Abstract
3D bioprinting is an emerging technology that enables the fabrication of three-dimensional organised cellular constructs. One of the major challenges in 3D bioprinting is to develop a material to meet the harsh requirements (cell-compatibility, printability, structural stability post-printing and bio-functionality to regulate cell behaviours) suitable for printing. Gelatin methacryloyl (GelMA) has recently emerged as an attractive biomaterial in tissue engineering because it satisfies the requirements of bio-functionality and mechanical tunability. However, poor rheological property such as low viscosity at body temperature inhibits its application in 3D bioprinting. In this work, an enzymatic crosslinking method triggered by Ca2+-independent microbial transglutaminase (MTGase) was introduced to catalyse isopeptide bonds formation between chains of GelMA, which could improve its rheological behaviours, specifically its viscosity. By combining enzymatic crosslinking and photo crosslinking, it is possible to tune the solution viscosity and quickly stabilize the gelatin macromolecules at the same time. The results showed that the enzymatic crosslinking can increase the solution viscosity. Subsequent photo crosslinking could aid in fast stabilization of the structure and make handling easy.
Keywords: microbial transglutaminase, enzymatic crosslinking, photo crosslinking, viscosity
1. Introduction
Organ shortage[1] calls for a great need for the development of new biological substitutes. Tissue engineering has emerged as an attractive method to meet this need. The classic tissue engineering strategy is to seed specific cells isolated from a biopsy onto a three-dimensional (3D) scaffold, occasionally incorporating growth factors, to provide a temporal support for cell proliferation, differentiation and eventually formation of neotissue[2]. One major limitation of this strategy is the lack of precision in cell placement due to manual cell seeding; it is difficult to place different cell types at certain position depending on the type and function of a tissue[3]. To overcome this drawback, an automated and precise technology known as 3D bioprinting has gained scientists’ interest in recent years. It is a computer-controlled process to produce 3D constructs layer by layer, in which cells mixed with biomaterials can be distributed in a certain position[4]. This direct method makes it an attractive tool for the development of 3D-organised cellular constructs with special biological and mechanical properties[5].
One major challenge of 3D bioprinting is to develop a printing material to meet a repertoire of characteristics suitable for printing. The printing materials should have suitable physiochemical properties such as shear thinning, high viscosity, as well as post-printing structural stability[6,7]. Moreover, the materials should provide a desirable environment for cells to encapsulate, migrate, proliferate and differentiate[8]. Hydrogels exert great potential as printing materials due to their cell-encapsulating ability and their mimicking of physical and chemical properties of the extracellular matrix (ECM)[9]. The difficulty lies in the delicate balance between printability and biological properties of hydrogels towards 3D bioprinting. Increasing the polymer concentration results in a highly viscous hydrogel precursor and a quick gelation into a crosslinked hydrogel, which provides good printability and high shape fidelity[10,11], but a dense polymer network can inhibit the formation of new ECM and matrix remodelling as well as cell migration[12,13]. Therefore, the development of a hydrogel system with appropriate balance of printability and cell support will promote hydrogel application in 3D bioprinting.
Large numbers of natural-or synthetic-derived hydrogels have been studied for 3D bioprinting such as alginate[14], collagen[15], gelatin[16] and poly(ethylene glycol) diacrylate[17]. Among those materials, gelatin is an attractive material with biological cues containing cell-adhesion motifs (arginine-glycine-aspartic acid (RGD) sequences) and target sites for matrix metalloproteinase (MMP) in cell remodelling and degradation[18]. The thermally sensitive ability of gelatin can support the printing process[19-22]. Moreover, gelatin can be modified with methacrylamide and a minority of methacrylate groups, resulting in a photo-crosslinkable material-gelatin methacryloyl (GelMA)[23]. GelMA retains biofunctionality from gelatin[18] and its photocrosslinkable property enables quick formation of a covalently crosslinked hydrogel, which maintains the printed construct permanently, thus becoming stable under physiological temperature[24].
GelMA has been demonstrated as a suitable printing material for 3D bioprinting. Printing GelMA requires relatively high polymer concentrations due to low viscosity at 37 °C[24]; however, previous work has shown that the high polymer concentration could compromise cell viability[24-26]. Nichol et al. studied cell viability of NIH 3T3 fibroblasts encapsulated in 5%-15% GelMA, and high cell viability (>80%) was generally observed in below 10% GelMA[27]. Additionally, to improve the printability of GelMA, precise control of the nozzle temperature and the cooling down the platform have been conducted to successfully print GelMA, but then the hardware becomes important[28]. Thus, the development of a smart system with improved rheological properties is imperative for using GelMA in 3D bioprinting.
In this work, an enzymatic crosslinking process triggered by a Ca2+-independent microbial transglutaminase (MTGase), a nontoxic crosslinker with high specific activity[29], was introduced to catalyse the isopeptide formation between the y-carboxamides of glutamine residues and e-primary amino of lysine residues in chains of GelMA[30]. We hypothesize that this enzymatic crosslinking method could improve the rheological properties and printability. We examined the gelling behaviour and viscosity of 10% GelMA solution treated with MTGase, as well as the mechanical properties of hydrogels formed by enzymatic crosslinking and photo crosslinking.
2. Materials and Method
2.1 Materials
Methacrylic anhydride (MAAnh), Irgacure 2959 (I2959), deuterium oxide (D2O) and gelatin (gel strength ~ 175 g Bloom, Type A, from porcine skin) were purchased from Sigma-Aldrich (St. Louis, MO, USA). MAAnh is a reactant for synthesizing gelatin methacryloyl. I2959 is the most reported photo initiator for GelMA due to its water solubility and relatively low cytotoxicity compared with other photo initiators[31]. The microbial transglutaminase (MTGase) was obtained from Ajinomoto (Tokyo, Japan). The microbial transglutaminase powder with sodium caseinate and maltodextrin additives has an enzymatic activity of 100 U/g.
2.2 Synthesis of GelMA
The synthesis of GelMA was carried out under the optimized condition according to the previous work[32,33]. Briefly, GelMA was prepared by reaction of type A gelatin with methacrylic anhydride as in the following: 7.95 g of Na2CO3 and 14.65 g of NaHCO3 were dissolved in 1 L distilled water to produce 0.25 mol/L carbonate-bicarbonate (CB) buffer solution. Following that, 50 g of gelatin was dissolved into 500 mL of the as-prepared buffer. The pH value of the gelatin solution was gradually adjusted to 9 by adding 5 mol/L NaOH solution in a dropwise manner. MAAnh was added to the solution to achieve an MAAnh:gelatin ratio of 0.05 mL/g. The reaction proceeded at 50 °C for 3 h. 1 mol/L HCl was added and the reaction was stopped when the pH value of the solution was adjusted to 7.4. The crude product was filtered and dialyzed using Minimate TFF system (Pall Corporation, New York, NY, USA) with a 10K MWCO cassette to remove any unreacted MAAnh and methacrylic acid by-product. Finally, GelMA was lyophilized to obtain a dried product and stored at -20 °C for future use.
2.3 1H NMR Characterization
The methacryloylation of gelatin was measured by using 1H NMR spectroscopy. The GelMA solution had a concentration of 50 mg/mL in D2O and 1H NMR spectra were repetitively collected for three times. Purely absorptive signals were corrected by phase correction. The areas of the peaks were integrated after baseline correction. The degree of methacryloylation (DM) was defined by Equation 1 where the percentage of e-amino groups of gelatin modified with methacryloyl groups was calculated.
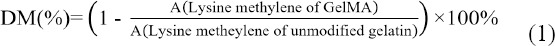
2.4 Preparation of Enzymatic Crosslinked GelMA (MTGase-GelMA) Solutions
GelMA solution was prepared by dissolving the lyophilized GelMA at a concentration of 10% (w/v) in phosphate-buffered saline (PBS) solution. Different amounts of MTGase were separately added to the as-prepared GelMA solutions so that the final concentrations were 1, 3 and 5 U/mL. The incubation temperature was 37 °C.
2.5 Preparation of Hydrogels with Different Crosslinking
The enzymatic crosslinked hydrogels were prepared by pouring 200 μL 10% (w/v) GelMA with 3 U/mL MTGase into a cylindrical mould, and sealed and incubated at 37 °C for 12 h. The photo-crosslinked hydrogels were prepared by pouring 200 μL of 10% (w/ v) GelMA solution containing 0.1% (w/v) Irgacure 2959 into a cylindrical mould at 37 °C and exposed to UV light (λ = 365 nm with an intensity of 1.5 mW/cm2) for 5 min. The dual crosslinked hydrogels were prepared by a mixture of the above steps; after incubating the MTGase-GelMA solution at 37 °C for 12 h, the samples were then UV-cured for 5 min. All the samples were taken out of the mould for further experiments, of which the dimensions were 8 mm in diameter and 3 mm in height.
2.6 Rheological Properties
The rheological properties of the enzymatically crosslinked hydrogels were tested by a rheometer (MCR 501, Anton Paar Germany GmbH, Ostfildern, Germany) with a 25-mm cone-plate geometry and with an angle of 2°. To study the formation of gel network, the time sweep test was performed where storage modulus (G’) and loss modulus (G”) were monitored as a function of time at a fixed frequency of 1 Hz and strain of 3%. To avoid the evaporation of water, the MTGase-GelMA solutions were sealed in the tube and incubated at 37 °C, then sequentially loaded onto the rheometer at an interval of 1 hour and tested for 1 min. During testing, measurements were taken every second, and the average of the 60 data points represented the average modulus of the sample at different incubation time periods. The time-dependent viscosity during the enzymatic crosslinking proceeding was tested at 37 °C under the shear rate of 100 s-1, in a similar way of data collection described earlier. The flow behaviour of solutions was examined within the range of shear rate from 0.1 to 1000 s-1 at 37 °C.
Frequency sweep was carried out to test the viscoelastic properties of the hydrogels by using a parallel plate geometry with 10-mm diameter. The mechanical spectra were recorded with 2% strain over a frequency range from 0.1 to 10 Hz at 37 °C.
3. Results and Discussion
3.1 Methacryloylation of Gelatin
The chemical structures of unmodified gelatin and GelMA are shown in Figure 1A and B. Compared with the spectrum of unmodified gelatin, GelMA sample formed new functional groups, marked as green “a” and blue “c” in Figure 1B, which can be confirmed by the 1H NMR spectra (Figure 1C). The peaks at around chemical shifts (δ) of 5.3 and 5.6 ppm were assigned to the acrylic protons (2H) of the grafted methacryloyl group, and another peak at δ = 1.9 ppm was attributed to the methyl group (3H) of the grafted methacryloyl group. Meanwhile, there was a decrease of intensity at 2.9 < δ < 3.1 ppm, which was assigned to the lysine methylene (2H) and marked as pink “b”. As lysine is the reaction site, this trend could be used to quantify DM, which yielded to be 53.5% ± 0.9%. The remaining lysine groups could be utilized for enzymatic crosslinking as it is an acyl acceptor.
Figure 1.
The chemical structures of (A) unmodified gelatin and (B) GelMA, and (C) their respective 1H-NMR spectra. Green “a” and blue “c” represent the signals of the methyl group and acrylic protons of the grafted methacrylic group respectively, and pink “b” indicates the signal of lysine methylene.
3.2 Rheological Characterization
3.2.1 Gelling Period of GelMA Incubated with MTGase
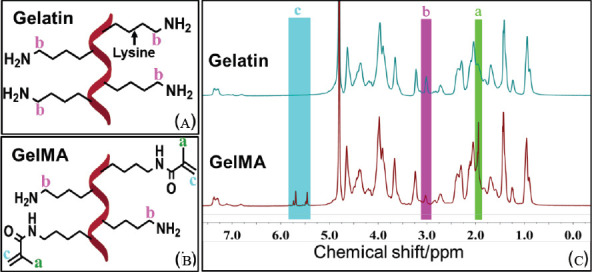
The mechanism of enzymatic crosslinking is that MTGase catalyses the inter-and intra-molecular bonds formation between the γ-carboxamides of glutamine residues and e-primary amino of lysine residues in the chains of GelMA (Figure 2). The growth of a connected structure will eventually lead to formation of a chemical gel.
Figure 2.
The crosslinking mechanism of the MTGase-GelMA hydrogel
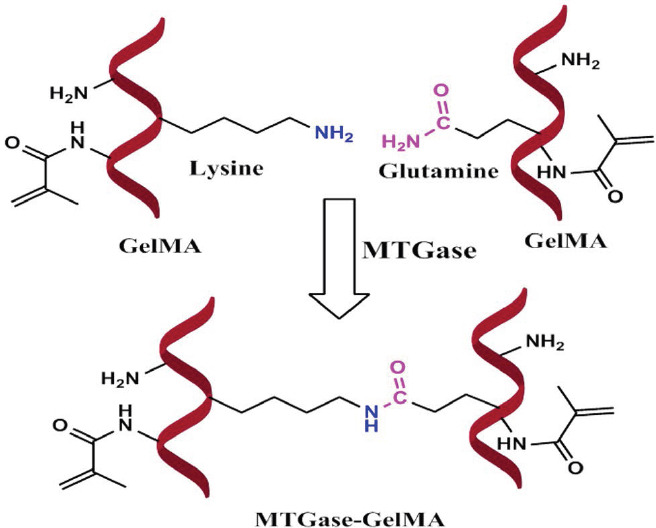
To determine that the reaction took place, a time sweep test was performed at the incubation temperature (37 °C). The effect of the MTGase concentration on the gel formation of 10% GelMA was examined. There were two moduli generated from these experiments: G’ representing the deformation energy stored, and G” being the energy dissipated during shear. They are sensitive to molecular structure evolution, especially the formation of network.
Figure 3 shows that, at the beginning, the GelMA solution with MTGase behaved liquid-like, where G” is higher than G’. When gelation takes place, there is a crossover of G’ and G” (whereby the value of G’ is higher), which could be seen for the 10% GelMA incubated with 5 U/mL MTGase. The effect of the concentration of MTGase on the gelling period is summarized in Table 1. There was no gel formation observed for GelMA solution containing 1 U/mL MTGase within 4 h. Above 1 U/mL MTGase, gelling periods were detected when the MTGase concentration was increased: 3-4 h for 3 U/mL MTGase and 1-2 h for 5 U/mL MTGase. Additionally, the gelling period of hydrogels was confirmed by the tube inversion method (Figure 3, inset). Those results reveal that MTGase does exhibit a crosslinking action; the gelling times for the MTGase-GelMA hydrogels are shortened by raising the MTGase concentration due to the enhanced catalytic activity.
Figure 3.
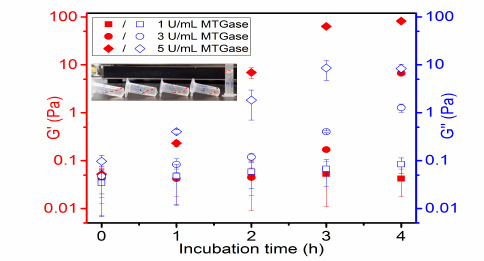
Effect of the MTGase concentration on the gelling period of 10% GelMA solutions at incubation temperature (37 °C). The inset photo shows representative images of transition from a liquid to a chemical gel of 10% GelMA treated with 3 U/ mL MTGase at 37 °C.
Table 1.
Gelling period of enzyme-catalysed MTGase-GelMA with various concentrations of MTGase at 37 °C with a fixed GelMA concentration of 10% (w/v)
| MTGase Concentration (U/mL) | Gelling period (within 4 hours) |
|---|---|
| 1 | - |
| 3 | 3-4 h |
| 5 | 1-2 h |
It has been reported that MTGase catalyses the conversion of gelatin solutions into hydrogels, and gelling times depends on the type and concentration of gelatin[34]. Type A gelatin was selected in the study because Type A gelatin prepared by acid treatment is more effective for enzymatic crosslinking than Type B gelatin prepared by base treatment, as base treatment can hydrolyse the amide groups of glutamine residues and suppress enzymatic crosslinking.
3.2.2 Viscosity During Incubation with MTGase
Viscosity and shear thinning behaviour are important properties which affect the extrusion process in 3D printing[6]. The nozzle is easily clogged when the viscosity is too high within the nozzle tip during extrusion[35]; however, a relatively high viscosity is required to avoid the surface tension-driven droplet formation and the collapse of post-extrusion structure[36]. Thus, a material with shear thinning behaviour and a suitable viscosity will be favoured for 3D printing. Murphy et al. summarized that a range of viscosity (30 mPa-s to 6 x 107 mPa.s) would be suitable for extrusion-based printing[5]. The viscosity of 10% GelMA solution without MTGase treatment was 5.9 mPa-s (Figure 4A) under the shear rate of 100 s-1 (which was reported as the shear rate of materials experienced in the needle tip[37,38]), which was far below the aforementioned range of printing viscosity. Literature has shown that the viscosity of gelatin solution increases after transglutaminase induced-enzymatic crosslinking[39], so for the current study, it was intended to alter the viscosity of 10% GelMA solution with MTGase treatment, and thus the viscosities of 10% GelMA solutions incubated with different concentrations of MTGase at 37 °C were investigated under the shear rate of 100 s-1. Additionally, the flow behaviours of the solutions were studied within the range of shear rates from 0.1 to 1000 s-1, as shown in Figure 4B.
Figure 4.
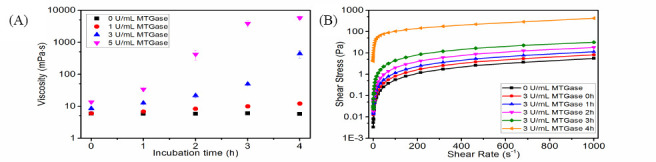
(A) The time-dependent viscosity of 10% GelMA solution incubated with different concentration of MTGase at 37 °C under the shear rate of 100 s-1; (B) the flow behaviour of 10% GelMA solution incubated with 3 U/mL MTGase at 37 °C
Figure 4A shows that the addition of MTGase resulted in a net increase in viscosity after incubation at 37 °C. The rate of viscosity increment was higher with increasing MTGase concentration. At a concentration of 1 U/ mL MTGase, the viscosity of 10% GelMA solution increased from 5.9 mPa-s (0 h) to 12.1 mPa-s (4 h), while there was a great change in viscosity for 10% GelMA containing 3 U/mL MTGase and 5 U/mL MTGase, from 8.4 mPa-s (0 h) to 438.5 mPa-s (4 h) and 13.6 mPa-s (0 h) to 5776.1 mPa-s (4 h), respectively. The addition of MTGase enzyme catalyses the covalent crosslinking action in gelatin, resulting in an increased molecular weight and crosslinking degree[40], and most likely contributing to higher viscosity when added to GelMA. Though higher viscosity values can reach the threshold value suitable for printing, the continued increment in viscosity (Figure 4A) may eventually lead to the clogging of nozzle over time. Thus, a suitable incubation time should be optimized in the future. Notably, the solutions exhibited shear-thinning behaviour at certain incubation times (Figure 4B), which would facilitate the printing process.
3.3 The Effect of Different Crosslinking Methods on Viscoelastic Properties of Hydrogels
While sufficient viscosity is required for printability during printing, further gelation is necessary for handling and maintaining the final constructed shape. Thus, frequency sweep test was performed to investigate the effects of single (either enzymatic or photo-crosslinking) or dual (both enzymatic and photo-) crosslinking on viscoelastic properties. The enzymatic crosslinked hydrogels formed during incubation with MTGase. Subsequently, enzymatic crosslinked hydrogels were photocured on exposure to UV light to support long-term stability of GelMA constructs. Such dual crosslinked hydrogels exhibited enhanced viscoelastic properties when compared with single crosslinked samples, as evident from Figure 5; the enzymatic crosslinked hydrogel had the lowest G’ when compared to photo-and dual-crosslinked hydrogels, with the latter exhibiting the largest G’ values. Figure 6 shows structural integrity, in increasing order, during the handling of the constructs with the type of crosslinking (single or dual).
Figure 5.
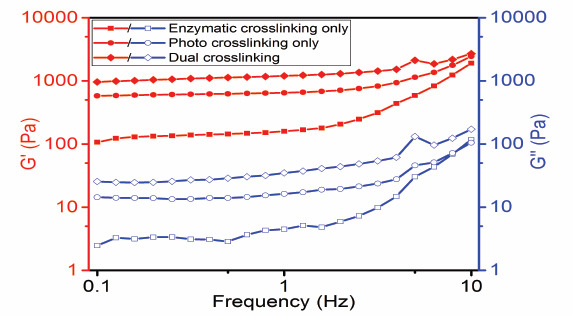
Viscoelastic properties of different crosslinked hydrogels. Enzymatic crosslinking hydrogel was formed by incubating 10% GelMA with 3 U/mL MTGase for 12 h. Photo crosslinking hydrogel was formed by curing 10% GelMA at 1.5 mW/cm2 for 5 min. Dual crosslinking hydrogel was formed by enzymatic crosslinking and then by photo crosslinking.
Figure 6.
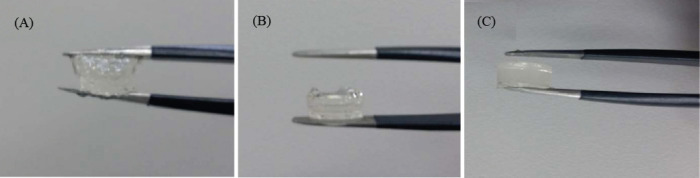
Handling of (A) enzymatic, (B) photo-and (C) dual crosslinked hydrogels
4. Conclusion
In this study, an enzymatic crosslinking method was introduced to GelMA system to improve its rheological properties for 3D printing application. It was found that MTGase could catalyse the bond formation in GelMA. The viscosity of GelMA solution could be increased by increasing the enzyme concentration and incubation time, and the solutions exhibited shear-thinning behaviour. Subsequently, fast photo-crosslinking of GelMA aided in maintaining its structural integrity. Such dual crosslinked hydrogels could achieve higher mechanical stability. Thus, MTGase treatment on GelMA could be a practical method to facilitate the application of GelMA in 3D printing.
Conflict of Interest and Funding
No conflict of interest was reported by the authors. The authors gratefully acknowledge the National Research Foundation (NRF) Competitive Research Programme (NRF-CRP10-2012-07) for the financial support.
References
- 1.Abouna G M. Organ shortage crisis:Problems and possible solutions. Transplantation Proceedings. 2008;40(1):34–38. doi: 10.1016/j.transproceed.2007.11.067. http://dx.doi.org/10.1016/j.transproceed.2007.11.067. [DOI] [PubMed] [Google Scholar]
- 2.Ikada Y. Vol. 8. San Diego, USA: 2006. Tissue engineering: Fundamentals and applications. [Google Scholar]
- 3.Wust S, Muller R, Hofmann Controlled positioning of cells in biomaterials—Approaches towards 3D tissue printing. Journal of Functional Biomaterials. 2011;2(3):119–154. doi: 10.3390/jfb2030119. http://dx.doi.org/10.3390/jfb2030119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gu B K, Choi D J, Park S J, et al. 3-dimensional bioprinting for tissue engineering applications. Biomaterials Research. 2016;20(1):12. doi: 10.1186/s40824-016-0058-2. http://dx.doi.org/10.1186/s40824-016-0058-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Murphy S V, and Atala A. 3D bioprinting of tissues and organs. Nature Biotechnology. 2014;32(8):773–785. doi: 10.1038/nbt.2958. http://dx.doi.org/10.1038/nbt.2958. [DOI] [PubMed] [Google Scholar]
- 6.Jungst T, Smolan W, Schacht K, et al. Strategies and molecular design criteria for 3D printable hydrogels. Chemical Review. 2016;116(3):1496–1539. doi: 10.1021/acs.chemrev.5b00303. http://dx.doi.org/10.1021/acs.chemrev.5b00303. [DOI] [PubMed] [Google Scholar]
- 7.Holzl K, Lin S, Tytgat L, et al. Bioink properties before, during and after 3D bioprinting. Biofabrication. 2016;8(3):032002. doi: 10.1088/1758-5090/8/3/032002. http://dx.doi.org/10.1088/1758-5090/8/3/032002. [DOI] [PubMed] [Google Scholar]
- 8.Carrow J K, et al. Polymers for bioprinting. In: Atala A, Yoo J J, editors. Essentials of 3D Biofabrication and Translation. Oxford, UK: Academic Press; 2015. [Google Scholar]
- 9.Peppas N A, Hilt JZ, Khademhosseini A, et al. Hydrogels in biology and medicine: From molecular principles to bionanotechnology. Advanced Materials. 2006;18(11):13451360. http://dx.doi.org/10.1002/adma.200501612. [Google Scholar]
- 10.Gaetani R, Doevendans P A, Metz C H, et al. Cardiac tissue engineering using tissue printing technology and human cardiac progenitor cells. Biomaterials. 2012;33(6):1782–1790. doi: 10.1016/j.biomaterials.2011.11.003. http://dx.doi.org/10.1016/j.biomaterials.2011.11.003. [DOI] [PubMed] [Google Scholar]
- 11.Khalil S, Sun W. Bioprinting endothelial cells with alginate for 3D tissue constructs. Journal of Biomechanical Engineering. 2009;131(11):111002. doi: 10.1115/1.3128729. http://dx.doi.org/10.1115/L3128729. [DOI] [PubMed] [Google Scholar]
- 12.Brandl F, Sommer F, Goepferich A. Rational design of hydrogels for tissue engineering: Impact of physical factors on cell behavior. Biomaterials. 2007;28(2):134–146. doi: 10.1016/j.biomaterials.2006.09.017. http://dx.doi.org/10.1016/j.biomaterials.2006.09.017. [DOI] [PubMed] [Google Scholar]
- 13.DeForest C A, KS Anseth. Advances in bioactive hydrogels to probe and direct cell fate. Annual Review of Chemical andBiomolecular Engineering. 2012;3:421–444. doi: 10.1146/annurev-chembioeng-062011-080945. http://dx.doi.org/10.1146/annurev-chembioeng-062011-080945. [DOI] [PubMed] [Google Scholar]
- 14.Markstedt K, Mantas A, Tournier I, et al. 3D bioprinting human chondrocytes with nanocellulose-alginate bioink for cartilage tissue engineering applications. Biomacromolecules. 2015;16(5):1489–1496. doi: 10.1021/acs.biomac.5b00188. http://dx.doi.org/10.1021/acs.biomac.5b00188. [DOI] [PubMed] [Google Scholar]
- 15.Park J Y, Choi J C, Shim J H, et al. A comparative study on collagen type I and hyaluronic acid dependent cell behavior for osteochondral tissue bioprinting. Biofabrication. 2014;6(3):035004. doi: 10.1088/1758-5082/6/3/035004. http://dx.doi.org/10.1088/1758-5082/6/3/035004. [DOI] [PubMed] [Google Scholar]
- 16.Duan B, Hockaday L A, Kang K H, et al. 3D bioprinting of heterogeneous aortic valve conduits with alginate/gelatin hydrogels. Journal of Biomedical Materials Research Part A. 2013;101A(5):1255–1264. doi: 10.1002/jbm.a.34420. http://dx.doi.org/10.1002/jbm.a.34420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shanjani Y, Pan C C, Elomaa L, et al. A novel bioprinting method and system for forming hybrid tissue engineering constructs. Biofabrication. 2015;7(4):045008. doi: 10.1088/1758-5090/7/4/045008. http://dx.doi.org/10.1088/1758-5090/7/4/045008. [DOI] [PubMed] [Google Scholar]
- 18.Klotz BJ, Gawlitta D, Rosenberg AJ, et al. Gelatin-methacryloyl hydrogels: Towards biofabrication-based tissue repair. Trends in Biotechnology. 2016;34(5):394–407. doi: 10.1016/j.tibtech.2016.01.002. http://dx.doi.org/10.1016/j.tibtech.2016.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang X, Yan Y, Pan Y, et al. Generation of three-dimensional hepatocyte/gelatin structures with rapid prototyping system. Tissue Engineering. 2006;12(1):83–90. doi: 10.1089/ten.2006.12.83. http://dx.doi.org/10.1089/ten.2006.12.83. [DOI] [PubMed] [Google Scholar]
- 20.Yan Y, Wang X, Pan Y, et al. Fabrication of viable tissue-engineered constructs with 3D cell-assembly technique. Biomaterials. 2005;26(29):5864–5871. doi: 10.1016/j.biomaterials.2005.02.027. http://dx.doi.org/10.1016/j.biomaterials.2005.02.027. [DOI] [PubMed] [Google Scholar]
- 21.Zhang T, Yan Y, Wang X, et al. Three-dimensional gelatin and gelatin/hyaluronan hydrogel structures for traumatic brain injury. Journal of Bioactive and Compatible Polymers. 2007;22(1):19–29. http://dx.doi.org/10.1177/0883911506074025. [Google Scholar]
- 22.Yan Y, Wang X, Xiong Z, et al. Direct construction of a three-dimensional structure with cells and hydrogel. Journal of Bioactive and Compatible Polymers. 2005;20(3):259–269. http://dx.doi.org/10.1177/0883911505053658. [Google Scholar]
- 23.Yue K, Trujillo-de Santiago G, Alvarez M M, et al. Synthesis, properties, and biomedical applications of gelatin methacryloyl (GelMA) hydrogels. Biomaterials. 2015;73:254271. doi: 10.1016/j.biomaterials.2015.08.045. http://dx.doi.org/10.1016/j.biomaterials.2015.08.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schuurman W, Levett P A, Pot M W, et al. Gelatin-methacrylamide hydrogels as potential biomaterials for fabrication of tissue -engineered cartilage constructs. Macromolecular Bioscience. 2013;13(5):551–561. doi: 10.1002/mabi.201200471. http://dx.doi.org/10.1002/mabi.201200471. [DOI] [PubMed] [Google Scholar]
- 25.Du Y, Lo E, Ali S, et al. Directed assembly of cell-laden microgels for fabrication of 3D tissue constructs. Proceedings of the National Academy of Sciences. 2008;105(28):9522–9527. doi: 10.1073/pnas.0801866105. http://dx.doi.org/10.1073/pnas.0801⇕05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yeh J, Ling Y, Karp J M, et al. Micromolding of shape-controlled, harvestable cell-laden hydrogels. Biomaterials. 2006;27(31):5391–5398. doi: 10.1016/j.biomaterials.2006.06.005. http://dx.doi.org/10.1016/j.biomaterials.2006.06.005. [DOI] [PubMed] [Google Scholar]
- 27.Nichol J W, Koshy S T, Bae H, et al. Cell-laden micro-engineered gelatin methacrylate hydrogels. Biomaterials. 2010;31(21):5536–5544. doi: 10.1016/j.biomaterials.2010.03.064. http://dx.doi.org/10.1016/j.biomaterials.2010.03.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Billiet T, Gevaert E, De Schryver T, et al. The 3D printing of gelatin methacrylamide cell-laden tissue-engineered constructs with high cell viability. Biomaterials. 2014;35(1):49–62. doi: 10.1016/j.biomaterials.2013.09.078. http://dx.doi.org/10.1016/j.biomaterials.2013.09.078. [DOI] [PubMed] [Google Scholar]
- 29.Zhao L, Lib X, Zhao J, et al. A novel smart injectable hydrogel prepared by microbial transglutaminase and humanlike collagen:Its characterization and biocompatibility. Materials Science and Engineering: C. 2016;68(1):317–326. doi: 10.1016/j.msec.2016.05.108. http://dx.doi.org/10.1016/j.msec.2016.05.108. [DOI] [PubMed] [Google Scholar]
- 30.Kieliszek M, Misiewicz A. Microbial transglutaminase and its application in the food industry. A review Folia Microbiologica. 2014;59(3):241–250. doi: 10.1007/s12223-013-0287-x. http://dx.doi.org/10.1007/s12223-013-0287-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Williams C G, Malik A N, Kim T K, et al. Variable cytocompatibility of six cell lines with photoinitiators used for polymerizing hydrogels and cell encapsulation. Biomaterials. 2005;26(11):1211–1218. doi: 10.1016/j.biomaterials.2004.04.024. http://dx.doi.org/10.1016/j.biomaterials.2004.04.024. [DOI] [PubMed] [Google Scholar]
- 32.Shirahama H, Lee BH, Tan LP, et al. Precise tuning of facile one-pot gelatin methacryloyl (GeLMA) synthesis. Scientific Reports. 2016;6:31036. doi: 10.1038/srep31036. http://dx.doi.org/10.1038/srep31036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lee B H, Shirahama H, Cho N J, et al. Efficient and controllable synthesis of highly substituted gelatin methacrylamide for mechanically stiff hydrogels. RSC Advances. 2015;5(128):106094–106097. http://dx.doi.org/10.1039/C5RA22028A. [Google Scholar]
- 34.McDermott M K, Chen T, Williams C M, et al. Mechanical properties of biomimetic tissue adhesive based on the microbial transglutaminase-catalyzed crosslinking of gelatin. Biomacromolecules. 2004;5(4):1270–1279. doi: 10.1021/bm034529a. http://dx.doi.org/10.1021/bm034529a. [DOI] [PubMed] [Google Scholar]
- 35.Wust S, Godla M E, Muller R, et al. Tunable hydrogel composite with two-step processing in combination with innovative hardware upgrade for cell-based three-dimensional bioprinting. Acta Biomaterialia. 2014;10(2):630–640. doi: 10.1016/j.actbio.2013.10.016. http://dx.doi.org/10.1016Zj.actbio.2013.10.016. [DOI] [PubMed] [Google Scholar]
- 36.Malda J, Visser J, Melchels F P, et al. 25th anniversary article:Engineering hydrogels for biofabrication. Advanced Materials. 2013;25(36):5011–5028. doi: 10.1002/adma.201302042. http://dx.doi.org/10.1002/adma.201302042. [DOI] [PubMed] [Google Scholar]
- 37.Das S, Pati F, Chameettachal S, et al. Enhanced redifferentiation of chondrocytes on microperiodic silk/gelatin scaffolds:Toward tailor-made tissue engineering. Biomacromolecules. 2013;14(2):311–321. doi: 10.1021/bm301193t. http://dx.doi.org/10.1021/bm301193t. [DOI] [PubMed] [Google Scholar]
- 38.Li H, S Liu, L Lin. Rheological study on 3D printability of alginate hydrogel and effect of graphene oxide. International Journal of Bioprinting. 2016;2(2):54–66. http://dx.doi.org/10.18063/IJB.2016.02.007. [Google Scholar]
- 39.Yi J, Kim Y T, Bae H J, et al. Influence of transgluta-minase-induced cross-linking on properties of fish gelatin films. Journal of Food Science. 2006;71(9):E376–E383. http://dx.doi.org/10.1111/j.1750-3841.2006.00191.x. [Google Scholar]
- 40.Bae H J, Darby D O, Kimmel R M, et al. Effects of transglutaminase-induced cross-linking on properties of fish gelatin-nanoclay composite film. Food Chemistry. 2009;114(1):180–189. http://dx.doi.org/10.1016/j.foodchem.2008.09.057. [Google Scholar]


