Summary
Purpose Apatinib, a new tyrosine kinase inhibitor targeting vascular endothelial growth factor receptor-2, has shown promising efficacy against several solid cancers, but evidence of its efficacy against relapsed and refractory nasopharyngeal carcinoma is limited. We investigated the efficacy and safety of apatinib for relapsed and refractory nasopharyngeal carcinoma in an open-label, single-arm, phase II clinical trial. Fifty-one patients with relapsed and refractory nasopharyngeal carcinoma in the First Affiliated Hospital, Zhengzhou University, who met the inclusion criteria were enrolled in the study. All patients received apatinib at an initial dose of 500 mg daily (1 cycle = 28 days). The primary and secondary endpoints were overall response rate, progression-free survival, and overall survival. We evaluated treatment effects and recorded apatinib-related adverse events by performing regular follow-ups and workup. The overall response rate (complete and partial responses) was 31.37% (16/51). The median overall survival and progression-free survival were 16 (95% CI, 9.32–22.68) and 9 months (95% CI, 5.24–12.76), respectively. Most patients tolerated treatment-related adverse events of grades 1 and 2; hypertension (29, 56.86%), proteinuria (25, 49.02%), and hand–foot syndrome (27, 52.94%) were the most common adverse events. There were no treatment-related deaths. Apatinib showed good efficacy and safety in patients with relapsed and refractory NPC.
Keywords: Apatinib, Efficacy, Nasopharyngeal carcinoma, Targeted therapy
Introduction
Nasopharyngeal carcinoma (NPC) is a common malignant tumor of the head and neck with a distinct regional and racial prevalence in southern China, especially among people of Cantonese ancestry. Radiotherapy is the main treatment for NPC [1, 2]. Despite intensive treatment, 30%–40% of patients with NPC show progressive disease [3, 4], and the treatment of patients with relapsed and refractory NPC remains a major challenge. Although palliative chemotherapy can confer median progression-free survival (PFS) for 3–9 months in patients with a recurrent disease, the overall survival (OS) remains low [5]. Hence, patients with relapsed and refractory NPC are recommended to participate in clinical trials.
Angiogenesis is an important step in the development of several malignant tumors. Apatinib, a new vascular endothelial growth factor receptor-2 (VEGFR-2) tyrosine kinase inhibitor, selectively targets intracellular ATP-binding site and has shown efficacy against various solid tumors, especially advanced gastric carcinoma [5–8]. In a previous study [9], VEGF was overexpressed in more than 60% of clinical biopsy samples of NPC. Co-expression of tumor VEGF and hypoxia-related growth factors in NPC is associated with poor prognosis, and it serves as potential evidence to explore the effectiveness of apatinib against relapsed and recurrent NPC. However, clinical data to help assess the antitumor activity against recurrent and refractory NPC are limited. Hence, in the present study, we evaluated the safety and efficacy of apatinib in 51 patients with relapsed and refractory NPC.
Materials and methods
Ethical statement
The patient data used in the study were anonymized. All procedures were in agreement with the Declaration of Helsinki.
Patients and eligibility criteria
Fifty-one patients in the First Affiliated Hospital of Zhengzhou University, Henan, China, were enrolled between December 2016 and January 2019. The inclusion criteria were as follows: 1) patients aged between 18 and 70 years with an Eastern Cooperative Oncology Group (ECOG) performance status of 0–3; 2) patients with histologically confirmed NPC who did not respond to the first-line platinum-containing chemotherapy and second-line single or combined chemotherapy before apatinib treatment according to the National Comprehensive Cancer Network guideline; and 3) patients with at least one measurable lesion according to the Response Evaluation Criteria in Solid Tumors (RECIST) and with acceptable hematologic, hepatic, and renal functions. Patients were excluded if they had other malignant tumors; cardiac insufficiency or arrhythmia; uncontrolled complications such as diabetes mellitus, coagulation disorders, and urine protein ≥ ++; or were pregnant or breastfeeding.
Study design
Each patient received apatinib 500 mg at 30 min after lunch, at the same time, once daily as an initial dose until disease progression or intolerable toxicity was noted (1 treatment cycle = 28 days). Dose interruptions and reductions were allowed in cases of grade 3/4 toxicities. For each treatment cycle, interruptions were allowed for a maximum of 7 days continuously or cumulatively, and dose reductions from 500 to 375 mg daily and then to 250 mg daily were allowed for a maximum of two times. For intolerable grade 2 toxicities, one dose reduction was considered when the investigators deemed necessary.
Assessments and statistical analysis
Overall response rate (ORR) was defined as the ratio of patients achieving a complete response (CR) or partial response (PR) according to RECIST1.1 [10, 11]. PFS was defined as the time from enrollment to documentation of disease progression and OS was defined as the time from assignment to death from any cause. Both PFS and OS were estimated using the Kaplan–Meier method with 95% confidence intervals (CIs). Baseline evaluation before treatment included a physical examination, the ECOG status assessment, antecedent blood pressure measurement, complete blood count (CBC), blood chemistry panel, coagulation function test, routine urinalysis, routine stool test consisting of occult blood (OB) testing, echocardiography (ECG), and baseline lesion evaluation by contrast-enhanced computerized tomography (CT). The following tests were performed at follow-up: physical examination, CBC, biochemical profiling, and dynamic contrast-enhanced CT or magnetic resonance imaging (MRI). Treatment efficacy was evaluated after every two cycles by contrast-enhanced CT. Adverse events (AEs) were assessed according to the Common Terminology Criteria for Adverse Events (CTCAE) version 5.0 and related data were collected during outpatient clinic visit and follow-up [12].
Results
Baseline characteristics
Fifty-five patients with relapsed and refractory NPC were registered in the study after obtaining signed informed consent (Table 1). During the screening stage, one patient did not meet the inclusion criteria and three patients were excluded from the per-protocol population because their post-baseline efficacy assessment data were not available. The remaining 51 patients were included in the safety and activity analyses (Table 1).
Table 1.
Baseline characteristics of 51 patients
| Characteristics | Number of patients (%) |
|---|---|
| Age, years | |
| Median age | 50 |
| Range | (18 to 70) |
| Sex | |
| Male | 43 (84.31) |
| Female | 8 (15.69) |
| ECOG performance status | |
| ≤2 | 31 (60.78) |
| >2 | 20 (39.22) |
| Pathological type (WHO) | |
| WHO I | 9 (17.65) |
| WHO II/III | 42 (82.35) |
| EBER | |
| Negative | 10 (19.61) |
| Positive | 41 (80.39) |
| bFGF | |
| Positive | 12 (23.53) |
| Negative | 39 (76.47) |
| Ki67 | |
| <25% | 10 (19.61) |
| >25% | 41 (80.39) |
| Hb (g/L) | |
| ≥120 | 39 (76.47) |
| <120 | 12 (23.53) |
| Lactate dehydrogenase (IU/L) | |
| ≤245 | 35 (68.63) |
| >245 | 16 (31.37) |
| Alkaline phosphatase (IU/L) | |
| ≤110 | 30 (58.82) |
| >110 | 21 (41.18) |
| T stage (UICC/AJCC 8th edition) | |
| T1–2 | 7 (13.73) |
| T3–4 | 44 (86.27) |
| N stage (UICC/AJCC 8th edition) | |
| N1–2 | 10 (19.61) |
| N3–4 | 41 (80.39) |
| Clinical stages | |
| III | 20 (39.22) |
| IV | 31 (60.78) |
| Distant metastasis | |
| Yes | 39 (76.47) |
| No | 12 (23.53) |
| Radiotherapy of the primary tumor | |
| Yes | 48 (94.12) |
| No | 3 (5.88) |
| Metastatic lesion | |
| Bone | 20 (39.22) |
| Liver | 11 (21.57) |
| Lung | 13 (25.49) |
| Distant lymph nodes, mediastinum, and others | 7 (13.73) |
Abbreviations: ECOG, Eastern Cooperative Oncology Group; LDH, lactate dehydrogenase; Hb, hemoglobin; ALP, alkaline phosphatase
Efficacy
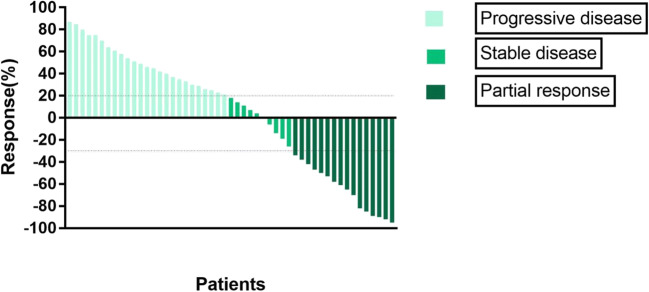
The data collection cut-off time for the primary analysis was May 2019. By the last follow-up, 16 (31.37%) patients had achieved ORR, with 0 cases of CR and 16 cases (31.37%) of PR (Table 2). Twenty-five of the 51 (49.02%) patients showed disease progression, and five of them showed progression during the initial two-cycle evaluation. The median OS was 16 months (95% CI, 9.32–22.68) and the median PFS was 9 months (95% CI, 5.24–12.76) (Figs. 1, 2 and 3). Twenty patients (39.22%) died before the cutoff date; among these, eight (15.69%) showed PR and 12 (23.53%) showed disease progression. Twenty of the remaining patients showed tumor shrinkage from the baseline findings at their last follow-up. The percentage changes from the baseline are shown in Fig. 1.
Table 2.
Treatment responses
| Effective evaluation | N (%) |
|---|---|
| CR | 0 |
| PR | 16 (31.37) |
| SD | 10 (19.61) |
| PD | 25 (49.02) |
| ORR (CR + PR) | 16 (31.37) |
| DCR (CR + PR + SD) | 26 (50.98) |
CR, complete response; PR, partial response; SD, stable disease; PD, progressive disease; ORR, overall response rate; DCR, disease control rate
Fig. 1.
Waterfall plot for the best percentage change in target lesion size
Fig. 2.
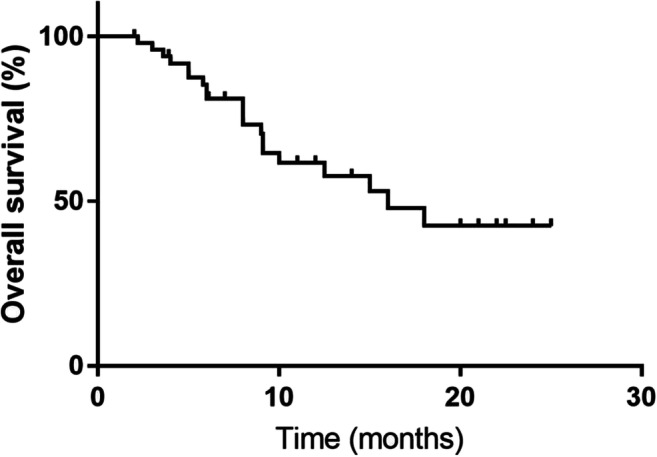
Kaplan–Meier graph for overall survival (n = 51)
Fig. 3.
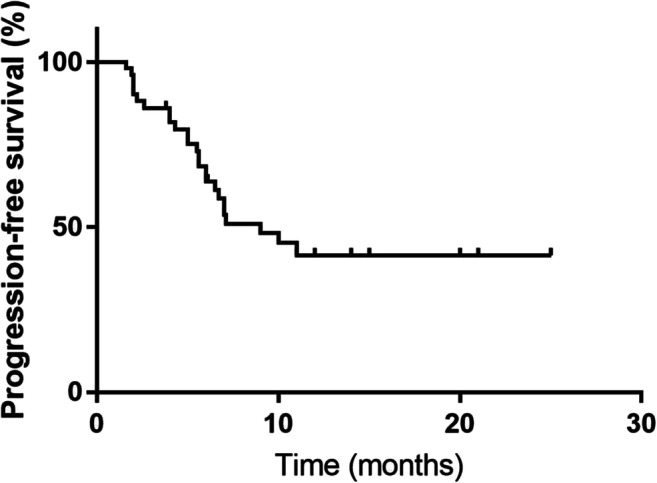
Kaplan–Meier graph for progression-free survival (n = 51)
Safety
The incidence of AEs of any grade, regardless of causality, was 100%. These included hematological and non-hematological toxicities (Table 3). Notably, simultaneous occurrence of multiple AEs in patients was common. Most patients showed treatment-related AEs of grades 1–2, with proteinuria (25 patients, 49.02%), hypertension (29 patients, 56.86%), and hand–foot syndrome (27 patients, 52.94%) being the most common. AEs of grades 3–4 were noted in 18 patients. Fatigue was a common symptom among patients with leucopenia. One patient was admitted to the hospital to receive treatment for severe hemorrhinia, which was considered possibly treatment related. There was no grade 5 AE.
Table 3.
Major treatment-related adverse events, n (%)
| Adverse events | Grade 1–2, n (%) | Grade 3, n (%) | Grade 4, n (%) | Grade 5, n (%) | ALL, n (%) |
|---|---|---|---|---|---|
| Non-hematologic | |||||
| Hypertension | 23 (45.10) | 3 (5.88) | 3 (5.88) | 0 | 29 (56.86) |
| Hand–foot syndrome | 25 (49.02) | 2 (3.92) | 0 | 0 | 27 (52.94) |
| Proteinuria | 24 (47.06) | 1 (1.96) | 0 | 0 | 25 (49.02) |
| Mucositis | 10 (19.61) | 0 | 0 | 0 | 10 (19.61) |
| Fatigue | 18 (35.29) | 1 (1.96) | 1 (1.96) | 0 | 20 (39.22) |
| Myalgia/arthralgia | 5 (9.80) | 0 | 0 | 0 | 5 (9.80) |
| Hyperbilirubinemia | 1 (1.96) | 0 | 0 | 0 | 1 (1.96) |
| Aminotransferase increased | 2 (3.92) | 0 | 0 | 0 | 2 (3.92) |
| Vomiting | 9 (17.65) | 1 (1.96) | 0 | 0 | 10 (19.61) |
| Erythra | 2 (3.92) | 0 | 0 | 0 | 2 (3.92) |
| Nausea | 7 (13.73) | 1 (1.96) | 0 | 0 | 8 (15.69) |
| Headache | 6 (11.76) | 2 (3.92) | 0 | 0 | 8 (15.69) |
| Pain | 3 (5.88) | 0 | 0 | 0 | 3 (5.88) |
| Alopecia | 2 (3.92) | 0 | 0 | 0 | 2 (3.92) |
| hemorrhage | 7 (13.73) | 0 | 2 (3.92) | 0 | 9 (17.65) |
| Hematologic | |||||
| Leucopenia | 7 (13.73) | 0 | 0 | 0 | 7 (13.73) |
| Neutropenia | 18 (35.29) | 1 (1.96) | 0 | 0 | 19 (37.25) |
| Thrombocytopenia | 6 (11.76) | 0 | 0 | 0 | 6 (11.76) |
| Anemia | 15 (29.41) | 1 (1.96) | 1 (1.96) | 0 | 17 (33.33) |
Dose adjustments
Dose adjustments were necessary (refer to Study design) for 31 (60.78%) patients. For 23 patients (45.10%), one dose reduction from 500 mg per day to 375 mg was required, and for eight patients (15.69%), the dose was reduced to 250 mg per day. Treatment was temporarily interrupted for 27 patients (52.94%) and was resumed after an interval of fewer than 7 days or rational dose adjustments were performed (Table 4). Treatment was interrupted permanently owing to grade 4 hypertension in 4 patients (7.84%) and due to severe hemorrhinia in 1 patient. The symptoms in these patients were resolved after support treatment.
Table 4.
Dose adjustments (n, %)
| Dose interrupted permanently | 4 (7.84) |
|---|---|
| Dose interrupted temporarily | 27 (52.94) |
| Dose Adjustments | |
| No dose adjustments (500 mg) | 20 (39.22) |
| 375 mg per day | 23 (45.10) |
| 250 mg per day | 8 (15.69) |
Discussion
Cisplatin combined with 5-fluorouracil is a common first-line regimen for advanced NPC, with the associated response rates of 66%–78% and median survival of 11–14 months [13–15]. Taxane- or gemcitabine-based doublet combination regimen has also been commonly employed, with a response rate of 22%–75% [16–18]. Treatment for patients with recurrent NPC is challenging because there is no standard second-line treatment regimens after the failure of the first-line platinum-based chemotherapy. This group of patients represents those with the most urgent unmet therapeutic need.
Molecular-targeted therapy has been broadly explored and evaluated against one or several genetic mutations, aberrant growth factor pathways, or angiogenesis in NPC. To the best of our knowledge, this is the first study to report the efficacy and safety of apatinib for treating relapsed and refractory NPC. As an oral anti-angiogenesis drug for advanced tumors, apatinib showed potential in our study. We observed objective responses in 31.37% patients, achieving a median PFS of 9 months and a median OS of 16 months, superior to those associated with several popular novel agents (Table 5). Nivolumab is a human monoclonal antibody that targets programmed death receptor-1 (PD-1); it has been approved for the treatment of various types of cancer in several countries. Early-stage clinical trials of anti-PD-1 therapies have shown promising outcomes with objective response rates (ORRs) of 21%–34% in recurrent or metastatic NPC [19, 20]. Axitinib, another VEGFR inhibitor, has also been reported to help achieve an ORR of 19% among 37 evaluable patients, including 1 who showed PR, 6 who showed unconfirmed PR, and 22 who showed a stable disease. However, the median time to progression was 5.0 months and median OS was 10.4 months, indicating an inferior curative effect compared with that of apatinib [21]. Adoptive T cell therapy has emerged as a strategy to treat human cancers. Despite a preferable median OS of 38.1 months [22], which superior to that observed in our study, the feasibility and generalization of adoptive T cell therapy are limited by extremely high cost and complexity of the associated laboratory techniques. Thus, significant challenges in solid cancer management remain.
Table 5.
Comparison of clinical characteristics of apatinib and other novel antigens for relapsed and refractory NPC
| Drug | Study | N | ORR | PFS | OS |
|---|---|---|---|---|---|
| Axitinib | Hui et al. | 37 | 19% | 5 months | 10.4 months |
| Nivolumab | Ma et al. [20] | 32 | 13% |
3.5 months 3-month PFS, 64.2% |
3-month OS, 87.5% OS was not reached |
| Pazopanib | Lim et al. [29] | 33 | 6.1% | 4.4 months | 1-year OS, 32% |
| Interleukin-2 | Chi et al. [30] | 14 | 0 | – | 9 months |
| Adoptive T cell therapy | Smith et al. [22] | 29 | – | 5.5 months | 38.1 months |
Angiogenesis is mainly affected by the microenvironment, and VEGFR is one of the most important angiogenesis factors. Apatinib acts by selectively competing for ATP-binding sites of VEGFR-2. VEGFR-2 is autophosphorylated at its carboxyl terminal tail and kinase insert region, leading to the activation of its kinase activity and binding of phospholipase C-γ plus the adaptor molecules TSAd, Sck, and Shb [23, 24]. This triggers the downstream RAS-RAF-MEK-ERK and PI3K pathways [25]. The vital role of angiogenesis in malignancies has been confirmed. However, to the best of our knowledge, there are no studies on the efficacy and safety of VEGF-2 inhibitors for relapsed and refractory NPC.
Hypertension, proteinuria, and hand–foot syndrome are regarded as the most common AEs of anti-angiogenic agents (Table 3). In our study, most cases of hypertension, proteinuria, and hand–foot syndrome were of grades 1 and 2, with incidences of 56.86%, 49.02%, and 52.94%, respectively. These findings were generally consistent with the results of previous studies on other solid cancers [26, 27]. It is worth noting that one patient showed severe hemorrhinia, possibly related to the treatment, which could indicate that VEGFR inhibitor drugs may increase bleeding risk in patients. This could be due to drug-induced platelet dysfunction and reduced synthesis of vascular endothelial tissue factor, damaging vascular integrity. VEGF can stimulate the proliferation of vascular endothelial cells, maintain the integrity of blood vessels, and ensure the normal regulation of coagulation function. Inhibition of the VEGFR transduction pathway can reduce the regeneration ability of vascular endothelial cells, expose procoagulant phospholipids under the matrix, and cause platelet dysfunction, leading to hemorrhage or thrombosis [28].
Apatinib administered orally, without the need for hospitalization of patients or an infusion pump for administration, might result in improved patient compliance and economic feasibility. To the best of our knowledge, this is the first study to evaluate the efficacy and safety of apatinib in patients with relapsed and refractory NPC. The antitumor effects and reverse drug resistance may be improved by combining apatinib treatment with chemotherapy or other targeted drugs, including anti-angiogenesis agents with different action mechanisms. However, this study had some limitations. This was a single-arm, retrospective study with no control group for comparison, and thus selection bias could not be ruled out because of the non-randomized design. Second, the study population was relatively small. Nonetheless, based on our results, apatinib appears to be safe and highly efficacious against relapsed and refractory NPC, which strongly indicates the need for further research to confirm its efficacy against nasopharyngeal cancer.
Funding information
This work was supported by the national natural science foundation of China (No. 81570204).
Compliance with ethical standards
Conflict of interest
The authors declare no conflicts of interest.
Consent to participate
Informed consent was obtained from all participants included in the study.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Ling Li and Fei Kong contributed equally to the work.
Contributor Information
Ling Li, Email: lingl510@126.com.
Mingzhi Zhang, Email: mingzhi_zhang@126.com.
References
- 1.Feng BJ, Huang W, Shugart YY, Lee MK, Zhang F, Xia JC, Wang HY, Huang TB, Jian SW, Huang P, Feng QS, Huang LX, Yu XJ, Li D, Chen LZ, Jia WH, Fang Y, Huang HM, Zhu JL, Liu XM, Zhao Y, Liu WQ, Deng MQ, Hu WH, Wu SX, Mo HY, Hong MF, King MC, Chen Z, Zeng YX. Genome-wide scan for familial nasopharyngeal carcinoma reveals evidence of linkage to chromosome 4. Nat Genet. 2002;31:395–399. doi: 10.1038/ng932. [DOI] [PubMed] [Google Scholar]
- 2.Xu Z, Fang S, Zuo Y, Zhang Y, Cheng R, Wang Q, Yang Z, Cai W, Ma J, Yang X, Gao G. Combination of pigment epithelium-derived factor with radiotherapy enhances the antitumor effects on nasopharyngeal carcinoma by downregulating vascular endothelial growth factor expression and angiogenesis. Cancer Sci. 2011;102:1789–1798. doi: 10.1111/j.1349-7006.2011.02013. [DOI] [PubMed] [Google Scholar]
- 3.Hwang HN (1983) Nasopharyngeal carcinoma in the People's Republic of China: incidence, treatment, and survival rates. Radiology 149:305–309. https://doi.org/10.1148/radiology.149.1.6412281 [DOI] [PubMed]
- 4.Jin T, Li B, Chen XZ. A phase II trial of Endostar combined with gemcitabine and cisplatin chemotherapy in patients with metastatic nasopharyngeal carcinoma (NCT01612286) Oncol Res. 2013;21:317–323. doi: 10.1016/j.tranon.2018.01.002. [DOI] [PubMed] [Google Scholar]
- 5.Chan OS, Ngan RK. Individualized treatment in stage IVC nasopharyngeal carcinoma. Oral Oncol. 2014;50:791–797. doi: 10.1016/j.oraloncology.2014.01.004. [DOI] [PubMed] [Google Scholar]
- 6.Brower V. Apatinib in treatment of refractory gastric cancer. Lancet Oncol. 2016;17:e137. doi: 10.1016/S1470-2045(16)00138-8. [DOI] [PubMed] [Google Scholar]
- 7.Fornaro L, Vasile E, Falcone A. Apatinib in advanced gastric cancer: A doubtful step forward. J Clin Oncol. 2016;34:3822–3823. doi: 10.1200/JCO.2016.68.6931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li J, Qin S, Xu J, Guo W, Xiong J, Bai Y, Sun G, Yang Y, Wang L, Xu N, Cheng Y, Wang Z, Zheng L, Tao M, Zhu X, Ji D, Liu X, Yu H. Apatinib for chemotherapy-refractory advanced metastatic gastric cancer: results from a randomized, placebo-controlled, parallel-arm, phase II trial. J Clin Oncol. 2013;31:3219–3225. doi: 10.1200/JCO.2013.48.8585.k. [DOI] [PubMed] [Google Scholar]
- 9.Hui EP, Chan AT, Pezzella F, Turley H, To KF, Poon TC, Zee B, Mo F, Teo PM, Huang DP, Gatter KC, Johnson PJ, Harris AL. Coexpression of hypoxia-inducible factors 1alpha and 2alpha, carbonic anhydrase IX, and vascular endothelial growth factor in nasopharyngeal carcinoma and relationship to survival. Clin Cancer Res. 2002;8:2595–2604. [PubMed] [Google Scholar]
- 10.Roy C, Addison CL, Mazzarello S, Kuchuk I, Hutton B, Clemons M (2014) Assessment of therapeutic response through clinical assessment measures. In: Vassiliou V, Chow E, Kardamakis D (eds) bone metastases. Cancer metastasis - biology and treatment, vol. 21. Springer, Dordrecht
- 11.Sclanders B, Serednicka K, Riddell AM. Measuring tumour response: RECIST 1.1 and beyond. Imaging. 2013;22:20110083. doi: 10.1259/imaging.20110083. [DOI] [Google Scholar]
- 12.Schoen MW, Basch E, Hudson LL, Chung AE, Mendoza TR, Mitchell SA, St Germain D, Baumgartner P, Sit L, Rogak LJ, Shouery M, Shalley E, Reeve BB, Fawzy MR, Bhavsar NA, Cleeland C, Schrag D, Dueck AC, Abernethy AP. Software for administering the National Cancer Institute’s Patient-Reported Outcomes Version of the Common Terminology Criteria for Adverse Events: Usability study. JMIR Hum Factors. 2018;5:e10070. doi: 10.2196/10070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Au E, Ang PT. A phase II trial of 5-fluorouracil and cisplatinum in recurrent or metastatic nasopharyngeal carcinoma. Ann Oncol. 1994;5:87–88. doi: 10.1093/oxfordjournals.annonc.a058703. [DOI] [PubMed] [Google Scholar]
- 14.Gao Y, Huang HQ, Bai B, Cai QC, Wang XX, Cai QQ. Treatment outcome of docetaxel, capecitabine and cisplatin regimen for patients with refractory and relapsed nasopharyngeal carcinoma who failed previous platinum-based chemotherapy. Expert Opin Pharmacother. 2014;15:163–171. doi: 10.1517/14656566.2014.866652. [DOI] [PubMed] [Google Scholar]
- 15.Wang TL, Tan YO. Cisplatin and 5-fluorouracil continuous infusion for metastatic nasopharyngeal carcinoma. Ann Acad Med Singap. 1991;20:601–603. [PubMed] [Google Scholar]
- 16.Chua DTT, Sham JST, Au GKH. Induction chemotherapy with cisplatin and gemcitabine followed by reirradiation for locally recurrent nasopharyngeal carcinoma. Am J Clin Oncol. 2005;28:464–471. doi: 10.1097/01.coc.0000180389.86104.68. [DOI] [PubMed] [Google Scholar]
- 17.Peng PJ, Ou XQ, Chen ZB, Liao H, Peng YL, Wang SY, Zhang HY, Lin Z. Multicenter phase II study of capecitabine combined with nedaplatin for recurrent and metastatic nasopharyngeal carcinoma patients after failure of cisplatin-based chemotherapy. Cancer Chemother Pharmacol. 2013;72:323–328. doi: 10.1007/s00280-013-2203-0. [DOI] [PubMed] [Google Scholar]
- 18.Wang CC, Chang JY, Liu TW, Lin CY, Yu YC, Hong RL. Phase II study of gemcitabine plus vinorelbine in the treatment of cisplatin-resistant nasopharyngeal carcinoma. Head Neck. 2006;28:74–80. doi: 10.1002/hed.20310. [DOI] [PubMed] [Google Scholar]
- 19.Hsu C, Lee SH, Ejadi S, Even C, Cohen RB, le Tourneau C, Mehnert JM, Algazi A, van Brummelen E, Saraf S, Thanigaimani P, Cheng JD, Hansen AR. Safety and antitumor activity of pembrolizumab in patients with programmed death-ligand 1-positive nasopharyngeal carcinoma: Results of the KEYNOTE-028 study. J Clin Oncol. 2017;35:4050–4056. doi: 10.1200/JCO.2017.73.3675. [DOI] [PubMed] [Google Scholar]
- 20.Ma BBY, Lim WT, Goh BC, Hui EP, Lo KW, Pettinger A, Foster NR, Riess JW, Agulnik M, Chang AYC, Chopra A, Kish JA, Chung CH, Adkins DR, Cullen KJ, Gitlitz BJ, Lim DW, To KF, Chan KCA, Lo YMD, King AD, Erlichman C, Yin J, Costello BA, Chan ATC. Antitumor activity of nivolumab in recurrent and metastatic nasopharyngeal carcinoma: An international, multicenter study of the Mayo Clinic Phase 2 Consortium (NCI-9742) J Clin Oncol. 2018;36:1412–1418. doi: 10.1200/JCO.2017.77.0388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hui EP, Ma BBY, Loong HHF, Mo F, Li L, King AD, Wang K, Ahuja AT, Chan CML, Hui CWC, Wong CH, Chan ATC. Efficacy, safety and pharmacokinetics of axitinib in nasopharyngeal carcinoma: a preclinical and phase 2 correlative study. Clin Cancer Res. 2018;24:1030–1037. doi: 10.1158/1078-0432.CCR-17-1667. [DOI] [PubMed] [Google Scholar]
- 22.Smith C, Lee V, Schuessler A, et al. Pre-emptive and therapeutic adoptive immunotherapy for nasopharyngeal carcinoma: Phenotype and effector function of T cells impact on clinical response. Oncoimmunology. 2017;6:e1273311. doi: 10.1080/2162402X.2016.1273311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kang Y, Ryu M, Park SR et al (2016) A phase II study of apatinib, a highly selective inhibitor of VEGFR-2, in patients with metastatic solid tumors without standard treatment options. Ann Oncol 27(suppl_6)
- 24.Sun D, Hou H, Zhang C, Zhang X. The efficacy and safety of apatinib for refractory malignancies: a review and meta-analysis. Onco Targets Ther. 2018;11:6539–6554. doi: 10.2147/OTT.S176429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhao D, Hou H, Zhang X. Progress in the treatment of solid tumors with apatinib: a systematic review. Onco Targets Ther. 2018;11:4137–4147. doi: 10.2147/OTT.S172305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lin Y, Wu Z, Zhang J, et al. Apatinib for metastatic breast cancer in non-clinical trial setting: Satisfying efficacy regardless of previous anti-angiogenic treatment. Tumor Biol. 2017;39:101042831771103. doi: 10.1177/1010428317711033. [DOI] [PubMed] [Google Scholar]
- 27.Zhang Y, Han C, Li J, Zhang L, Wang L, Ye S, Hu Y, Bai L. Efficacy and safety for Apatinib treatment in advanced gastric cancer: a real world study. Sci Rep. 2017;7:13208. doi: 10.1038/s41598-017-13192-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.McFee RM, Artac RA, McFee RM, Clopton DT, Smith RA, Rozell TG, Cupp AS. Inhibition of vascular endothelial growth factor receptor signal transduction blocks follicle progression but does not necessarily disrupt vascular development in perinatal rat ovaries. Biol Reprod. 2009;81:966–977. doi: 10.1095/biolreprod.109.078071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lim WT, Ng QS, Ivy P, Leong SS, Singh O, Chowbay B, Gao F, Thng CH, Goh BC, Tan DS, Koh TS, Toh CK, Tan EH. A phase II study of pazopanib in Asian patients with recurrent/metastatic nasopharyngeal carcinoma. Clin Cancer Res. 2011;17:5481–5489. doi: 10.1158/1078-0432.CCR-10-3409. [DOI] [PubMed] [Google Scholar]
- 30.Chi KH, Myers JN, Chow KC, Chan WK, Tsang YW, Chao Y, Yen SH, Lotze MT. Phase II trial of systemic recombinant interleukin-2 in the treatment of refractory nasopharyngeal carcinoma. Oncology. 2001;60:110–115. doi: 10.1159/000055306. [DOI] [PubMed] [Google Scholar]