Abstract
Variability in habitat selection can lead to differences in fitness; however limited research exists on how habitat selection of mid-ranking predators can influence population-level processes in multi-predator systems. For mid-ranking, or mesopredators, differences in habitat use might have strong demographic effects because mesopredators need to simultaneously avoid apex predators and acquire prey. We studied spatially-explicit survival of cheetahs (Acinonyx jubatus) in the Mun-Ya-Wana Conservancy, South Africa, to test hypotheses related to spatial influences of predation risk, prey availability, and vegetation complexity, on mesopredator survival. For each monitored cheetah, we estimated lion encounter risk, prey density, and vegetation complexity within their home range, on short-term (seasonal) and long-term (lifetime) scales and estimated survival based on these covariates. Survival was lowest for adult cheetahs and cubs in areas with high vegetation complexity on both seasonal and lifetime scales. Additionally, cub survival was negatively related to the long-term risk of encountering a lion. We suggest that complex habitats are only beneficial to mesopredators when they are able to effectively find and hunt prey, and show that spatial drivers of survival for mesopredators can vary temporally. Collectively, our research illustrates that individual variation in mesopredator habitat use can scale-up and have population-level effects.
Subject terms: Community ecology, Population dynamics
Introduction
Understanding how individual habitat use can influence fitness can offer insight into the structure of food webs and predator–prey interactions1. Spatial and temporal variation in resources, as well as the ability of individuals to find and use these resources, can lead to differences in individual survival and reproduction, which in turn can scale up to population-level effects2,3. In predator–prey systems, environmental features that influence the predator’s ability to find or kill prey can affect the survival or reproduction of the predator4,5. On the other hand, environmental features that influence predator avoidance, predator detection, the prey’s ability to escape predators, or the prey’s ability to find food, can affect the survival or reproduction of the prey6–8. Identifying the connections between habitat use and demography is important for understanding the fitness costs and benefits of habitats6, although this understanding is lacking for systems with multiple predators.
In systems with multiple predators, the interaction between habitat use and demography could be particularly complex. Subordinate predators, or mesopredators9, need to select habitats that will allow them to obtain prey, while still avoiding predation by top predators9. In some systems, apex predators and mesopredators occur in the same general habitats, but mesopredators use fine-scale spatial or temporal partitioning to reduce encounter rates10,11. Although this partitioning might reduce short-term mortality risk for mesopredators, the longer-term risk of co-occurring with apex predators could reduce the fitness of the mesopredator through non-consumptive effects, such as reduced foraging opportunities or shifts into non-optimal habitats12. Many previous studies have investigated how apex predators can affect the abundances13–15, habitat use16,17, and behavior of mesopredators18,19, but considerably less research has focused on how mesopredator-apex predator cooccurrence can scale-up and affect mesopredator demographic rates, especially across longer-term time scales.
Additionally, habitat characteristics such as vegetation complexity can modulate the habitat-survival relationship in systems with multiple predators. Theory predicts that mesopredators will experience reduced mortality from apex predators (i.e., intraguild predation or intraspecific killing) in areas with high habitat complexity, because of lower encounter rates20. Most previous research on the role of habitat complexity on survival has been conducted in experimental systems with aquatic or insect species20,21, whereas less research has focused on wild mesopredator populations. The effects of vegetation complexity on mesopredator in wild population might be particularly complicated because of tradeoffs between hunting and protection from apex predators in different habitat types1. A greater understanding of how habitat characteristics such as vegetation complexity can influence mesopredator survival, is critical.
We studied the interaction between habitat use and mesopredator demography, using the cheetah (Acinonyx jubatus) as our focal species. Cheetahs are subordinate to lions, and the majority of cheetah mortality is from lion predation22. In addition to direct predation, lions can steal prey from cheetahs23, and can affect the habitat use and behavior of cheetahs24,25. In turn, these non-consumptive effects related to predation risk might affect the long-term survival and fitness of cheetahs. Previous research suggests that cheetahs and lions exhibit high levels of home range overlap, but that cheetah use fine-scale spatial partitioning to avoid the short-term risk of lion predation10,26,27. However, how this partitioning might indirectly affect the survival of cheetahs in the long-term is unknown. In addition, dense vegetation is hypothesized as another mechanism of lion-cheetah coexistence by acting as a predation refuge28, but limited research has focused on the linkages between vegetation and cheetah survival, particularly in the southern portion of their range29.
We investigated support for three competing hypotheses of spatial drivers on mesopredator survival: (1) mesopredator survival would be driven by the risk of encountering top predators (top-down spatial regulation), (2) mesopredator survival would be driven by prey densities (bottom-up spatial regulation), and (3) mesopredator survival would be driven by vegetation complexity (habitat complexity risk mediation hypothesis). Under the spatial top-down hypothesis we predicted that cheetah survival would be negatively related to the probability of encountering lions. Under the spatial bottom-up hypothesis we predicted that cheetah survival would be positively related to spatial prey density. Under the habitat complexity risk mediation hypothesis, we predicted that cheetah survival would be positively related to vegetation complexity. Because the strength of spatial drivers might depend on the temporal scale at which they are assessed, we tested our three hypotheses in relation to both short-term and long-term habitat use. By studying spatial influences of mesopredator demography, we can better understand factors that structure food webs with multiple predators, and in turn prioritize habitat features that promote the coexistence of multiple predator species.
Results
Short-term spatial drivers of survival
We included 133 cheetahs in our survival analyses for a total of 110 months. Within these cheetahs, 28 individuals were only in the adult state, 78 individuals were only in the cub state, and 27 individuals were included in the models as both in the cub and adult states.
Cheetah survival was most sensitive to short-term environmental conditions within the 50% home range contour and the same top model was supported regardless of home range contour (Appendix S2). Thus, we only present the results from the 50% HR models. At the short-term time scale, cheetahs exhibited variation in environmental conditions within their home range with regards to EVI (mean = 0.29; range = 0.14–0.44), lion encounter risk (mean = 0.36; range = 0.16–0.90), and prey density (mean = 37.8 prey/km2, range = 24.0–80.3 prey/km2).
For our short-term survival models, survival was best described by the average EVI within the core of a cheetah’s home range (Table 1). In contrast to our predictions under the habitat complexity risk mediation hypothesis, for both adults and cubs, EVI had a negative influence on survival, with higher survival occurring at the lowest EVI values (Fig. 1). At the lowest EVI values, adult monthly survival was 0.99 (85% CI = 0.98–1.00) and cub monthly survival was 0.98 (85% CI = 0.97–0.99), whereas at the highest EVI values, adult monthly survival was 0.97 (85% CI = 0.94–0.98) and cub monthly survival was 0.84 (85% CI = 0.75–0.91). Cub survival was more sensitive than adult survival to changes in EVI, with the probability of surviving decreasing 2.8% for every 1-unit increase in EVI.
Table 1.
Model selection results for multi-state joint live-encounter dead-recovery spatial-explicit survival models for cheetahs with seasonal spatial covariates, Mun-Ya-Wana Conservancy, KwaZulu-Natal, South Africa, 2008–2018.
| Model | AICc | ΔAICC | − 2 × ln(L)a | wb | kc |
|---|---|---|---|---|---|
| S(state:EVI) | 3601.15 | 0 | 3583.02 | 0.55 | 9 |
| S(state:lion + state:EVI) | 3603.51 | 2.36 | 3581.32 | 0.17 | 11 |
| S(state:lion * state:EVI) | 3604.06 | 2.91 | 3577.80 | 0.13 | 13 |
| S(state:prey + state:EVI) | 3604.60 | 3.45 | 3582.41 | 0.10 | 11 |
| S(state:EVI + state:lion + state:prey) | 3607.08 | 5.93 | 3580.82 | 0.03 | 13 |
| S(state:prey * state:EVI) | 3607.31 | 6.16 | 3581.05 | 0.03 | 13 |
| S(state:lion * state:prey) | 3613.35 | 12.20 | 3587.09 | 0.00 | 13 |
| S(state:prey) | 3617.96 | 16.81 | 3599.84 | 0.00 | 9 |
| S(state) | 3619.33 | 18.19 | 3603.23 | 0.00 | 8 |
| S(state:lion) | 3620.62 | 19.47 | 3602.49 | 0.00 | 9 |
| S(state:lion + state:prey) | 3621.51 | 20.36 | 3599.32 | 0.00 | 11 |
States in the model include cubs (juveniles dependent on their mothers) and adults (non-juveniles). All models include effects of year on recovery rates and season on survival rates.
aLog-likelihood.
bAkaike model weight.
cNumber of model parameters.
Figure 1.
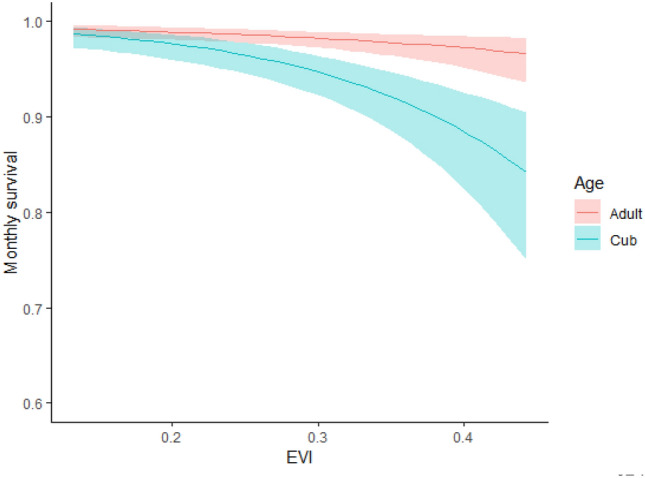
Monthly survival of adult and cheetah cubs in relation to short term (seasonal) average Enhanced Vegetation Index (EVI) within an individual’s home range, Mun-Ya-Wana Conservancy, KwaZulu-Natal, South Africa, 2008–2018. Shaded regions represent 85% CI.
Long-term spatial drivers of survival
Similar to short-term survival, we assessed lifetime environmental covariates using the 50% HR contour. At the lifetime scale, cheetahs exhibited variation in environmental conditions within their home range with regards to EVI (mean = 0.3; range = 0.19–0.42), lion encounter risk (mean = 0.37; range = 0.13–0.84), and prey density (mean = 40.4 prey/km2, range = 25.8–75.7 prey/km2).
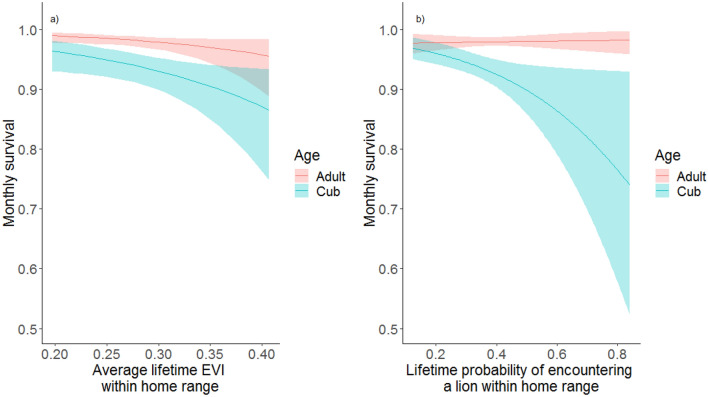
When considering environmental covariates across individuals’ lifetimes, survival was best described by a model including the average EVI and the average risk of encountering a lion within a cheetahs’ home range during their lifetime (Table 2). Models including EVI and lion encounter risk separately were also competitive (Table 2), however we only present results from the top model given that the covariate relationships were similar in all competitive models. In contrast to our predictions under the habitat complexity risk mediation hypothesis, lifetime EVI had a negative influence on adult and cub survival. At the lowest lifetime EVI values, adult monthly survival was 0.99 (85% CI = 0.98–1.00) and cub monthly survival was 0.96 (85% CI = 0.93–0.98), whereas at the highest lifetime EVI values, adult monthly survival was 0.96 (85% CI = 0.89–0.98) and cub monthly survival was 0.87 (85% CI = 0.75–0.93). As predicted by the spatial top down hypothesis, lifetime lion encounter risk had a negative influence on cub survival, although there was not a significant effect of lifetime lion encounter risk on adult survival (Fig. 2). For cubs, at the lowest lifetime lion encounter risk, monthly survival was 0.97 (85% CI = 0.95–0.99), whereas at the highest lifetime lion encounter risk, monthly survival was 0.74 (85% CI = 0.52–0.96).
Table 2.
Model selection results for multi-state joint live-encounter dead-recovery spatial-explicit survival models for cheetahs with spatial covariates averaged across individual cheetahs’ lifetimes, Mun-Ya-Wana Conservancy, KwaZulu-Natal, South Africa, 2008–2018.
| Model | AICc | ΔAICC | − 2 × ln(L)a | wb | kc |
|---|---|---|---|---|---|
| S(state:lion + state:EVI) | 3608.85 | 0 | 3586.66 | 0.30 | 11 |
| S(state:EVI) | 3609.06 | 0.21 | 3590.93 | 0.27 | 9 |
| S(state:lion) | 3610.45 | 1.60 | 3592.32 | 0.13 | 9 |
| S(state:prey * state:EVI) | 3611.52 | 2.67 | 3585.26 | 0.08 | 13 |
| S(state:lion * state:EVI) | 3611.82 | 2.97 | 3585.56 | 0.07 | 13 |
| S(state:EVI + state:lion + state:prey) | 3612.36 | 3.51 | 3586.10 | 0.05 | 13 |
| S(state:prey + state:EVI) | 3612.44 | 3.59 | 3590.25 | 0.05 | 11 |
| S(state:lion * state:prey) | 3613.40 | 4.55 | 3587.14 | 0.03 | 13 |
| S(state:lion + state:prey) | 3614.41 | 5.56 | 3592.22 | 0.02 | 11 |
| S(state:prey) | 3618.74 | 9.89 | 3600.61 | 0.00 | 9 |
| S(state) | 3619.33 | 10.49 | 3603.23 | 0.00 | 8 |
States in the model include cubs (juveniles dependent on their mothers) and adults (non-juveniles). All models include effects of year on recovery rates and season on survival rates.
aLog-likelihood.
bAkaike model weight.
cNumber of model parameters.
Figure 2.
Monthly survival of adult and cheetah cubs in relation to (a) long-term (lifetime) average Enhanced Vegetation Index (EVI) within an individual’s home range while holding lion density constant at an average value, and (b) long-term (lifetime) average probability of encountering a lion within an individual’s home range while holding EVI constant at an average value, Mun-Ya-Wana Conservancy, KwaZulu-Natal, South Africa, 2008–2018. Shaded regions represent 85% CI.
Discussion
We evaluated support for effects of spatial influences of top-down predation risk, bottom-up prey availability, and habitat complexity on cheetah survival, and found the most consistent support for survival being influenced by habitat complexity across multiple temporal scales. However, our results contradict the habitat complexity risk mediation hypothesis, which predicts that mesopredators should experience increased survival in areas of high habitat complexity20. Instead, our results show that subordinate predators do not always benefit from structurally complex habitats, potentially because subordinate predators might only benefit from habitat complexity if they are able to avoid predation, and effectively obtain prey, in complex habitats. In predator–prey systems, using specific areas as refuges from predation can come at a cost to the prey, because although they might reduce predation risk, the resource availability of the refuge might be lower than non-refuge areas because of increased competition or sub-optimal conditions30,31. In systems of multiple predators, the quality of a predation refuge habitat might be related to the subordinate predator’s ability to find and hunt prey, which is a function of the subordinate predator’s hunting mode32.
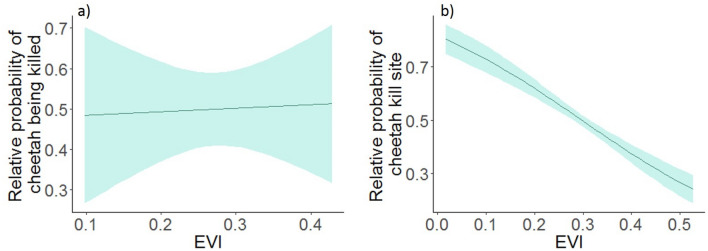
There are two main explanations as to why we did not find support for the habitat complexity risk mediation hypothesis in our study system. First, vegetation complexity might increase or reduce the probability of cheetahs being predated upon. Ambush predators in a variety of systems have been found to have enhanced hunting abilities in structurally-complex areas because of decreased sight lines for prey33,34. Although lions use a variety of habitat types, they kill prey more frequently in areas of dense vegetation35,36. Closed habitat types could act as a predation refuge for cheetahs by providing cover to enhance concealment from lions, but these habitats could also increase predation risk because of the hunting preferences of lions. Conversely, open habitat types might reduce the probability of predation by improving cheetahs’ ability to detect nearby lions, compared to closed habitats37. However, when we analyzed locations where cheetahs were killed by other predators (Appendix S3), we did not find evidence to suggest that vegetation complexity increased the risk of cheetahs being predated upon (Fig. 3a). Therefore, it seems that cheetahs experience predation independent of vegetation complexity, and higher predation in areas of higher EVI might not be the mechanism driving our observed patterns of spatial survival.
Figure 3.
Relative probability of (a) cheetahs being killed by other predator species and (b) cheetah kill site occurrence in relation to Enhanced Vegetation Index (EVI), Mun-Ya-Wana Conservancy, KwaZulu-Natal, South Africa, 2008–2018. Shaded regions represent 85% CI.
Second, vegetation complexity might influence cheetahs’ hunting ability, which in turn could affect survival. Cheetahs are coursing predators, as opposed to ambush predators, and can reach high speeds when chasing prey38. Therefore, open areas could improve cheetah hunting success by allowing cheetahs to see prey easier and facilitating high-speed chases. Although cheetahs are able to hunt in areas of dense vegetation39, they are more likely to initiate hunts, and have higher hunting success, in open habitats40. Prey availability can be an important driver of carnivore demography41, so the use of areas to facilitate hunting, rather than the density of prey themselves42, could influence mesopredator survival. Indeed, when we analyzed cheetah kill site locations (Appendix S3) we found that cheetah kill sites were more likely to be located in areas with low EVI (Fig. 3b). For other mesopredators that rely on cover for hunting, survival might be higher in areas of dense vegetation; thus, future research should focus on how hunting behaviors and vegetation complexity interact to influence mesopredator survival.
In addition to habitat complexity affecting cheetah survival, we found that cheetah survival was also influenced by duration of exposure to top-down predation risk. Our results indicate that long-term risk of encountering a lion, rather than short-term risk of encountering a lion, influenced cheetah survival, with cubs having a lower probability of survival if there was a higher probability of encountering lions in their home range during the entire time when they were a cub. We likely did not observe effects of lion encounter risk on short-term cheetah survival because cheetahs have adapted behaviors to minimize short-term predation risk25. For example, cheetahs use the same general areas as apex predators such as lions and use fine-scale spatial partitioning to reduce the probability of lion encounters10,26,27. The use of spatial or temporal partitioning by mesopredators affected by apex predators has been found in a variety of other systems, such as red foxes (Vulpes vulpes) avoiding coyotes (Canis latrans) in North America43, and European badgers (Meles meles) avoiding wolves (Canis lupus) in Italy11.
Although fine-scale partitioning or other predator avoidance behaviors might be beneficial to reduce short-term risk for mesopredators, our results show that the long-term risk of co-occurring with an apex predator can negatively influence mesopredator survival. In the long-term, the risk of encountering lions could be associated with the direct effects of predation, with an increased probability of antagonistic encounters44. Long-term risk can also be associated with non-consumptive effects of predation related to reduced foraging45,46, or reduced parental care47. We could not explicitly investigate whether long-term exposure to predation risk reduced cub survival through direct or indirect effects. The majority of cub mortality in our study system is a result of predation48, but cubs also experience non-predation mortality such as starvation or injury22,48, and indirect effects of predation risk could have reduced cub body condition, which might increase susceptibility to predation. In the absence of direct predation, the long-term risk of predation has been found to cause changes in the morphology49, behavior50,51, physiology52,53, and demography54,55 of a variety of species. Our results build on the growing literature on long-term risk of predation to demonstrate how long-term predation risk can affect the demography of mesopredators.
Understanding spatial variation in survival can help inform wildlife conservation actions by focusing efforts on environmental factors that improve the survival of imperiled species. Specific for cheetahs in southern Africa, bush encroachment has caused the transition from open grasslands to closed habitats dominated by woody plants56. Bush encroachment can be caused by a number of factors including climate, fire, and herbivore distributions, but is predicted to increase based on future climate change models57. Based on our results, increased bush encroachment could be detrimental to cheetah populations that do not have adequate open areas for hunting. Thus, the persistence of this species in the southern portion of their range could be improved by prescribed burning or mechanical vegetation removal in order to maintain open habitats58,59. One limitation of our study was that we focused on only one cheetah population. Cheetahs experience variable conditions throughout their range, including differences in habitat composition, predator and prey communities, and conservation practices60,61. Therefore, further research is needed to better understand range-wide variability in spatial drivers of cheetah survival.
Our research shows how individual space use of mesopredators can scale-up and influence population-level processes, and illustrates the importance of understanding spatial drivers of survival on different temporal scales62,63. We propose that, in systems with apex predators and mesopredators, the survival of mesopredators in the short-term is driven by vegetative complexity likely associated with prey acquisition, whereas long-term survival depends on both top-down and bottom-up influences. Additionally, our results show that complex habitats might only be beneficial for mesopredators when they allow mesopredators to avoid apex predators, and effectively find and hunt prey, at the same time. Understanding how individual space use can influence population-level processes of mesopredators can offer insight into how communities with multiple predators are structured and can provide recommended conservation actions to ensure the future persistence of mesopredator species.
Methods
Study area
We studied cheetah survival in Mun-Ya-Wana Conservancy (Phinda Private Game Reserve), in northern KwaZulu-Natal, South Africa, from 2008–2018. The dominant vegetation type is broad-leaf woodland (42% of the conservancy), with open grasslands (31% of the conservancy), and semi-open wooded-grasslands (27% of the conservancy) interspersed throughout the reserve. The elevation of the Conservancy ranges from 4 to 201 m above sea level. The climate is subtropical with warm, dry winters (April–September) and hot, humid summers (October–March), with the majority of rain falling in the summer64. The Mun-Ya-Wana Conservancy is surrounded by electrified game fencing, and has grown in size as adjacent reserves have joined the Conservancy. From 2008–2017 the study area was 235 km2 in area, after which internal fences were removed and the Conservancy expanded to 285 km2. Cheetahs and lions were reintroduced into the reserve in 1992 and have been monitored since48,65. Throughout the study, lion densities ranged from 0.06 to 0.17 lions/km2 and cheetah densities ranged from 0.06 to 0.18 cheetahs/km2. Although both leopards (Panthera pardus) and spotted hyenas (Crocuta crocuta) occur in the study area, lions are the dominant apex predator48,65. Common prey species include impala (Aepyceros melampus), nyala (Tragelaphus angasii), zebra (Equus quagga burchellii), and wildebeest (Connochaetes taurinus).
Carnivore monitoring
We monitored the cheetah and lion populations by subdividing the reserve into seven sections. Trained monitors usually drove the roads in each section at least once a week. In addition, monitors frequently followed-up on sightings reported by game rangers conducting game drives within the reserve. Cheetahs and lions can be individually recognized using their spot patterns, whisker spots, and scars, which allowed us to monitor the populations based on sightings alone66. We obtained an average of 40 ± 6 locations per individual cheetah during adult states and 27 ± 2 locations during cub states. When cheetahs or lions were observed, we recorded the location, behavior, and number of individuals present.
Cheetah habitat use
We quantified coarse-scale cheetah habitat use by estimating lifetime home ranges for individual cheetahs. Although small-scale differences in habitat use might occur seasonally, cheetahs in our study area had stable home ranges across their lifetimes (Appendix S1). However, cheetahs will often shift home ranges when they become independent from their mothers, so for individuals that were included in the study as both cubs and adults, we estimated cub and adult home ranges separately. We estimated home ranges by calculating a utilization distribution (UD) using a fixed-kernel estimator and the plug-in method of bandwidth selection67. We only included cheetahs in our analyses with > 10 locations. For cubs died that before reaching the minimum number of locations, we used covariates associated with their mother’s home range, or the home range of their surviving littermates. For each cheetah’s home range, we extracted time-varying covariates of lion encounter risk, prey spatial density, and vegetation complexity (see below). To account for temporal differences in spatial drivers of survival, we extracted covariates within home ranges corresponding to each season, and also averaged covariates within home ranges across the lifetime of individual cheetahs. For cheetahs that were included in the analyses as both cubs and adults, we calculated separate “lifetime” covariate values for cub and adult periods separately. To identify the spatial scale most influential to survival, we extracted these covariates within the 50%, 75%, and 95% UD isopleths.
Lion encounter risk
We estimated lion encounter risk by analyzing the spatial distribution of lions in each season68,69. We collected sightings data on the location of lion prides, rather than individual lions, from 2000–2019. For each pride of lions in a given season, we calculated a utilization distribution (UD) using a fixed-kernel estimator using the plug-in method of bandwidth selection67. To account for differences in pride size, we multiplied the UD for each pride by the average number of lions in that pride within a specific season70. To obtain a reserve-level measure of lion encounter risk, we added the individual pride UDs and rescaled the resulting values such that a value of 0 indicated no risk of encounter, and 1 indicated the highest risk of encounter.
Prey spatial density
We estimated spatial variation in prey density in the reserve by collecting distance sampling data on impala, and nyala during the dry season (April–September), and the wet season (October–March) from 2010–201548. We limited our prey analyses to these species because they comprised 82% of cheetah kills in the study area65. We estimated prey abundance using hierarchical distance sampling models with spatial covariates on both the abundance and detection processes, and used our top model to extrapolate prey abundance over our entire study period48. Our resulting prey density rasters depicted the average number of prey within 400 m2 cells for each season of each year.
Vegetation complexity
We incorporated spatial variation in vegetation complexity into our cheetah survival models. Enhanced Vegetation Index (EVI) has been found to be correlated with vegetation structure in Africa, with open areas having low EVI values, and areas with dense vegetation having high EVI values71. In addition, EVI is sensitive to changes in rainfall. Thus, using EVI as an index for vegetation complexity also allowed us to incorporate changes in greenness, which could affect visibility. We obtained (EVI) data at a 250 m resolution (https://lpdaac.usgs.gov/data_access/data_pool) and calculated seasonal EVI values on a yearly basis by averaging EVI values across the entirety of a season.
Cheetah spatially-explicit survival
We analyzed spatial drivers of cheetah monthly survival from February 2009 to March 2018. When a dead cheetah was discovered, we tried to determine the cause of death by checking the nearby area for tracks and scats, and examining the carcass. For each month, we recorded if each monitored cheetah was sighted or recovered dead as adults or cubs48. If a cheetah was removed from the reserve for management purposes, we censored that individual animal from analyses48. Because lion and cheetah density can vary greatly within a season, and because cubs can be born and become independent at any time during the year, we conducted our analysis on a monthly timescale to best reflect the conditions that might be driving survival48.
Because survival of cubs from the same litter might not independent, we first ran a Chi-square test of independent survival72 to test this assumption, with the null hypothesis being that survival of cubs is independent. To run this analysis, we randomly selected half (n = 19) of the monitored litters and ran a survival model (see below for information on model structure) without any individual covariates, to estimate monthly cub survival. We used the results of this model to estimate the expected number of living cubs at independence, which we defined as 16 months post-birth48. When then repeated this procedure 50 times and ran a Chi-square test on the observed vs. expected number of survived cubs. We found that fates of cubs within the same litter were independent (X2 = 34.3; p = 0.12), so for our subsequent survival models we treated each individual cheetah cub as an independent sample. Male cheetahs in the same coalition might also have non-independent fates, so we used the same Chi-square test of independent survival modeling framework with 50 replicates to test independence of males in coalitions (n = 11 coalitions). We found that that fates of males within the same coalition were independent (X2 = 6.5; p = 0.28), so for our subsequent analysis we treated each individual male cheetah as an independent sample.
Model structure
Similar to previous research on cheetah survival in this system48, we analyzed cheetah survival using multi-state joint live-encounter dead-recovery models73 using the rmark R package74. This model made use of our frequent re-sightings and mortality data, and allowed for survival estimation based on individuals with unknown fates. Additionally, because juvenile cheetahs stay with their mothers for variable amounts of time75, we could not incorporate a regular age structure into our models. Thus, we used a multi-state approach to estimate survival for both cubs and adults simultaneously48. We specified the two model states as cub (juvenile cheetahs dependent on their mother) and adult (cheetahs that were independent from their mother) and did not incorporate immigration or emigration because our population was a closed population.
Hypothesis testing
We previously determined that cheetah survival was best described using a structural model with resighting rate varying by year, and survival varying by season48. Therefore, we used the same structural model for these analyses to test for the effects of spatial covariates on cheetah survival. We used a two-stage approach to evaluate our hypotheses of interest. We first ran models to determine the spatial scale most influential to survival by running models with spatial covariates corresponding to the 50%, 75%, and 95% UD isopleths (Appendix S2). We considered spatial covariates on two temporal scales: short-term (seasonal), and long-term (spatial covariates within a home range averaged over an individual’s lifetime). The spatial scale associated with the best-fit model at both the short-term and long-term scales was retained and used for the hypothesis-testing portion of our analysis (Appendix S2).
To test our hypotheses of interest, we developed 11 a priori models that included covariates of average lion encounter risk, average prey density, and average EVI, as well as additive and multiplicative models with the same covariates. Similar to the first stage of our analysis, we considered spatial covariates both at the short-term (seasonal) and long-term (spatial covariates within a home range averaged over an individual’s lifetime) temporal scales. Because adults and cubs are known to have different survival rates75, we did not consider any models in which state was not included. We compared models separately for each temporal scale using Akaike’s Information Criterion corrected for sample size (AICc;76), considered models within 2 ΔAICc of the top model to be competitive, and evaluated if covariates were informative by calculating 85% confidence intervals77.
Supplementary information
Acknowledgements
We thank Mun-Ya-Wana Conservancy and Phinda Private Game Reserve for allowing us to conduct research on their land, the numerous employees and volunteers who assisted in the data collection for this research, and J. Janse van Rensburg for providing some of the prey data. This research was partially funded by a Panthera Kaplan Graduate Award to LG.
Author contributions
L.G., D.J. and R.S. conceived the ideas; L.H., J.F. and C.S.‐D. collected the data; L.G. analyzed the data; L.G. and D.J. led the writing of the manuscript. All authors contributed critically to the drafts and gave final approval for publication.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
is available for this paper at 10.1038/s41598-020-73318-3.
References
- 1.Schmitz OJ, Miller JRB, Trainor AM, Abrahms B. Toward a community ecology of landscapes: predicting multiple predator–prey interactions across geographic space. Ecology. 2017;98:2281–2292. doi: 10.1002/ecy.1916. [DOI] [PubMed] [Google Scholar]
- 2.van Noordwijk AJ, de Jong G. Acquisition and allocation of resources: their influence on variation in life history. Am. Nat. 1986;128:137–142. doi: 10.1086/284547. [DOI] [Google Scholar]
- 3.Gaillard J-M, et al. Habitat-performance relationships: finding the right metric at a given spatial scale. Philos. Trans. R. Soc. Lond. B. Biol. Sci. 2010;365:2255–2265. doi: 10.1098/rstb.2010.0085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mosser, A., Fryxell, J. M., Eberly, L. & Packer, C. Serengeti real estate: Density vs. fitness-based indicators of lion habitat quality. Ecol. Lett.12, 1050–1060 (2009). [DOI] [PubMed]
- 5.Kosterman MK, Squires JR, Holbrook JD, Pletscher DH, Hebblewhite M. Forest structure provides the income for reproductive success in a southern population of Canada lynx. Ecol. Appl. 2018;28:1032–1043. doi: 10.1002/eap.1707. [DOI] [PubMed] [Google Scholar]
- 6.DeCesare NJ, et al. Linking habitat selection and predation risk to spatial variation in survival. J. Anim. Ecol. 2014;83:343–352. doi: 10.1111/1365-2656.12144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hebblewhite M, Merrill EH, McDonald TL. Spatial decomposition of predation risk using resource selection functions: An example in a wolf-elk predator-prey system. Oikos. 2005;111:101–111. doi: 10.1111/j.0030-1299.2005.13858.x. [DOI] [Google Scholar]
- 8.McLoughlin PD, Dunford JS, Boutin S. Relating predation mortality to broad-scale habitat selection. J. Anim. Ecol. 2005;74:701–707. doi: 10.1111/j.1365-2656.2005.00967.x. [DOI] [Google Scholar]
- 9.Ritchie EG, Johnson CN. Predator interactions, mesopredator release and biodiversity conservation. Ecol. Lett. 2009;12:982–998. doi: 10.1111/j.1461-0248.2009.01347.x. [DOI] [PubMed] [Google Scholar]
- 10.Vanak AT, et al. Moving to stay in place: behavioral mechanisms for coexistence of African large carnivores. Ecology. 2013;94:2619–2631. doi: 10.1890/13-0217.1. [DOI] [PubMed] [Google Scholar]
- 11.Torretta E, Serafini M, Puopolo F, Schenone L. Spatial and temporal adjustments allowing the coexistence among carnivores in Liguria (N–W Italy) Acta Ethol. 2016;19:123–132. doi: 10.1007/s10211-015-0231-y. [DOI] [Google Scholar]
- 12.Preisser EL, Bolnick DI, Benard MF. Scared to death? The effects of intimidation and consumption in predator–prey interactions. Ecology. 2005;86:501–509. doi: 10.1890/04-0719. [DOI] [Google Scholar]
- 13.Swanson A, et al. Cheetahs and wild dogs show contrasting patterns of suppression by lions. J. Anim. Ecol. 2014;83:1418–1427. doi: 10.1111/1365-2656.12231. [DOI] [PubMed] [Google Scholar]
- 14.Levi T, Wilmers C. Wolves—coyotes—foxes: a cascade among carnivores. Ecology. 2012;93:921–929. doi: 10.1890/11-0165.1. [DOI] [PubMed] [Google Scholar]
- 15.Henke SE, Bryant FC. Effects of coyote removal on the faunal community in western Texas. J. Wildl. Manag. 1999;63:1066. doi: 10.2307/3802826. [DOI] [Google Scholar]
- 16.Gehrt SD, Prange S. Interference competition between coyotes and raccoons: a test of the mesopredator release hypothesis. Behav. Ecol. 2007;18:204–214. doi: 10.1093/beheco/arl075. [DOI] [Google Scholar]
- 17.St-Pierre C, Ouellet JP, Crête M. Do competitive intraguild interactions affect space and habitat use by small carnivores in a forested landscape? Ecography (Cop.) 2006;29:487–496. doi: 10.1111/j.0906-7590.2006.04395.x. [DOI] [Google Scholar]
- 18.Shores CR, Dellinger JA, Newkirk ES, Kachel SM, Wirsing AJ. Mesopredators change temporal activity in response to a recolonizing apex predator. Behav. Ecol. 2019;30:1324–1335. doi: 10.1093/beheco/arz080. [DOI] [Google Scholar]
- 19.Allen ML, Elbroch LM, Wilmers CC, Wittmer HU. The comparative effects of large carnivores on the acquisition of carrion by scavengers. Am. Nat. 2015;185:822–833. doi: 10.1086/681004. [DOI] [PubMed] [Google Scholar]
- 20.Janssen A, Sabelis MW, Magalhães S, Van T. Habitat structure affects intraguild predation. Ecology. 2007;88:2713–2719. doi: 10.1890/06-1408.1. [DOI] [PubMed] [Google Scholar]
- 21.Finke DL, Denno RF. Intraguild predation diminished in complex-structured vegetation: Implication for prey suppression. Ecology. 2002;83:643–652. doi: 10.1890/0012-9658(2002)083[0643:IPDICS]2.0.CO;2. [DOI] [Google Scholar]
- 22.Laurenson MK. High juvenile mortality in cheetahs (Acinonyx jubatus) and its consequences for maternal care. J. Zool. Soc. Lond. 1994;234:387–408. doi: 10.1111/j.1469-7998.1994.tb04855.x. [DOI] [Google Scholar]
- 23.Hunter JS, Durant SM, Caro TM. To flee or not to flee: predator avoidance by cheetahs at kills. Behav. Ecol. Sociobiol. 2007;61:1033–1042. doi: 10.1007/s00265-006-0336-4. [DOI] [Google Scholar]
- 24.Hilborn, A. et al. Cheetahs modify their prey handling behavior depending on risks from top predators. Behav. Ecol. Sociobiol.72, 74 (2018).
- 25.Swanson A, Arnold T, Kosmala M, Forester J, Packer C. In the absence of a “landscape of fear”: How lions, hyenas, and cheetahs coexist. Ecol. Evol. 2016;6:8534–8545. doi: 10.1002/ece3.2569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dröge, E., Creel, S., Becker, M. S. & M’soka, J. Spatial and temporal avoidance of risk within a large carnivore guild. Ecol. Evol.7, 189–199 (2016). [DOI] [PMC free article] [PubMed]
- 27.Broekhuis F, Cozzi G, Valeix M, Mcnutt JW, Macdonald DW. Risk avoidance in sympatric large carnivores: Reactive or predictive? J. Anim. Ecol. 2013;82:1098–1105. doi: 10.1111/1365-2656.12077. [DOI] [PubMed] [Google Scholar]
- 28.Mills MGL, Mills MEJ. Cheetah cub survival revisited: a re-evaluation of the role of predation, especially by lions, and implications for conservation. J. Zool. 2014;292:136–141. doi: 10.1111/jzo.12087. [DOI] [Google Scholar]
- 29.Broekhuis F. Natural and anthropogenic drivers of cub recruitment in a large carnivore. Ecol. Evol. 2018;8:6748–6755. doi: 10.1002/ece3.4180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Orrock JL, Preisser EL, Grabowski JH, Trussell GC. The cost of safety: refuges increase the impact of predation risk in aquatic systems. Ecology. 2013;94:573–579. doi: 10.1890/12-0502.1. [DOI] [PubMed] [Google Scholar]
- 31.Donelan SC, Grabowski JH, Trussell GC. Refuge quality impacts the strength of nonconsumptive effects on prey. Ecology. 2016;98:403–411. doi: 10.1002/ecy.1647. [DOI] [PubMed] [Google Scholar]
- 32.Miller JRB, Ament JM, Schmitz OJ. Fear on the move: predator hunting mode predicts variation in prey mortality and plasticity in prey spatial response. J. Anim. Ecol. 2014;83:214–222. doi: 10.1111/1365-2656.12111. [DOI] [PubMed] [Google Scholar]
- 33.Michel MJ, Adams MM. Differential effects of structural complexity on predator foraging behavior. Behav. Ecol. 2009;20:313–317. doi: 10.1093/beheco/arp005. [DOI] [Google Scholar]
- 34.Blake LW, Gese EM. Resource selection by cougars: influence of behavioral state and season. J. Wildl. Manag. 2016;80:1205–1217. doi: 10.1002/jwmg.21123. [DOI] [Google Scholar]
- 35.Hopcraft JGC, Sinclair ARE, Packer C. Planning for success: Serengeti lions seek prey accessibility rather than abundance. J. Anim. Ecol. 2005;74:559–566. doi: 10.1111/j.1365-2656.2005.00955.x. [DOI] [Google Scholar]
- 36.Davies AB, Tambling CJ, Kerley GIH, Asner GP. Effects of vegetation structure on the location of lion kill sites in African thicket. PLoS ONE. 2016;11:1–20. doi: 10.1371/journal.pone.0149098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Camp MJ, Rachlow JL, Woods BA, Johnson TR, Shipley LA. When to run and when to hide: the influence of concealment, visibility, and proximity to refugia on perceptions of risk. Ethology. 2012;118:1010–1017. doi: 10.1111/eth.12000. [DOI] [Google Scholar]
- 38.Wilson AM, et al. Locomotion dynamics of hunting in wild cheetahs. Nature. 2013;498:185–189. doi: 10.1038/nature12295. [DOI] [PubMed] [Google Scholar]
- 39.Rostro-García S, Kamler JF, Hunter LTB. To kill, stay or flee: the effects of lions and landscape factors on habitat and kill site selection of cheetahs in South Africa. PLoS ONE. 2015;10:e0117743. doi: 10.1371/journal.pone.0117743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mills MGL, Broomhall LS, du Toit JT, Toit JT. Cheetah Acinonyx jubatus feeding ecology in the Kruger National Park and a comparison across African savanna habitats: is the cheetah only a successful hunter on open grassland plains? Wildl. Biol. 2004;10:177–186. doi: 10.2981/wlb.2004.024. [DOI] [Google Scholar]
- 41.Fuller, T. & Sievert, P. Carnivore demography and the consequences of changes in prey availability. in Carnivore conservation (eds. Gittleman, J. L., Funk, S. M., Macdonald, D. & Wayne, R. K.) 163–179 (Cambridge University Press, Cambridge 2001).
- 42.Balme G, Hunter LTB, Slotow R. Feeding habitat selection by hunting leopards Panthera pardus in a woodland savanna: prey catchability versus abundance. Anim. Behav. 2007;74:589–598. doi: 10.1016/j.anbehav.2006.12.014. [DOI] [Google Scholar]
- 43.Gosselink TE, Van Deelen TR, Warner RE, Joselyn MG. Temporal habitat partitioning and spatial use of coyotes and red foxes in East-Central Illinois. J. Wildl. Manag. 2003;67:90–103. doi: 10.2307/3803065. [DOI] [Google Scholar]
- 44.Palomares F, Caro TM. Interspecific killing among mammalian carnivores. Am. Nat. 1999;153:492–508. doi: 10.1086/303189. [DOI] [PubMed] [Google Scholar]
- 45.Brown JS. Vigilance, patch use and habitat selection: foraging under predation risk. Evol. Ecol. Res. 1999;1:49–71. [Google Scholar]
- 46.Creel, S. The control of risk hypothesis: reactive vs. proactive antipredator responses and stress-mediated vs. food-mediated costs of response. Ecol. Lett.21, 947–956 (2018). [DOI] [PubMed]
- 47.Dudeck BP, Clinchy M, Allen MC, Zanette LY. Fear affects parental care, which predicts juvenile survival and exacerbates the total cost of fear on demography. Ecology. 2018;99:127–135. doi: 10.1002/ecy.2050. [DOI] [PubMed] [Google Scholar]
- 48.Gigliotti LC, et al. Context-dependency of top-down, bottom-up, and density-dependent influences on cheetah demography. J. Anim. Ecol. 2020;2:449–459. doi: 10.1111/1365-2656.13099. [DOI] [PubMed] [Google Scholar]
- 49.Relyea RA. Morphological and behavioral plasticity of larval anurans in response to different predators. Ecology. 2001;82:523–540. doi: 10.1890/0012-9658(2001)082[0523:MABPOL]2.0.CO;2. [DOI] [Google Scholar]
- 50.Suraci JP, Clinchy M, Dill LM, Roberts D, Zanette LY. Fear of large carnivores causes a trophic cascade. Nat. Commun. 2016;7:1–7. doi: 10.1038/ncomms10698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Valeix M, et al. Behavioral adjustments of African herbivores to predation risk by lions: Spatiotemporal variations influence habitat use. Ecology. 2009;90:23–30. doi: 10.1890/08-0606.1. [DOI] [PubMed] [Google Scholar]
- 52.Sheriff MJ, Krebs CJ, Boonstra R. The sensitive hare: sublethal effects of predator stress on reproduction in snowshoe hares. J. Anim. Ecol. 2009;78:1249–1258. doi: 10.1111/j.1365-2656.2009.01552.x. [DOI] [PubMed] [Google Scholar]
- 53.Clinchy M, et al. Multiple measures elucidate glucocorticoid responses to environmental variation in predation threat. Oecologia. 2011;166:607–614. doi: 10.1007/s00442-011-1915-2. [DOI] [PubMed] [Google Scholar]
- 54.Travers M, Clinchy M, Zanette L, Boonstra R, Williams TD. Indirect predator effects on clutch size and the cost of egg production. Ecol. Lett. 2010;13:980–988. doi: 10.1111/j.1461-0248.2010.01488.x. [DOI] [PubMed] [Google Scholar]
- 55.LaManna JA, Martin TE. Costs of fear: behavioural and life-history responses to risk and their demographic consequences vary across species. Ecol. Lett. 2016;19:403–413. doi: 10.1111/ele.12573. [DOI] [PubMed] [Google Scholar]
- 56.Roques KG, O’Connor TG, Watkinson AR. Dynamics of shrub encroachment in an African savanna: Relative influences of fire, herbivory, rainfall and density dependence. J. Appl. Ecol. 2001;38:268–280. doi: 10.1046/j.1365-2664.2001.00567.x. [DOI] [Google Scholar]
- 57.Tews J, Jeltsch F. Modelling the impact of climate change on woody plant population dynamics in South African savanna. BMC Ecol. 2004;4:1–12. doi: 10.1186/1472-6785-4-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Joubert DF, Smit GN, Hoffman MT. The role of fire in preventing transitions from a grass dominated state to a bush thickened state in arid savannas. J. Arid Environ. 2012;87:1–7. doi: 10.1016/j.jaridenv.2012.06.012. [DOI] [Google Scholar]
- 59.Lohmann D, Tietjen B, Blaum N, Joubert DF, Jeltsch F. Prescribed fire as a tool for managing shrub encroachment in semi-arid savanna rangelands. J. Arid Environ. 2014;107:49–56. doi: 10.1016/j.jaridenv.2014.04.003. [DOI] [Google Scholar]
- 60.Durant SM, et al. The global decline of cheetah Acinonyx jubatus and what it means for conservation. Proc. Natl. Acad. Sci. 2017;114:528–533. doi: 10.1073/pnas.1611122114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Weise FJ, et al. The distribution and numbers of cheetah (Acinonyx jubatus) in southern Africa. PeerJ. 2017;5:e4096. doi: 10.7717/peerj.4096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Prugh LR, et al. Designing studies of predation risk for improved inference in carnivore-ungulate systems. Biol. Conserv. 2019;232:194–207. doi: 10.1016/j.biocon.2019.02.011. [DOI] [Google Scholar]
- 63.Moll RJ, et al. The many faces of fear: a synthesis of the methodological variation in characterizing predation risk. J. Anim. Ecol. 2017;86:749–765. doi: 10.1111/1365-2656.12680. [DOI] [PubMed] [Google Scholar]
- 64.Janse van Rensburg, J., McMillan, M., Giżejewska, A. & Fattebert, J. Rainfall predicts seasonal home range size variation in nyala. Afr. J. Ecol.56, 418–423 (2018).
- 65.Hunter, L. T. B. The behavioural ecology of reintroduced lions and cheetahs in the Phinda Resource Reserve, Kwazulz-Natal, South Africa. Phd thesis 1–206 (1998).
- 66.Caro, T. M. Cheetahs of the Serengeti Plains. (The University of Chicago Press, Chicago, 1994).
- 67.Gitzen RA, Millspaugh JJ, Kernohan BJ. Bandwidth selection for fixed-kernel analysis of animal utilization distributions. J. Wildl. Manag. 2006;70:1334–1344. doi: 10.2193/0022-541X(2006)70[1334:BSFFAO]2.0.CO;2. [DOI] [Google Scholar]
- 68.Thaker M, et al. Minimizing predation risk in a landscape of multiple predators: effects on the spatial distribution of African ungulates. Ecology. 2011;92:398–407. doi: 10.1890/10-0126.1. [DOI] [PubMed] [Google Scholar]
- 69.Moll RJ, Killion AK, Montgomery RA, Tambling CJ, Hayward MW. Spatial patterns of African ungulate aggregation reveal complex but limited risk effects from reintroduced carnivores. Ecology. 2016;97:1123–1134. doi: 10.1890/15-0707.1. [DOI] [PubMed] [Google Scholar]
- 70.Kauffman MJ, et al. Landscape heterogeneity shapes predation in a newly restored predator-prey system. Ecol. Lett. 2007;10:690–700. doi: 10.1111/j.1461-0248.2007.01059.x. [DOI] [PubMed] [Google Scholar]
- 71.Tsalyuk M, Kelly M, Getz WM. Improving the prediction of African savanna vegetation variables using time series of MODIS products. ISPRS J. Photogramm. Remote Sens. 2017;131:77–91. doi: 10.1016/j.isprsjprs.2017.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Winterstein SR. Chi-square tests for intrabrood independence when using the Mayfield method. J. Wildl. Manage. 1992;56:398–402. doi: 10.2307/3808842. [DOI] [Google Scholar]
- 73.Barker RJ, White GC, McDougall M. Movement of Paradise Shelduck between molt sites: a joint multistate-dead recovery mark-recapture model. J. Wildl. Manage. 2005;69:1194–1201. doi: 10.2193/0022-541X(2005)069[1194:MOPSBM]2.0.CO;2. [DOI] [Google Scholar]
- 74.Laake, J. L. RMark: An R Interface for Analysis of Capture-Recapture Data with MARK. Version 2.2.4. (2013).
- 75.Kelly MJ, et al. Demography of the Serengeti cheetah (Acinonyx jubatus) population: the first 25 years. J. Zool. 1998;224:473–488. doi: 10.1111/j.1469-7998.1998.tb00053.x. [DOI] [Google Scholar]
- 76.Burnham KP, Anderson DR. Model Selection and Multimodel Inference: A Practical Information-Theoretic Approach. Berlin: Springer; 2002. [Google Scholar]
- 77.Arnold TW. Uninformative parameters and model selection using Akaike’s Information Criterion. J. Wildl. Manag. 2010;74:1175–1178. doi: 10.1111/j.1937-2817.2010.tb01236.x. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.