Abstract
We assessed the accuracy of semi-automated tumor volume maps of plexiform neurofibroma (PN) generated by a deep neural network, compared to manual segmentation using diffusion weighted imaging (DWI) data. NF1 Patients were recruited from a phase II clinical trial for the treatment of PN. Multiple b-value DWI was imaged over the largest PN. All DWI datasets were registered and intensity normalized prior to segmentation with a multi-spectral neural network classifier (MSNN). Manual volumes of PN were performed on 3D-T2 images registered to diffusion images and compared to MSNN volumes with the Sørensen-Dice coefficient. Intravoxel incoherent motion (IVIM) parameters were calculated from resulting volumes. 35 MRI scans were included from 14 subjects. Sørensen-Dice coefficient between the semi-automated and manual segmentation was 0.77 ± 0.016. Perfusion fraction (f) was significantly higher for tumor versus normal tissue (0.47 ± 0.42 vs. 0.30 ± 0.22, p = 0.02), similarly, true diffusion (D) was significantly higher for PN tumor versus normal (0.0018 ± 0.0003 vs. 0.0012 ± 0.0002, p < 0.0001). By contrast, the pseudodiffusion coefficient (D*) was significantly lower for PN tumor versus normal (0.024 ± 0.01 vs. 0.031 ± 0.005, p < 0.0001). Volumes generated by a neural network from multiple diffusion data on PNs demonstrated good correlation with manual volumes. IVIM analysis of multiple b-value diffusion data demonstrates significant differences between PN and normal tissue.
Subject terms: Diagnostic markers, Computational biology and bioinformatics, Peripheral neuropathies, Cancer, Cancer imaging
Introduction
Neurofibromatosis type 1 (NF1) is a common genetic neurocutaneous disease, affecting 1 in 3000 newborn infants1. There are over 1000 identified mutations of the NF1 gene which produces a tumor suppressor protein called neurofibromin2. A prototypical NF1 tumor is the plexiform neurofibroma (PN), a neurofibroma variant with tumor cells that spread along multiple nerve fascicles, resulting in an extensive mass of thickened nerve bundles in a proteinaceous matrix. These PNs affect 25–50% of NF1 patients and can occur anywhere there are nerve fibers leading to significant morbidity and mortality depending on the size and location adjacent to vital structures3. Approximately 10% of PNs can transform to become malignant peripheral nerve sheath tumors over the lifetime of the patient3. Currently, surgical resection is the only available therapy, but is infrequently curative due to extensive invasion, making complete resection difficult3. No effective medical therapy currently exists; however, clinical trials are underway to evaluate chemotherapy which shrink tumor or limit growth.
Therefore, accurate measurements of tumor burden are necessary to assess for tumor response. Two dimensional measurements are limited in assessing treatment change because of the common irregularity and extensiveness of PNs. Considerable intra- and interobserver variation has also been demonstrated in two- or three-dimensional manual measurements4. Furthermore, in cases with large, extensive PNs throughout the entire body, user fatigue can result in measurement errors5. Ideally, automated segmentation of PNs from normal tissue with limited user interaction would decrease intra- and interobserver variability as well as user fatigue. The purpose of this project is to develop a neural network deep learning algorithm to perform semi-automated volume segmentation of PNs based on multiple b-value diffusion weighted MRI and to assess the accuracy of the tumor volume maps by comparing to manual segmentation. Post-segmentation, intravoxel incoherent motion (IVIM) parameters generated from the multiple b-value modeling of data are also compared between PN and background normal tissue.
Methods
The Indiana University Institutional Review Board approved this prospective study. All NF1 subjects participated with informed consent in a phase II clinical trial of imatinib mesylate (Imatinib) for the treatment of PNs6. Inclusion criteria were NF1 patients with at least one plexiform neurofibroma greater than 1 cm in a single dimension, an MRI scan with multiple b-value diffusion weighted imaging (DWI) at baseline, and at least one follow-up MRI at 6-month intervals. All MRI sequences were performed on a 1.5 T scanner (Magnetom Avanto, Siemens Healthineers, Erlangen, Germany) and included 3D T2-weighted spectral attenuated inversion recovery (SPAIR) sequences in the coronal plane (TR = 2200, TE = 200, 1 mm slice thickness, 0 mm gap, FA = 120°, matrix = 448 × 450) as well as diffusion weighted sequences in the axial or sagittal plane with multiple b-values (b = 0, 50, 150, 200, 400, 600, TR = 2137, TE = 68.4, ST = 5 mm, gap = 6.5 mm, FA = 90°) focused on the largest PN. Sagittal or axial orientation was chosen on diffusion weighted sequences for maximum in-plane coverage of the largest PN. For the purposes of comparison to diffusion weighted datasets, modified SPAIR images were reformatted in the same plane as the diffusion dataset at matching 5 mm slice thickness and truncated to match the diffusion anatomical coverage.
Neural network machine learning
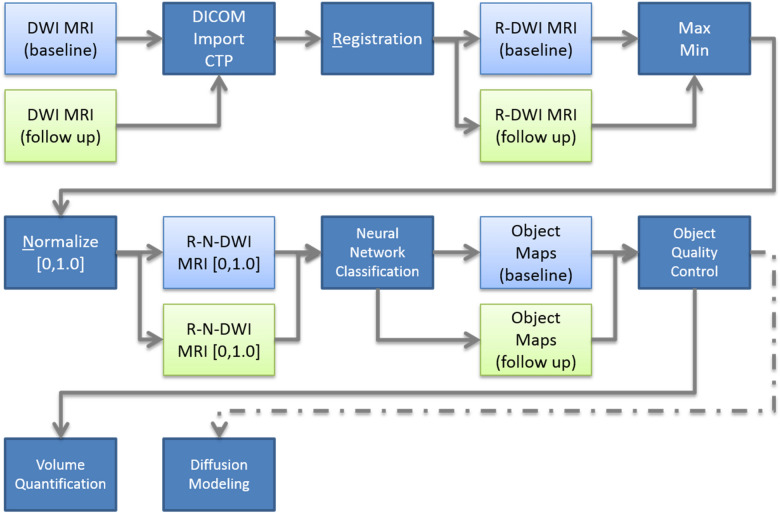
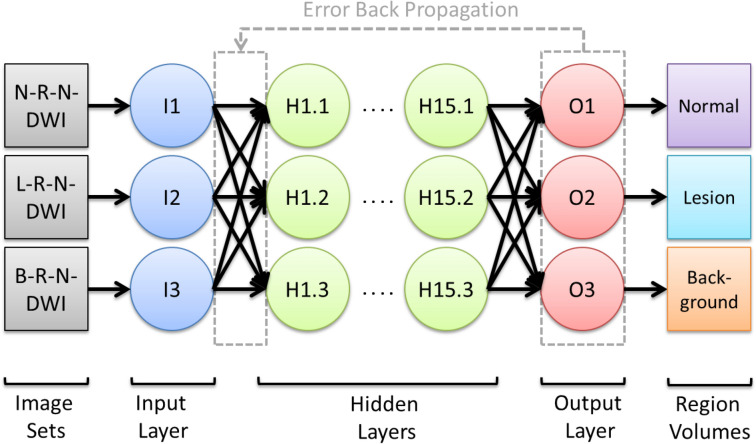
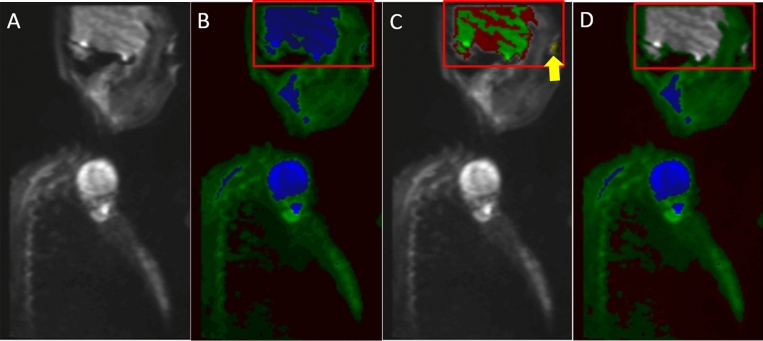
All diffusion datasets were registered to the baseline time point for each subject using the normalized entropy mutual information method described previously7. Images were intensity normalized over the interval [0.0, 1.0] by dividing each image volume by the maximum value of the B0 image set (Fig. 1). Using the multispectral neural network (MSNN) modeling tool available in Analyze 12.0 (AnalyzeDirect, Overland Park, KS)8, model priors for each “class” were constructed by defining regions of interest for normal (i.e. non-tumor) tissue, NF1 lesions, and “background” (i.e. remainder) that were extracted across all registered normalized multi-b value (0, 50, 100, 150, 200, 400, 600) DWI images. The selection of hidden layers was based on an empirical analysis of the model, where the minimum probability was fixed at 0.1, alpha probability was set to 0.25, and epochs set at 200 according to the guidance from the manufacturer. Under these conditions, the number of hidden layers was varied systematically from 3 to 20 layers and the degree of segmentation assessed for over classification. Once the number of layers were selected, the number of epochs were systematically varied from 100 to 1000, keeping the number of hidden layers, minimum probability, and alpha probability constant. Once optimized, the multi-b value voxel series for each class were then applied to a feed-forward neural network consisting of 15 hidden layers each having 3 nodes (Appendix Fig. 8), for training purposes, with 200 epochs. In addition, the recommended minimum probability of 0.1 and an alpha probability of 0.25 were used for voxel classification. The minimum probability represents the likelihood that any given voxel belongs to a defined class; voxels with values less than this probability are left unclassified. Similarly, the alpha probability is a confidence-weighting parameter which controls the strength of the relaxation during iterative optimization. Due to design limitations of the MSNN tool, only a single training set could be defined as part of the model priors; therefore, we randomly selected an NF1 subject from the population to serve as the training set for all subsequent segmentation, and was excluded from the remaining analysis. Post-training, classification of the remaining normalized multi-b value images for each subject were then performed generating 3D object maps for each region in the image series. Each resulting volume map identified as tumor by the neural network was then reviewed and manually edited for obvious erroneous anatomy by a trained imaging scientist (SP) which commonly included bladder, brain, eyes, and spinal fluid (Fig. 2). These misclassified anatomical structures were excluded from the tumor volume.
Figure 1.
Image processing workflow for Multi-Spectral Neural Network classification. DWI MRI image series from baseline and follow up scans are imported into the Clinical Trials Processor for de-identification and then registered using the normalized entropy mutual information method described previously7. Post registration, maximum and minimum intensities were computed, images intensity normalized over the interval [0.0, 1.0], and images were segmented using the MSNN method. Object maps were then individually inspected for classification errors, cleaned (if required), and then submitted to volumetric and IVIM diffusion modeling.
Figure 8.
Multi-Spectral Neural Network (MSNN) Classifier Diagram. Normalized Diffusion Weighted Images (DWI) b-Value (0, 50, 100, 150, 200, 400, 600) serve as inputs to the model, where nodal weights are applied to the hidden and output layers to yield output voxels which are classified as normal, tumor, or background once the model is trained. In all cases, training sets represented a random NF1 subject from the population which has been manually segmented by a trained expert. In the input layer N-, L-, and B-prefixes represent the Normal, Lesion, and Background inputs, respectively, while R-N in each set refers to Registered-Normalized images.
Figure 2.
Representative normalized sagittal images of cervical DWI MRI: (A) b = 0 DWI image (unclassified); (B) Initial Classified Object Map based on the MSNN model described in Fig. 1; normal tissue (green) and tumor (blue); (C) Misclassified structures such as Brain (red) and Orbits (yellow arrow) which require exclusion from tumor volume; and (D) Quality control corrected normal (green) and tumor (blue).
Manual segmentation analysis
As a comparison to manual segmentation, a neuroradiology fellow (JK) manually traced all plexiform neurofibromas on the registered SPAIR images in consensus with the senior neuroradiologist (CH). Measurements were made on a commercially available image analysis software (MIM Encore 6.9.3, MIM Software INC, Cleveland, OH, https://www.mimsoftware.com/nuclear_medicine/encore) using a freehand ROI tool. The volumes of the manual measurements were summated for each MRI exam and used for analysis. Bland–Altman plots were performed between the MSNN volume results and corresponding manual segmentation results. Linear regression and Sørensen–Dice coefficient was used to analyze correlation and concordance between manual tumor volumes and neural network results.
IVIM analysis
Neural network generated volume maps were also used to generate average b-value series for IVIM analysis according to the bi-exponential model proposed by Le Bihan et al.9:
| 1 |
where S, b, f, D*, and D are the raw signal, b-value, perfusion fraction, pseudo-diffusion (mixed diffusion) coefficient (sec/mm2), and true molecular diffusion (slow diffusion) coefficient (sec/mm2), respectively. Calculation of IVIM parameters were performed on a molecular kinetic modeling software (eLucidate, Paul R. Territo, Ph.D, Indiana University, Indianapolis, USA), where the uniformly weighted sum-of squares difference (SSD) between the objective function (Eq. 1) and normalized b-value series was optimized via Simulated Annealing (relative SSD, tolerance 1e−15). IVIM parameters were compared across available scanning time points at baseline, 6 months and 12 months, between PN tumors and normal structures excluding the bladder, brain, eyes and spinal fluid. Significant differences of IVIM parameters were assessed between all PN tumor and normal tissue using a two-tailed paired T-test, while a One-way ANOVA was used to assess for significant changes in IVIM parameters for PNs between baseline, 6-month and 12-month scans. In all cases, significance was taken at the p ≤ 0.05 level.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Written informed consent was obtained from all individual participants included in the study.
Results
A total of 35 MRI scans were included from 14 subjects (mean age 22.6 years; range 2–51 years; 11 females, 3 males). Location for the largest PN included: head and neck (9 subjects), chest (3 subjects), abdomen and pelvis (1 subject), and right thigh (1 subject).
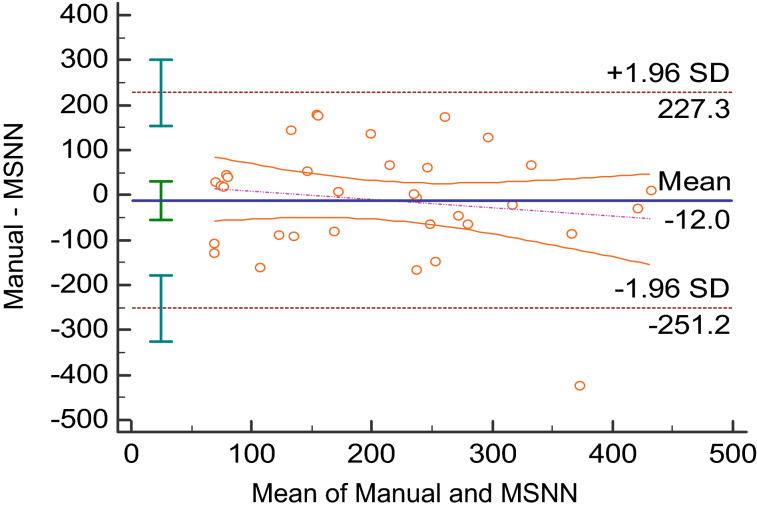
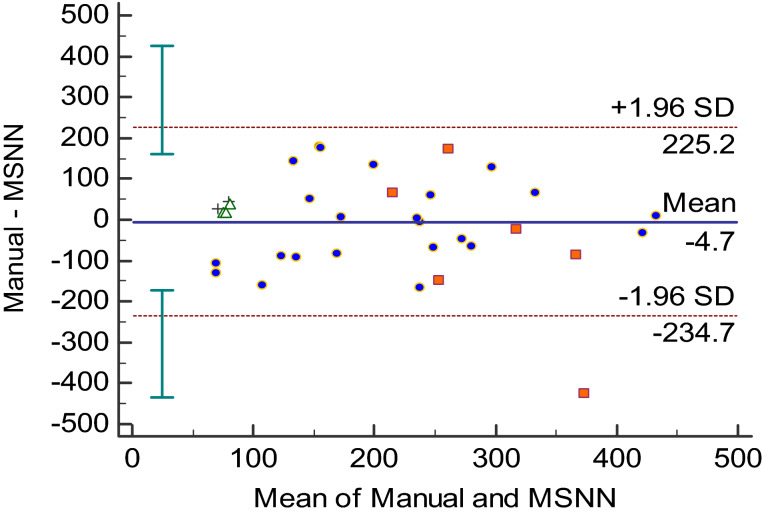
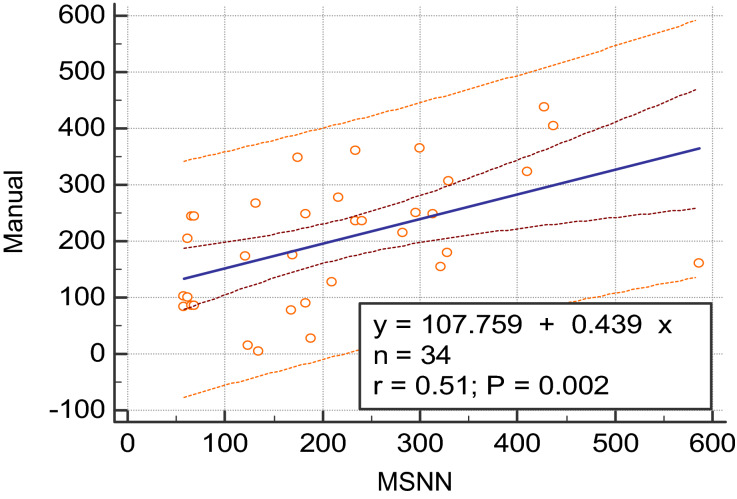
On average, a neural network case including manual definition of tumor classes with removal of obvious normal anatomy took 6 min, while manually measuring the PNs took an average of 18 min. Bland–Altman plots of the MSNN versus manual segmentation results are also shown in Figs. 3 and 4. Raw values for the Bland Altman plots are given in Table 1. Linear regression (Fig. 5) showed significant correlation (r = 0.51, p = 0.002). The Sørensen–Dice coefficient was 0.77 ± 0.016 (± SEM, range 0.54–0.96).
Figure 3.
Bland–Altman analysis of MSNN and manual segmentation volumes in milliliters. The purple dot and dash line represents the linear regression of the differences with 95% confidence interval lines.
Figure 4.
Bland–Altman analysis between MSNN and manual segmentation volumes in milliliters. Each data point represents an anatomic region for the measured PNs. Cross = abdomen/ pelvis, triangle = thigh, circle = head/neck and square = chest.
Table 1.
Individual subject volumes in cubic millimeters for the manual and MSNN segmentation methods.
| MRI scan (#) | Manual (mm3) | MSNN (mm3) | Average (mm3) | Difference (mm3) | % Difference | Region |
|---|---|---|---|---|---|---|
| 1 | 84,116 | 57,239 | 70,678 | 26,877 | 38 | Abd/Pelvis |
| 2 | 102,200 | 57,267 | 79,734 | 44,933 | 56 | Abd/Pelvis |
| 3 | 172,495 | 120,543 | 146,519 | 51,952 | 35 | Head/Neck |
| 4 | 175,431 | 169,303 | 172,367 | 6127 | 4 | Head/Neck |
| 5 | 100,013 | 62,270 | 81,142 | 37,743 | 47 | Thigh |
| 6 | 85,172 | 65,845 | 75,508 | 19,327 | 26 | Thigh |
| 7 | 85,172 | 68,423 | 76,798 | 16,748 | 22 | Thigh |
| 8 | 204,618 | 62,270 | 133,444 | 142,348 | 107 | Head/Neck |
| 9 | 242,986 | 65,845 | 154,416 | 177,141 | 115 | Head/Neck |
| 10 | 242,827 | 68,423 | 155,625 | 174,403 | 112 | Head/Neck |
| 11 | 365,620 | 299,650 | 332,635 | 65,970 | 20 | Head/Neck |
| 12 | 405,175 | 437,408 | 421,292 | − 32,232 | − 8 | Head/Neck |
| 13 | 437,522 | 427,553 | 432,538 | 9969 | 2 | Head/Neck |
| 14 | 305,228 | 328,925 | 317,077 | − 23,697 | − 7 | Chest |
| 15 | 323,213 | 410,551 | 366,882 | − 87,339 | − 24 | Chest |
| 16 | 77,969 | 168,348 | 123,159 | − 90,379 | − 73 | Head/Neck |
| 17 | 89,170 | 182,879 | 136,024 | − 93,709 | − 69 | Head/Neck |
| 18 | 27,533 | 188,696 | 108,114 | − 161,162 | − 149 | Head/Neck |
| 19 | 276,930 | 216,959 | 246,944 | 59,971 | 24 | Head/Neck |
| 20 | 234,409 | 240,454 | 237,432 | − 6045 | − 3 | Head/Neck |
| 21 | 236,166 | 234,250 | 235,208 | 1916 | 1 | Head/Neck |
| 22 | 178,562 | 328,011 | 253,287 | − 149,449 | − 59 | Chest |
| 23 | 160,167 | 586,325 | 373,246 | − 426,159 | − 114 | Chest |
| 24 | 266,998 | 132,493 | 199,746 | 134,504 | 67 | Head/Neck |
| 25 | 360,918 | 234,274 | 297,596 | 126,643 | 43 | Head/Neck |
| 26 | 347,271 | 175,144 | 261,207 | 172,127 | 66 | Chest |
| 27 | 247,957 | 183,138 | 215,547 | 64,820 | 30 | chest |
| 28 | 215,193 | 282,491 | 248,842 | − 67,298 | − 27 | Head/Neck |
| 29 | 247,391 | 314,026 | 280,709 | − 66,635 | − 24 | Head/Neck |
| 30 | 249,562 | 296,156 | 272,859 | − 46,594 | − 17 | Head/Neck |
| 31 | 127,238 | 210,280 | 168,759 | − 83,043 | − 49 | Head/Neck |
| 32 | 154,225 | 322,093 | 238,159 | − 167,868 | − 70 | Head/Neck |
| 33 | 84,116 | 57,239 | 70,678 | 26,877 | 38 | Abd/Pelvis |
| 34 | 4138 | 133,978 | 69,058 | − 129,840 | − 188 | Head/Neck |
| 35 | 14,717 | 123,338 | 69,027 | − 108,621 | − 157 | Head/Neck |
In addition, the average, difference and percent difference between the segmentation methods used in the Bland–Altman analysis are provided as well for comparison.
Figure 5.
Linear regression analysis of manual versus MSNN volumes with trend line and 95% confidence intervals.
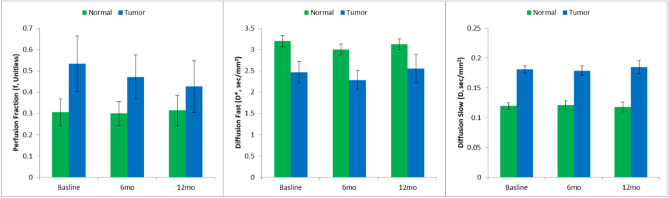
All IVIM modeling showed a very high degree of fit across the three time points studied for normal (non-tumor; R = 0.999 ± 0.0001, 0.999 ± 0.0002, 0.998 ± 0.0002) and PN tumors (R2 = 0.999 ± 0.0001, 0.999 ± 0.0004, 0.998 ± 0.0005). Moreover, there was a significant difference for all IVIM parameters between normal tissue and PN tumors. Perfusion fraction (i.e. f) was significantly higher for tumor versus normal tissue (0.47 ± 0.42 vs. 0.30 ± 0.22, p = 0.02), similarly, D was significantly higher for PN tumor versus normal tissue (0.0018 ± 0.0003 vs. 0.0012 ± 0.0002, p < 0.0001). By contrast, D* was significantly lower for PN tumor versus normal tissue (0.024 ± 0.01 vs. 0.031 ± 0.005, p < 0.0001). Despite the differences in tissue IVIM parameters, there was no significant change from Imatinib treatment baseline (pre-treatment) when comparing tissues through time (Fig. 6).
Figure 6.
Results for IVIM Kinetic Modeling: Perfusion Fraction (f, unitless); Diffusion Fast (D*, s/mm2); Diffusion Slow (D, s/mm2) for baseline, 6 and 12 months post Imatinib treatment in normal tissue and NF1 tumors.
Discussion
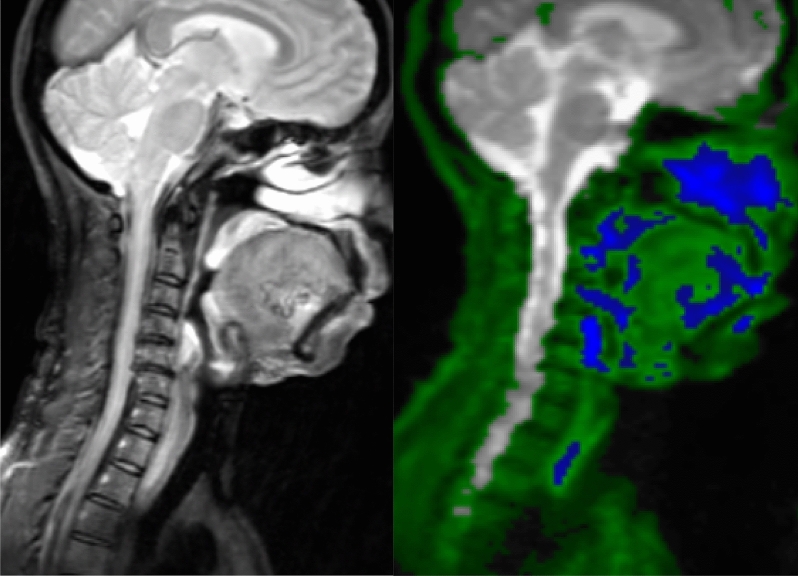
We evaluated the accuracy of semi-automated tumor volumes generated by a deep neural network based on diffusion weighted imaging with multiple b-values. The semi-automated volumes had a 77% concordance rate from Sørensen–Dice analysis compared to manually measured volumes. The Bland Altman plot by scan anatomy showed that discordance was greatest in several of the head and neck and chest cases. This is likely caused by PNs which were in close proximity with the lungs, skull base, and paranasal sinuses, locations highly prone to susceptibility artifact on echo planar imaging (Fig. 7). Although we were able to achieve a good concordance rate with Sørensen–Dice analysis, the inclusion of these areas likely prevented even higher concordance. Similarly, spinal and paraspinal tumors may also be affected by artifact on echo planar imaging, however, several neck and abdomen/pelvis exams had paraspinal location tumors which had better concordance.
Figure 7.
51-year-old female with NF1. (A) Sagittal SPAIR image of the head and neck. (B) Sagittal neural network map shows erroneous identification of nasal mucosa, palatine tonsils and airway as tumor tissue (blue).
Previous semi-automated segmentation of PNs focused on signal intensity from STIR images with either a signal intensity or histogram-based algorithm to identify tumor borders with or without additional spatial connectivity algorithms. Poussaint et al.10 used a commercially available image analysis software which requires a user to click on the center of a lesion and then drag until the ROI encompasses the whole lesion. The software is able to compute the edge based on best-guess of the lesion border where signal intensity values are expected to change rapidly; however, in cases where the intensity gradient is low, the edge detection will fail resulting in inaccurate tumor volume10. Moreover, since this approach is slice based, it requires repeated application of the seed value for every slice. Finally, the user must then edit the borders with a separate tool. Combined, this approach is both labor intensive and subject to human error, and in has limited use in a clinical setting. Solomon et al.11 used a histogram matching method that requires initial user ROI placement on a tumor for each slice, which again is labor intensive. To overcome these limitations, Cai et al.12 used a dynamic threshold level set technique which requires users to identify each lesion which then automatically expand in three-dimensional space based on lesion versus background histograms. This approach, which overcomes the slice limitations of previous investigators, still requires that a trained expert identify each tumor in the image volume. Finally, Weizman et al.13 also uses a STIR histogram matching algorithm which requires a one-time training requiring manual volumetric analysis of a tumor and identifying of a small frame including most of the tumor and a large frame which includes tumor and background. In the segmentation phase, the user is required to scribble over the tumor, which can then propagate in 3D space; however, like all the described methods, manual error correction of STIR hyper-intense normal structures adjacent to tumor is required.
Comparatively, our method using a deep learning neural network does require a single training phase but afterwards does not require user input in identifying tumors. However, manual user correction of normal anatomy is required, particularly for head and neck cases as brain, eyes and spinal fluid were consistently erroneously classified as tumor despite the training phase (Fig. 2). Diffusion characteristics for these normal structures were too similar to PNs to be properly classified as normal background anatomy. The time necessary for a trained imaging scientist to process the images and delete normal anatomy took on average 6 min compared to an average of 18 min for manual volumetric analysis. Furthermore, Weizman et al. determined that for complex cases, up to 35 min per case would be required for manual segmentation13. If one expands this to the typical case load encountered by radiologists at our institution, this amounts to 0.007–0.014 FTE, or $3500–7000/case in annualized cost savings.
Previous works on this topic measured inter-rater volume concordance and repeatability for method assessment. We were not able to perform repeatability because of the lack of user input in the creation of tumor volumes. Once trained, the neural network will generate identical volume maps for the same case, leading to perfect repeatability and concordance for the same case. We did not assess inter-rater manual concordance as measuring large PN volumes in our datasets would likely lead to greater discordance from user fatigue5,14. Ultimately no true gold standard exists unless manual volume measurements were performed by a trained expert resistant to fatigue. In clinical trials, change in the volume of tumor burden may be more helpful than accurate tumor volumes, provided consistent inclusion of false positive volumes. Although we did have follow-up MRI scans, these were not performed with the same exact tumor coverage or at the same time and had 6-month intervals with ongoing chemotherapy which would limit a test for method precision for different scans on the same subject.
An ideal gold standard in PN segmentation for clinical trials would require accuracy and precision in addition to a minimal amount of user input to lessen user fatigue. With advances in processor technology and big data, computer deep learning has been applied to various categories of medical imaging including anatomy identification and segmentation15, as well as computer aided detection and diagnosis16. Our study is the first and only to our knowledge to apply deep learning for semi-automated volume segmentation for PNs in the NF1 population using multiple b-value DWI data. Further investigation in limiting the false positive volumes identified may be accomplished using spin echo-based diffusion techniques without susceptibility distortion. Other quantitative data may also be helpful such as signal intensity, histogram analysis, perfusion or a combined multi-parametric approach for deep learning as have been used for other oncology applications17.
Our study is also the first to our knowledge in the literature to assess for IVIM parameters on PN tumors. We were not able to detect treatment effects with imatinib mesylate in IVIM parameters of PN across the timepoints of baseline, 6 month and 12 months after treatment, which suggests that this chemotherapeutic agent may not influence tissue diffusion at the time points studied. Again, a comparison with volumetric analysis using the neural network data was not possible due to lack of consistent inclusion of anatomy on diffusion sequence acquisition. However, we did detect significant differences for all IVIM parameters compared with normal (non-tumor) tissue after removal of brain, bladder, eyes, and spinal fluid from the object maps. Normal background tissue was primarily connective tissue, muscle, bone, solid organs and bowel. Interestingly, PN tumor had significantly higher f, but lower D* compared to normal tissues. The f correlates with blood volume and the D* theoretically correlates with blood flow18, suggesting that PNs have a higher total blood volume but slower overall blood flow19. These data are consistent with pathological reports of biopsy samples taken from PN subjects when stained for smooth muscle actin, with diffuse plexiform neurofibromas demonstrating the highest vessel density compared to other subtypes of neurofibromas20. Although the qualitative detection of PNs compared to background normal tissue is not usually difficult for a trained expert on STIR images, the ability of IVIM to quantitatively differentiate PNs from background tissue may be an alternative approach to aid the neuroradiologist in determining optimal treatment planning and response to therapy.
Finally, the current approach has some limitations. The MSNN model implementation is fixed, limiting its extension to more advanced modeling approaches. For instance, convolutional neural networks have been developed and used successfully for image segmentation21. In addition, the current established tool also fixes the training model to a single set of images (i.e. one multi-b value DWI set), preventing additional models and thus limits accuracy. Since the current tool limits the number of model training sets to a single series, one approach may be to perform iterative random selection of the subject to serve as the training set, and then repeat the analysis to evaluate biases that result from the model (i.e. “jack-knifing”). In addition, the MSNN tool does not permit selection of alternative activation functions (i.e. sigmoid, ELU, ReLU, leaky ReLU, tanh, etc.), or the ability to control the level of connections and/or recursions across layers, which limits the broad application of this tool for complex imaging studies. Despite these limitations, the current tool was chosen based on its broad availability, computational efficiency, ease of use for establishing training and analysis sets, and ability to translate to clinical research operations. Based on the initial promising results, these studies can then serve as the basis for custom software tools that overcome the aforementioned limitations. These factors, along with the reasonable high accuracy, suggest that this approach has merit and warrants additional investigation as a time saving tool in clinical practice.
Conclusion
IVIM parameters can significantly differentiate PNs from normal background tissue. Deep learning of multiple b-value DWI data from a neural network successfully generated volume maps in a semi-automated process. When compared with manual measurements, the semiautomated volumes had a Sørensen–Dice coefficient of 77%. However, PNs in anatomy typically affected by susceptibility artifact within the head, neck and chest limit greater concordance and further work is needed to develop this technology into a routine clinical tool.
Abbreviations
- PN
Plexiform neurofibroma
- NF1
Neurofibromatosis type 1
- MSNN
Multispectral neural network
- IVIM
Intravoxel incoherent motion
Appendix
See Fig. 8 .
Author contributions
C.H. wrote the main and revised manuscript text, performed project design, and manual segmentation. J.K. performed manual segmentation and manuscript review. S.P. performed data quality control, segmentation, and data keeping. S.K. performed manuscript review and editing. K.R. performed patient recruitment, clinical data acquisition and trial design with manuscript review. P.T. performed project design, neural network analysis, manuscript review and revision.
Funding
A portion of this work was supported by a grant from the Department of Defense: “Pilot Study of Gleevec/ Imatinib Mesylate (STI-571, NSC 716051) in Neurofibromatosis (NF1) Patients with Plexiform Neurofibromas”.
Competing interests
Kent Robertson is the recipient of the funding from the Department of Defense. Chang Ho, John Kindler, Scott Persohn, Stephen Kralik and Paul Territo have no conflicts of interest.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Evans DG, et al. Birth incidence and prevalence of tumor-prone syndromes: estimates from a UK family genetic register service. Am. J. Med. Genet. A. 2010;152A:327. doi: 10.1002/ajmg.a.33139. [DOI] [PubMed] [Google Scholar]
- 2.Jett K, Friedman JM. Clinical and genetic aspects of neurofibromatosis 1. Genet. Med. 2010;12:1–11. doi: 10.1097/GIM.0b013e3181bf15e3. [DOI] [PubMed] [Google Scholar]
- 3.Prada CE, et al. Pediatric plexiform neurofibromas: Impact on morbidity and mortality in neurofibromatosis type 1. J. Pediatr. 2012;160:461–467. doi: 10.1016/j.jpeds.2011.08.051. [DOI] [PubMed] [Google Scholar]
- 4.Harris GJ, et al. Three-dimensional volumetrics for tracking vestibular schwannoma growth in neurofibromatosis type II. Neurosurgery. 2008;62:1314–1319. doi: 10.1227/01.neu.0000333303.79931.83. [DOI] [PubMed] [Google Scholar]
- 5.Stec N, Arje D, Moody AR, Krupinski EA, Tyrrell PN. A systematic review of fatigue in radiology: Is it a problem? AJR Am. J. Roentgenol. 2018;210:799–806. doi: 10.2214/AJR.17.18613. [DOI] [PubMed] [Google Scholar]
- 6.Robertson KA, et al. Imatinib mesylate for plexiform neurofibromas in patients with neurofibromatosis type 1: A phase 2 trial. Lancet Oncol. 2012;13:1218–1224. doi: 10.1016/S1470-2045(12)70414-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Studholme, C., Hawkes, D.J., Hill, D. Normalized entropy measure for multimodality image alignment in Proceedings of the International Society for Optics and Photonics Volume 3338, Medical Imaging: Image Processing (1998).
- 8.Kramer AH, Sangiovanni-Vincentelli A. Efficient parallel learning algorithms for neural networks. In: Touretzky DS, editor. Advances in Neural Information Processing Systems 1. London: Morgan Kaufmann Publishers; 1989. pp. 40–48. [Google Scholar]
- 9.Le Bihan D, et al. MR imaging of intravoxel incoherent motions: Application to diffusion and perfusion in neurologic disorders. Radiology. 1986;161:401–407. doi: 10.1148/radiology.161.2.3763909. [DOI] [PubMed] [Google Scholar]
- 10.Poussaint TY, Jaramillo D, Chang Y, Korf B. Interobserver reproducibility of volumetric MR imaging measurements of plexiform neurofibromas. AJR Am. J. Roentgenol. 2003;180:419–423. doi: 10.2214/ajr.180.2.1800419. [DOI] [PubMed] [Google Scholar]
- 11.Solomon J, et al. Automated detection and volume measurement of plexiform neurofibromas in neurofibromatosis 1 using magnetic resonance imaging. Comput. Med. Imaging Graph. 2004;28:257–265. doi: 10.1016/j.compmedimag.2004.03.002. [DOI] [PubMed] [Google Scholar]
- 12.Cai W, et al. Tumor burden in patients with neurofibromatosis types 1 and 2 and schwannomatosis: determination on whole-body MR images. Radiology. 2009;250:665–673. doi: 10.1148/radiol.2503080700. [DOI] [PubMed] [Google Scholar]
- 13.Weizman L, et al. PNist: interactive volumetric measurements of plexiform neurofibromas in MRI scans. Int J Comput Assist Radiol Surg. 2014;9:683–693. doi: 10.1007/s11548-013-0961-0. [DOI] [PubMed] [Google Scholar]
- 14.Waite S, et al. Tired in the reading room: The influence of fatigue in radiology. J. Am. Coll. Radiol. 2017;14:191–197. doi: 10.1016/j.jacr.2016.10.009. [DOI] [PubMed] [Google Scholar]
- 15.Akkus Z, et al. Deep learning for brain MRI segmentation: State of the art and future directions. J. Digit. Imaging. 2017;30:449–459. doi: 10.1007/s10278-017-9983-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shen D, Wu G, Suk H-I. Deep learning in medical image analysis. Annu. Rev. Biomed. Eng. 2017;19:221–248. doi: 10.1146/annurev-bioeng-071516-044442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hosny A, Parmar C, Quackenbush J, Schwartz LH, Aerts HJWL. Artificial intelligence in radiology. Nat. Rev. Cancer. 2018;18:500–510. doi: 10.1038/s41568-018-0016-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Koh DM, Collins DJ, Orton MR. Intravoxel incoherent motion in body diffusion-weighted MRI: Reality and challenges. AJR Am. J. Roentgenol. 2011;196:1351–1361. doi: 10.2214/AJR.10.5515. [DOI] [PubMed] [Google Scholar]
- 19.Wirestam R, et al. Perfusion-related parameters in intravoxel incoherent motion MR imaging compared with CBV and CBF measured by dynamic susceptibility-contrast MR technique. Acta Radiol. 2011;42:123–128. doi: 10.1080/028418501127346459. [DOI] [PubMed] [Google Scholar]
- 20.Friedrich RE, Behrendt CA, Glatzel M, Hagel C. Vascular innervation in benign neurofibromas of patients with neurofibromatosis type 1. Anticancer Res. 2015;35:6509–6516. [PubMed] [Google Scholar]
- 21.Rawat W, Wang Z. Deep convolutional neural networks for image classification: A comprehensive review. Neural Comput. 2017;29:2352–2449. doi: 10.1162/neco_a_00990. [DOI] [PubMed] [Google Scholar]