Abstract
Bioprinting has been introduced as a new technique in tissue engineering for more than a decade. However, characteristics of bioprinted part are still distinct from native human tissue and organ in terms of both shape fidelity and functionality. Recently, the combination of at least two hydrogels or “multi-materials/multi-nozzles” bioprinting enables simultaneous deposition of both model and support materials, thus advancing the complexity of bioprinted shapes from 2.5D lattice into micro-channeled 3D structure. In this article, a perspective on the roles of second bioinks or support materials is presented and future outlook of sacrificial materials is discussed.
Keywords: hydrogel, support materials, bioprinting, additive manufacturing, tissue engineering
1. Introduction
Bioprinting is an emerging technology that shows potential for regenerative medicine and other biomedical applications[1-3]. Unlike other 3D printing techniques which print non-living materials, bioprinting incorporates living materials during the printing process. However, the bioprinted structures are still different from complex native human tissue or organ. One reason is that “Bioink” which mostly refers to hydrogels has a relatively low mechanical integrity compared to other 3D printing materials such as metals, ceramics and polymers[4-7]. Some of the hydrogels are highly biocompatible and even able to promote tissue growth and tissue formation, but hydrogels that have good biocompatibility usually have low printability and low mechanical strength before and during printing. For example, collagen and gelatin-methacrylate (GelMA) has good bio- compatibility, but their printability is poor and the mechanical strength is low. The viscosities of both hydrogels are low at the human body temperature (or before crosslinking stage). This may favor direct printing of cell-hydrogel suspensions without any change in environment or equipment such as temperature control unit[2,8], but it is difficult to print them into 3D shapes without using strength enhancement strategy (e.g. chemical crosslinking or adding thickener). A second reason is that there is a lack of sufficient support materials suitable for bioink. Support, also known as sacrificial material or structure, is a basic but important concept in 3D printing. It allows the fabrication of overhang features and complex internal structures. Similarly, in 3D bioprinting, it is difficult to print 3D complex shapes and geometries without using support. Therefore, at the current stage, bioprinting of complex hollow structures that can completely mimic human’s vascular systems or hollow organs such as heart or kidney is very challenging. Single material printing will not be sufficient to provide all the required properties (including bio- compatibility, mechanical integrity and printability)[1-9] for bioprinted 3D complex hollow structures.
2. Roles of Support Materials in Bioprinting
Recently, printing beyond 2.5D (2.5D shape is the shape that comes from repeatedly printing a 2D pattern in Z direction without changing the pattern in any layer) grid structure and simple tubes into the micro-channeled and hollow structures represent a great advancement in bioprinting[10,11]. It is no longer stacking layer by layer in the same pattern. To form small channels or hollow tubes, a s econd “bioink” is used. The second bioink acts as support material or sacrificial material. As shown in Figure 1, the second ink acts as mold or sacrificial materials to create a hollow structure or track for perfusion purpose. This technique normally composes of chemically cros- slinked hydrogel especially UV crosslinked hydrogel such as GelMA[11] or polyethylene glycol diacrylate (PEGDA)[12] as model materials and usually thermo-responsive hydrogels such as pluronic F127 or agarose as support materials[2]. By incorporating both types of hydrogels, the complex hollow or complex track pattern in 2.5D level or microfluidic level is possible to be fabricated.
Figure 1.
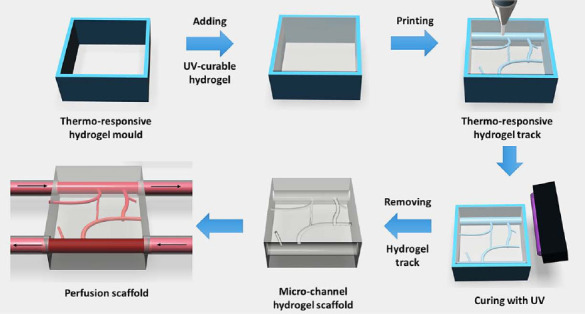
Schematic of hollow structure or track fabrication using two different types of hydrogels. UV curable hydrogel is model material whereas thermo-responsive hydrogel is sacrificial materials.
Even though the complex track has more advancement compared to just 2.5D lattice structure, in the reality, the real human organ is never in 2.5D plane. Rather, human organ is an intricate 3D structure which has a very complex shape, fine details and topography. Therefore, the technique in Figure 1 is not sufficiently adequate to bring 3D bioprinting to the level of printing a human organ. This is due to the fact that only one of the materials, either model or support materials (mostly support material), has the good mechanical strength which is responsible for the shape stability and shape integrity.
Another recent advancement is the development of freeform reversible embedding of suspended hydro- gels or “FRESH” technique, which allows 3D structure to be fabricated by using gelatin bath as the support material with many types of other hydrogels as model materials such as alginate with calcium chloride (CaCl2) or fibrinogen and thrombin[13]. However, this technique needs precision control in temperature in addition to positional control. Gelatin is thermo-responsive which will start to melt when the temperature is above 30 °C[2]. Therefore, temperature control is critical for “FRESH”. Moreover, the “FRESH” technique is batch by batch specific so the crosslinked agent must be pre-mixed with gelatin bath first. Lastly, because of gelatin bath, the printing temperature needs to be lower than 30 °C (around 22-25 °C) which is not ideal for printing cells over a prolonged period. To date, “FRESH” is considered one of the most advanced techniques. To sum up, a list of support materials for bioprinting is shown in Table 1.
Table 1.
Common support hydrogels in bioprinting
| Support hydrogel | Model hydrogel | Bioprinted form | References |
|---|---|---|---|
| Gelatin and derivatives | Alginate, Fibrin and collagen | 3D hollow structure | [13] |
| Agarose | GelMA, SPELA*, PEGDMA** and PEGDA | 2.5D complex track | [14] |
| Pluronic and derivatives | GelMA and Agarose | 2.5D complex track and micro-pattern | [11,15] |
Star poly(ethylene glycol-co-lactide) acrylate
Poly(ethylene glycol) dimethacrylate
Here comes the question, what is the next advancement for support material in bioprinting? In 3D printing, support materials always play an important role to create overhanging and hollow structures. As shown in Figure 2 which is an anatomical heart model, the overhanging part (Figure 2 A) needs a support to hold even in other 3D printing techniques (Figure 2B). It is almost impossible for bioprinting to fabricate this structure without using support. However, comparing the materials that have been used in fused deposition modelling (FDM) with hydrogels or bioinks for bio- printer, the mechanical strength of the FDM materials is typically stronger[4-16]. The current hydrogels that have been used with bioprinter are too soft to hold the shape. Moreover, with the high water content, osmosis pressure will affect the interaction between model and support materials (e.g. water travels by osmosis pressure to another hydrogel which will lead to change in concentration, viscosity and mechanical properties). Thus, the requirements needed in bioprinting for fabricating 3D complex hollow structures are: (i) the material (including both model and support) must have sufficient shape integrity and can be printed in a cell friendly environment. To ensure biocompatibility properties, the bio-derived materials or even extracellular matrix (ECM) derived materials are preferred. Furthermore, to enhance mechanical properties, strong bio-derived materials such as silk or hydroxyapa- tite can be integrated with the model hydrogel as the composite natural materials; (ii) there is no or minimal reaction between model and support materials; and (iii) support materials must be easily removable without sacrificing cell viability. In order to achieve this property, either the concentration of model and support materials need to be similar or there is an interaction to create wall between model and support materials. For example, if model material contains Ca2+ and support contains alginate, the wall can be created by semi physical crosslinked reaction of alginate and calcium ion. All the mentioned techniques need to involve with the advancement of materials science and chemistry to understand the nature of materials and reaction mechanism.
Figure 2.
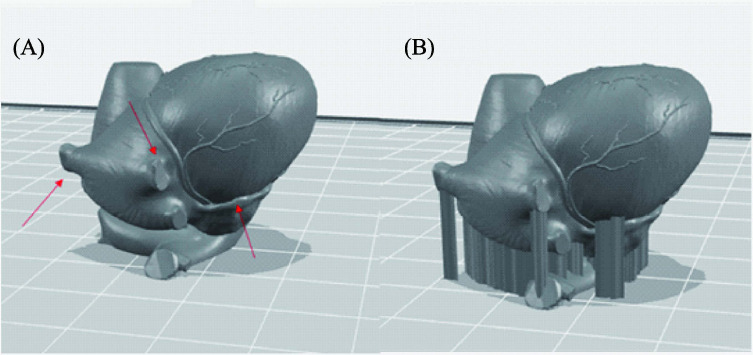
(A) Computer model of anatomical heart (red arrows pointed the overhanging parts) and (B) Computer model of anatomical heart with support structure.
3. Conclusion
It is evident that support materials are essential for both 3D printing and bioprinting. In 3D printing, the support materials have been used to fabricate complex structures with high resolution, which should be applicable to bioprinitng as well. The support materials may also be applied for upscale printing, organ printing and the advancement in tissue model for drug delivery and other related biomedical applications. In terms of achieving 3D complex hollow structures, it is challenging to rely on single material to accomplish it. The 2.5D complex track by using sacrificial materials has proved its application for lab-on-a chip and organ-on-a chip level. Nevertheless, in order to scale up beyond micro-level, further improvements in materials research such as new biocomposite material or novel derived natural materials for both model and support materials for bioprinters are needed.
Conflict of Interest and Funding
No conflict of interest was reported by the authors.
Acknowledgements
Singapore Centre for 3D Printing (SC3DP) is supported by the Singapore National Research Foundation (NRF).
References
- 1.Murphy S V, Atala A. 3D bioprinting of tissues and organs. Nature Biotechnology. 2014;32:779–785. doi: 10.1038/nbt.2958. https://dx.doi.org/10.1038/nbt.2958. [DOI] [PubMed] [Google Scholar]
- 2.Suntornnond R, An J, Chua C K. Bioprinting of thermoresponsive hydrogels for next generation tissue engineering: A review. Macromolecular Materials and Engineering. 2016 https://doi.org/10.1002/mame.201600266. [Google Scholar]
- 3.Lee J M, Yeong W Y. Design and printing strategies in 3D bioprinting of cell-hydrogels: A review. Advanced Healthcare Material. 2016 doi: 10.1002/adhm.201600435. https://doi.org/10.1002/adhm.201600435. [DOI] [PubMed] [Google Scholar]
- 4.Schuurman W, Khristov V, Pot M W, et al. Bioprinting of hybrid tissue constructs with tailorable mechanical properties. Biofabrication. 2011;3:021001. doi: 10.1088/1758-5082/3/2/021001. https://doi.org/10.1088/1758-5082/3/2/021001. [DOI] [PubMed] [Google Scholar]
- 5.Cheah C M, Leong K F, Chua C K, et al. Characterization of microfeatures in selective laser sintered drug delivery devices. Proceedings of the Institution of Mechanical Engineers, Part H: Journal of Engineering in Medicine. 2002;216:369–383. doi: 10.1243/095441102321032166. https://doi.org/10.1243/095441102321032166. [DOI] [PubMed] [Google Scholar]
- 6.Yeong W Y, Chua C K, Leong K F, et al. Comparison of drying methods in the fabrication of collagen scaffold via indirect rapid prototyping. Journal of Bio- medical Materials Research —Part B Applied Biomate- rials. 2007;82:260–266. doi: 10.1002/jbm.b.30729. https://doi.org/10.1002/jbm.b.30729. [DOI] [PubMed] [Google Scholar]
- 7.Kok Y H, Tan X P, Loh N H, et al. Geometry dependence of microstructure and microhardness for selective electron beam-melted Ti-6Al-4V parts. Virtual and Physical Prototyping. 2016;11:183–191. https://doi.org/10.1080/17452759.2016.1210483. [Google Scholar]
- 8.Ozbolat I T, Hospodiuk M. Current advances and future perspectives in extrusion-based bioprinting. Biomaterials. 2016;76:321–343. doi: 10.1016/j.biomaterials.2015.10.076. https://dx.doi.org/10.1016/j .biomaterials.2015.10.076. [DOI] [PubMed] [Google Scholar]
- 9.Murphy S V, Skardal A, Atala A. Evaluation of hydrogels for bio-printing applications. Journal of Biomedical Materials Research Part A. 2013;101A:272–284. doi: 10.1002/jbm.a.34326. https://doi.org/10.1002/jbm.a.34326. [DOI] [PubMed] [Google Scholar]
- 10.Kucukgul C, Ozler S B, Inci I, et al. 3D bioprinting of biomimetic aortic vascular constructs with self- supporting cells. Biotechnology and Bioengineering. 2015;112:811–821. doi: 10.1002/bit.25493. https://doi.org/10.1002/bit.25493. [DOI] [PubMed] [Google Scholar]
- 11.Kolesky D B, Truby R L, Gladman A S, et al. 3D bioprinting of vascularized, heterogeneous cell-laden tissue constructs. Advanced Materials. 2014;26:3124, 3130. doi: 10.1002/adma.201305506. https://doi.org/10.1002/adma.201305506. [DOI] [PubMed] [Google Scholar]
- 12.Hoch E, Tovar G E M, Borchers K. Bioprint- ing of a rtificial blood vessels: Current approaches towards a demanding goal. European Journal of Car- dio-Thoracic Surgery. 2014;46:767–778. doi: 10.1093/ejcts/ezu242. https://doi.org/10.1093/ejcts/ezu242. [DOI] [PubMed] [Google Scholar]
- 13.Hinton T J, Jallerat Q, Palchesko R N, et al. Three-dimensional printing of complex biological structures by freeform reversible embedding of suspended hydrogels. Science Advances. 2015;1 doi: 10.1126/sciadv.1500758. https://doi.org/10.1126/sciadv.1500758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bertassoni L E, Cecconi M, Manoharan V, et al. Hydrogel bioprinted microchannel networks for vascularization of tissue engineering constructs. Lab on a Chip. 2014;14:2202–2211. doi: 10.1039/c4lc00030g. https://doi.org/10.1039/C4LC00030G. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Muller M, Becher J, Schnabelrauch M, et al. Printing thermoresponsive reverse molds for t he creation of patterned two-component hydrogels for 3D cell culture. Journal of Visualized Experiments. 2013:e50632–e50632. doi: 10.3791/50632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Boparai K, Singh R, Singh H. Comparison of tri- bological behaviour for Nylon6-Al-Al2O3and ABS parts fabricated by fused deposition modelling. Virtual and Physical Prototyping. 2015;10:59–66. https://doi.org/10.1080/17452759.2015.1037402. [Google Scholar]


