Abstract
Semaphorins have been traditionally known as axon guidance proteins that negatively regulate axonal growth. However, in the past couple of decades, their versatile role in so many other biological processes has come to prominence as well. One such example is their role in cancer. In this review article, the focus was on the tumor proliferative and tumor suppressive role of all 20 semaphorin family members under the 7 semaphorin classes found in vertebrates and invertebrates as well as the ongoing and emerging therapeutic approaches to combat semaphorin-mediated cancers. Except sema6C, 19 of the 20 non-viral semaphorin family members have been discovered to be associated with cancer in one way or another. Eleven semaphorin family members have been discovered to be tumor proliferative and 8 to be tumor suppressive. Six therapeutic avenues and their safety profiles have been discussed which are currently at use or at the various stages of development. Finally, perspectives on which approach is the best for treating cancers associated with semaphorins have been given.
Keywords: Semaphorin, Protein, Cancer, Tumor proliferation, Tumor suppression, Therapeutics
Introduction
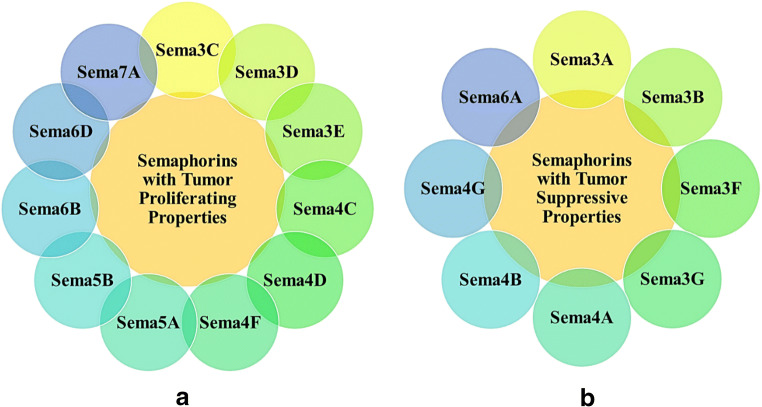
Semaphorins are a group of proteins which were initially identified as proteins involved in axon guidance in a repulsive manner (Mitsogiannis et al. 2017). However, its role in angiogenesis (Huber et al. 2003; Butti et al. 2018), organogenesis (Tran et al. 2007), homeostasis (Orr et al. 2017), bone remodeling (Li et al. 2017; Worzfeld and Offermanns 2014), immune system (Potiron et al., n.d.; Takamatsu and Kumanogoh 2012; Takamatsu et al. 2010; Yazdani and Terman 2006), and so on have also been discovered. Among 19 non-viral semaphorin family members, 11 show tumor proliferative activity (Fig. 1a) and the rest are tumor suppressive (Fig. 1b) (Neufeld and Kessler 2008; Tamagnone 2012; Neufeld et al. 2016). Every class of semaphorin has a sema domain in common which contains 500 residues. Class 2 and 3 semaphorins are found as secreted proteins, classes 4–6 are transmembrane proteins, and classes 1, 4, 5, and 6 contain transmembrane and short cytoplasmic domains. Sema7 remains bound to the cellular membrane by a gpi (glycosylphosphatidylinositol) membrane anchor. Semaphorins bind to and signal through two types of receptors, namely plexin and neuropilin (Tamagnone et al. 1999). Other proteins including vascular endothelial growth factor receptor-2 (vegfr-2), met, and erbb-2 act as co-receptors for semaphorins by combining with plexins and neuropilins (Toyofuku 2004; Bellon et al. 2010).
Fig. 1.
Semaphorin family members categorized based on their tumor proliferative or tumor suppressive properties. Eleven belong to the tumor proliferative (A), and 8 to the tumor suppressive (B) category
Some semaphorins play a role in tumor progression through a number of biological mechanisms such as sustained cell proliferation, evasion of apoptosis, oxidative stress regulation, tumoral neo-angiogenesis, invasion and metastasis, pro-tumorigenic inflammation, and escaping the immune surveillance by the immune system (Rehman and Tamagnone 2013). Contrarily, some semaphorins exhibit tumor suppressive activity through reverse signaling mechanism, inhibition of matrix metalloproteinase-9 (mmp-9), which promotes invasion of cancer and tumor metastasis, modulating vascular endothelial growth factor (vegf) signaling, reducing integrin αvβ3 protein which plays a crucial part in metastasis, etc. (Wu et al. 2011; Segarra et al. 2012; Yu, 2012; Kapil et al. 2017). In the following sections of this review article, the role of various semaphorin family members in tumor proliferation and tumor suppression and also a number of therapeutic endeavors that are being made targeting semaphorins have been discussed. Apart from that, insights from systems biology have been taken into consideration for identifying the protein-protein interactions of the two groups of semaphorins (tumor proliferative and suppressive) and the most important targets for therapy. Fig. 1 shows semaphorin family members divided into two catagories—(A) tumor proliferative and (B) tumor suppressive.
Semaphorins with tumor proliferative properties
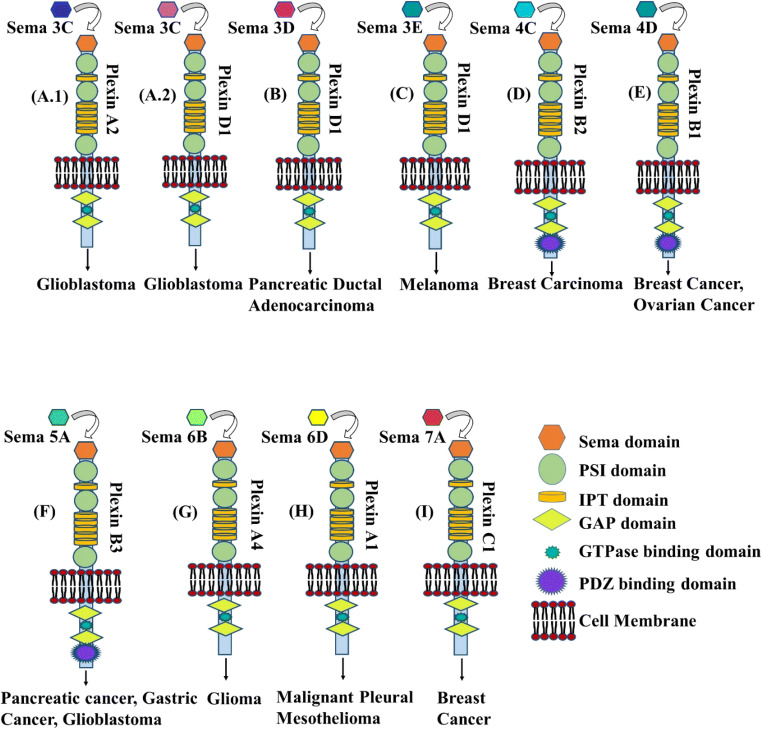
Tumor proliferative semaphorins interact with certain receptors and carry out their functions. Fig. 2 shows the semaphorin family members and the specific type of receptors they interact and how they promote tumor proliferation as a result. There are 11 semaphorin family members (Fig. 1a) that have tumor proliferative activity. They are discussed below:
Fig. 2.
Semaphorins with tumor proliferative properties, the receptors they interact with and the outcome. (A.1, A.2) sema3C interacts with plexinA2 and plexinD1 and promotes glioblastoma. (B) sema3D interacts with plexin D1 and promotes pancreatic ductal adenocarcinoma. (C) sema3E interacts with plexinD1 and promotes melanoma. (D) sema4C interacts with plexinB2 and promotes melanoma. (E) sema4D interacts with plexinB1 and promotes breast and ovarian cancer. (F) sema5A interacts with plexinB3 and promotes pancreatic cancer, gastric cancer, and glioblastoma. (G) sema6B interacts with plexinA4 and promotes glioma. (H) sema6D interacts with plexinA1 and promotes malignant pleural mesothelioma. (I) sema7A interacts with plexinC1 and promotes breast cancer
Sema3C
Sema3C is a secreted glycoprotein and is a member of the class 3 semaphorins. It possesses an N-terminal sema domain, integrin and immunoglobulin-like domains, and a C-terminal basic domain (which is an alpha helix rich in basic amino acids). For proper functioning of sema3C, homodimerization and proteolytic cleavage of the C-terminal propeptide are absolutely mandatory. It signals through two subtypes of neuorpilin receptors, namely neuropilin-1 and neuropilin-2, and act as an attractive axon guidance protein (Goshima et al. 2002). Sema3C is expressed primarily in various organs such as the heart, skeletal muscle, colon, small intestine, ovary, testis, and prostate. Moderate and ubiquitous level of expression is also observed in other organs including the brain (Fagerberg et al., 2013). Various studies on gastric cancer about the part that sema3C plays on tumor progression were monitored. In neoplastic cells, sema3C level was detected to be elevated which is associated with promotion of tumor differentiation. A vital role relating to the morphological changes that happen in cancer cells which ultimately leads to excessive growth and spreading has been suggested for sema3C (Malik et al. 2016). Overexpression of metalloproteases called adants-1 results in the release of sema3C by cleaving the propeptide at the C-terminal, and consequently induces cancer cell migration. One group of researchers that used orthotopic model found that the sema3C silencing led to significant degree of suppression of cancer in nude mice (Miyato et al. 2012). As cell adherence is one of the properties of cancer cell progression, another research group has observed that the inhibition of sema3C activity reduces the adhesion of the cancer cell, and thus decreases the invasion. sema3C has been observed to be associated with gliobalstoma along with its receptors plexinA1 and plexinD1 (Fig. 2(A.1, 2A.2)) (Man et al. 2014).
Sema3D
Sema3D is a secreted axon guidance protein that belongs to the semaphorin III family and exerts its function through neuropilin receptor (Feiner et al. 1997). Sema3D is found to be expressed mainly in the surface ectoderm among ectoderm-derived tissues. It is also expressed in the lens, nasal placodes, diencephalon, dorsal neural tube, and optical and otic vesicles (Bao and Jin 2006).
In one study, sema3D has been found to be involved with the promotion of pancreatic ductal adenocarcinoma through plexinD1 (Fig. 2(B)). Sema3D promotes metastasis in these cells either via an autocrine signaling mechanism, or via a paracrine signaling between dorsal root ganglion and the pancreatic cancer cells. Knocking down of sema3D exhibited a decline in the invasive and metastatic capabilities in vitro and in vivo (Foley et al. 2015; Jurcak et al. 2018).
Sema3E
Sema3E has a critical part to play in cell signaling through its receptor plexinD1. It is involved in many biological activities such as the mediation of reorganization of the actin cytoskeleton, which leads to the retraction of cell projections, promotion of focal adhesion disassembly, and inhibition of adhesion of endothelial cells to the extracellular matrix; regulation of angiogenesis, both during embryogenesis and after birth. Despite being expressed in 144 tissues, its highest expression is observed in the lung (Ota et al., 2003).
Overexpression of sema3E was observed to be associated with progression of tumor and cell proliferation via the mapk/erk pathway in pancreatic cancer. On the other hand, reduction in in vitro migration and in vivo cell proliferation, and tumor incidence and size has been observed as a result of knockout of sema3E (Yong et al. 2016). Elevated levels of sema3E have been found to be involved in the development of gastric cancer as well (Maejima et al. 2016). Another study has discovered that sema3E in combination with its receptor plexinD1 drives cell invasiveness and metastasis in melanoma cells (Fig. 2(C)) (Casazza et al. 2010).
Sema4C
Sema4C is single-pass type I membrane protein. It signals through its receptors plexinB1 and plexinB2 (Paldy et al. 2017; Williamson et al., 2015). It is found in the cell junction, synapse, postsynaptic cell membrane, postsynaptic density, cytoplasmic vesicle, secretory vesicle, and synaptic vesicle membrane. It is expressed in 234 tissues but the peak expression is observed in trigeminal ganglion (Ota et al., 2003).
Sema4C/plexinB2 signaling has been demonstrated to be essential for the growth of breast carcinoma. Upregulation of sema4C in luminal-type breast carcinoma leads to increased migration and invasiveness. Downregulation of sema4C in various types of mammary cancer cells leads to dramatic growth inhibition. It has been demonstrated that a sema4C/plexinB2/larg-dependent signaling cascade is required to maintain critical level of rhoA-GTP in cancer cells (Fig. 2(D)) (Gurrapu et al. 2018).
Sema4D
Sema4D is a single-pass type I membrane protein found on the cell membrane. It interacts with the cell surface receptors plexinB1 and plexinB2. It is involved in the control of gabaergic synapse development, promotion of inhibitory synaptic growth, modulation of the complexity and arborization of developing neurites in hippocampal neurons, and promotion of migration of cerebellar granule cells. Although it is expressed in 211 tissues, the biggest expression level is observed in cervical spinal cord’s C1 segment (Ota et al., 2003).
Membrane-bound sema4D binds to plexinB1 which then activates oncogenic receptor met, a single-pass tyrosine kinase. Increased levels of sema4D expression are observed in breast cancer cell lines. Various treatments have been applied to target the metastatic aspect of tumor progression. In bone-homing malignancies, bisphosphonates have been used to stop skeletal metastases, but it inhibits osteoclasts and halts bone remodeling which results in fragile bones and jaw osteonecrosis. It is proposed that inhibiting the activity of sema4D is a potential alternative, as it is not involved that much in bone remodeling and so is able to yield better therapeutic outcome without or less harmful side effects (Tamagnone and Comoglio 2004).
In an another study, it has been seen that the lateral migration ability of the cells are significantly decreased in response to the downregulation of sema4D expression (Jiang et al. 2016). Furthermore, downregulation of sema4D has been identified as a promoter of apoptosis of some types of cancer cells.
A group of scientists revealed the role of sema4D in angiogenesis where they observed that rho-dependent mechanism through plexinB1 is involved in the process (Basile et al. 2004; Basile et al. 2005; Basile et al. 2007). Moreover, 3 type-B plexins can form spontaneous complexes with the tyrosine kinase receptors met and ron (Conrotto et al. 2004). Although met acts as a receptor for the angiogenic factor, hepatocyte growth factor (HGF), ron acts as a receptor for macrophage stimulating protein. Activation of these receptors promotes “invasive growth,” which encompasses tissue morphogenesis and tumor progression and metastasis. It has been proven through experiments that the coexpression of met and plexinB1 increases the chance of tumor progression in human breast and ovarian cancer (Fig. 2(E)) (Valente et al. 2009). Experiments show that the soluble form of sema4D increases endothelial cell migration and helps with their arraying in tubes that resemble capillaries. This process mimicks the important chain of actions that occur in vivo during the angiogenic process (Conrotto et al. 2005).
In breast cancer cells, plexinB1 and plexinB2 can also conjugate with erbb-2 protein. Sema4D and sema4C are capable of causing phosphorylation of erbb-2 protein by binding to plexinB1 or plexinB2 (Swiercz et al. 2004). In such cases, when sema4D binds to plexinB1 in conjucation with erbb-2, it promotes cellular migration and metastasis (Fazzari et al. 2007).
Sema4F
Sema4F is a single-pass type I membrane protein found in the cell membrane, cell junction, synapse, postsynaptic cell membrane, and postsynaptic density. It acts as a ligand for the receptors plexinB1–3, C1, and D1 (Alto and Terman, 2016). Despite being expressed in 159 tissues, the highest expression level is observed in the frontal cortex (Ota et al., 2003).
Sema4F has been identified as a biomarker of aggressive prostate cancer since it is significantly involved in human prostate cancer progression. It has been found to be a main controller of what goes on between the nerves found within the tumor microenvironment and the cancer cells. Due to the relationship between nerves and cancer, it can be evaluated as a significant therapeutic target (Ding et al. 2013). Sema4F has been implicated in cancer-induced neurogenesis but the nature of receptors involved in the signaling mechanism remains unknown (Ayala et al. 2008).
Sema5A
Sema5A is a single-pass type I membrane protein found on the cellular membrane. It acts as the ligand for plexinB3 protein. Stimulation of plexinB3 by sema5A in glioma cells results in the disassembly of F-actin stress fibers, disruption of focal adhesions, and cellular collapse. It has been observed to be expressed in 218 tissues. The highest expression level is observed in metanephric glomerulus (Ota et al., 2003).
Sema5A has been identified as a well-known marker for tumors of aggressive nature in the pancreas. In sema5A-negative panc-1 cells, ectopic expression of mouse sema5A of full length has been discovered to significantly (p < 0.05) increase tumorigenesis, metastasis, and growth in vivo and also the proliferation, invasiveness, and homotypic aggregation in vitro (Sadanandam et al. 2010). Secreted sema5A has been observed to increase metastasis and endothelial cell proliferation (Sadanandam et al. 2012). One study revealed that the sema5A expression at the protein and mRNA level was the least in normal gastric mucosa, moderate in primary gastric carcinoma, and at its peak in lymph nodes that contains metastatic gastric carcinoma. Besides, plexinB3 and sema5A were seen to be expressed in a closely correlated fashion. It shows that the expression of sema5A and plexinB3 which is the receptor for sema5A increases in a gradual way as the cancer progresses. This suggests that sema5A may play a crucial part when it comes to invasion and metastasis of gastric cancer (Fig. 2(F)) (Pan et al. 2009).
In contrast, a study on lung cancer patients who are all female and who do not smoke in Taiwan shows that the axon guidance signaling pathway was seriously dysregulated and the expression level of sema5A had an impact on the success rate in treatment of lung cancer. Immunohistochemistry tests unveiled that the female patients with lower amounts of sema5A protein had lower survival rates. One interesting discovery was that the link between the sema5A expression and therapeutic success is applicable to women exclusively. It indicates that sema5A can be used as gender-specific prognostic biomarker of patients suffering from lung cancer (Lu et al. 2010).
Sema5B
Sema5B is a single-pass type III membrane protein. It mainly acts as an attractive axon guidance cue. Sema3F signals through its receptor plexinA3 to erase synaptic contacts between dentate gyrus mossy fibers and cornu ammonis (CA)3 pyramidal cells which are inappropriate in nature during the development of hippocampus (Liu et al. 2005). Despite being expressed in 125 tissues, its highest expression level is observed in the cerebral cortex (Ota et al., 2003).
The role of sema5B in cancer has not been widely studied. The relation between sema5B and cancer proliferation has been clearly established only in one area which is renal cell carcinoma. In one study, sema5B has been discovered to be greatly trans-activated in renal cell carcinoma. The relation is further illustrated by the fact that knocking down the expression of sema5B in renal cell carcinoma cells through the use of siRNAs significantly reduces renal cell carcinoma viability (Hirota et al. 2006). But the receptors through which sema5B exerts its cancer proliferative activity are still not clear. Sema5B has been identified as a protumerigenic factor by a recent study and the data suggests that its expression is controlled by the transcription suppressive activity of two tumor suppressive genes, namely vhl and prdm16 (Kundu et al. 2018).
Sema6B
Sema6B is a single-pass type I membrane protein found in the cell membrane. It is implicated mainly in the peripheral and central nervous system development. It induces inhibitory responses in hippocampal and sympathetic neurons after binding to their receptor plexinA4 (Liu et al. 2005). Although it is expressed in 132 tissues, its highest expression level is observed in the dorsolateral prefrontal cortex (Ota et al., 2003).
Evidence for the transduction of proliferative signals induced by sema6B in autocrine manner has been detected in one study where effects of plexinA4 silencing have been mimicked by silencing the expression of sema6B in human primary glioblastoma and in endothelial cells. It also prevented tumor formation (Kigel et al. 2011). In breast cancer tissues, however, sema6B expression has been observed to be decreased significantly (D’Apice et al. 2013). Sema6B is known to promote glioma through interaction with plexinA4 receptor (Fig. 2(G)) (Correa et al. 2001).
Sema6D
Sema6D has 6 isoforms, 5 of whom are single-pass type I membrane protein found in the cell membrane and the other is found in the cytoplasm. Binding of sema6D to its receptor plexinA4 generates anti-inflammatory polarization of macrophages (Kang et al. 2018). It is expressed in 199 tissues but the peak expression level is observed in the forebrain (Ota et al., 2003).
Sema6D was thought to be involved in angiogenesis in vivo. Experiments determining the level of expression of sema6D at the mRNA and protein level in gastric carcinoma vs normal gastric mucosa showed significant increase in the sema6D expression in gastric carcinoma compared with its normal counterpart. It indicates that sema6D has a critical part to play in promoting gastric carcinoma (Zhao et al. 2006). In one study done on gastric cancer, elevated expression of sema6D and plexinA1 has been observed in vascular epithelial cells, and a positive correlation with vegfr-2 has also been shown. Sema6D forms complex with its plexin receptor and vegfr-1 and thus activate the vegfr-2 signaling pathway which consequently enhances angiogenesis in tumor cells. A strong correlation between the distribution patterns of vegfr-2 and elevated sema6D expression level has been revealed by representative fluorescence images in tumor tissue. (Lu et al. 2016). Malignant mesothelioma cells are seen to frequently express sema6D and its receptors plexinA1. Both of them are necessary for sustaining anchorage-independent growth of these malignant cells (Fig. 2(H)) (Catalano et al. 2009).
Sema7A
Sema7A is found in the cell membrane where it acts as lipid anchor and gpi anchor in the extracellular side. On the cell membrane of basal and supra-basal skin keratinocytes, it has been detected in a punctate form. It has a critical role in integrin-mediated signaling. It is involved in the regulation of cell migration and also immune responses as well as axon guidance. Sema7A interacts with its receptor plexinCl and promotes the formation of olfactory synapses in an activity-dependent manner (Inoue et al. 2018). It is expressed in 195 tissues, but the highest expression level is found in the one of the segments of the cervical spinal cord called the C1 segment (Ota et al., 2003).
Sema7A has been observed to promote of tumor metastasis and growth when it comes to human oral cancer especially when it involves matrix metalloproteases and the control of G1 cell cycle (Saito et al. 2015). Another study has demonstrated that sema7A plays a critical role in progression of mammary tumor. In a murine model of advanced breast carcinoma, suppression of tumor-derived sema7A and genetic ablation of host-derived sema7A have been observed to impair tumor progression (Fig. 2(I)) (Garcia-Areas et al. 2017). Sema7A promotes macrophage production of molecules associated with angiogenesis in breast cancer (Garcia-Areas et al. 2014).
Semaphorins with tumor suppressive properties
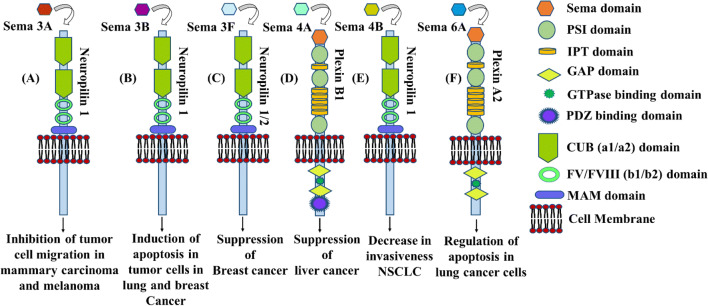
Tumor suppressive semaphorins interact with certain receptors in order to carry out their functions. Fig. 3 shows the semaphorin family members and the specific type of receptors they interact with and the outcome that helps in tumor suppression. Eight semaphorin family members (Fig. 1b) show tumor suppressive activity. They are discussed below:
Fig. 3.
Semaphorins with tumor suppressive properties, the receptors they interact with, and the outcome. (A) sema3A interacts with neuropilin-1 and inhibits tumor cell migration in mammary carcinoma and melanoma cells. (B) sema3B interacts with neuropilin-1 and induces apoptosis in lung and breast cancer cells. (C) sema3F interacts with neuropilin-1/2 and suppresses tumor proliferation in breast cancer. (D) sema4A interacts with plexinB1 suppresses liver cancer cells. (E) sema4B interacts with neuropilin and decreases the invasiveness of NSCLC. (F) sema6A interacts with plexinA2 and regulates apoptosis in lung cancer cells
Sema3A
Sema3A is found in the secret form. It has a critical part to play in the control of puberty through neurons and in the olfactory system growth. It also induces the collapse and paralysis of growth cones of neurons. Sema3A uses neuropilin-1 and plexinA1 as its receptors for carrying out axonal repulsion and abolition of growth cone collapse respectively (Takahashi et al. 1999; Castellani et al. 2000). Despite being expressed in 128 tissues, its biggest expression level is observed in the intestine (Ota et al., 2003).
Sema3A has been reported to drastically suppress angiogenesis and tumor growth in tongue squamous cancer cells in mice (Huang et al. 2017). Another study revealed that sema3A restricts tumor growth through differential control of the tumor-associated macrophage proliferation (Wallerius et al. 2016). But in some cases, for example in pancreatic cancer, the situation is reversed for sema3A, as it promotes rather than inhibits tumor progression (Müller et al. 2007). Sema3A can also directly affect tumor cells. It prevents the metastasis of breast cancer cells (mda-mb-231) and the invasiveness of prostate cancer (Bachelder et al. 2003; Herman and Meadows 2007). In mammary carcinoma and melanoma cells, sema3A exhibited inhibition of tumor cell migration by signaling through neuropilin-1 receptors (Fig. 3(A)) (Casazza et al. 2011).
Sema3B
Sema3B is found in secreted form and it accumulates mainly in the endoplasmic reticulum. It acts as a repulsive axon guidance cue through giving local signals to mark areas inaccessible for growing axons. Sema3B signals through both neuropilin-1 and neuropilin-2 and promotes the growth of mouse neonatal cortical neurons (Julien et al. 2005).It is expressed in 212 tissues, but its highest expression level is observed in the tibial nerves (Ota et al., 2003).
Some have predicted that the sema domain of sema3B was responsible for tumor suppression (Nakamura et al. 2000). One experiment revealed that the reduction in sema3B expression correlates with the methylation of CpG islands in the putative sema3B promoter region. Sema3B was seen to inhibit lung cancer cell growth but mutated sema3B failed to show this effect on tumor growth. Interestingly, these mutants were only one amino acid different from the wild type but resulted in difference in their tumor suppressive ability (Tomizawa et al. 2001). It also shows anti-tumor activity by binding to neuropilin-1 in breast and lunch carcinoma (Fig. 3(B)) (Castro-Rivera et al. 2008).
Sema3F
Sema3F is found in secreted form. It is involved in cell motility and cell adhesion. Sena3F uses both neuropilin-1 and neuropilin-2 as its receptor but its affinity for the latter is about 10 times greater than that for neuropilin-1 (Chen et al. 1997). Although it is expressed in 237 tissues, its highest expression level is observed in cervix squamous epithelium (Ota et al., 2003).
Sema3F has a vital role in suppressing the progression of oral squamous cell carcinoma (Liu et al. 2017). In the case of colorectal cancer, sema3F was found to control cell proliferation in a negative manner. Another observation was that the highly metastatic cancer cells expressed reduced amount of sema3F. Increase in sema3F level produced decrease in integrin αvβ3 and knockdown of sema3F did the opposite. The cells with reduced sema3F levels showed higher cell migration capability than controls and sema3F-overexpressing cells. All these evidences suggest that sema3F suppresses tumor. Sema3F exerts tumor suppressive effect in motile breast cancer cells through its interactions with neuropilin-1 and neuropilin-2 receptors (Fig. 3(C)) (Nasarre et al. 2005).
Sema3G
Sema3G is found in secreted form. It acts as a chemorepulsive axon guidance protein in sympathetic axons. It also acts as ligand of neuropillin-2 protein. It binds to neuropilin-2 and repels sympathetic axons (Taniguchi et al. 2005). It is expressed in 214 tissues, but its highest level of is observed in adipose tissue of abdominal region (Ota et al., 2003).
Sema3G has been reported to prevent migration and invasion of tumor cells. It was discovered that overexpression of sema3G leads to inhibition of the migratory and invasive behavior of glioma cells. Additionally, it also inhibits mmp-2 activity which is an index of tumor invasion ability (Yu, 2012). However, not much has been exposed about the receptors which mediate tumor suppression by sema3G.
Sema4A
Sema4A is a single-pass type I membrane protein found in the cell membrane. It is involved in the control of synapse development in glutamatergic and gabaergic neurons. It also plays a role in inhibitory synapse development. In the immune system, it helps prime antigen-specific T cells and promote differentiation of Th1 T helper cells. Sema4A interacts with neuropilin-1 and plays a crucial role in the differentiation of regulatory T (Treg) cells, survival, stability, and function (Delgoffe et al. 2013). Although it is expressed in 174 tissues, its highest level of expression is found in blood (Ota et al., 2003).
One study has unearthed that sema4A can function like a receptor instead of a ligand. As a result, it can transduce signals which have been triggered by plexinB1 binding. The protein Scrib has been identified as the effector protein of sema4A which works downstream in this reverse signaling mechanism. It was shown that plexin-B1-sema4A binding helps with the interaction of sema4A with Scrib (Sun et al., 2016), Scrib has been identified as a protein that suppresses tumor in liver cancer cells (Fig. 3(D)) (Kapil et al. 2017).
Sema4B
Sema4B is a single-pass type I membrane protein found on membranes. It is involved in the inhibition of axonal growth by providing signals locally in order to mark territories inaccessible for growing axons. Sema4B binds to postsynaptic density protein also called psd-95 and assists in the formation or function of synaptic specializations (Burkhardt et al. 2005). It is expressed in 166 tissues, but the peak expression level is observed in the ectocervix (Ota et al., 2003).
Sema4B has been seen to inhibit non-small cell lung cancer growth both in vitro and in vivo (Jian et al. 2015). It prevents metastasis of non-small cell lung cancer through inhibition of mmp-9. Overexpression of sema4B has been found to significantly decrease mmp-9 level which decreased the potential of NSCLC invasiveness (Fig. 3(E)) (Jian et al. 2014).
Sema4G
Sema4G is a single-pass type I membrane protein found on the cell membrane. It is an axon guidance protein that acts as a receptor for plexinB2 residing on the cell surface. Despite being expressed in 146 tissues, its highest expression level is observed in the mucosa of transverse colon (Ota et al., 2003).
In colorectal cancer tissues, the expression of sema4G has been observed to be downregulated significantly, which proved that it is a tumor suppressor but the receptors and signaling mechanism involved remain largely unknown (Lu et al. 2012; Wang et al., 2008).
Sema6A
Sema6A is a single-pass type I membrane protein found on the cell membrane. It acts as a receptor for plexinA2 found on the cell surface which is critical in cell-cell signaling. It is needed for the migration of normal granule cell in the growing cerebellum. It also takes part in the reorganization of the actin cytoskeleton. But its main role is axon guidance in the growing central nervous system as a repulsive axon guidance cue. Despite being expressed in 221 tissues, its highest expression level is observed in the adrenal cortex (Ota et al., 2003).
Sema6A control angiogenesis through the modulation of vegf signaling. In one study, adult sema6A-null mice exhibited reduced tumor compared with controls (Segarra et al. 2012). In an in vivo study, the sema6A ectodomain has been observed to inhibit tumor formation in kidney cancer cells (Dhanabal et al. 2005). From all these studies, it becomes apparent that sema6A is mainly a tumor suppressive protein. However, in another study, forced sema6A overexpression induced anchorage-independent growth and a considerable rise in invasiveness in melanoma cells (Loria et al., 2014). Sema6A interacts with plexinA2 and regulates apoptosis in lung cancer cells (Fig. 3(F)) (Shen et al. 2018).
Therapeutic approaches targeting semaphorins
Use of mutation
The proteolytic fragment of sema3E called p61 is responsible for its pro-metastatic activity (Christensen et al. 1998). It mediates metastasis through transactivation of its co-receptor tyrosine kinase erbb-2 which combines with the plexinD1 in cancer cells (Casazza et al. 2010). A variant of sema3E (Uncl-sema3E) that is mutated and uncleavable binds with plexinD1 in the same way as p61-sema3E does; however, it did not promote erbb-2 activation, and thus it also prevented the downstream signaling pathways. This reduced metastatic spreading (Casazza et al. 2012).
Use of anti-sema antibody
In a recent study, it has been demonstrated that an anti-sema3A antibody can suppress glioblastoma tumor growth (Lee et al. 2018). Sema4D acts as a guidance molecule that impedes the movement of tumoricidal immune cells from entering the tumor microenvironment (Delaire et al. 2001). Blocking sema4D using antibodies suppresses a number of tumor-associated macrophages and promotes treatment efficacy of checkpoint inhibitors anti-pd-1 and anti-ctla4. Combining anti-sema4D antibody with anti ctla-4 antibody acts synergistically and helps in complete tumor rejection and survival (Evans et al. 2015).
Use of tumor suppressive semaphorins
One study has revealed that overexpression of sema3A can drastically suppress tumor growth through inhibition of angiogenesis (Huang et al. 2017). In other studies, significant inhibition of tumor progression in multiple mouse models had been observed after delivery of sema3A in a systemic fashion (Casazza et al. 2011). When tumor-infiltrating monocytes delivered the sema3A, decrease in tumor angiogenesis was observed. For better results, sema3B and sema3F combination could also be tried. Sema3D and sema3E has exhibited strong anti-angiogenic effects in a glioma tumor model in mice (Sabag et al. 2012).
Through targeting semaphorin receptors
Neuropilins
The significance of neuropilins in cancer has been well established (Geretti et al. 2008; Pellet-Many et al. 2008; Guttmann-Raviv et al. 2006; Staton et al. 2007). Wide expression of neuropilins has been found in various human tumors as well as tumor-associated vessels (Grandclement and Borg 2011). They are found to be overexpressed in tumor cells and lead to poor prognosis. In endothelial cells, nrp-1 and vegfr-2 act through stimulation of PI3K activation (Staton et al. 2007). Moreover, vegf works as an autocrine survival factor in tumor cells that overexpress neuropilin (Lee et al. 2007; Barr et al. 2008). Shorter disease-free and overall survival status were correlated with elevated expression of neuropilin-1 in lung cancer patients (Barr et al. 2008).
Neuropilin-blocking antibodies, nrp-blocking peptides, and neuropilin soluble forms have been used to treat cancer as neuropilins have been considered as co-receptors for vegf protein which promotes angiogenesis in tumor cells (Geretti et al. 2008). Antibodies against neuropilin showed inhibition of tumor angiogenesis in animal models. When used in combination with an anti-vegf antibody, it showed even better inhibitor activity against cancer (Liang et al. 2007; Pan et al. 2007). Use of peptides to block vegf-neuropilin interaction has been demonstrated to be successful in tumor inhibition (Barr et al. 2005; Hong et al. 2007; Wronski et al., 2005; Vander-Kooi et al. 2007; Starzec et al. 2006). Internalization of neuropilin-1 is another way of inhibiting it. As recently shown, addition of sulfur groups to polysaccharides like dextran sulfate and fucoidan decreases endothelial cell surface levels of neuropilins. It also does the same to vegfr-1 and vegfr-2 to a limited extent. In this way, they block the binding and functioning of sema3A and vegf (Narazaki et al. 2008).
Plexins
In serous ovarian cancer cells, plexinB1 protein was found to be significantly highly expressed compared with normal ovarian cells or benign ovarian neoplasms. A positive correlation between plexinB1 expression with lymphatic metastasis has been observed in ovarian cancer cells (Narazaki et al. 2008).
A specific peptidic antagonist capable of disrupting oligomerization mediated by the transmembrane domain of the plexinA1 inhibited the signaling and functional activity of plexinA1 and inhibited the growth of brain tumor and angiogenesis related to tumor. In various human glioblastoma models such as glioma cancer stem cells, the anti-tumor activity exhibited by this peptide was observed in vivo (Jacob et al. 2016).
In one experiment, cancer cell invasiveness was reduced using siRNA against plexinB1 and erbb-2 which interact with sema4A. Invasive capability was massively reduced when wild-type plexinB1 was replaced by a mutant form through reduction in rhoA/rhoC activity. Similar outcome was achieved when an anti-plexinB1 antibody was used which hindered the erbb-2-plexinB1 interaction (Worzfeld et al. 2012).
Use of targeting semaphorin co-receptors
Transmembrane receptors contribute to various cell signaling activities. When the ligand joins with the extracellular domain, intracellular signaling cascades become activated or inhibited. A common feature of these signaling events is the formation of complexes between multiple proteins (Cebecauer et al. 2010; Lemmon and Schlessinger 2010). The formation of such complexes is initiated by the dimerization/oligomerization of the receptors. Examples of such complexes include the heterodimer between erbb-2 and plexinB1 (Jacob et al. 2016).
PlexinB1 acts as one of the receptors for sema4D (Jacob et al. 2016). It also combines with their co-receptor, erbb-2. Upon sema4D binding to plexinB1, tyrosine kinase domain of erbb-2 becomes activated and it leads to the autophosphorylation of erbb-2 and also the activation of plexinB1 and downstream cell signaling pathways (Janssen et al. 2010; Swiercz et al. 2004). In cancer cells, promotion of angiogenesis by rho-dependent mechanisms is one of the downstream consequence of sema4D-plexinB1 binding (Basile et al. 2005; Basile et al. 2007; Swiercz et al. 2004). In majority of aggressive breast cancer tumors, erbb-2/her-2 is found to be overexpressed. This makes erbb-2 a good therapeutic target (Badache and Gonçalves 2006). Erbb-2 is also expressed at increased levels in colon cancers where it also considered as a target for inhibition (Pectasides and Bass 2015). Lapatinib, a small molecule tyrosine kinase inhibitor, has been proven to be efficacious and safe for treatment of locally advance and metastatic breast cancer (Gomez et al. 2008). Trastuzumab is an anti-cancer agent used in breast cancer patients. It is a humanized monoclonal antibody that is designed against the extracellular domain of erbb-2 (Valabrega et al. 2007). Other emerging small inhibitors of erbb-2 include AST-1306, AEE-788, CI-1033 (canertinib), TAK-285, PF299804, PF299 (dacomitinib), and EKB-569 (perlitinib). Many of them are in different stages of clinical trial. (Schroeder et al. 2014). Natural products which also bind to tyrosine kinase domain and inhibit the signaling by erbb-2 are also in consideration for use as potential drugs in future (Ahammad et al. 2019; Yang et al. 2011; Li et al. 2016).
Use of microRNAs and siRNAs
In one study on human oral cancer cells, when transfected with microRNA-203, the expression of sema6A was reduced and tumor suppression was achieved (Lim et al. 2017). In another study, siRNA sequences which specifically target sema3C were built and passed into breast cancer cells and it significantly downregulated the expression of sema3C and which in turn significantly suppressed tumor migration and proliferation (Zhu et al. 2017).
Safety of therapeutic approaches involving semaphorins
When injected in an intraocular fashion, sema3E not only shows anti-angiogenic activity towards tumor-associated vessels but also negatively affects normal vessels which poses a chance of bleeding in some tissues (Sakurai et al. 2010; Meyer et al. 2016). One study has shown that when sema3E is injected intravitreally, it exclusively inhibits the extraretinal vascular outgrowth but lets the process of regeneration of the retinal vasculature run unaffected (Fukushima et al. 2011). This type of selective inhibition generated through different modes of administration might be the solution to the side effects caused by semaphorin administration for therapeutic purposes.
It has been predicted that crossing the blood brain barrier might not be easy for sema3D and sema3E when administered locally or systemically which means that there is little chance of neurotoxicity in the central nervous systems in the case of such therapeutic approaches. One study has demonstrated that semaphorins that are bound by the membranes can be produced at the right place, in the right cells, and at the right concentration for having the desired therapeutic impact (Rogalewski et al. 2010; Meyer et al. 2016).
For secreted semaphorins having such selective impact might be a bit more challenging due to autocrine effects or gradient-mediated effects which might produce cell type–specific and opposing results. Sema3A exemplifies this challenge as it can stimulate glioma cell dispersion as a side effect when it is delivered systemically for the inhibition of breast tumor growth (Casazza et al. 2011). After reviewing a wide range of approaches citing the use of semaphorins for targeting cancer, we have not found any direct correction between use of semaphorins and neurotoxicity.
Conclusions and perspectives
Similar review articles on the topic have focused mainly on the role various semaphorins play on the progression or suppression of cancers, the role of their receptors, biological mechanisms etc. (Gu and Giraudo 2013). Many others have shed light on role of specific members of semaphorin family on cancer or their role in specific types of cancer (Shen et al. 2018; Xiao et al. 2018; Hao and Yu 2018; Lontos et al. 2018; Drabkin et al. 2014). The current review article discusses the role semaphorins play in cancer as well as the therapeutic approaches that target these proteins.
Even though initially discovered as axon guidance molecules, versatile roles of semaphorins have been well established in the past couple of decades, especially their roles in cancer. Promotion of cancer through semaphorin is mediated via diverse mechanisms. Out of the 20 semaphorin family members, 19 have been observed to have some degree of association with cancers. Only exception was sema6C with whom no clear relation with cancer has been established so far. Out of the 19 that have association with cancers, 11 have been known to be tumor proliferative and 8 to be tumor suppressive. When it comes to their mechanism of action, diverse set of mediators are involved in the process. Targeting these mechanisms and their mediators, a number of different therapeutic approaches have been developed. Ranging from the usage of antibodies against specific semaphorins to targeting their receptors and co-receptors have been applied. Increased role of natural products and usage of inhibitors of multiple receptors of semaphorins and co-receptors can enrich the therapeutic arsenal of scientists fighting semaphorin-mediated cancers. Screening of natural products that can block the activity of tumor proliferative semaphorins and their receptors as well as co-receptors can help us find a way to curtail the effects of certain cancers. Using them in combination with already approved drugs might produce a synergistic effect and provide at a faster rate of recovery for cancer patients. As discussed in this paper, there are a number of therapeutic approaches to targeting semaphorins for the treatment of cancer which includes the use of mutation, anti-sema antibody, and tumor suppressive semaphorins, targeting semaphorin receptors such as neuropilins and plexins, targeting semaphorin co-receptors, and the use of microRNAs and siRNAs. Among them, finding out the most promising strategy depends on the type of tumor, its mode of proliferation, and most importantly the member of the semaphorin family that is involved as a mediator. For example, for preventing metastasis promoted by sema3E, mutation has been found to be the most efficient process (Casazza et al. 2012). On the other hand, use of anti-sema4D antibodies is most effective against sema4D which impedes the movement of tumoricidal immune cells (Evans et al. 2015). If the mode of proliferation of tumor is through angiogenesis, then the use of tumor suppressive semaphorins, especially sema3A, sema3D, and sema3E, and combination of sema3B and sema3F are the most efficacious and proven form of treatment (Neufeld et al. 2011; Huang et al. 2017; Casazza et al. 2011). Since semaphorins also act as axon guidance molecules, we believe that rather than targeting themselves, targeting their receptors such as neuropilins and plexins, and co-receptors, such as erbb-2 which are overexpressed in case of various types of cancer, offer more specificity which is crucial for the success of treatment. Use of certain microRNAs and siRNAs to regulate the expression of semaphorins and their receptors is a recent endeavor in cancer therapeutics. The advantage offered by microRNA or siRNA-based therapeutics is that they are highly specific and so are more efficient. Designing peptides against semaphorin receptors is an effective weapon against oncogenic signaling. Apart from all these ways, adoption of an interdisciplinary approach can be a useful strategy for targeting semaphorin-mediated cancers.
Acknowledgments
Availability of data and material
Not applicable.
Code availability
Not applicable.
Compliance with ethical standards
Conflict of interest
The author declare that they have no conflict of interest.
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Ahammad I, Sarker MRI, Khan AM, Islam S, Hossain M (2019) Virtual screening to identify novel inhibitors of Pan ERBB family of proteins from natural products with known anti-tumorigenic properties. Int J Pept Res Ther
- Alto, L.T. and Terman, J.R. (2016). Semaphorins and their signaling mechanisms. Methods in Molecular Biology, [online] pp.1–25. Available at: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5538787. Accessed 11 Jun 2020 [DOI] [PMC free article] [PubMed]
- Ayala GE, Dai H, Powell M, Li R, Ding Y, Wheeler TM, Shine D, Kadmon D, Thompson T, Miles BJ, Ittmann MM, Rowley D. Cancer-related axonogenesis and neurogenesis in prostate cancer. Clin Cancer Res. 2008;14(23):7593–7603. doi: 10.1158/1078-0432.CCR-08-1164. [DOI] [PubMed] [Google Scholar]
- Bachelder, R.E., Lipscomb, E.A., Lin, X., Wendt, M.A., Chadborn, N.H., Eickholt, B.J. and Mercurio, A.M. (2003). Competing autocrine pathways involving alternative neuropilin-1 ligands regulate chemotaxis of carcinoma cells. Cancer Research, 63(17), pp.5230–5233. Available at: http://www.ncbi.nlm.nih.gov/pubmed/14500350. Accessed 11 Jun 2020 [PubMed]
- Badache A, Gonçalves A. The ErbB2 signaling network as a target for breast cancer therapy. J Mammary Gland Biol Neoplasia. 2006;11(1):13–25. doi: 10.1007/s10911-006-9009-1. [DOI] [PubMed] [Google Scholar]
- Bao Z-Z, Jin Z. Sema3D andSema7A have distinct expression patterns in chick embryonic development. Dev Dyn. 2006;235(8):2282–2289. doi: 10.1002/dvdy.20882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barr MP, Byrne AM, Duffy AM, Condron CM, Devocelle M, Harriott P, Bouchier-Hayes DJ, Harmey JH. A peptide corresponding to the neuropilin-1-binding site on VEGF165 induces apoptosis of neuropilin-1-expressing breast tumour cells. Br J Cancer. 2005;92(2):328–333. doi: 10.1038/sj.bjc.6602308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barr, M.P., Bouchier-Hayes, D.J. and Harmey, J.J. (2008). Vascular endothelial growth factor is an autocrine survival factor for breast tumour cells under hypoxia. International Journal of Oncology, 32(1), pp.41–48. Available at: http://www.ncbi.nlm.nih.gov/pubmed/18097541 Accessed 11 Jun. 2020 [PubMed]
- Basile, J.R., Barac, A., Zhu, T., Guan, K.-L. and Gutkind, J.S. (2004). Class IV Semaphorins promote angiogenesis by stimulating rho-initiated pathways through Plexin-B. Cancer Research 64(15), pp.5212–5224. Available at: http://cancerres.aacrjournals.org/content/64/15/5212.short [Accessed 11 Jun. 2020] [DOI] [PubMed]
- Basile JR, Afkhami T, Gutkind JS. Semaphorin 4D/Plexin-B1 induces endothelial cell migration through the activation of PYK2, Src, and the phosphatidylinositol 3-kinase-Akt pathway. Mol Cell Biol. 2005;25(16):6889–6898. doi: 10.1128/MCB.25.16.6889-6898.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basile JR, Holmbeck K, Bugge TH, Gutkind JS. MT1-MMP controls tumor-induced angiogenesis through the release of semaphorin 4D. J Biol Chem. 2007;282(9):6899–6905. doi: 10.1074/jbc.M609570200. [DOI] [PubMed] [Google Scholar]
- Bellon A, Luchino J, Haigh K, Rougon G, Haigh J, Chauvet S, Mann F. VEGFR2 (KDR/Flk1) signaling mediates axon growth in response to semaphorin 3E in the developing brain. Neuron. 2010;66(2):205–219. doi: 10.1016/j.neuron.2010.04.006. [DOI] [PubMed] [Google Scholar]
- Burkhardt C, Müller M, Badde A, Garner CC, Gundelfinger ED, Püschel AW. Semaphorin 4B interacts with the post-synaptic density protein PSD-95/SAP90 and is recruited to synapses through a C-terminal PDZ-binding motif. FEBS Lett. 2005;579(17):3821–3828. doi: 10.1016/j.febslet.2005.05.079. [DOI] [PubMed] [Google Scholar]
- Butti R, Kumar TV, Nimma R, Kundu GC. Impact of semaphorin expression on prognostic characteristics in breast cancer. Breast Cancer: Targets and Therapy. 2018;10:79–88. doi: 10.2147/BCTT.S135753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casazza A, Finisguerra V, Capparuccia L, Camperi A, Swiercz JM, Rizzolio S, Rolny C, Christensen C, Bertotti A, Sarotto I, Risio M, Trusolino L, Weitz J, Schneider M, Mazzone M, Comoglio PM, Tamagnone L. Sema3E–Plexin D1 signaling drives human cancer cell invasiveness and metastatic spreading in mice. J Clin Investig. 2010;120(8):2684–2698. doi: 10.1172/JCI42118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casazza A, Fu X, Johansson I, Capparuccia L, Andersson F, Giustacchini A, Squadrito ML, Venneri MA, Mazzone M, Larsson E, Carmeliet P, De Palma M, Naldini L, Tamagnone L, Rolny C. Systemic and targeted delivery of semaphorin 3A inhibits tumor angiogenesis and progression in mouse tumor models. Arterioscler Thromb Vasc Biol. 2011;31(4):741–749. doi: 10.1161/ATVBAHA.110.211920. [DOI] [PubMed] [Google Scholar]
- Casazza A, Kigel B, Maione F, Capparuccia L, Kessler O, Giraudo E, Mazzone M, Neufeld G, Tamagnone L. Tumour growth inhibition and anti-metastatic activity of a mutated furin-resistant semaphorin 3E isoform. EMBO Molecular Medicine. 2012;4(3):234–250. doi: 10.1002/emmm.201100205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castellani V, Chédotal A, Schachner M, Faivre-Sarrailh C, Rougon G. Analysis of the L1-deficient mouse phenotype reveals cross-talk between Sema3A and L1 signaling pathways in axonal guidance. Neuron. 2000;27(2):237–249. doi: 10.1016/s0896-6273(00)00033-7. [DOI] [PubMed] [Google Scholar]
- Castro-Rivera E, Ran S, Brekken RA, Minna JD. Semaphorin 3B inhibits the phosphatidylinositol 3-kinase/Akt pathway through neuropilin-1 in lung and breast cancer cells. Cancer Res. 2008;68(20):8295–8303. doi: 10.1158/0008-5472.CAN-07-6601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catalano A, Lazzarini R, Di Nuzzo S, Orciari S, Procopio A. The plexin-A1 receptor activates vascular endothelial growth factor-receptor 2 and nuclear factor-κB to mediate survival and anchorage-independent growth of malignant mesothelioma cells. Cancer Res. 2009;69(4):1485–1493. doi: 10.1158/0008-5472.CAN-08-3659. [DOI] [PubMed] [Google Scholar]
- Cebecauer M, Spitaler M, Serge A, Magee AI. Signalling complexes and clusters: functional advantages and methodological hurdles. J Cell Sci. 2010;123(3):309–320. doi: 10.1242/jcs.061739. [DOI] [PubMed] [Google Scholar]
- Chen H, Chédotal A, He Z, Goodman CS, Tessier-Lavigne M. Neuropilin-2, a novel member of the neuropilin family, is a high affinity receptor for the semaphorins Sema E and Sema IV but not Sema III. Neuron. 1997;19(3):547–559. doi: 10.1016/s0896-6273(00)80371-2. [DOI] [PubMed] [Google Scholar]
- Christensen, C.R., Klingelhöfer, J., Tarabykina, S., Hulgaard, E.F., Kramerov, D. and Lukanidin, E. (1998). Transcription of a novel mouse semaphorin gene, M-semaH, correlates with the metastatic ability of mouse tumor cell lines. Cancer Research, 58(6), pp.1238–1244. Available at: http://www.ncbi.nlm.nih.gov/pubmed/9515811 Accessed 11 Jun. 2020 [PubMed]
- Conrotto P, Corso S, Gamberini S, Comoglio PM, Giordano S. Interplay between scatter factor receptors and B plexins controls invasive growth. Oncogene. 2004;23(30):5131–5137. doi: 10.1038/sj.onc.1207650. [DOI] [PubMed] [Google Scholar]
- Conrotto P, Valdembri D, Corso S, Serini G, Tamagnone L, Comoglio PM, Bussolino F, Giordano S. Sema4D induces angiogenesis through met recruitment by plexin B1. Blood. 2005;105(11):4321–4329. doi: 10.1182/blood-2004-07-2885. [DOI] [PubMed] [Google Scholar]
- Correa RG, Sasahara RM, Bengtson MH, Katayama MLH, Salim ACM, Brentani MM, Sogayar MC, de Souza SJ, Simpson AJG. Human Semaphorin 6B [(HSA)SEMA6B], A novel human class 6 semaphorin gene: alternative splicing and all-trans-retinoic acid-dependent downregulation in glioblastoma cell lines. Genomics. 2001;73(3):343–348. doi: 10.1006/geno.2001.6525. [DOI] [PubMed] [Google Scholar]
- D’Apice L, Costa V, Valente C, Trovato M, Pagani A, Manera S, Regolo L, Zambelli A, Ciccodicola A, De Berardinis P. Analysis of SEMA6B gene expression in breast cancer: identification of a new isoform. Biochim Biophys Acta Gen Subj. 2013;1830(10):4543–4553. doi: 10.1016/j.bbagen.2013.05.003. [DOI] [PubMed] [Google Scholar]
- Delaire, S., Billard, C., Tordjman, R., Chédotal, A., Elhabazi, A., Bensussan, A. and Boumsell, L. (2001). Biological activity of soluble CD100. II. Soluble CD100, similarly to H-SemaIII, inhibits immune cell migration. Journal of Immunology (Baltimore, Md.: 1950), [online] 166(7), pp.4348–4354. Available at: http://www.ncbi.nlm.nih.gov/pubmed/11254688 Accessed 11 Jun. 2020 [DOI] [PubMed]
- Delgoffe, G.M., Woo, S.-R., Turnis, M.E., Gravano, D.M., Guy, C., Overacre, A.E., Bettini, M.L., Vogel, P., Finkelstein, D., Bonnevier, J., Workman, C.J. and Vignali, D.A.A. (2013). Stability and function of regulatory T cells is maintained by a neuropilin-1–semaphorin-4a axis. Nature 501(7466), pp.252–256. Available at: https://www.nature.com/articles/nature12428?page=6 Accessed 22 Mar. 2020 [DOI] [PMC free article] [PubMed]
- Dhanabal, M., Wu, F., Alvarez, E., McQueeney, K.D., Jeffers, M., MacDougall, J., Boldog, F.L., Hackett, C., Shenoy, S., Khramtsov, N., Weiner, J., Lichenstein, H.S. and LaRochelle, W.J. (2005). Recombinant semaphorin 6A-1 ectodomain inhibits in vivo growth factor and tumor cell line-induced angiogenesis. Cancer Biology & Therapy, 4(6), pp.659–668. Available at: http://www.ncbi.nlm.nih.gov/pubmed/15917651 Accessed 11 Jun. 2020 [DOI] [PubMed]
- Ding Y, He D, Florentin D, Frolov A, Hilsenbeck S, Ittmann M, Kadmon D, Miles B, Rowley D, Ayala G. Semaphorin 4F as a critical regulator of neuroepithelial interactions and a biomarker of aggressive prostate cancer. Clin Cancer Res. 2013;19(22):6101–6111. doi: 10.1158/1078-0432.CCR-12-3669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drabkin, H., Nasarre, P. and Gemmill, R. (2014). The emerging role of class-3 semaphorins and their neuropilin receptors in oncology. OncoTargets and Therapy, p.1663. Available at: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4181631/pdf/ott-7-1663.pdf Accessed 19 May 2020 [DOI] [PMC free article] [PubMed]
- Evans EE, Jonason AS, Bussler H, Torno S, Veeraraghavan J, Reilly C, Doherty MA, Seils J, Winter LA, Mallow C, Kirk R, Howell A, Giralico S, Scrivens M, Klimatcheva K, Fisher TL, Bowers WJ, Paris M, Smith ES, Zauderer M. Antibody blockade of semaphorin 4D promotes immune infiltration into tumor and enhances response to other immunomodulatory therapies. Cancer Immunology Research. 2015;3(6):689–701. doi: 10.1158/2326-6066.CIR-14-0171. [DOI] [PubMed] [Google Scholar]
- Fagerberg L, Hallström BM, Oksvold P, Kampf C, Djureinovic D, Odeberg J, Habuka M, Tahmasebpoor S, Danielsson A, Edlund K, Asplund A, Sjöstedt E, Lundberg E, Szigyarto CA-K, Skogs M, Takanen JO, Berling H, Tegel H, Mulder J, Nilsson P, Schwenk JM, Lindskog C, Danielsson F, Mardinoglu A, Sivertsson Å, von Feilitzen K, Forsberg M, Zwahlen M, Olsson I, Navani S, Huss M, Nielsen J, Ponten F, Uhlén M. Analysis of the human tissue-specific expression by genome-wide integration of transcriptomics and antibody-based proteomics. Mol Cell Proteomics. 2013;13(2):397–406. doi: 10.1074/mcp.M113.035600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fazzari P, Penachioni J, Gianola S, Rossi F, Eickholt BJ, Maina F, Alexopoulou L, Sottile A, Comoglio P, Flavell RA, Tamagnone L. Plexin-B1 plays a redundant role during mouse development and in tumour angiogenesis. BMC Developmental Biology. 2007;7(1):55. doi: 10.1186/1471-213X-7-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feiner L, Koppel AM, Kobayashi H, Raper JA. Secreted chick semaphorins bind recombinant neuropilin with similar affinities but bind different subsets of neurons in situ. Neuron. 1997;19(3):539–545. doi: 10.1016/s0896-6273(00)80370-0. [DOI] [PubMed] [Google Scholar]
- Foley K, Rucki AA, Xiao Q, Zhou D, Leubner A, Mo G, Kleponis J, Wu AA, Sharma R, Jiang Q, Anders RA, Iacobuzio-Donahue CA, Hajjar KA, Maitra A, Jaffee EM, Zheng L. Semaphorin 3D autocrine signaling mediates the metastatic role of annexin A2 in pancreatic cancer. Science Signaling. 2015;8(388):ra77–ra77. doi: 10.1126/scisignal.aaa5823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukushima Y, Okada M, Kataoka H, Hirashima M, Yoshida Y, Mann F, Gomi F, Nishida K, Nishikawa S-I, Uemura A. Sema3E-PlexinD1 signaling selectively suppresses disoriented angiogenesis in ischemic retinopathy in mice. J Clin Investig. 2011;121(5):1974–1985. doi: 10.1172/JCI44900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Areas R, Libreros S, Amat S, Keating P, Carrio R, Robinson P, Blieden C, Iragavarapu-Charyulu V (2014) Semaphorin7A promotes tumor growth and exerts a pro-angiogenic effect in macrophages of mammary tumor-bearing mice. Front Physiol 5 [DOI] [PMC free article] [PubMed]
- Garcia-Areas R, Libreros S, Simoes M, Castro-Silva C, Gazaniga N, Amat S, Jaczewska J, Keating P, Schilling K, Brito M, Wojcikiewicz EP, Iragavarpu-Charyulu V. Suppression of tumor-derived Semaphorin 7A and genetic ablation of host-derived Semaphorin 7A impairs tumor progression in a murine model of advanced breast carcinoma. Int J Oncol. 2017;51(5):1395–1404. doi: 10.3892/ijo.2017.4144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geretti E, Shimizu A, Klagsbrun M. Neuropilin structure governs VEGF and semaphorin binding and regulates angiogenesis. Angiogenesis. 2008;11(1):31–39. doi: 10.1007/s10456-008-9097-1. [DOI] [PubMed] [Google Scholar]
- Gomez HL, Doval DC, Chavez MA, Ang PC-S, Aziz Z, Nag S, Ng C, Franco SX, Chow LWC, Arbushites MC, Casey MA, Berger MS, Stein SH, Sledge GW. Efficacy and safety of lapatinib as first-line therapy for ErbB2-amplified locally advanced or metastatic breast Cancer. J Clin Oncol. 2008;26(18):2999–3005. doi: 10.1200/JCO.2007.14.0590. [DOI] [PubMed] [Google Scholar]
- Goshima, Y., Ito, T., Sasaki, Y. and Nakamura, F. (2002). Semaphorins as signals for cell repulsion and invasion. The Journal of Clinical Investigation 109(8), pp.993–998. Available at: https://www.jci.org/articles/view/15467 Accessed 11 Jun. 2020 [DOI] [PMC free article] [PubMed]
- Grandclement C, Borg C. Neuropilins: a new target for cancer therapy. Cancers. 2011;3(2):1899–1928. doi: 10.3390/cancers3021899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu C, Giraudo E. The role of semaphorins and their receptors in vascular development and cancer. Exp Cell Res. 2013;319(9):1306–1316. doi: 10.1016/j.yexcr.2013.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurrapu S, Pupo E, Franzolin G, Lanzetti L, Tamagnone L. Sema4C/PlexinB2 signaling controls breast cancer cell growth, hormonal dependence and tumorigenic potential. Cell Death & Differentiation. 2018;25(7):1259–1275. doi: 10.1038/s41418-018-0097-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guttmann-Raviv N, Kessler O, Shraga-Heled N, Lange T, Herzog Y, Neufeld G. The neuropilins and their role in tumorigenesis and tumor progression. Cancer Lett. 2006;231(1):1–11. doi: 10.1016/j.canlet.2004.12.047. [DOI] [PubMed] [Google Scholar]
- Hao J, Yu J. Semaphorin 3C and its receptors in cancer and cancer stem-like cells. Biomedicines. 2018;6(2):42. doi: 10.3390/biomedicines6020042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herman, J.G. and Meadows, G.G. (2007). Increased class 3 semaphorin expression modulates the invasive and adhesive properties of prostate cancer cells. International Journal of Oncology, 30(5), pp.1231–1238. Available at: http://www.ncbi.nlm.nih.gov/pubmed/17390026 Accessed 11 Jun. 2020 [PubMed]
- Hirota E, Yan L, Tsunoda T, Ashida S, Fujime M, Shuin T, Miki T, Nakamura Y, Katagiri T (2006) Genome-wide gene expression profiles of clear cell renal cell carcinoma: identification of molecular targets for treatment of renal cell carcinoma. Int J Oncol [PubMed]
- Hong T-M, Chen Y-L, Wu Y-Y, Yuan A, Chao Y-C, Chung Y-C, Wu M-H, Yang S-C, Pan S-H, Shih J-Y, Chan W-K, Yang P-C. Targeting Neuropilin 1 as an antitumor strategy in lung cancer. Clin Cancer Res. 2007;13(16):4759–4768. doi: 10.1158/1078-0432.CCR-07-0001. [DOI] [PubMed] [Google Scholar]
- Huang C, Wang Y, Huang J-H, Liu W (2017) Sema3A drastically suppresses tumor growth in oral cancer xenograft model of mice. BMC Pharmacol Toxicol 18(1) [DOI] [PMC free article] [PubMed]
- Huber AB, Kolodkin AL, Ginty DD, Cloutier J-F. Signaling at thegrowthcone: ligand-receptor complexes and the control of axon growth and guidance. Annu Rev Neurosci. 2003;26(1):509–563. doi: 10.1146/annurev.neuro.26.010302.081139. [DOI] [PubMed] [Google Scholar]
- Inoue N, Nishizumi H, Naritsuka H, Kiyonari H, Sakano H (2018) Sema7A/PlxnCl signaling triggers activity-dependent olfactory synapse formation. Nat Commun 9(1) [DOI] [PMC free article] [PubMed]
- Jacob L, Sawma P, Garnier N, Meyer LAT, Fritz J, Hussenet T, Spenlé C, Goetz J, Vermot J, Fernandez A, Baumlin N, Aci-Sèche S, Orend G, Roussel G, Crémel G, Genest M, Hubert P, Bagnard D. Inhibition of PlexA1-mediated brain tumor growth and tumor-associated angiogenesis using a transmembrane domain targeting peptide. Oncotarget. 2016;7(36):57851–57865. doi: 10.18632/oncotarget.11072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janssen BJC, Robinson RA, Pérez-Brangulí F, Bell CH, Mitchell KJ, Siebold C, Jones EY. Structural basis of semaphorin–plexin signalling. Nature. 2010;467(7319):1118–1122. doi: 10.1038/nature09468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jian H, Zhao Y, Liu B, Lu S. SEMA4b inhibits MMP9 to prevent metastasis of non-small cell lung cancer. Tumor Biol. 2014;35(11):11051–11056. doi: 10.1007/s13277-014-2409-8. [DOI] [PubMed] [Google Scholar]
- Jian H, Zhao Y, Liu B, Lu S. SEMA4B inhibits growth of non-small cell lung cancer in vitro and in vivo. Cell Signal. 2015;27(6):1208–1213. doi: 10.1016/j.cellsig.2015.02.027. [DOI] [PubMed] [Google Scholar]
- Jiang H, Chen C, Sun Q, Wu J, Qiu L, Gao C, Liu W, Yang J, Jun N, Dong J. The role of semaphorin 4D in tumor development and angiogenesis in human breast cancer. OncoTargets and Therapy. 2016;9:5737–5750. doi: 10.2147/OTT.S114708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Julien F, Bechara A, Fiore R, Nawabi H, Zhou H, Hoyo-Becerra C, Bozon M, Rougon G, Grumet M, Püschel AW, Sanes JR, Castellani V. Dual functional activity of semaphorin 3B is required for positioning the anterior commissure. Neuron. 2005;48(1):63–75. doi: 10.1016/j.neuron.2005.08.033. [DOI] [PubMed] [Google Scholar]
- Jurcak NR, Fujiwara K, Rucki AA, Foley K, Murphy A, Muth S, Brittingham A, Jaffee EM, Zheng L (2018) Abstract 3026: role of AnnexinA2, Sema3D and PlexinD1 in mediating perineural invasion as a mechanism of metastasis in pancreatic ductal adenocarcinoma. Tumor Biol
- Kang S, Nakanishi Y, Kioi Y, Okuzaki D, Kimura T, Takamatsu H, Koyama S, Nojima S, Nishide M, Hayama Y, Kinehara Y, Kato Y, Nakatani T, Shimogori T, Takagi J, Toyofuku T, Kumanogoh A. Semaphorin 6D reverse signaling controls macrophage lipid metabolism and anti-inflammatory polarization. Nat Immunol. 2018;19(6):561–570. doi: 10.1038/s41590-018-0108-0. [DOI] [PubMed] [Google Scholar]
- Kapil S, Sharma BK, Patil M, Elattar S, Yuan J, Hou SX, Kolhe R, Satyanarayana A (2017) The cell polarity protein Scrib functions as a tumor suppressor in liver cancer. Oncotarget 8(16) [DOI] [PMC free article] [PubMed]
- Kigel B, Rabinowicz N, Varshavsky A, Kessler O, Neufeld G. Plexin-A4 promotes tumor progression and tumor angiogenesis by enhancement of VEGF and bFGF signaling. Blood. 2011;118(15):4285–4296. doi: 10.1182/blood-2011-03-341388. [DOI] [PubMed] [Google Scholar]
- Kundu, A., Kho, E.-Y., Shelar, S.B., Nam, H., Brinkley, G., Darshan, S., Tang, Y., Kirkman, R., Crossman, D.K., Varambally, S., Rowe, G.C., Wei, S., Buckhaults, P. and Sudarshan, S. (2018). Abstract 4483: functional implications ofPRDM16loss in kidney cancer. Molecular and Cellular Biology / Genetics
- Lee T-H, Seng S, Sekine M, Hinton C, Fu Y, Avraham HK, Avraham S. Vascular endothelial growth factor mediates intracrine survival in human breast carcinoma cells through internally expressed VEGFR1/FLT1. PLoS Medicine. 2007;4(6):e186. doi: 10.1371/journal.pmed.0040186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J, Shin YJ, Lee K, Cho HJ, Sa JK, Lee S-Y, Kim S-H, Lee J, Yoon Y, Nam D-H. Anti-SEMA3A antibody: a novel therapeutic agent to suppress glioblastoma tumor growth. Cancer Res Treat. 2018;50(3):1009–1022. doi: 10.4143/crt.2017.315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemmon MA, Schlessinger J. Cell signaling by receptor tyrosine kinases. Cell. 2010;141(7):1117–1134. doi: 10.1016/j.cell.2010.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, J., Wang, H., Li, J., Bao, J. and Wu, C. (2016). Discovery of a potential HER2 inhibitor from natural products for the treatment of HER2-positive breast cancer. International Journal of Molecular Sciences, 17(7), p.1055. Available at: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4964431/ Accessed 22 Jul. 2019 [DOI] [PMC free article] [PubMed]
- Li Z, Hao J, Duan X, Wu N, Zhou Z, Yang F, Li J, Zhao Z, Huang S (2017) The role of semaphorin 3A in bone remodeling. Front Cell Neurosci 11 [DOI] [PMC free article] [PubMed]
- Liang W-C, Dennis MS, Stawicki S, Chanthery Y, Pan Q, Chen Y, Eigenbrot C, Yin J, Koch AW, Wu X, Ferrara N, Bagri A, Tessier-Lavigne M, Watts RJ, Wu Y. Function blocking antibodies to neuropilin-1 generated from a designed human synthetic antibody phage library. J Mol Biol. 2007;366(3):815–829. doi: 10.1016/j.jmb.2006.11.021. [DOI] [PubMed] [Google Scholar]
- Lim HS, Kim CS, Kim JS, Yu SK, Go DS, Lee, SA, Moon SM, Chun, HS, Kim, SG Kim, DK (2017) Suppression of oral carcinoma oncogenic activity by microRNA-203 via down-regulation of SEMA6A. Anticancer Res 37(10):5425–5433 [DOI] [PubMed]
- Liu X-B (2005) Stereotyped axon pruning via plexin signaling is associated with synaptic complex elimination in the hippocampus. J Neurosci 25(40):9124–9134 [DOI] [PMC free article] [PubMed]
- Liu Y, Li R, Yin K, Ren G, Zhang Y (2017) The crucial role of SEMA3F in suppressing the progression of oral squamous cell carcinoma. Cellular & Molecular Biology Letters 22(1) [DOI] [PMC free article] [PubMed]
- Lontos, K., Adamik, J., Tsagianni, A., Galson, D.L., Chirgwin, J.M. and Suvannasankha, A. (2018). The role of semaphorin 4D in bone remodeling and cancer metastasis. Frontiers in Endocrinology, 9. Available at: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6018527/pdf/fendo-09-00322.pdf Accessed 28 Apr. 2020 [DOI] [PMC free article] [PubMed]
- Loria R, Bon G, Perotti V, Gallo E, Bersani I, Baldassari P, Porru M, Leonetti C, Di Carlo S, Visca P, Brizzi MF, Anichini A, Mortarini R, Falcioni R. Sema6A and Mical1 control cell growth and survival of BRAFV600E human melanoma cells. Oncotarget. 2014;6(5):2779–2793. doi: 10.18632/oncotarget.2995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu, T.-P., Tsai, M.-H., Lee, J.-M., Hsu, C.-P., Chen, P.-C., Lin, C.-W., Shih, J.-Y., Yang, P.-C., Hsiao, C.K., Lai, L.-C. and Chuang, E.Y. (2010). Identification of a novel biomarker, SEMA5A, for non–small cell lung carcinoma in nonsmoking women. Cancer Epidemiology and Prevention Biomarkers 19(10), pp.2590–2597. Available at: http://cebp.aacrjournals.org/content/19/10/2590.long Accessed 11 Jun. 2020 [DOI] [PubMed]
- Lu, T.-P., Tsai, M.-H., Hsiao, C.K., Lai, L.-C. and Chuang, E.Y. (2012). Expression and functions of semaphorins in cancer. Translational Cancer Research 1(2), pp.74–87. Available at: http://tcr.amegroups.com/article/view/388/759 Accessed 11 Jun. 2020
- Lu Y, Xu Q, Chen L, Zuo Y, Liu S, Hu Y, Li X, Li Y, Zhao X. Expression of semaphorin 6D and its receptor plexin-A1 in gastric cancer and their association with tumor angiogenesis. Oncol Lett. 2016;12(5):3967–3974. doi: 10.3892/ol.2016.5208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maejima R, Tamai K, Shiroki T, Yokoyama M, Shibuya R, Nakamura M, Yamaguchi K, Abue M, Oikawa T, Noguchi T, Miura K, Fujiya T, Sato I, Iijima K, Shimosegawa T, Tanaka N, Satoh K. Enhanced expression of semaphorin 3E is involved in the gastric cancer development. Int J Oncol. 2016;49(3):887–894. doi: 10.3892/ijo.2016.3593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malik, M.F.A., Satherley, L.K., Davies, E.L., Ye, L. and Jiang, W.G. (2016). Expression of semaphorin 3C in breast cancer and its impact on adhesion and invasion of breast cancer cells. Anticancer Research, [online] 36(3), pp.1281–1286. Available at: http://www.ncbi.nlm.nih.gov/pubmed/26977026 Accessed 11 Jun. 2020 [PubMed]
- Man J, Shoemake J, Zhou W, Fang X, Wu Q, Rizzo A, Prayson R, Bao S, Rich JN, Yu JS. Sema3C promotes the survival and tumorigenicity of glioma stem cells through Rac1 activation. Cell Rep. 2014;9(5):1812–1826. doi: 10.1016/j.celrep.2014.10.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer LAT, Fritz J, Pierdant-Mancera M, Bagnard D. Current drug design to target the semaphorin/neuropilin/plexin complexes. Cell Adhes Migr. 2016;10(6):700–708. doi: 10.1080/19336918.2016.1261785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitsogiannis MD, Little GE, Mitchell KJ (2017) Semaphorin-plexin signaling influences early ventral telencephalic development and thalamocortical axon guidance. Neural Dev 12(1) [DOI] [PMC free article] [PubMed]
- Miyato H, Tsuno NH, Kitayama J. Semaphorin 3C is involved in the progression of gastric cancer. Cancer Sci. 2012;103(11):1961–1966. doi: 10.1111/cas.12003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller MW, Giese NA, Swiercz JM, Ceyhan GO, Esposito I, Hinz U, Büchler P, Giese T, Büchler MW, Offermanns S, Friess H. Association of axon guidance factor semaphorin 3A with poor outcome in pancreatic cancer. Int J Cancer. 2007;121(11):2421–2433. doi: 10.1002/ijc.22949. [DOI] [PubMed] [Google Scholar]
- Nakamura, F., Kalb, R. G. and Strittmatter, S. M. (2000) ‘Molecular basis of semaphorin-mediated axon guidance’, Journal of Neurobiology. John Wiley & Sons, Inc., 44(2), pp. 219–229 [DOI] [PubMed]
- Narazaki M, Segarra M, Tosato G. Sulfated polysaccharides identified as inducers of neuropilin-1 internalization and functional inhibition of VEGF165 and semaphorin3A. Blood. 2008;111(8):4126–4136. doi: 10.1182/blood-2007-09-112474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nasarre P, Kusy S, Constantin B, Castellani V, Drabkin HA, Bagnard D, Roche J. Semaphorin SEMA3F has a repulsing activity on breast cancer cells and inhibits E-cadherin-mediated cell adhesion. Neoplasia. 2005;7(2):180–189. doi: 10.1593/neo.04481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neufeld G, Kessler O. The semaphorins: versatile regulators of tumour progression and tumour angiogenesis. Nat Rev Cancer. 2008;8(8):632–645. doi: 10.1038/nrc2404. [DOI] [PubMed] [Google Scholar]
- Neufeld G, Sabag AD, Rabinovicz N, Kessler O. Semaphorins in angiogenesis and tumor progression. Cold Spring Harbor Perspectives in Medicine. 2011;2(1):a006718–a006718. doi: 10.1101/cshperspect.a006718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neufeld G, Mumblat Y, Smolkin T, Toledano S, Nir-Zvi I, Ziv K, Kessler O. The role of the semaphorins in cancer. Cell Adhes Migr. 2016;10(6):652–674. doi: 10.1080/19336918.2016.1197478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orr BO, Fetter RD, Davis GW. Retrograde semaphorin–plexin signalling drives homeostatic synaptic plasticity. Nature. 2017;550(7674):109–113. doi: 10.1038/nature24017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ota T, Suzuki Y, Nishikawa T, Otsuki T, Sugiyama T, Irie R, Wakamatsu A, Hayashi K, Sato H, Nagai K, Kimura K, Makita H, Sekine M, Obayashi M, Nishi T, Shibahara T, Tanaka T, Ishii S, Yamamoto J, Saito K, Kawai Y, Isono Y, Nakamura Y, Nagahari K, Murakami K, Yasuda T, Iwayanagi T, Wagatsuma M, Shiratori A, Sudo H, Hosoiri T, Kaku Y, Kodaira H, Kondo H, Sugawara M, Takahashi M, Kanda K, Yokoi T, Furuya T, Kikkawa E, Omura Y, Abe K, Kamihara K, Katsuta N, Sato K, Tanikawa M, Yamazaki M, Ninomiya K, Ishibashi T, Yamashita H, Murakawa K, Fujimori K, Tanai H, Kimata M, Watanabe M, Hiraoka S, Chiba Y, Ishida S, Ono Y, Takiguchi S, Watanabe S, Yosida M, Hotuta T, Kusano J, Kanehori K, Takahashi-Fujii A, Hara H, Tanase T, Nomura Y, Togiya S, Komai F, Hara R, Takeuchi K, Arita M, Imose N, Musashino K, Yuuki H, Oshima A, Sasaki N, Aotsuka S, Yoshikawa Y, Matsunawa H, Ichihara T, Shiohata N, Sano S, Moriya S, Momiyama H, Satoh N, Takami S, Terashima Y, Suzuki O, Nakagawa S, Senoh A, Mizoguchi H, Goto Y, Shimizu F, Wakebe H, Hishigaki H, Watanabe T, Sugiyama A, Takemoto M, Kawakami B, Yamazaki M, Watanabe K, Kumagai A, Itakura S, Fukuzumi Y, Fujimori Y, Komiyama M, Tashiro H, Tanigami A, Fujiwara T, Ono T, Yamada K, Fujii Y, Ozaki K, Hirao M, Ohmori Y, Kawabata A, Hikiji T, Kobatake N, Inagaki H, Ikema Y, Okamoto S, Okitani R, Kawakami T, Noguchi S, Itoh T, Shigeta K, Senba T, Matsumura K, Nakajima Y, Mizuno T, Morinaga M, Sasaki M, Togashi T, Oyama M, Hata H, Watanabe M, Komatsu T, Mizushima-Sugano J, Satoh T, Shirai Y, Takahashi Y, Nakagawa K, Okumura K, Nagase T, Nomura N, Kikuchi H, Masuho Y, Yamashita R, Nakai K, Yada T, Nakamura Y, Ohara O, Isogai T, Sugano S. Complete sequencing and characterization of 21,243 full-length human cDNAs. Nat Genet. 2003;36(1):40–45. doi: 10.1038/ng1285. [DOI] [PubMed] [Google Scholar]
- Paldy E, Simonetti M, Worzfeld T, Bali KK, Vicuña L, Offermanns S, Kuner R (2017) Semaphorin 4C Plexin-B2 signaling in peripheral sensory neurons is pronociceptive in a model of inflammatory pain. Nat Commun 8(1) [DOI] [PMC free article] [PubMed]
- Pan Q, Chanthery Y, Liang W-C, Stawicki S, Mak J, Rathore N, Tong RK, Kowalski J, Yee SF, Pacheco G, Ross S, Cheng Z, Le Couter J, Plowman G, Peale F, Koch AW, Wu Y, Bagri A, Tessier-Lavigne M, Watts RJ. Blocking Neuropilin-1 function has an additive effect with anti-VEGF to inhibit tumor growth. Cancer Cell. 2007;11(1):53–67. doi: 10.1016/j.ccr.2006.10.018. [DOI] [PubMed] [Google Scholar]
- Pan G-Q, Ren H-Z, Zhang S-F, Wang X-M, Wen J-F. Expression of semaphorin 5A and its receptor plexin B3 contributes to invasion and metastasis of gastric carcinoma. World Journal of Gastroenterology. 2009;15(22):2800. doi: 10.3748/wjg.15.2800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pectasides E, Bass AJ. ERBB2 emerges as a new target for colorectal cancer. Cancer Discovery. 2015;5(8):799–801. doi: 10.1158/2159-8290.CD-15-0730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pellet-Many C, Frankel P, Jia H, Zachary I. Neuropilins: structure, function and role in disease. Biochem J. 2008;411(2):211–226. doi: 10.1042/BJ20071639. [DOI] [PubMed] [Google Scholar]
- Potiron V, Nasarre P, Roche J, Healy C, Boumsell L (n.d.) Semaphorin signaling in the immune system. Advances in Experimental Medicine and Biology:132–144 [DOI] [PubMed]
- Rehman M, Tamagnone L. Semaphorins in cancer: biological mechanisms and therapeutic approaches. Semin Cell Dev Biol. 2013;24(3):179–189. doi: 10.1016/j.semcdb.2012.10.005. [DOI] [PubMed] [Google Scholar]
- Rogalewski A, Dittgen T, Klugmann M, Kirsch F, Krüger C, Pitzer C, Minnerup J, Schäbitz W-R, Schneider A. Semaphorin 6A improves functional recovery in conjunction with motor training after cerebral ischemia. PLoS ONE. 2010;5(5):e10737. doi: 10.1371/journal.pone.0010737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabag, A.D., Bode, J., Fink, D., Kigel, B., Kugler, W. and Neufeld, G. (2012). Semaphorin-3D and semaphorin-3E inhibit the development of tumors from glioblastoma cells implanted in the cortex of the brain. PLoS ONE, 7(8), p.e42912 [DOI] [PMC free article] [PubMed]
- Sadanandam A, Varney ML, Singh S, Ashour AE, Moniaux N, Deb S, Lele SM, Batra SK, Singh RK. High gene expression of semaphorin 5A in pancreatic cancer is associated with tumor growth, invasion and metastasis. Int J Cancer. 2010;127(6):1373–1383. doi: 10.1002/ijc.25166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadanandam A, Sidhu SS, Wullschleger S, Singh S, Varney ML, Yang C-S, Ashour AE, Batra SK, Singh RK. Secreted semaphorin 5A suppressed pancreatic tumour burden but increased metastasis and endothelial cell proliferation. Br J Cancer. 2012;107(3):501–507. doi: 10.1038/bjc.2012.298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito T, Kasamatsu A, Ogawara K, Miyamoto I, Saito K, Iyoda M, Suzuki T, Endo-Sakamoto Y, Shiiba M, Tanzawa H, Uzawa K. Semaphorin7A promotion of tumoral growth and metastasis in human oral cancer by regulation of G1 cell cycle and matrix metalloproteases: possible contribution to tumoral angiogenesis. PLOS ONE. 2015;10(9):e0137923. doi: 10.1371/journal.pone.0137923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakurai A, Gavard J, Annas-Linhares Y, Basile JR, Amornphimoltham P, Palmby TR, Yagi H, Zhang F, Randazzo PA, Li X, Weigert R, Gutkind JS. Semaphorin 3E initiates antiangiogenic signaling through plexin D1 by regulating Arf6 and R-Ras. Mol Cell Biol. 2010;30(12):3086–3098. doi: 10.1128/MCB.01652-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroeder R, Stevens C, Sridhar J. Small molecule tyrosine kinase inhibitors of ErbB2/HER2/Neu in the treatment of aggressive breast cancer. Molecules. 2014;19(9):15196–15212. doi: 10.3390/molecules190915196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segarra M, Ohnuki H, Maric D, Salvucci O, Hou X, Kumar A, Li X, Tosato G. Semaphorin 6A regulates angiogenesis by modulating VEGF signaling. Blood. 2012;120(19):4104–4115. doi: 10.1182/blood-2012-02-410076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen C-Y, Chang Y-C, Chen L-H, Lin W-C, Lee Y-H, Yeh S-T, Chen H-K, Fang W, Hsu C-P, Lee J-M, Lu T-P, Hsiao P-W, Lai L-C, Tsai M-H, Chuang EY (2018) The extracellular SEMA domain attenuates intracellular apoptotic signaling of semaphorin 6A in lung cancer cells. Oncogenesis 7(12) [DOI] [PMC free article] [PubMed]
- Starzec A, Vassy R, Martin A, Lecouvey M, Di Benedetto M, Crépin M, Perret GY. Antiangiogenic and antitumor activities of peptide inhibiting the vascular endothelial growth factor binding to neuropilin-1. Life Sci. 2006;79(25):2370–2381. doi: 10.1016/j.lfs.2006.08.005. [DOI] [PubMed] [Google Scholar]
- Staton C, Kumar I, Reed M, Brown N. Neuropilins in physiological and pathological angiogenesis. J Pathol. 2007;212(3):237–248. doi: 10.1002/path.2182. [DOI] [PubMed] [Google Scholar]
- Sun T, Yang L, Kaur H, Pestel J, Looso M, Nolte H, Krasel C, Heil D, Krishnan RK, Santoni M-J, Borg J-P, Bünemann M, Offermanns S, Swiercz JM, Worzfeld T. A reverse signaling pathway downstream of Sema4A controls cell migration via Scrib. J Cell Biol. 2016;216(1):199–215. doi: 10.1083/jcb.201602002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swiercz JM, Kuner R, Offermanns S. Plexin-B1/RhoGEF–mediated RhoA activation involves the receptor tyrosine kinase ErbB-2. J Cell Biol. 2004;165(6):869–880. doi: 10.1083/jcb.200312094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi T, Fournier A, Nakamura F, Wang L-H, Murakami Y, Kalb RG, Fujisawa H, Strittmatter SM. Plexin-neuropilin-1 complexes form functional semaphorin-3A receptors. Cell. 1999;99(1):59–69. doi: 10.1016/s0092-8674(00)80062-8. [DOI] [PubMed] [Google Scholar]
- Takamatsu H, Kumanogoh A. Diverse roles for semaphorin−plexin signaling in the immune system. Trends Immunol. 2012;33(3):127–135. doi: 10.1016/j.it.2012.01.008. [DOI] [PubMed] [Google Scholar]
- Takamatsu H, Okuno T, Kumanogoh A. Regulation of immune cell responses by semaphorins and their receptors. Cellular & Molecular Immunology. 2010;7(2):83–88. doi: 10.1038/cmi.2009.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamagnone L. Emerging role of semaphorins as major regulatory signals and potential therapeutic targets in cancer. Cancer Cell. 2012;22(2):145–152. doi: 10.1016/j.ccr.2012.06.031. [DOI] [PubMed] [Google Scholar]
- Tamagnone L, Comoglio PM. To move or not to move? EMBO Rep. 2004;5(4):356–361. doi: 10.1038/sj.embor.7400114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamagnone L, Artigiani S, Chen H, He Z, Ming G, Song H, Chedotal A, Winberg ML, Goodman CS, Poo M, Tessier-Lavigne M, Comoglio PM. Plexins are a large family of receptors for transmembrane, secreted, and GPI-anchored semaphorins in vertebrates. Cell. 1999;99(1):71–80. doi: 10.1016/s0092-8674(00)80063-x. [DOI] [PubMed] [Google Scholar]
- Taniguchi M, Masuda T, Fukaya M, Kataoka H, Mishina M, Yaginuma H, Watanabe M, Shimizu T. Identification and characterization of a novel member of murine semaphorin family. Genes Cells. 2005;10(8):785–792. doi: 10.1111/j.1365-2443.2005.00877.x. [DOI] [PubMed] [Google Scholar]
- Tomizawa Y, Sekido Y, Kondo M, Gao B, Yokota J, Roche J, Drabkin H, Lerman MI, Gazdar AF, Minna JD. Inhibition of lung cancer cell growth and induction of apoptosis after reexpression of 3p21.3 candidate tumor suppressor gene SEMA3B. Proc Natl Acad Sci. 2001;98(24):13954–13959. doi: 10.1073/pnas.231490898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toyofuku T. Dual roles of Sema6D in cardiac morphogenesis through region-specific association of its receptor, plexin-A1, with off-track and vascular endothelial growth factor receptor type 2. Genes Dev. 2004;18(4):435–447. doi: 10.1101/gad.1167304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tran TS, Kolodkin AL, Bharadwaj R. Semaphorin regulation of cellular morphology. Annu Rev Cell Dev Biol. 2007;23(1):263–292. doi: 10.1146/annurev.cellbio.22.010605.093554. [DOI] [PubMed] [Google Scholar]
- Valabrega G, Montemurro F, Aglietta M. Trastuzumab: mechanism of action, resistance and future perspectives in HER2-overexpressing breast cancer. Ann Oncol. 2007;18(6):977–984. doi: 10.1093/annonc/mdl475. [DOI] [PubMed] [Google Scholar]
- Valente, G., Nicotra, G., Arrondini, M., Castino, R., Capparuccia, L., Prat, M., Kerim, S., Tamagnone, L. and Isidoro, C. (2009). Co-expression of plexin-B1 and met in human breast and ovary tumours enhances the risk of progression. Cellular Oncol 31(6), pp.423–436. Available at: https://pubmed.ncbi.nlm.nih.gov/19940359/ Accessed 11 Jun. 2020 [DOI] [PMC free article] [PubMed]
- Vander-Kooi CW, Jusino MA, Perman B, Neau DB, Bellamy HD, Leahy DJ. Structural basis for ligand and heparin binding to neuropilin B domains. Proc Natl Acad Sci. 2007;104(15):6152–6157. doi: 10.1073/pnas.0700043104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallerius, M., Wallmann, T., Bartish, M., O stling, J., Mezheyeuski, A., Tobin, N.P., Nygren, E., Pangigadde, P., Pellegrini, P., Squadrito, M.L., Ponten, F., Hartman, J., Bergh, J., De Milito, A., De Palma, M., Ostman, A., Andersson, J. and Rolny, C. (2016). Guidance molecule SEMA3A restricts tumor growth by differentially regulating the proliferation of tumor-associated macrophages. Cancer Res 76(11), pp.3166–3178. Available at: https://cancerres.aacrjournals.org/content/76/11/3166.article-info Accessed 5 Feb. 2020 [DOI] [PubMed]
- Wang, X., Zbou, C., Qiu, G., Fan, J., Tang, H. and Peng, Z. (2008). Screening of new tumor suppressor genes in sporadic colorectal cancer patients. Hepato-Gastroenterology, 55(88), pp.2039–2044. Available at: http://www.ncbi.nlm.nih.gov/pubmed/19260473 Accessed 11 Jun. 2020 [PubMed]
- Williamson M, de Winter P, Masters JR. Plexin-B1 signalling promotes androgen receptor translocation to the nucleus. Oncogene. 2015;35(8):1066–1072. doi: 10.1038/onc.2015.160. [DOI] [PubMed] [Google Scholar]
- Worzfeld, T. and Offermanns, S. (2014). Semaphorins and plexins as therapeutic targets. Nature Reviews Drug Discovery 13(8), pp.603–621. Available at: https://www.nature.com/articles/nrd4337#ref-CR61 Accessed 3 Feb. 2020 [DOI] [PubMed]
- Worzfeld T, Swiercz JM, Looso M, Straub BK, Sivaraj KK, Offermanns S. ErbB-2 signals through Plexin-B1 to promote breast cancer metastasis. J Clin Investig. 2012;122(4):1296–1305. doi: 10.1172/JCI60568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wronski MA, Raju N, Pillai R, Bogdan NJ, Marinelli ER, Nanjappan P, Ramalingam K, Arunachalam T, Eaton S, Linder KE, Yan F, Pochon S, Tweedle MF, Nunn AD. Tuftsin binds neuropilin-1 through a sequence similar to that encoded by exon 8 of vascular endothelial growth factor. J Biol Chem. 2005;281(9):5702–5710. doi: 10.1074/jbc.M511941200. [DOI] [PubMed] [Google Scholar]
- Wu, F., Zhou, Q., Yang, J., Duan, G., Ou, J., Zhang, R., Pan, F., Peng, Q., Tan, H., Ping, Y., Cui, Y., Qian, C., Yan, X. and Bian, X. (2011). Endogenous axon guiding chemorepulsant semaphorin-3F inhibits the growth and metastasis of colorectal carcinoma. Clinical Cancer Research 17(9), pp.2702–2711. Available at: http://clincancerres.aacrjournals.org/content/17/9/2702.long Accessed 11 Jun. 2020 [DOI] [PubMed]
- Xiao J-B, Li X-L, Liu L, Wang G, Hao S-N, Dong H-J, Wang X-M, Zhang Y-F, Liu H-D (2018) The association of semaphorin 5A with lymph node metastasis and adverse prognosis in cervical cancer. Cancer Cell Int 18(1) [DOI] [PMC free article] [PubMed]
- Yang S-C, Chang S-S, Chen CY-C. Identifying HER2 inhibitors from natural products database. PLoS ONE. 2011;6(12):e28793. doi: 10.1371/journal.pone.0028793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yazdani U, Terman JR. The semaphorins. Genome Biology. 2006;7(3):211. doi: 10.1186/gb-2006-7-3-211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yong L-K, Lai S, Liang Z, Poteet E, Chen F, van Buren G, Fisher W, Mo Q, Chen C, Yao Q. Overexpression of Semaphorin-3E enhances pancreatic cancer cell growth and associates with poor patient survival. Oncotarget. 2016;7(52):87431–87448. doi: 10.18632/oncotarget.13704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu R (2012) Effects of SEMA3G on migration and invasion of glioma cells. Oncol Rep [DOI] [PubMed]
- Zhao X-Y. Expression of semaphorin 6D in gastric carcinoma and its significance. World Journal of Gastroenterology. 2006;12(45):7388. doi: 10.3748/wjg.v12.i45.7388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu X, Zhang X, Ye Z, Chen Y, Lv L, Zhang X, Hu H (2017) Silencing of semaphorin 3C suppresses cell proliferation and migration in MCF-7 breast cancer cells. Oncology Letters [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.