in SLE seems to have a protective role (clearance of apoptotic cells and immune complexes) but also a pathogenic role (amplification of an inflammatory response), and genetic analysis showed that an intact classical pathway is important to protect from development of lupus nephritis. Genetic defects in the alternative pathway genes also have been associated with SLE and lupus nephritis (FH, factor I, large deletions in CFHR1-CFHR3 possibly through anti-FH antibodies).70 Although there is evidence of genetic abnormalities of the classical and alternative pathway in development of lupus nephritis, the role of genetic variants in complement genes has not been studied in detail in patients with TMA in lupus. In patients with persistent TMA despite treatment of lupus nephritis, the use of eculizumab in 43 patients with SLE/APS showed a positive outcome in hematological indexes in 40 (93%) patients and kidney function improvement in 29 (72.5%) patients,17,71 suggesting involvement of complement pathways. Unfortunately, genetic analysis of complement genes was not performed in most patients in this study.
APS can occur in isolation (primary) or in association with other autoimmune diseases such as SLE (secondary). Catastrophic APS (CAPS), defined as intravascular thrombosis in patients with persistent antiphospholipid antibodies affecting 3 or more systems simultaneously or within 1 week,72 occurs in less than 1% of patients with APS and has a high mortality rate (approximately 30%) despite treatment with steroids, anticoagulation, plasma exchange, intravenous immunoglobulin, and rituximab. Renal involvement is commonly found in CAPS, and a frequent finding is TMA.73 Regardless of whether TMA is noted in APS, a role for complement activation on endothelial cells in the hypercoagulability status of patients with antiphospholipid antibodies74 has been identified, and eculizumab has been used to treat refractory cases of CAPS. A systematic review on the use of eculizumab in 6 patients with CAPS found improvement or stabilization in all cases.75 Elevated soluble C5b-9 and other complement products upstream of C5 were found in a patient with CAPS who was treated with eculizumab with good response to complement inhibition in hours.76
In scleroderma, the presence of kidney injury, thrombocytopenia, and microangiopathic hemolytic anemia, with or without malignant hypertension, is known as scleroderma renal crisis (SRC)77 and occurs in less than 5% of patients with systemic sclerosis (2.4% in a cohort of 637 patients). Steroid use is one risk factor,78 and viral infections, such as influenza B, may precipitate SRC.79 Differentiating SRC from other causes of TMA may be difficult, with poor kidney and patient outcomes80 despite standard treatment (plasma exchange, angiotensin converting enzyme inhibitors),81 even in normotensive patients.66 The pathophysiology is still not completely unraveled and comprises Major histocompatibility complex class I haplotypes, B and T cells, antibodies to angiotensin 2 receptor 1, and RNA polymerase III,77 which may have a role in endothelial cell activation, overexpression of endothelin-1 and complement activation as seen with C4d deposition in peritubular capillaries82 and C3b staining in kidney biopsies of patients with SRC.83 Recurrence rate after kidney transplantation is low (<2%) and there may be a role for mTOR inhibitors.78 Good response to eculizumab in resistant cases even with negative complement genetic findings reinforce the role of complement activation in SRC.84, 85, 86
In summary, the presence of underlying genetic variants in complement genes seems rare in patients with systemic diseases and TMA. Some patients, however, demonstrate a good response to eculizumab that suggests a secondary complement involvement.
TMA and Pregnancy
The differential diagnosis of TMA in pregnancy includes cases of eclampsia, preeclampsia, and HELLP syndrome, which can show an overlap of signs and symptoms. aHUS should be suspected when despite delivery, TMA fails to improve and extends beyond 72 h, which would be the expected time for recovery in cases of eclampsia, preeclampsia, and HELLP syndrome.87 In 2010, Fakhouri et al.29 found that among 100 women with aHUS, 21 cases were associated with pregnancy (P-aHUS), and 79% of the cases occurred in the postpartum period. In this series, genetic defects in alternative complement pathway were detected in 18 of 21 patients. Without specific treatment, the prognosis in these cases was reserved because 76% of patients progressed to ESRD. The authors also observed a higher risk of having TMA in a second pregnancy. In another cohort of 29 patients with secondary TMA treated with eculizumab,17 2 patients presented P-aHUS with good response to eculizumab despite absence of detectable pathogenic variants.
Complement-mediated disorders in pregnancy were elegantly reviewed in a recent work,88 reinforcing that TMA associated with pregnancy, especially in the postpartum period, is highly associated with genetic complement abnormalities and most patients benefit from eculizumab.
TMA and IgAN
IgAN is the most common primary glomerulonephritis worldwide. In a Brazilian cohort, from 118 patients with IgAN, 21 (17.8%) had histologic features of TMA. These patients presented more frequently hypertension, hematuria, low serum C3 levels, and worse kidney function, leading more often to renal replacement therapy than patients without TMA features on biopsy (71.4% vs. 21.6%).89
In another cohort, of 128 cases of IgAN, 68 (53.1%) had features of TMA on the kidney biopsy. Similarly, 71% of the TMA had uncontrolled hypertension, and on follow up doubling of serum creatinine or ESRD developed in all patients with laboratory evidence of TMA. The kidney biopsy findings were similar to that seen in malignant hypertension. No genetic mutations in complement genes were identified in the 11 patients studied with severe TMA in this series.90 Similarly, no mutations in CFH were identified in another large series of IgAN.91 Taken together, genetic variants of complement genes do not appear to play a role in TMA in the setting of IgAN.
TMA and Infections
TMA in the context of infectious diseases have been extensively reviewed,92,93 with findings as follows:
-
•
Shiga toxin-producing enterobacteria: E. coli is the most common; others include Shigella dysenteriae, Campylobacter jejuni, and Moraxella osloensis
-
•
Viral infections: cytomegalovirus, Epstein-Barr virus, varicella zoster virus, parvovirus B19, human immunodeficiency virus, influenza virus
-
•
Respiratory tract infection agents: Bordetella pertussis, Streptococcus pneumoniae,16 Mycoplasma pneumoniae94
-
•
Protozoa: Toxoplasma gondii
-
•
Rare agents: erlichiosis,95 Capnocytophaga canimorsus,96,97 Plasmodium vivax (malaria),98 snake bites (Bothrops jararaca),99 dengue fever,100, 101, 102 West Nile vírus,103 Chikungunya fever104,105
-
•
Severe acute respiratory syndrome coronavirus type 2 (SARS-CoV-2)
Mechanisms of TMA-associated with infectious diseases are complex and include direct endothelial injury, development of ADAMTS13 inhibitors, and complement activation of different levels and magnitude. As an illustration, putative mechanisms of TMA in influenza infection are production of neuraminidase (although lower than seen with Streptococcus pneumoniae), direct infection of endothelial cells leading to apoptosis, activation of platelets, and generation of thrombin. Severe deficiency of ADAMTS13 has been detected in patients with influenza infection, HIV-associated TMA,106 and dengue fever.102 In experimental models, terminal complement activation during influenza infections can lead to lung injury and neutrophil recruitment. Furthermore, elevated soluble C5b-9 was detected in patients with H1N1 infection during the 2009 pandemic.107 Complement activation in patients with STEC-HUS has been demonstrated,108 and eculizumab can be considered in patients with STEC-HUS and severe organ involvement.109 Le Clech et al.65 found 18 patients with secondary TMA due to infections (among 110 patients with secondary TMA) and only 1 patient with E. coli HUS also had a pathogenic variant in CFH. Genetic variants of alternative pathway complement proteins are variable in patients with infection-related TMA (Table 1).
Table 1.
Case reports and series of patients with infection-related thrombotic microangiopathy who carried pathogenic variants in complement genes
| Author | Pts | Genetic findings | Infection/trigger | Treatment and outcome |
|---|---|---|---|---|
| Bitzan et al.107 | N = 30 | Seven of 8 patients tested presented variants in complement genes (C3, MCP, MCP combined with CFB, clusterin) | Influenza A (20 pts) Influenza B (4 pts) Influenza vaccine (5 pts) 1 not described |
20 of 24 pts recovered with PE/PI 2 pts received eculizumab 3 pts died |
| Mittal et al.118 | 16 y | MCP (chr.1:207930883A>G 2bp upstream exon 3) | Influenza B | oseltamivir, PE, steroids; good outcome |
| Okano et al.119 | 23 y | C3 missense (p.I1157T) | Influenza A | Eculizumab; good outcome |
| Szilagyi et al.16 | 37 mo 18 mo 29 mo |
Three of 5 pts had positive genetic findings: CFH Arg114949X Arg1131X CFI Pro50Ala Pro32Ala THBD Thr44Ile Thr26 Ile |
Streptococcus pneumoniae | PE/PD/no sequelae Support/no sequelae PD/no sequelae |
| Tong et al.120 | 6 mo | S. pneumoniae | ||
| Berner et al.121 | NB | Altered FH in Western blot | Bordetella pertussis | Deceased |
| Obando et al.122 | NB | Homozygous MCPggaac risk haplotype | B. pertussis | Plasma infusion; good outcome |
| Kwon et al.123 | 5 y 4 y |
Heterozygous MCP exon 2 (c.191G>T) Anti-FH antibody |
Varicella zoster virus | Supportive; good outcome PE; good outcome |
| Agrawal et al.124 | 5 y | Two SNPs in CFH gene, exon-7 rs1061147 (p.Ala243Ala) and exon-9 rs1061170 (p.His402Tyr) | Malaria vivax | Artesunate, primaquine, dialysis; CKD |
| Westra et al.36 | 26 STEC 11 aHUS |
STEC: 7/25 (28%) – CFH, C3, anti-factor H Ab aHUS: 7/11 (63.3%) – CD46, C3, anti-factor H Ab | STEC-HUS | Variable |
| Çelakil et al.125 | 6 y |
CFH (p.Glu936Asp) CFB (p.Arg32Trp) |
STEC-HUS | Eculizumab; CKD |
| Aldridge et al.126 | 55 IR HUS cases | 1 CFH 1 post-transplant recurrence of HUS |
STEC-HUS S. pneumoniae |
Variable; 19% progressed to ESRD |
| Fremeaux-Bacchi et al.127 | 108 pts with STEC | 3/75 (4%) had variants with MAF <0.1% vs. 0.8% in controls | STEC-HUS | 1 patient progressed to ESRD (CFH pathogenic variant) |
| Dowen et al.128 | 16 y | C3 (c.4855A>G) (p.Ser1619Arg) | STEC-HUS | Post-transplant recurrence resolved with eculizumab |
aHUS, atypical hemolytic uremic syndrome; CKD, chronic kidney disease; ESRD, end-stage renal disease; FH, factor H; HUS, hemolytic uremic syndrome; IR, infection related; NB, newborn; PD, peritoneal dialysis; PE, plasma exchange; PI, plasma infusion; SNP, single-nucleotide polymorphisms; STEC-HUS, Shiga toxin hemolytic uremic syndrome.
In 2020, the world is being challenged with the COVID-19 pandemic, a disease caused by the SARS-CoV-2. Previously, experimental studies in mice had identified the complement system as an important mediator of SARS-CoV tissue injury after the 2002–2003 SARS outbreak.110 There is growing evidence of complement activation orchestrated with coagulation disorders111 as one of the mechanisms of tissue injury and endothelial cell damage from SARS-CoV-2 infection,112 and autopsy reports of patients with COVID-19 infection have identified evidence of TMA in the pulmonary vasculature.113 The bulk of complement components such as C5a may derive from uncontrolled complement regulation triggered directly by SARS-CoV-2 infection. Also, there appears to be a role for lectin pathway overstimulation on the pathogenesis of thrombotic events, and these latter findings may have therapeutic implications.114 Jhaveri et al.115 described a patient with TMA who presented normal coagulation tests, normal ADAMTS13 activity, elevated soluble C5b-9 and soluble CBb, low serum FH activity, and positive antiphospholipid IgM antibody. This patient received 1 dose of eculizumab but had a fatal outcome 2 days later. Although antiphospholipid antibodies have been found in patients with COVID-19, the pathophysiological significance is still undetermined.116 Preliminary data of eculizumab use in 4 patients with confirmed SARS-CoV-2 infection who received noninvasive mechanical ventilation and multiple associated drug therapy showed good results,117 a larger ongoing trial (SOLID-C19 NCT 04288713) may confirm the benefit of eculizumab in patients with COVID-19. Currently, 13 clinical trials addressing complement inhibition in COVID-19 infection are underway.
In summary, genetic variants in complement proteins can be present in TMA associated with infections. The type and incidence of genetic variants depends on the infection. The role of complement inhibitors in TMA-associated with infections is unclear.
Monoclonal Gammopathy and TMA
Monoclonal gammopathy has been shown to be associated with C3G, a disease associated with abnormalities of the complement pathway.129 Recently, monoclonal gammopathy was also shown to be associated with TMA. Complement evaluation has not yet been evaluated in patients in this group.130
Conclusion
The severity and extent of genetic complement abnormalities in secondary TMA is variable. Malignant hypertension and pregnancy-associated TMA are more likely to be associated with genetic complement abnormalities and appear similar to those in aHUS, whereas autoimmune diseases and drug-associated TMA are less likely to have genetic complement abnormalities. Genetic complement defects in infection-associated TMA are variable, and infections may trigger aHUS. We propose early use of complement inhibition in patients with secondary TMA refractory to traditional therapy, provided there is significant organ dysfunction. Determining the extent of complement involvement in secondary TMA may help in the decision to use complement inhibitors in early presentation. The duration of complement inhibition should be tailored based on presence of complement abnormalities and response to therapy, and there is an urgent need for randomized controlled trials regarding complement inhibition in secondary TMA.
Disclosure
LMPP is a speaker for Alexion Pharma Brazil and a scientific consultant for C3 Glomerulopathy and OrphanDC Brazil. All the other authors declared no competing interests.
Acknowledgments
The authors thank Dr. Richard Smith, University of Iowa, for reviewing and providing valuable input for the aHUS portion of the manuscript.
References
- 1.Moake J.L. Thrombotic microangiopathies. N Engl J Med. 2002;347:589–600. doi: 10.1056/NEJMra020528. [DOI] [PubMed] [Google Scholar]
- 2.Laurence J., Haller H., Mannucci P.M. Atypical hemolytic uremic syndrome (aHUS): essential aspects of an accurate diagnosis. Clin Adv Hematol Oncol. 2016;14(suppl 11):2–15. [PubMed] [Google Scholar]
- 3.George J.N., Nester C.M. Syndromes of thrombotic microangiopathy. N Engl J Med. 2014;371:1847–1848. doi: 10.1056/NEJMc1410951. [DOI] [PubMed] [Google Scholar]
- 4.Scully M., Cataland S., Coppo P. Consensus on the standardization of terminology in thrombotic thrombocytopenic purpura and related thrombotic microangiopathies. J Thromb Haemost. 2017;15:312–322. doi: 10.1111/jth.13571. [DOI] [PubMed] [Google Scholar]
- 5.Fox L.C., Cohney S.J., Kausman J.Y., Shortt J., Hughes P.D., Wood E.M. Consensus opinion on diagnosis and management of thrombotic microangiopathy in Australia and New Zealand. Nephrology (Carlton) 2018;23:507–517. doi: 10.1111/nep.13234. [DOI] [PubMed] [Google Scholar]
- 6.Moake J.L., Rudy C.K., Troll J.H. Unusually large plasma factor VIII:von Willebrand factor multimers in chronic relapsing thrombotic thrombocytopenic purpura. N Engl J Med. 1982;307:1432–1435. doi: 10.1056/NEJM198212023072306. [DOI] [PubMed] [Google Scholar]
- 7.Noris M., Caprioli J., Bresin E. Relative role of genetic complement abnormalities in sporadic and familial aHUS and their impact on clinical phenotype. Clin J Am Soc Nephrol. 2010;5:1844–1859. doi: 10.2215/CJN.02210310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fremeaux-Bacchi V., Fakhouri F. Genetics and outcome of atypical hemolytic uremic syndrome: a nationwide French series comparing children and adults. Clin J Am Soc Nephrol. 2013;8:554–562. doi: 10.2215/CJN.04760512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jodele S., Zhang K., Zou F. The genetic fingerprint of susceptibility for transplant-associated thrombotic microangiopathy. Blood. 2016;127:989–996. doi: 10.1182/blood-2015-08-663435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Asif A., Nayer A., Haas C.S. Atypical hemolytic uremic syndrome in the setting of complement-amplifying conditions: case reports and a review of the evidence for treatment with eculizumab. J Nephrol. 2017;30:347–362. doi: 10.1007/s40620-016-0357-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schaefer F., Ardissino G., Ariceta G. Clinical and genetic predictors of atypical hemolytic uremic syndrome phenotype and outcome. Kidney Int. 2018;94:408–418. doi: 10.1016/j.kint.2018.02.029. [DOI] [PubMed] [Google Scholar]
- 12.Bendapudi P.K., Hurwitz S., Fry A. Derivation and external validation of the PLASMIC score for rapid assessment of adults with thrombotic microangiopathies: a cohort study. Lancet Haematol. 2017;4:e157–e164. doi: 10.1016/S2352-3026(17)30026-1. [DOI] [PubMed] [Google Scholar]
- 13.Menne J., Nitschke M., Stingele R. Validation of treatment strategies for enterohaemorrhagic Escherichia coli O104:H4 induced haemolytic uraemic syndrome: case–control study. BMJ. 2012;345:e4565. doi: 10.1136/bmj.e4565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Keir L.S. Shiga toxin associated hemolytic uremic syndrome. Hematol Oncol Clin North Am. 2015;29:525–539. doi: 10.1016/j.hoc.2015.01.007. [DOI] [PubMed] [Google Scholar]
- 15.Wijnsma K.L., van Bommel S.A., van der Velden T. Fecal diagnostics in combination with serology: best test to establish STEC-HUS. Pediatr Nephrol. 2016;31:2163–2170. doi: 10.1007/s00467-016-3420-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Szilagyi A., Kiss N., Bereczki C. The role of complement in Streptococcus pneumoniae–associated haemolytic uraemic syndrome. Nephrol Dial Transplant. 2013;28:2237–2245. doi: 10.1093/ndt/gft198. [DOI] [PubMed] [Google Scholar]
- 17.Cavero T., Rabasco C., Lopez A., Roman E., Avila A., Sevillano A. Eculizumab in secondary atypical haemolytic uraemic syndrome. Nephrol Dial Transplant. 2017;32:466–474. doi: 10.1093/ndt/gfw453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Praga M., Rodriguez de Cordoba S. Secondary atypical hemolytic uremic syndromes in the era of complement blockade. Kidney Int. 2019;95:1298–1300. doi: 10.1016/j.kint.2019.01.043. [DOI] [PubMed] [Google Scholar]
- 19.Skattum L. Clinical complement analysis—an overview. Transfus Med Rev. 2019;33:207–216. doi: 10.1016/j.tmrv.2019.09.001. [DOI] [PubMed] [Google Scholar]
- 20.Angioi A., Fervenza F.C., Sethi S. Diagnosis of complement alternative pathway disorders. Kidney Int. 2016;89:278–288. doi: 10.1016/j.kint.2015.12.003. [DOI] [PubMed] [Google Scholar]
- 21.Sethi S., Fervenza F.C. Pathology of renal diseases associated with dysfunction of the alternative pathway of complement: C3 glomerulopathy and atypical hemolytic uremic syndrome (aHUS) Semin Thromb Hemost. 2014;40:416–421. doi: 10.1055/s-0034-1375701. [DOI] [PubMed] [Google Scholar]
- 22.Fan X., Yoshida Y., Honda S. Analysis of genetic and predisposing factors in Japanese patients with atypical hemolytic uremic syndrome. Mol Immunol. 2013;54:238–246. doi: 10.1016/j.molimm.2012.12.006. [DOI] [PubMed] [Google Scholar]
- 23.Lee J.M., Park Y.S., Lee J.H. Atypical hemolytic uremic syndrome: Korean pediatric series. Pediatr Int. 2015;57:431–438. doi: 10.1111/ped.12549. [DOI] [PubMed] [Google Scholar]
- 24.Szarvas N., Szilagyi A., Csuka D. Genetic analysis and functional characterization of novel mutations in a series of patients with atypical hemolytic uremic syndrome. Mol Immunol. 2016;71:10–22. doi: 10.1016/j.molimm.2016.01.003. [DOI] [PubMed] [Google Scholar]
- 25.Zhang T., Lu J., Liang S. Comprehensive analysis of complement genes in patients with atypical hemolytic uremic syndrome. Am J Nephrol. 2016;43:160–169. doi: 10.1159/000445127. [DOI] [PubMed] [Google Scholar]
- 26.Osborne A.J., Breno M., Borsa N.G. Statistical validation of rare complement variants provides insights into the molecular basis of atypical hemolytic uremic syndrome and C3 glomerulopathy. J Immunol. 2018;200:2464–2478. doi: 10.4049/jimmunol.1701695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Thergaonkar R.W., Narang A., Gurjar B.S. Targeted exome sequencing in anti-factor H antibody negative HUS reveals multiple variations. Clin Exp Nephrol. 2018;22:653–660. doi: 10.1007/s10157-017-1478-6. [DOI] [PubMed] [Google Scholar]
- 28.Kavanagh D., Goodship T.H., Richards A. Atypical hemolytic uremic syndrome. Semin Nephrol. 2013;33:508–530. doi: 10.1016/j.semnephrol.2013.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fakhouri F., Roumenina L., Provot F. Pregnancy-associated hemolytic uremic syndrome revisited in the era of complement gene mutations. J Am Soc Nephrol. 2010;21:859–867. doi: 10.1681/ASN.2009070706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Merinero H.M., Garcia S.P., Garcia-Fernandez J. Complete functional characterization of disease-associated genetic variants in the complement factor H gene. Kidney Int. 2018;93:470–481. doi: 10.1016/j.kint.2017.07.015. [DOI] [PubMed] [Google Scholar]
- 31.Richards S., Aziz N., Bale S. Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet Med. 2015;17:405–424. doi: 10.1038/gim.2015.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Holmes L.V., Strain L., Staniforth S.J. Determining the population frequency of the CFHR3/CFHR1 deletion at 1q32. PLoS ONE. 2013;8 doi: 10.1371/journal.pone.0060352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bhattacharjee A., Reuter S., Trojnar E. The major autoantibody epitope on factor H in atypical hemolytic uremic syndrome is structurally different from its homologous site in factor H-related protein 1, supporting a novel model for induction of autoimmunity in this disease. J Biol Chem. 2015;290:9500–9510. doi: 10.1074/jbc.M114.630871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Roumenina L.T., Loirat C., Dragon-Durey M.A. Alternative complement pathway assessment in patients with atypical HUS. J Immunol Methods. 2011;365:8–26. doi: 10.1016/j.jim.2010.12.020. [DOI] [PubMed] [Google Scholar]
- 35.Cataland S.R., Holers V.M., Geyer S. Biomarkers of terminal complement activation confirm the diagnosis of aHUS and differentiate aHUS from TTP. Blood. 2014;123:3733–3738. doi: 10.1182/blood-2013-12-547067. [DOI] [PubMed] [Google Scholar]
- 36.Westra D., Volokhina E.B., van der Molen R.G. Serological and genetic complement alterations in infection-induced and complement-mediated hemolytic uremic syndrome. Pediatr Nephrol. 2017;32:297–309. doi: 10.1007/s00467-016-3496-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Prohaszka Z., Varga L., Fust G. The use of 'real-time' complement analysis to differentiate atypical haemolytic uraemic syndrome from other forms of thrombotic microangiopathies. Br J Haematol. 2012;158:424–425. doi: 10.1111/j.1365-2141.2012.09168.x. [DOI] [PubMed] [Google Scholar]
- 38.Sridharan M., Go R.S., Abraham R.S. Diagnostic utility of complement serology for atypical hemolytic uremic syndrome. Mayo Clin Proc. 2018;93:1351–1362. doi: 10.1016/j.mayocp.2018.07.008. [DOI] [PubMed] [Google Scholar]
- 39.Noris M., Galbusera M., Gastoldi S. Dynamics of complement activation in aHUS and how to monitor eculizumab therapy. Blood. 2014;124:1715–1726. doi: 10.1182/blood-2014-02-558296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Palomo M., Blasco M., Molina P. Complement activation and thrombotic microangiopathies. Clin J Am Soc Nephrol. 2019;14:1719–1732. doi: 10.2215/CJN.05830519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cremer A., Amraoui F., Lip G.Y. From malignant hypertension to hypertension-MOD: a modern definition for an old but still dangerous emergency. J Hum Hypertens. 2016;30:463–466. doi: 10.1038/jhh.2015.112. [DOI] [PubMed] [Google Scholar]
- 42.Isles C.G., McLay A., Jones J.M. Recovery in malignant hypertension presenting as acute renal failure. Q J Med. 1984;53:439–452. [PubMed] [Google Scholar]
- 43.Timmermans S., Werion A., Damoiseaux J. Diagnostic and risk factors for complement defects in hypertensive emergency and thrombotic microangiopathy. Hypertension. 2020;75:422–430. doi: 10.1161/HYPERTENSIONAHA.119.13714. [DOI] [PubMed] [Google Scholar]
- 44.van den Born B.J., Honnebier U.P., Koopmans R.P., van Montfrans G.A. Microangiopathic hemolysis and renal failure in malignant hypertension. Hypertension. 2005;45:246–251. doi: 10.1161/01.HYP.0000151620.17905.ee. [DOI] [PubMed] [Google Scholar]
- 45.Totina A., Iorember F., El-Dahr S.S., Yosypiv I.V. Atypical hemolytic-uremic syndrome in a child presenting with malignant hypertension. Clin Pediatr (Phila) 2013;52:183–186. doi: 10.1177/0009922811412942. [DOI] [PubMed] [Google Scholar]
- 46.Timmermans S., Abdul-Hamid M.A., Vanderlocht J. Patients with hypertension-associated thrombotic microangiopathy may present with complement abnormalities. Kidney Int. 2017;91:1420–1425. doi: 10.1016/j.kint.2016.12.009. [DOI] [PubMed] [Google Scholar]
- 47.Timmermans S., Abdul-Hamid M.A., Potjewijd J. C5b9 formation on endothelial cells reflects complement defects among patients with renal thrombotic microangiopathy and severe hypertension. J Am Soc Nephrol. 2018;29:2234–2243. doi: 10.1681/ASN.2018020184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cavero T., Arjona E., Soto K. Severe and malignant hypertension are common in primary atypical hemolytic uremic syndrome. Kidney Int. 2019;96:995–1004. doi: 10.1016/j.kint.2019.05.014. [DOI] [PubMed] [Google Scholar]
- 49.Tsai H.M. Does anticomplement therapy have a role in the management of malignant hypertension? J Clin Hypertens (Greenwich) 2016;18:359–360. doi: 10.1111/jch.12664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chen F.Y., Chen C.H., Lin C.C. Hypertensive crisis and refractory hypertension caused by atypical hemolytic uremic syndrome and effect of eculizumab. Acta Cardiol Sin. 2018;34:446–449. doi: 10.6515/ACS.201809_34(5).20180326D. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hunt R., Yalamanoglu A., Tumlin J. A mechanistic investigation of thrombotic microangiopathy associated with IV abuse of Opana ER. Blood. 2017;129:896–905. doi: 10.1182/blood-2016-08-736579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Eremina V., Jefferson J.A., Kowalewska J. VEGF inhibition and renal thrombotic microangiopathy. N Engl J Med. 2008;358:1129–1136. doi: 10.1056/NEJMoa0707330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Page E.E., Little D.J., Vesely S.K., George J.N. Quinine-induced thrombotic microangiopathy: a report of 19 patients. Am J Kidney Dis. 2017;70:686–695. doi: 10.1053/j.ajkd.2017.05.023. [DOI] [PubMed] [Google Scholar]
- 54.Al-Nouri Z.L., Reese J.A., Terrell D.R. Drug-induced thrombotic microangiopathy: a systematic review of published reports. Blood. 2015;125:616–618. doi: 10.1182/blood-2014-11-611335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Reese J.A., Bougie D.W., Curtis B.R. Drug-induced thrombotic microangiopathy: experience of the Oklahoma Registry and the BloodCenter of Wisconsin. Am J Hematol. 2015;90:406–410. doi: 10.1002/ajh.23960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Grange S., Coppo P., Centre de reference des microangiopathies Thrombotic microangiopathies and antineoplastic agents. Nephrol Ther. 2017;13(suppl 1):S109–S113. doi: 10.1016/j.nephro.2017.01.016. [DOI] [PubMed] [Google Scholar]
- 57.Bharthuar A., Egloff L., Becker J. Rituximab-based therapy for gemcitabine-induced hemolytic uremic syndrome in a patient with metastatic pancreatic adenocarcinoma: a case report. Cancer Chemother Pharmacol. 2009;64:177–181. doi: 10.1007/s00280-008-0900-x. [DOI] [PubMed] [Google Scholar]
- 58.Ritchie G.E., Fernando M., Goldstein D. Rituximab to treat gemcitabine-induced hemolytic-uremic syndrome (HUS) in pancreatic adenocarcinoma: a case series and literature review. Cancer Chemother Pharmacol. 2017;79:1–7. doi: 10.1007/s00280-016-3123-6. [DOI] [PubMed] [Google Scholar]
- 59.Rogier T., Gerfaud-Valentin M., Pouteil-Noble C. [Clinical efficacy of eculizumab as treatment of gemcitabine-induced thrombotic microangiopathy: a case report] Rev Med Interne. 2016;37:701–704. doi: 10.1016/j.revmed.2015.12.027. [in French] [DOI] [PubMed] [Google Scholar]
- 60.Lopez Rubio M.E., Rodado Martinez R., Illescas M.L. Gemcitabine-induced hemolytic-uremic syndrome treated with eculizumab or plasmapheresis: two case reports. Clin Nephrol. 2017;87:100–106. doi: 10.5414/CN108838. [DOI] [PubMed] [Google Scholar]
- 61.Krishnappa V., Gupta M., Shah H., Das A. The use of eculizumab in gemcitabine induced thrombotic microangiopathy. BMC Nephrol. 2018;19:9. doi: 10.1186/s12882-018-0812-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Martin K., Roberts V., Chong G. Eculizumab therapy in gemcitabine-induced thrombotic microangiopathy in a renal transplant recipient. Oxf Med Case Reports. 2019;2019:omz048. doi: 10.1093/omcr/omz048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Facchini L., Lucchesi M., Stival A. Role of eculizumab in a pediatric refractory gemcitabine-induced thrombotic microangiopathy: a case report. J Med Case Rep. 2017;11:209. doi: 10.1186/s13256-017-1373-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Daviet F., Rouby F., Poullin P. Thrombotic microangiopathy associated with gemcitabine use: presentation and outcome in a national French retrospective cohort. Br J Clin Pharmacol. 2019;85:403–412. doi: 10.1111/bcp.13808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Le Clech A., Simon-Tillaux N., Provot F. Atypical and secondary hemolytic uremic syndromes have a distinct presentation and no common genetic risk factors. Kidney Int. 2019;95:1443–1452. doi: 10.1016/j.kint.2019.01.023. [DOI] [PubMed] [Google Scholar]
- 66.Babar F., Cohen S.D. Thrombotic microangiopathies with rheumatologic involvement. Rheum Dis Clin North Am. 2018;44:635–649. doi: 10.1016/j.rdc.2018.06.010. [DOI] [PubMed] [Google Scholar]
- 67.Thurman J.M., Frazer-Abel A., Holers V.M. The evolving landscape for complement therapeutics in rheumatic and autoimmune diseases. Arthritis Rheumatol. 2017;69:2102–2113. doi: 10.1002/art.40219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hernandez-Molina G., Garcia-Trejo L.P., Uribe N., Cabral A.R. Thrombotic microangiopathy and poor renal outcome in lupus patients with or without antiphospholipid syndrome. Clin Exp Rheumatol. 2015;33:503–508. [PubMed] [Google Scholar]
- 69.Song D., Wu L.H., Wang F.M. The spectrum of renal thrombotic microangiopathy in lupus nephritis. Arthritis Res Ther. 2013;15:R12. doi: 10.1186/ar4142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Noris M., Remuzzi G. Genetics of immune-mediated glomerular diseases: focus on complement. Semin Nephrol. 2017;37:447–463. doi: 10.1016/j.semnephrol.2017.05.018. [DOI] [PubMed] [Google Scholar]
- 71.Kello N., Khoury L.E., Marder G. Secondary thrombotic microangiopathy in systemic lupus erythematosus and antiphospholipid syndrome, the role of complement and use of eculizumab: case series and review of literature. Semin Arthritis Rheum. 2019;49:74–83. doi: 10.1016/j.semarthrit.2018.11.005. [DOI] [PubMed] [Google Scholar]
- 72.Cervera R., Rodriguez-Pinto I., Espinosa G. The diagnosis and clinical management of the catastrophic antiphospholipid syndrome: a comprehensive review. J Autoimmun. 2018;92:1–11. doi: 10.1016/j.jaut.2018.05.007. [DOI] [PubMed] [Google Scholar]
- 73.Turrent-Carriles A., Herrera-Felix J.P., Amigo M.C. Renal involvement in antiphospholipid syndrome. Front Immunol. 2018;9:1008. doi: 10.3389/fimmu.2018.01008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Erkan D., Salmon J.E. The role of complement inhibition in thrombotic angiopathies and antiphospholipid syndrome. Turk J Haematol. 2016;33:1–7. doi: 10.4274/tjh.2015.0197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Tinti M.G., Carnevale V., Inglese M. Eculizumab in refractory catastrophic antiphospholipid syndrome: a case report and systematic review of the literature. Clin Exp Med. 2019;19:281–288. doi: 10.1007/s10238-019-00565-8. [DOI] [PubMed] [Google Scholar]
- 76.Barratt-Due A., Floisand Y., Orrem H.L. Complement activation is a crucial pathogenic factor in catastrophic antiphospholipid syndrome. Rheumatology (Oxford) 2016;55:1337–1339. doi: 10.1093/rheumatology/kew040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ghossein C., Varga J., Fenves A.Z. Recent developments in the classification, evaluation, pathophysiology, and management of scleroderma renal crisis. Curr Rheumatol Rep. 2016;18:5. doi: 10.1007/s11926-015-0551-y. [DOI] [PubMed] [Google Scholar]
- 78.Woodworth T.G., Suliman Y.A., Li W. Scleroderma renal crisis and renal involvement in systemic sclerosis. Nat Rev Nephrol. 2016;12:678–691. doi: 10.1038/nrneph.2016.124. [DOI] [PubMed] [Google Scholar]
- 79.Shimizu T., Iwamoto N., Okamoto M. Scleroderma renal crisis complicated with thrombotic microangiopathy triggered by influenza B virus infection. Intern Med. 2019;58:441–445. doi: 10.2169/internalmedicine.1441-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Mouthon L., Bussone G., Berezne A. Scleroderma renal crisis. J Rheumatol. 2014;41:1040–1048. doi: 10.3899/jrheum.131210. [DOI] [PubMed] [Google Scholar]
- 81.Abudiab M., Krause M.L., Fidler M.E., Nath K.A., Norby S.M. Differentiating scleroderma renal crisis from other causes of thrombotic microangiopathy in a postpartum patient. Clin Nephrol. 2013;80:293–297. doi: 10.5414/CN107465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Batal I., Domsic R.T., Shafer A. Renal biopsy findings predicting outcome in scleroderma renal crisis. Hum Pathol. 2009;40:332–340. doi: 10.1016/j.humpath.2008.08.001. [DOI] [PubMed] [Google Scholar]
- 83.Okroj M., Johansson M., Saxne T. Analysis of complement biomarkers in systemic sclerosis indicates a distinct pattern in scleroderma renal crisis. Arthritis Res Ther. 2016;18:267. doi: 10.1186/s13075-016-1168-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Uriarte M.H., Larrarte C., Rey L.B. Scleroderma renal crisis debute with thrombotic microangiopathy: a successful case treated with eculizumab. Case Rep Nephrol. 2018;2018:6051083. doi: 10.1155/2018/6051083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Thomas C.P., Nester C.M., Phan A.C. Eculizumab for rescue of thrombotic microangiopathy in PM-Scl antibody-positive autoimmune overlap syndrome. Clin Kidney J. 2015;8:698–701. doi: 10.1093/ckj/sfv101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Devresse A., Aydin S., Le Quintrec M. Complement activation and effect of eculizumab in scleroderma renal crisis. Medicine (Baltimore) 2016;95:e4459. doi: 10.1097/MD.0000000000004459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Fakhouri F., Vercel C., Fremeaux-Bacchi V. Obstetric nephrology: AKI and thrombotic microangiopathies in pregnancy. Clin J Am Soc Nephrol. 2012;7:2100–2106. doi: 10.2215/CJN.13121211. [DOI] [PubMed] [Google Scholar]
- 88.Amari Chinchilla K., Vijayan M., Taveras Garcia B., Jim B. Complement-mediated disorders in pregnancy. Adv Chronic Kidney Dis. 2020;27:155–164. doi: 10.1053/j.ackd.2020.01.002. [DOI] [PubMed] [Google Scholar]
- 89.Neves P.D.M.M., Souza R.A., Torres F.M. Evidences of histologic thrombotic microangiopathy and the impact in renal outcomes of patients with IgA nephropathy. PLoS One. 2020;15(11) doi: 10.1371/journal.pone.0233199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.El Karoui K., Hill G.S., Karras A. A clinicopathologic study of thrombotic microangiopathy in IgA nephropathy. J Am Soc Nephrol. 2012;23:137–148. doi: 10.1681/ASN.2010111130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Edey M., Strain L., Ward R. Is complement factor H a susceptibility factor for IgA nephropathy? Mol Immunol. 2009;46:1405–1408. doi: 10.1016/j.molimm.2008.12.002. [DOI] [PubMed] [Google Scholar]
- 92.Riedl M., Fakhouri F., Le Quintrec M. Spectrum of complement-mediated thrombotic microangiopathies: pathogenetic insights identifying novel treatment approaches. Semin Thromb Hemost. 2014;40:444–464. doi: 10.1055/s-0034-1376153. [DOI] [PubMed] [Google Scholar]
- 93.Gavriilaki E., Anagnostopoulos A., Mastellos D.C. Complement in thrombotic microangiopathies: unraveling Ariadne’s thread into the labyrinth of complement therapeutics. Front Immunol. 2019;10:337. doi: 10.3389/fimmu.2019.00337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Caeiro Alves F., Aguiar R., Pessegueiro P., Pires C. Thrombotic microangiopathy associated with Mycoplasma pneumoniae infection. BMJ Case Rep. 2018;2018 doi: 10.1136/bcr-2017-222582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Hassan W., Talwar M., Balaraman V., Molnar M.Z. Ehrlichiosis infection mimicking thrombotic microangiopathy syndrome early after kidney transplantation. Transpl Infect Dis. 2020 doi: 10.1111/tid.13305. [DOI] [PubMed] [Google Scholar]
- 96.Smeets N.J.L., Fijnheer R., Sebastian S., De Mast Q. Secondary thrombotic microangiopathy with severely reduced ADAMTS13 activity in a patient with Capnocytophaga canimorsus sepsis: a case report. Transfusion. 2018;58:2426–2429. doi: 10.1111/trf.14829. [DOI] [PubMed] [Google Scholar]
- 97.Tani N., Nakamura K., Sumida K. An immunocompetent case of Capnocytophaga canimorsus infection complicated by secondary thrombotic microangiopathy and disseminated intravascular coagulation. Intern Med. 2019;58:3479–3482. doi: 10.2169/internalmedicine.3110-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Nair R.K., Rao K.A., Mukherjee D. Acute kidney injury due to acute cortical necrosis following vivax malaria. Saudi J Kidney Dis Transpl. 2019;30:960–963. doi: 10.4103/1319-2442.265474. [DOI] [PubMed] [Google Scholar]
- 99.Malaque C.M.S., Duayer I.F., Santoro M.L. Acute kidney injury induced by thrombotic microangiopathy in two cases of Bothrops envenomation. Clin Toxicol (Phila) 2019;57:213–216. doi: 10.1080/15563650.2018.1510129. [DOI] [PubMed] [Google Scholar]
- 100.Bhargava V., Gupta P., Kauntia R., Bajpai G. Dengue fever–induced thrombotic microangiopathy: an unusual cause of renal failure. Indian J Nephrol. 2017;27:321–323. doi: 10.4103/0971-4065.202837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Nieto-Rios J.F., Alvarez Barreneche M.F., Penagos S.C. Successful treatment of thrombotic microangiopathy associated with dengue infection: a case report and literature review. Transpl Infect Dis. 2018;20 doi: 10.1111/tid.12824. [DOI] [PubMed] [Google Scholar]
- 102.Gogireddy R.R., Kumar V., Ranjit S., Natraj R., Venkatachalapathy P., Jayakumar I. Thrombotic thrombocytopenic purpura in a 2.5-year-old boy with dengue infection: a rare complication. Paediatr Int Child Health. 2020;40:135–138. doi: 10.1080/20469047.2019.1706299. [DOI] [PubMed] [Google Scholar]
- 103.Zanoni F., Alfieri C., Moroni G. Delayed diagnosis of West Nile virus infection in a kidney transplant patient due to inaccuracies in commonly available diagnostic tests. Exp Clin Transplant. 2020;18:385–389. doi: 10.6002/ect.2018.0107. [DOI] [PubMed] [Google Scholar]
- 104.Epelboin L., Bidaud B., Mosnier E. Fatal case of chikungunya and concomitant thrombotic thrombocytopenic purpura in French Guiana during air flight medical evacuation. J Travel Med. 2017;24 doi: 10.1093/jtm/tax028. [DOI] [PubMed] [Google Scholar]
- 105.Kumar V., Jain R., Kumar A. Chikungunya fever presenting as life threatening thrombotic thrombocytopenic purpura. J Assoc Physicians India. 2017;65:96–100. [PubMed] [Google Scholar]
- 106.Swart L., Schapkaitz E., Mahlangu J.N. Thrombotic thrombocytopenic purpura: a 5-year tertiary care centre experience. J Clin Apher. 2019;34:44–50. doi: 10.1002/jca.21673. [DOI] [PubMed] [Google Scholar]
- 107.Bitzan M., Zieg J. Influenza-associated thrombotic microangiopathies. Pediatr Nephrol. 2018;33:2009–2025. doi: 10.1007/s00467-017-3783-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Bowen E.E., Coward R.J. Advances in our understanding of the pathogenesis of hemolytic uremic syndromes. Am J Physiol Renal Physiol. 2018;314:F454–F461. doi: 10.1152/ajprenal.00376.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Lapeyraque A.L., Malina M., Fremeaux-Bacchi V. Eculizumab in severe Shiga-toxin-associated HUS. N Engl J Med. 2011;364:2561–2563. doi: 10.1056/NEJMc1100859. [DOI] [PubMed] [Google Scholar]
- 110.Gralinski L.E., Sheahan T.P., Morrison T.E. Complement activation contributes to severe acute respiratory syndrome coronavirus pathogenesis. mBio. 2018;9 doi: 10.1128/mBio.01753-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.D’Alessandro A., Thomas T., Dzieciatkowska M. Serum proteomics in COVID-19 patients: altered coagulation and complement status as a function of IL-6 level. J Proteome Res. 2020 doi: 10.1021/acs.jproteome.0c00365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Noris M., Benigni A., Remuzzi G. The case of complement activation in COVID-19 multiorgan impact. Kidney Int. 2020;19:4417–4427. doi: 10.1016/j.kint.2020.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Fox S.E., Akmatbekov A., Harbert J.L. Pulmonary and cardiac pathology in African American patients with COVID-19: an autopsy series from New Orleans. Lancet Respir Med. 2020;8:P681–P686. doi: 10.1016/S2213-2600(20)30243-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Lo M.W., Kemper C., Woodruff T.M. COVID-19: complement, coagulation, and collateral damage. J Immunol. 2020 doi: 10.4049/jimmunol.2000644. [epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Jhaveri K.D., Meir L.R., Flores Chang B.S. Thrombotic microangiopathy in a patient with COVID-19. Kidney Int. 2020;98:509–512. doi: 10.1016/j.kint.2020.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Zhang Y., Xiao M., Zhang S. Coagulopathy and antiphospholipid antibodies in patients with Covid-19. N Engl J Med. 2020;382:e38. doi: 10.1056/NEJMc2007575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Diurno F., Numis F.G., Porta G. Eculizumab treatment in patients with COVID-19: preliminary results from real life ASL Napoli 2 Nord experience. Eur Rev Med Pharmacol Sci. 2020;24:4040–4047. doi: 10.26355/eurrev_202004_20875. [DOI] [PubMed] [Google Scholar]
- 118.Mittal N., Hartemayer R., Jandeska S., Giordano L. Steroid responsive atypical hemolytic uremic syndrome triggered by influenza B infection. J Pediatr Hematol Oncol. 2019;41:e63–e67. doi: 10.1097/MPH.0000000000001180. [DOI] [PubMed] [Google Scholar]
- 119.Okano M., Matsumoto T., Nakamori Y. [Atypical hemolytic uremic syndrome with C3 p.I1157T missense mutation successfully treated with eculizumab] Rinsho Ketsueki. 2018;59:178–181. doi: 10.11406/rinketsu.59.178. [in Japanese] [DOI] [PubMed] [Google Scholar]
- 120.Tong Y.T., Al-Salihi S., Belousova T. A case of suspected Streptococcus pneumoniae hemolytic uremic syndrome (pHUS) with utilization of minor crossmatching for platelet blood products lead to a diagnosis of atypical hemolytic uremic syndrome (aHUS) Ann Clin Lab Sci. 2018;48:797–800. [PubMed] [Google Scholar]
- 121.Berner R., Krause M.F., Gordjani N. Hemolytic uremic syndrome due to an altered factor H triggered by neonatal pertussis. Pediatr Nephrol. 2002;17:190–192. doi: 10.1007/s00467-001-0798-6. [DOI] [PubMed] [Google Scholar]
- 122.Obando I., Camacho M.S., Falcon-Neyra D. Atypical hemolytic uremic syndrome associated with Bordetella pertussis infection. Pediatr Infect Dis J. 2012;31:1210. doi: 10.1097/INF.0b013e31826153fb. [DOI] [PubMed] [Google Scholar]
- 123.Kwon T., Belot A., Ranchin B., Baudouin V. Varicella as a trigger of atypical haemolytic uraemic syndrome associated with complement dysfunction: two cases. Nephrol Dial Transplant. 2009;24:2752–2754. doi: 10.1093/ndt/gfp166. [DOI] [PubMed] [Google Scholar]
- 124.Agrawal P., Kumar A., Parwaiz A. Complement factor H gene polymorphisms and vivax malaria associated thrombotic microangiopathy. Saudi J Kidney Dis Transpl. 2019;30:540–544. doi: 10.4103/1319-2442.256865. [DOI] [PubMed] [Google Scholar]
- 125.Celakil M.E., Yucel B.B., Bek K. CFH and CFB mutations in Shiga toxin-associated haemolytic uraemic syndrome in a 6-year-old boy. Paediatr Int Child Health. 2020;40:129–131. doi: 10.1080/20469047.2019.1616458. [DOI] [PubMed] [Google Scholar]
- 126.Aldridge M., Burke J. Follow-up of children with infection-associated haemolytic uraemic syndrome 1979–1995: would eculizumab have improved prognosis? J Paediatr Child Health. 2020;56:577–580. doi: 10.1111/jpc.14685. [DOI] [PubMed] [Google Scholar]
- 127.Fremeaux-Bacchi V., Sellier-Leclerc A.L., Vieira-Martins P. Complement gene variants and Shiga toxin-producing Escherichia coli-associated hemolytic uremic syndrome: retrospective genetic and clinical study. Clin J Am Soc Nephrol. 2019;14:364–377. doi: 10.2215/CJN.05830518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Dowen F., Wood K., Brown A.L. Rare genetic variants in Shiga toxin-associated haemolytic uraemic syndrome: genetic analysis prior to transplantation is essential. Clin Kidney J. 2017;10:490–493. doi: 10.1093/ckj/sfx030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Ravindran A., Fervenza F.C., Smith R.J.H. C3 glomerulopathy associated with monoclonal Ig is a distinct subtype. Kidney Int. 2018;94:178–186. doi: 10.1016/j.kint.2018.01.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Ravindran A., Go R.S., Fervenza F.C. Thrombotic microangiopathy associated with monoclonal gammopathy. Kidney Int. 2017;91:691–698. doi: 10.1016/j.kint.2016.09.045. [DOI] [PubMed] [Google Scholar]