Abstract
3D printing is a technology well-suited for biomedical applications due to its ability to create highly complex and arbitrary structures from personalized designs with a fast turnaround. However, due to a limited selection of 3D-printable materials, the biofunctionality of many 3D-printed components has not been paid enough attention. In this perspective, we point out that post-3D printing modification is the solution that could close the gap between 3D printing technology and desired biomedical functions. We identify architectural reconfiguration and surface functionalization as the two main post-3D printing modification processes and discuss potential techniques for post-3D printing modification to achieve desired biofunctionality.
Keywords: 3D printing, biomedical, post-3D printing modification, 4D printing, 3D-printed microfluidics
1. Introduction
3D-printing technology has garnered significant attention from the public, academia and industry in recent years[1-5]. It has been gradually transformed from a prototyping and modeling tool to a fabrication technique that promises a great potential of revolutionizing manufacturing industry[6]. Two unique features of 3D-printing technology make it well-suited for biomedical applications. Firstly, 3D printing is able to monolithically fabricate highly complex and arbitrary structures from digital designs, which is particularly useful in the fabrication of organ models, tissue engineering scaffolds and bioimplant devices with highly irregular and hierarchical architectures that are difficult to produce using traditional manufacturing techniques. Secondly, 3D printing produces fully customized components with a fast turnaround from design to production, which would make personalized medicine a reality in the near future.
Current applications of 3D printing in biomedical field mainly fall into three categories. The first category is biomodeling. At organ level, organs or other large biological entities are constructed from imaging data using 3D printing[7,8]. Examples include liver model used for surgical planning[9]. At molecular level, biomolecular models are created based on crystallographic informational These visual models are helpful in studying molecular interactions and dynamics[2]. The second category is in vivo biomedical devices including 3D-printed tissue engineering scaffolds and 3D-printed prosthetics[1,3,4,12-15]. These devices are designed to temporarily or permanently replace damaged tissues or organs in the living body. The third category is in vitro biomedical platform such as microfluidic systems for molecular diagnostics and functional cell assays[16-21]. Currently, much effort in biomedical 3D printing has been devoted to creating desired 3D architecture. The role of 3D-printed components is typically limited to providing structural support. Their role as a biologically functional substrate is often unheeded, despite the fact that they are in constant interactions with biological entities. Even for 3D-printed tissue engineering scaffold which is designed to promote cell attachment and growth, its biological functions largely rely on the intrinsic property of the bulk materials, which imposes further constraints on the already limited selection of 3D-printable materials.
For 3D-printed components to achieve full potential in biomedical applications, a multiprocess 3D printing[22] that combines 3D printing and post-3D printing modification is highly coveted. The 3D-printed components could benefit substantially from the post-3D printing modification for improved biofunctionality. In this paper, we first summarize current approaches used for post-3D printing modification. Meanwhile, we identify the limitations of 3D-printed components for biomedical applications, and provide a perspective on how to close the gap using post-3D printing modification techniques.
2. Post-3D Printing Modification
Post-printing modification has two major effects. It is able to reconfigure the 3D architecture and/or chemically functionalize the surface of 3D-printed components, as shown in Figure 1.
Figure 1.
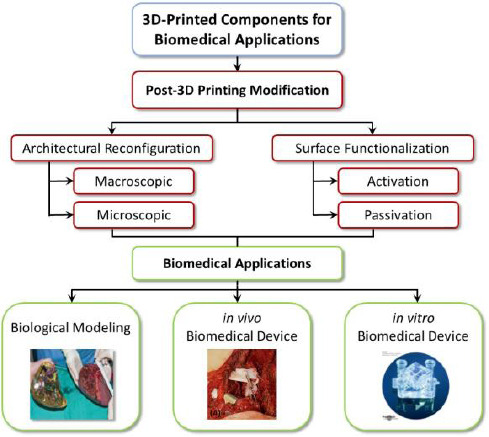
Post 3D-printing modification, which includes architectural reconfiguration and surface functionalization, improves biofunctionality of 3D-printed components. Inset figures reproduced[9,15,17] with permission from Wiley or under Creative Commons Attribution License.
2.1 Architectural Reconfiguration
2.1.1 Macroscopic
The architecture of 3D-printed components can be transformed post-printing. At macroscopic level, shape memory materials are incorporated in the 3D-printed components[23-25]. Under the designed transition conditions (e.g., temperature or solvent), the shape memory materials deform and transform the 3D-printed components into desired configuration. This concept, which is also known as 4D printing[26,27], creates a dynamic component that allows users to reconfigure the shape of 3D-printed components on demand. We consider the reconfiguration a post-3D printing modification method. The 4D printing technique has been demonstrated for soft robotics[28]. But we could expect more sophisticated biomedical applications using 4D printing technology. For example, 4D printing is well-suited for customized vascular stent. Once the pre-configured stent reaches the stricture, it could be induced to expand and open the stenotic vessel to restore blood flow.
The post-3D printing architectural reconfiguration could also enhance the performance of 3D-printed microfluidic devices. Microfluidic devices printed using conventional 3D-printing techniques such as stereolithography (SLA) or fused deposition modeling (FDM) have fairly poor lateral resolution. A more advanced technique, such as two-photon polymerization (2PP), is able to achieve sub-micro resolution[29], but it suffers from low throughput. So far, there is no 3D-printing technology that fabricates microfluidic devices with both high throughput and high resolution. Post-3D printing reconfiguration provides a potential solution. Earlier work by Khine et al. demonstrated a method to achieve high resolution lithography by creating large patterns and subsequently shrinking the device to reduce the pattern size (Figure 2))[30]. The same concept can be adopted in 3D printing. By printing a 3D microfluidic device with pre-stressed material and subsequently subjecting it to controlled shrinking, the reconfigured device could achieve high resolution despite the fact that the original device may have large features with a poor resolution.
Figure 2.
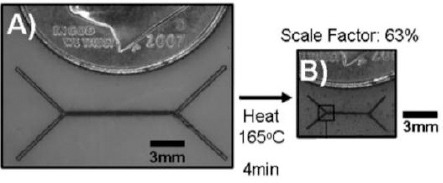
Reducing pattern size by shrinking for the fabrication of microfluidic devices. (A) Unshrunken pattern with laser printed master pattern. (B) The same master after being shrunken. Reproduced[30] with the permission from Royal Chemistry Society.
Surface smoothing is a seemingly trivial but actually a very important process in microfluidics. Because 3D printing produces components layer by layer, the surface usually appears uneven with evident layer lines. The resulting rough surface significantly alters the flow characteristics in microfluidics and leads to undesired outcome[18]. Commonly used surface smoothing techniques include sanding, blasting and vapor smoothing. These techniques have been readily applied to smoothen the exterior surface of 3D-printed components for aesthetic purposes. However, smoothen an interior surface such as microfluidic channels proves challenging. Vapor smoothing is perhaps the only technique applicable to microfluidics so far, but vapor cannot effectively enter the microfluidic channels on its own. Future work needs to look into how to actively pump vapor or liquid through the microfluidic channels to achieve desired surface architecture.
2.1.2 Microscopic
The microscopic architecture of 3D-printed components has been extensively studied, especially for 3D-printed tissue engineering scaffolds. These scaffolds are designed with microscopic porous architecture. The pore size, porosity and other microscopic topographic features of the scaffold are crucial for cell growth and scaffold degradation[3]. Techniques adopted from microfabrication, such as reaction ion etching and thin film deposition, can be applied to further fine-tune the microscopic architecture of 3D-printed components. The etching process could partially remove scaffold material on the surface and increases the pore size. The thin film deposited by e-beam evaporation or sputtering partially fills the microscopic pores and decreases the pore size. Chemical deposition techniques can also be employed to graft nanoscale structures on the surface. These nanostructures produce more hierarchical microscopic architecture that could enhance interactions with biological entities. In addition to the techniques mentioned above, gas foaming, solvent casting, particulate leaching, freeze drying and other techniques can also be implemented to reconfigure microscopic architecture during post-3D printing processing for various types of materials[31].
2.2 Surface Functionalization
Currently, many 3D-printed components are merely models that provide a more intuitive way of visualizing the architectural design. Some 3D-printed prototypes can also serve mechanical and structural functions. But the biological and chemical functions of 3D-printed components are frequently ignored. Current research on the biofunctionality of 3D-printed components still focuses on several conventional biocompatible materials that are adopted for 3D-printing, such as PGA, PLA and titanium. Due to the limited selection of 3D-printable materials and fabrication techniques, we believe that post-3D printing modification offers more options to functionalize 3D-printed components for biomedical applications. Depending on the purpose, different techniques may be employed to either activate or passivate the surface of 3D-printed components.
2.2.1 Surface Activation
Surface activation is accomplished by conjugating or depositing functional materials to the 3D-printed components. The functional materials include biologically active molecules such as growth factors, antibodies, peptides, nucleic acids or their derivatives. Alternatively, they can also be biocompatible or chemically active coating that promotes interaction between 3D-printed components and biological entities.
Surface activation techniques have already been used in 3D-printed tissue engineering scaffold. Lee et al. immobilized a growth factor called human bone morphogenic protein-2 (rhBMP-2) on the 3D-printed polycaprolactone (PCL) scaffold to promote osteogenic differentiation[32]. Yeh et al. immobilized an active compound extracted from a traditional Chinese medicine (TCM) herb known as Xu Duan onto 3D-printed PLA scaffold and discovered that both osteogenic and angiogenic markers of bone marrow stem cells were up-regulated[33]. Both groups used polydopamine chemistry to immobilize the biologically active materials. Polydopamine is bio-inspired material that has a strong adhesion to virtually any type of surface[34]. The polydopamine coating contains hydroxyl and amine functional groups which could covalently conjugate proteins and other active compounds. Polydopamine itself is also a biologically active material. Kao et al. coated PLA scaffold with polydopamine and noticed enhanced cell adhesion and proliferation on the scaffold[35]. Polydopamine also has sufficient reducing capability for electrodeless plating[34,36]. By dipping the 3D-printed components with a polydopamine coating in the solution containing gold salt, one can easily plate them with gold for the biomolecular self-assembly via gold-thio interactions[37]. The same process can be used to metalize the 3D-printed components for built-in electrochemical sensing. Polydopamine can also mediate the immobilization of a wide range of materials using a one-pot reaction to achieve versatile chemical functionality (Figure 3)[38]. Because of the simple coating procedure and versatile functions of polydopamine, it is an ideal technique for post-3D printing surface functionalization.
Figure 3.
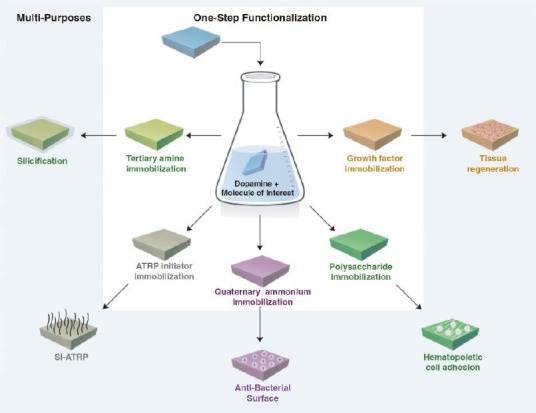
Schematics of polydopamine-mediated surface functionalization. Reproduced[38] with the permission from Wiley.
Besides polydopamine, other methods that add functional groups to 3D-printed components have also been proposed (Figure 4). Take initiator integrated 3D-printing for example[39]. The functional groups on Br-containing vinyl-terminated initiator. Reproduced[39] with the permission from Royal Chemistry Society.the 3D-printed components are made available by adding a functional compound to the 3D-printable resin. The 3D-printing process embeds the functional compound in the finished product. The authors have demonstrated surface-initiated atom transfer radical polymerization for post-printing surface modification by incorporating Br-containing vinyl-terminated initiator[39]. This technique could potentially enable a wide range of chemistry for surface modification of 3D-printed parts, if 3D-printable materials can be designed to carry carboxyl group for carbodiimide coupling[4] or other functional groups to conjugate proteins and other biomolecules to the surface via well-established click chemistry[41] with great ease.
Figure 4.
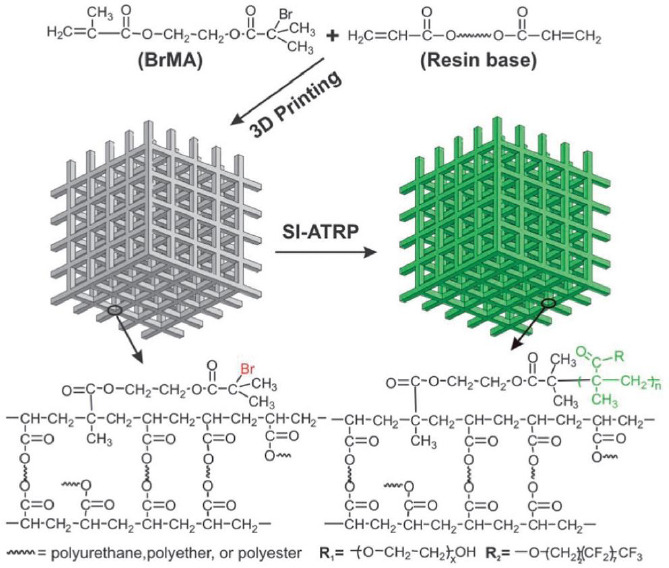
Schematics of initiator integrated 3D printing. Functional groups are embedded in the 3D-printed parts by incorporating
The post-3D printing modification described above focuses on in vivo biomedical devices. As a matter of fact, in vitro biomedical device could also benefit from post-3D printing modification. Many in vitro assays require functionalization with biologically active molecules. The polydopamine chemistry allows easy functionalization of microfluidic devices with antibodies or other binding agents for immunoassays. One may also functionalize 3D-printed devices with silica or nickel for solid phase nucleic acids and recombinant protein purification.
2.2.2 Surface Passivation
Compared to surface activation, post-3D printing passivation process has not been paid enough attention. Unlike tissue engineering scaffold, 3D-printed implants are meant to reside permanently in the living body. Therefore, they are complicated by problems such as protein fouling, bacteria fouling and foreign body reactions. Many surface modification techniques have been developed to reduce fouling and foreign body reactions. For example, polyethylene glycol (PEG) coating effectively prevents protein adsorption[4]. Silver nanoparticles are embedded in polymer coatings to form an antimicrobial film that prevents bacteria fouling[43]. Drug-loaded responsive polymer coating release drugs in a controlled fashion to provide long-term protection against foreign body reaction[44]. These techniques can all be readily applied to 3D-printed components. The same concept also applies to 3D-printed in vitro devices, which could incorporate these passivation techniques to prevent nonspecific adsorption of biomolecules, thereby reducing undesired background and improving detection sensitivity.
Many resins used in 3D printing, such as acrylate and its derivatives, are known to have cytotoxicity and cause developmental malformation and other adverse effects[45]. Although large-chain acrylate polymers that form the 3D-printed components are much less toxic, a significant amount of acrylate monomers and short-chain polymers are still present in the 3D-printed components. These small molecules may leach out and cause damages to biological entities. Post-3D printing modification by additional UV curing could crosslink the small molecules into long-chain polymer, thereby reducing the cytotoxicity of 3D-printed components. Future research needs to focus on biocompatibility of 3D-printable materials both before and after printing, and new methods to reduce the toxicity of 3D-printed components also need to be explored.
3. Conclusion
In conclusion, we have stressed the importance of post-3D printing modification. Because only a limited selection of 3D-printable materials and techniques are specifically designed for biomedical applications, post-3D printing modification provides a means to achieve desired architectural and functional properties. In this perspective, we have identified structural reconfiguration and surface functionalization as two main aspects of post-3D printing modification. We have discussed techniques that have already been implemented and suggested other potential post-3D printing modification techniques that can be employed to close the gap between 3D printing technology and requirements of biomedical application.
Conflict of Interest and Funding
No conflict of interest has been reported by the author. The author would like to thank the funding support by the startup grant from the School of Mechanical and Aerospace Engineering at Nanyang Technological University.
References
- 1.Leong K F, Cheah C M, Chua C K. Solid freeform fabrication of three-dimensional scaffolds for engineering replacement tissues and organs. Biomaterials. 2003;24(13):2363–2378. doi: 10.1016/s0142-9612(03)00030-9. https://dx.doi.org/10.1016/S0142-9612(03)00030-9. [DOI] [PubMed] [Google Scholar]
- 2.Utela B, Storti D, Anderson R, et al. A review of process development steps for new material systems in three dimensional printing (3DP) Journal of Manufacturing Processes. 2008;10(2):96–104. https://dx.doi.org/10.1016/jjmapro.2009.03.002. [Google Scholar]
- 3.Yang S, Leong K-F, Du Z, et al. The design of scaffolds for use in tissue engineering. Part II. Rapid prototyping techniques. Tissue Engineering. 2002;8(1):1–11. doi: 10.1089/107632702753503009. https://dx.doi.org/10.1089/107632702753503009. [DOI] [PubMed] [Google Scholar]
- 4.Yeong W-Y, Chua C-K, Leong K-F, et al. Rapid prototyping in tissue engineering: Challenges and potential. Trends in Biotechnology. 2004;22(12):643–652. doi: 10.1016/j.tibtech.2004.10.004. https://dx. doi. org/10.1016/j .tibtech.2004.10.004. [DOI] [PubMed] [Google Scholar]
- 5.Gross B C, Erkal J L, Lockwood S Y, et al. Evaluation of 3D printing and its potential impact on biotechnology and the chemical sciences. Analytical Chemistry. 2014;86(7):3240–3253. doi: 10.1021/ac403397r. https://dx. doi. org/10.1021/ac4033 97r. [DOI] [PubMed] [Google Scholar]
- 6.D'Aveni R. The 3-D printing revolution. Harvard Business Review. 2015;93(5):40–48. [Google Scholar]
- 7.Rengier F, Mehndiratta A, von Tengg-Kobligk H, et al. 3D printing based on imaging data: Review of medical applications. International Journal of Computer Assisted Radiology and Surgery. 2010;5(4):335–341. doi: 10.1007/s11548-010-0476-x. https://dx.doi.org/10T 007/s11548-010-0476-x. [DOI] [PubMed] [Google Scholar]
- 8.Singare S, Lian Q, Ping Wang W, et al. Rapid prototyping assisted surgery planning and custom implant design. Rapid Prototyping Journal. 2009;15(1):19–23. https://dx.doi.org/10.1108/13552540910925027. [Google Scholar]
- 9.Zein N N, Hanouneh I A, Bishop P D, et al. Three-dimensional print of a liver for preoperative planning in living donor liver transplantation. Liver Transplantation. 2013;19(12):1304–1310. doi: 10.1002/lt.23729. https://dx.doi.org/10.1002/lt.23729. [DOI] [PubMed] [Google Scholar]
- 10.Scalfani V F, Vaid T P. 3D printed molecules and extended solid models for teaching symmetry and point groups. Journal Chemical Education. 2014;91(8):1174–1180. https://dx.doi.org/10.1021/ed400887t. [Google Scholar]
- 11.Tibbits S, Falvello A. Adaptive Architecture. Waterloo, Canada: 2013. Biomolecular, chiral and irregular self-assemblies, inProceedings of the Association for Computer Aided Design in Architecture 2013; p. 2013. [Google Scholar]
- 12.Cheung H-Y, Lau K-T, Lu T-P, et al. A critical review on polymer-based bio-engineered materials for scaffold development. Composites Part B:Engineering. 2007;38(3):291–300. https://dx.doi.org/10.1016Zj.compositesb.2006.06.014. [Google Scholar]
- 13.Ho C M B, Ng S H, Yoon Y-J. A review on 3D printed bioimplants. International Journal of Precision Engineering and Manufacturing. 2015;16(5):1035–1046. https://dx.doi.org/10.1007/s12541-015-0134-x. [Google Scholar]
- 14.Peltola S M, Melchels F P W, Grijpma D W, et al. A review of rapid prototyping techniques for tissue engineering purposes. Annals of Medicine. 2008;40(4):268–280. doi: 10.1080/07853890701881788. https://dx.doi.org/10.1080/07⅚90701881788. [DOI] [PubMed] [Google Scholar]
- 15.Wong K C, Kumta S M, Geel N V, et al. One-step reconstruction with a 3D-printed, biomechanically evaluated custom implant after complex pelvic tumor resection. Computer Aided Surgery. 2015;20(1):14–23. doi: 10.3109/10929088.2015.1076039. https://dx.doi.org/10.3109/10929088.2015.1076039. [DOI] [PubMed] [Google Scholar]
- 16.Bhattacharjee N, Urrios A, Kang S, et al. The upcoming 3D-printing revolution in microfluidics. Lab on a Chip. 2016;16(10):1720–1742. doi: 10.1039/c6lc00163g. https://dx.doi.org/10.1039/C6LC00163G. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Au A K, Huynh W, Horowitz L F, et al. 3D-Printed microfluidics. Angewandte Chemie International Edition. 2016;55(12):3862–3881. doi: 10.1002/anie.201504382. https://dx.doi.org/10.1002/anie.201504382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lee J M, Zhang M, Yeong W Y. Characterization and evaluation of 3D printed microfluidic chip for cell processing. Microfluidics and Nanofluidics. 2016;20(1):1–15. https://dx.doi.org/10.1007/s10404-015-1688-8. [Google Scholar]
- 19.Au A K, Bhattacharjee N, Horowitz L F, et al. 3D-printed microfluidic automation. Lab on a Chip. 2015;15(8):1934–1941. doi: 10.1039/c5lc00126a. https://dx.doi.org/10.1039/c5lc00126a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Waheed S, Cabot J M, Macdonald N P, et al. 3D printed microfluidic devices: Enablers and barriers. Lab on a Chip. 2016;16(11):1993–2013. doi: 10.1039/c6lc00284f. https://dx.doi.org/10.1039/C6LC00284F. [DOI] [PubMed] [Google Scholar]
- 21.Ho C M B, Ng S H, Li K H H, et al. 3D printed microfluidics for biological applications. Lab on a Chip. 2015;15(18):3627–3637. doi: 10.1039/c5lc00685f. https://dx.doi.org/10.1039/C5LC00685F. [DOI] [PubMed] [Google Scholar]
- 22.MacDonald E, Wicker R. Multiprocess 3D printing for increasing component functionality. Science. 2016;353(6307):aaf2093. doi: 10.1126/science.aaf2093. https://dx.doi.org/10.1126/science.aaf2093. [DOI] [PubMed] [Google Scholar]
- 23.Ge Q, Sakhaei A H, Lee H, et al. Multimaterial 4D printing with tailorable shape memory polymers. Scientific Reports. 2016;6:31110. doi: 10.1038/srep31110. https://dx.doi.org/10.1038/srep31110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Khoo Z X, Teoh J E M, Liu Y, et al. 3D printing of smart materials: A review on recent progresses in 4D printing. Virtual and Physical Prototyping. 2015;10(3):103–122. https://dx.doi.org/10.1080/17452759.2015.1097054. [Google Scholar]
- 25.Gladman A S, Matsumoto E A, Nuzzo R G, et al. Biomimetic 4D printing. Nature Materials. 2016;15(4):413–418. doi: 10.1038/nmat4544. https://dx.doi.org/10.1038/nmat4544. [DOI] [PubMed] [Google Scholar]
- 26.An J, Chua C K, Mironov V. A perspective on 4D bioprinting. International Journal of Bioprinting. 2016;2(1):3–5. http://dx.doi.org/10.18063/IJB.2016.01.003. [Google Scholar]
- 27.Tibbits S. 4D printing:Multi-material shape change. Architectural Design. 2014;84(1):116–121. https://dx. doi. org/10.1002/ad. 1710. [Google Scholar]
- 28.Ilievski F, Mazzeo A D, Shepherd R F, et al. Soft robotics for chemists. Angewandte Chemie. 2011;50(8):18901895. doi: 10.1002/anie.201006464. https://dx.doi.org/10.1002/anie.201006464. [DOI] [PubMed] [Google Scholar]
- 29.Tan D, Li Y, Qi F, et al. Reduction in feature size of two-photon polymerization using SCR500. Applied Physics Letters. 2007;90(7):071106. https://dx.doi.org/10.1063/L2535504. [Google Scholar]
- 30.Grimes A, Breslauer D N, Long M, et al. Shrinky-Dink microfluidics:Rapid generation of deep and rounded patterns. Lab on a Chip. 2008;8(1):170–172. doi: 10.1039/b711622e. https://dx. doi. org/10.1039/b711622e. [DOI] [PubMed] [Google Scholar]
- 31.Chia H N, Wu B M. Recent advances in 3D printing of biomaterials. Journal of Biological Engineering. 2015;9(1):4. doi: 10.1186/s13036-015-0001-4. https://dx.doi.org/10.1186/s13036-015-0001-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pensa E, Cortes E, Corthey G n, et al. The chemistry of the sulfur-gold interface:In search of a unified model. Accounts of Chemical Research. 2012;45(8):11831192. doi: 10.1021/ar200260p. https://dx. doi. org/10.1021 /ar200260p. [DOI] [PubMed] [Google Scholar]
- 33.Kang S M, Hwang N S, Yeom J, et al. One-step multipurpose surface functionalization by adhesive catecholamine. Advanced Functional Materials. 2012;22(14):2949–2955. doi: 10.1002/adfm.201200177. https://dx. doi. org/10. 1002/adfm.201200177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lee S J, Lee D, Yoon T R, et al. Surface modification of 3D-printed porous scaffolds via mussel-inspired polydopamine and effective immobilization of rhBMP-2 to promote osteogenic differentiation for bone tissue engineering. Acta Biomaterialia. 2016;40:182–191. doi: 10.1016/j.actbio.2016.02.006. https://dx.doi.org/10.1016Zj.actbio.2016.02.006. [DOI] [PubMed] [Google Scholar]
- 35.Yeh C-H, Chen Y-W, Shie M-Y, et al. Poly(dopamine)-assisted immobilization of Xu Duan on 3D printed poly(lactic acid) scaffolds to up-regulate osteogenic and angiogenic markers of bone marrow stem cells. Materials. 2015;8(7):4299–4315. doi: 10.3390/ma8074299. https://dx.doi.org/10.3390/ma8074299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Liu Y, Ai K, and Lu L. Polydopamine and its derivative materials:Synthesis and promising applications in energy, environmental, and biomedical fields. Chemical Reviews. 2014;114(9):5057–5115. doi: 10.1021/cr400407a. https://dx.doi.org/10.1021/cr400407a. [DOI] [PubMed] [Google Scholar]
- 37.Kao C-T, Lin C-C, Chen Y-W, et al. Poly(dopamine) coating of 3D printed poly(lactic acid) scaffolds for bone tissue engineering. Materials Science and Engineering: C. 2015;56:165–173. doi: 10.1016/j.msec.2015.06.028. https://dx.doi.org/10.1016/j.msec.2015.06.028. [DOI] [PubMed] [Google Scholar]
- 38.Lee H, Dellatore S M, Miller W M, et al. Mussel-inspired surface chemistry for multifunctional coatings. Science. 2007;318:5849, 426–430. doi: 10.1126/science.1147241. https://dx.doi.org/10.1126/science. 1147241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wang X, Cai X, Guo Q, et al. 3DP, a robust 3D printing approach enabling genetic post-printing surface modification. Chemical Communications. 2013;49(86):10064–10066. doi: 10.1039/c3cc45817b. https://dx.doi.org/10.1039/c3cc45817b. [DOI] [PubMed] [Google Scholar]
- 40.Valeur E, Bradley M. Amide bond formation:beyond the myth of coupling reagents. Chemical Society Reviews. 2009;38(2):606–631. doi: 10.1039/b701677h. https://dx.doi.org/10.1039/B701677H. [DOI] [PubMed] [Google Scholar]
- 41.Kolb H C, Finn M G, Sharpless K B. Click chemistry:Diverse chemical function from a few good reactions. Angewandte Chemie International Edition. 2001;40(11):2004–2021. doi: 10.1002/1521-3773(20010601)40:11<2004::AID-ANIE2004>3.0.CO;2-5. https://dx.doi.org/10.1002/1521-3773(20010601)40:11<2 004::AID-ANIE2004>3.0.C0;2-5. [DOI] [PubMed] [Google Scholar]
- 42.Banerjee I, Pangule R C, Kane R S. Antifouling coatings: recent developments in the design of surfaces that prevent fouling by proteins, bacteria, and marine organisms. Advanced Materials. 2011;23(6):690–718. doi: 10.1002/adma.201001215. https://dx.doi.org/10.1002/adma.201001215. [DOI] [PubMed] [Google Scholar]
- 43.Sanchez-Salcedo S, Colilla M, Izquierdo-Barba I, et al. Preventing bacterial adhesion on scaffolds for bone tissue engineering. International Journal of Bioprinting. 2016;2(1):20–34. http://dx.doi.org/10.18063/IJB.2016.01.008. [Google Scholar]
- 44.Liu L, Chen G, Chao T, et al. Reduced foreign body reaction to implanted biomaterials by surface treatment with oriented osteopontin. Journal of Biomaterials Science, Polymer Edition. 2008;19(6):821–835. doi: 10.1163/156856208784522083. https://dx.doi.org/10.1163/156856208784522083. [DOI] [PubMed] [Google Scholar]
- 45.Oskui S M, Diamante G, Liao C, et al. Assessing and reducing the toxicity of 3D-printed parts. Environmental Science &Technology Letters. 2015;3(1):1–6. https://dx.doi.org/10.1021/acs.estlett.5b00249. [Google Scholar]


