Abstract
Cytochrome P450 monooxygenases (CYPs/P450s) are heme-thiolate proteins that are ubiquitously present in organisms, including non-living entities such as viruses. With the exception of self-sufficient P450s, all other P450 enzymes need electrons to perform their enzymatic activity and these electrons are supplied by P450 redox proteins. Different types of P450 redox proteins can be found in organisms and are classified into different classes. Bacterial P450s (class I) receive electrons from ferredoxins which are iron-sulfur cluster proteins. The presence of more than one copy and different types of ferredoxins within a bacterial species poses fundamental questions about the selectivity of P450s and ferredoxins in relation to each other. Apart from transferring electrons, ferredoxins have also been found to modulate P450 functions. Achieving an understanding of the interaction between ferredoxins and P450s is required to harness their biotechnological potential for designing a universal electron transfer protein. A brief overview of factors playing a role in ferredoxin and P450 interactions is presented in this review article.
Keywords: Cytochrome P450 monooxygenase, Ferredoxins, Interactions, Redox potentials, Evolution, Heme, Iron-sulfur cluster
Introduction
Cytochrome P450 monooxygenases (CYPs/P450s) represent one of the largest and oldest gene super-families found in all biological kingdoms (Nelson 2018), including non-living entities such as viruses (Lamb et al. 2019). P450s were named after their unusual spectrum with a peak of absorbance at 450 nm owing to the presence of heme-moiety (Klingenberg 1958). P450s activate molecular oxygen (White and Coon 1980) for the oxidative metabolism of a great variety of organic molecules (Bernhardt 2006; Fasan 2012; Isin and Guengerich 2007; Le-Huu et al. 2015; Sono et al. 1996; Syed et al. 2013). The primary reaction catalyzed by P450s is monooxygenation; i.e., one oxygen atom obtained from molecular oxygen is inserted into a substrate while the second oxygen atom undergoes reduction to water (White and Coon 1980). P450s need two electrons to perform their enzymatic reaction (White and Coon 1980). P450s receive these electrons from P450 redox proteins, with the exception of the so-called self-sufficient P450s that are fused to different types of P450 redox proteins (Guengerich and Munro 2013; Hannemann et al. 2007; Hlavica 2015; Lamb and Waterman 2013; Sello et al. 2015).
P450s have been classified into different categories based on the type of redox partners they employ for electrons (Hannemann et al. 2007). Bacterial P450s are considered class I P450s as they are soluble and receive electrons mostly from ferredoxins, soluble iron-sulfur (Fe-S) cluster proteins (Hannemann et al. 2007). It is well known that bacterial species have more than one ferredoxin in their genomes that belong to different types of iron-sulfur cluster such as [2Fe–2S], [3Fe–4S], [4Fe–4S], and a 7 Fe ferredoxin that contains a [3Fe–4S] and a [4Fe–4S] cluster (Campbell et al. 2019; Hannemann et al. 2007). The presence of more than one ferredoxin belonging to different iron-sulfur cluster types complicates the matter, as it is difficult to predict which ferredoxin is suitable for a P450 to ensure optimal activity. This furthermore poses the question of how a P450 or ferredoxin chooses its partner.
Our understanding of the interactions between ferredoxins and P450s has progressed with the identification of factors responsible for the interaction between ferredoxins and P450s have been identified. It has become clear that these interactions are to some degree specific between ferredoxins and P450s. These interactions exhibit both specificity and promiscuity (Ortega Ugalde et al. 2018; Sevrioukova et al. 2003; Sevrioukova et al. 1999; Sevrioukova and Poulos 2011; Zhang et al. 2018). Furthermore, studies have indicated that ferredoxins modulate bacterial P450 functions, thus, enhancing the P450 catalytic diversity (Li et al. 2020). A brief overview of factors playing a role in ferredoxins and P450 interactions is presented below.
Electrostatic interactions
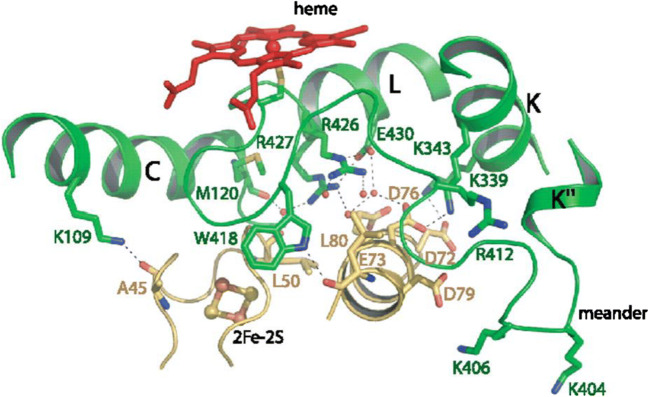
The charge-pairing mechanisms play a major role in attracting and complexation of redox partners, whereas hydrophobic and van der Waals cohesion forces play a minor role in docking events (Hlavica 2015). The electrostatic interactions between P450 and ferredoxin play a primary role in their recognition. The negatively charged amino acids on the iron-sulfur cluster site of ferredoxin and the positively charged residues located on the P450 proximal site play this role in pairing between P450 and ferredoxin. The proximal site of P450s consist of helices αC, αI, αJ, αK, and αL; the β1 strands; the meander loop; and the region around the heme-binding motif (Cys-pocket) (Graham and Peterson 1999) (Fig. 1). The meander loop is a structurally conserved region of the P450s situated in the middle of αK and the Cys-pocket. This region is covered by 7 to 10 amino acid residues, and it is believed to participate in heme-binding and stabilization of the tertiary structure (Sirim et al. 2010). It is noted that the αF-helix serves as an interacting domain between ferredoxins (adrenodoxin) and P450s (CYP11A1 from humans) (Fig. 1) (Sevrioukova et al. 1999).
Fig. 1.
Structural analysis of the CYP11A1 (green) and adrenodoxin (yellow) binding interface (Sevrioukova et al. 1999). The proximal site of CYP11A1 is interacting with the loop surrounding the adrenodoxin (2Fe–2S) cluster. Hydrogen bonds and water (represented with small red spheres)-mediated hydrogen bonds between amino acids are also shown
Analysis of the electrostatic surface of CYP101A1 (P450cam from Pseudomonas putida), CYP105AS-1 (from Amycolatopsis orientalis), CYP288A2 (CreJ from Corynebacterium glutamicum), CYP107E1 (MycG from Micromonospora griseorubida), CYP107L1 (PiKc from Streptomyces venezuelae), and CYP105A3 (P450sca2 from Streptomyces carbophilus) revealed that highly conserved arginine and histidine residues form a positively charged rim around a depression that is close to the center of the proximal surface (Zhang et al. 2018). These structural features are believed to be crucial for electrostatically steering the transient docking of the negatively charged ferredoxin, leading to the “encounter complex” to initiate the unidirectional electron transfer from ferredoxin to P450 (Hlavica et al. 2003; Page et al. 2003; Schilder and Ubbink 2013). On the other hand, a negatively charged surface surrounding the hydrophobic iron-sulfur cluster in ferredoxins was found to determine the high and wide-spectrum activities (Zhang et al. 2018). Ferredoxins with broad P450 electron transferability were found to have a classic anionic convex surface, which enables them to be docked with the basic concave surface of the P450 proximal site (Hlavica et al. 2003; Schilder and Ubbink 2013; Wada and Waterman 1992). The higher the negative charge in this region, the broader the P450 electron transfer capability found to be in ferredoxins and this charge was mainly contributed by the amino acids present in the helix 1-3 of ferredoxins. Although redox partners bind at the proximal region of P450s, some P450-ferredoxin partner complexes (CYP107L1-Fdx from Streptomyces coelicolor and CYP107L1-Fdx from Streptomyces elongatus) were exceptional, where ferredoxins were found to bind at distal regions of P450s (Zhang et al. 2018). However, this phenomenon was observed during in silico analysis of P450-ferredoxins interactions but has not, as yet, been confirmed by X-ray crystallography or NMR structures of P450-ferredoxin complex.
Redox potentials
Redox potentials are a measure of the tendency of a protein to give or receive electrons. Electrons flow from protein with more negative redox potential to protein having less negative redox potential. It is a well-known fact that the binding of substrate induces the shift of P450 heme-iron redox potential to a more favorable condition where electrons can be received from the redox protein (Table 1) (Hlavica 2015; Lewis and Hlavica 2000; Miles et al. 2000). It is also noted that different substrates induce different conformations in P450s, thus affecting their redox potentials differently and therefore their preferences for ferredoxins (Table 1) (Miles et al. 2000; Zhang et al. 2018). However, contrary to the substrate needed for a P450 to be reduced, some P450s are found to be reduced even in the absence of substrates, CYP3A4 and CYP102A1 (P450BM3) (Guengerich and Johnson 1997; Munro et al. 1995). Furthermore, no change in the redox potential has been observed in the presence of substrate for CYP102A1 (Fleming et al. 2003). Interestingly, the same phenomenon of no change in the redox potential was observed for CYP105AS-1 and CYP105AS3 in a recent study by Zhang and co-workers (Table 1) (Zhang et al. 2018). In addition to this, despite having the highest redox potential (− 438 mV), the electron transfer ability of ferredoxin Fdx0898 from Streptomyces elongatus PCC 7942 is very limited (Zhang et al. 2018). Based on these observations, the authors proposed that the redox potential of ferredoxins might not be the key factor in supporting P450 activity (Zhang et al. 2018). Considering these observations, it can be assumed that the general rule of this electron flow, such as substrate-induced lowering of P450 heme-iron redox potential, is not universal and each P450 behaves differently.
Table 1.
Analysis of redox potentials of P450s with and without substrate
| P450 | Redox potential (mV) | References | |
|---|---|---|---|
| Without substrate | With substrate | ||
| CYP101A1 (P450cam) | − 303 | − 173 | Lewis and Hlavica 2000 |
| CYP2B4 (P450LM2) | − 300 | − 225 | Lewis and Hlavica 2000 |
| CYP107E1 (MycG) | − 175 | − 92 | Zhang et al. 2018 |
| CYP107L1 (PikC) | − 123 | − 85 and − 110 | Zhang et al. 2018 |
| CYP288A2 (CreJ) | − 147 | − 133 | Zhang et al. 2018 |
| CYP105AS-1 | − 129 | − 130 | Zhang et al. 2018 |
| CYP105A3 (P450sca2) | − 250 | − 253 | Zhang et al. 2018 |
CYP107L1 (PikC) from Streptomyces venezuelae; P450cam (CYP101A1) from Pseudomonas putida; MycG (CYP107E1) from Micromonospora griseorubida; CreJ (CYP288A2) from Corynebacterium glutamicum; CYP105AS-1 from Amycolatopsis orientalis; P450sca2 (CYP105A3) from S. carbophilus. In the case of PikC, two redox potentials represented two different substrates: 12-membered ring macrolide YC-17 (− 85 mV) and 14-membered ring macrolide narbomycin (− 110 mV). These substrates influenced PikC differently and thus led to different redox potentials
Distance between P450 heme-iron and ferredoxin iron-sulfur cluster
The distance between the ferredoxins’ iron-sulfur cluster and P450s’ heme-iron center was found to be the key determinant of electron transfer (Liu et al. 2014; Page et al. 1999, 2003). Some progress has been made in understanding the effect of distance on electron transferability. Information on this aspect can be found in review articles (Hlavica 2015; Hlavica et al. 2003; Lewis and Hlavica 2000; Sevrioukova and Poulos 2011), articles detailing crystal/NMR structures of P450s and redox partners (Hiruma et al. 2013; Roncel et al. 2012; Sevrioukova et al. 1999; Strushkevich et al. 2011; Tripathi et al. 2013a), and articles detailing models of P450s and redox partners combining functional analysis (Liu et al. 2014; Sagadin et al. 2019; Sagadin et al. 2018; Zhang et al. 2018). In summary, the shorter the distance, the higher the electron transferability, whereas the longer the distance, the lower the electron transferability observed. Furthermore, the longer the distance, the more selective transfer of electrons to only specific P450s with less efficient electron transfer was also observed by redox partners (Zhang et al. 2018). A distance of 14 Å or less between redox centers is sufficient for highly robust transfer of electrons within milliseconds, whereas the shortest distance, found to be 6.2 Å, leads to electron transfer in nanoseconds (Liu et al. 2014; Page et al. 1999, 2003). Among self-sufficient P450s, the shorter the distance between heme and their redox domain, the better the efficiency with CYP102A1 found to be highly efficient compared with CYP116B6 (Zhang et al. 2020). This is due to the shorter distance between the heme domain and redox domain in CYP102A1 compared with CYP116B6 (Zhang et al. 2020). Analysis of distance between different P450s and redox proteins revealed that a distance of up to 27.1 Å between redox centers was able to support electron transfer capability (Table 2). However, to date, a distance that does not support electron transfer has not been reported.
Table 2.
Distance between heme-iron center of P450s and iron-sulfur cluster of ferredoxins that are able to support the activity of P450s. For some P450s, the general name or well-known names that were used in the articles were presented in parenthesis next to their systematic name. Since CYP102A1 is a model and highly efficient P450, the distance between its heme-iron center and FMN is also shown. In case of CYP116B6, self-sufficient P450, the distance between heme and its ferredoxin domain is presented
| Complexes | Distance (Å) | References |
|---|---|---|
| Cytochrome P450cam–putidaredoxin | 16 | Hiruma et al. 2013; Tripathi et al. 2013b |
| Cytochrome c550-Mn4Ca cluster | 22 | Roncel et al. 2012 |
| CYP106A2-Adx | 24 | Sagadin et al. 2019 |
| CYP11A1-Adx | 17.4 | Sevrioukova et al. 1999; Strushkevich et al. 2011 |
| CYP102A1 (P450BM3)-FMN | 18.4 | Sevrioukova et al. 1999 |
| CYP107L1-SelFdx1499 | 12.7 | Zhang et al. 2018 |
| CYP107L1-SelFdx0388 | 12.5 | Zhang et al. 2018 |
| CYP107L1-SelFdx2581 | 13 | Zhang et al. 2018 |
| CYP107L1-CglFdx0526 | 12.6 | Zhang et al. 2018 |
| CYP107L1-ScoFdx7676 | 27.1 | Zhang et al. 2018 |
| CYP107L1-SelFdx0898 | 24.1 | Zhang et al. 2018 |
| CYP101A1(P450cam)-SelFdx1499 | 17.9 | Zhang et al. 2018 |
| CYP288A2(CreJ)-SelFdx1499 | 14,1 | Zhang et al. 2018 |
| CYP105AS-1-SelFdx1499 | 15.4 | Zhang et al. 2018 |
| CYP105A3(P450sca2)-SelFdx1499 | 15.9 | Zhang et al. 2018 |
| CYP107A3(MycG)-SelFdx1499 | 14.7 | Zhang et al. 2018 |
| CYP107L1-SelFdx1499 | 12.7 | Zhang et al. 2018 |
| CYP116B46-[2Fe–2S] | 25.3 | Zhang et al. 2020 |
CYP107L1 (PikC) from Streptomyces venezuelae; CYP101A1 (P450cam) from Pseudomonas putida; CYP107E1 (MycG) from Micromonospora griseorubida; CYP288A2 (CreJ) from Corynebacterium glutamicum; CYP105AS-1 from Amycolatopsis orientalis; CYP105A3 (P450sca2) from S. carbophilus
Sco, S. coelicolor A3(2); Sel, S. elongatus PCC 7942; Cgl, C. glutamicum ATCC 13032; Adx, adrenodoxin; FdX, ferredoxin
Ultimately, the geometry of protein shapes, especially at the sites of P450s and redox partner binding, plays a role, as the tighter the binding, the shorter the distance between redox centers and thus the more efficient electron transfer capability (Hlavica 2015; Hlavica et al. 2003; Lewis and Hlavica 2000; Sevrioukova and Poulos 2011; Zhang et al. 2018).
Evolution
There has been speculation on the evolutionary preference of ferredoxins and P450s based on the available functional data and evolutionary patterns of different types of ferredoxins and P450s in organisms. Among ferredoxins, the 2Fe–2S ferredoxins were found to be efficient in transferring electrons to a large number of P450s (Ortega Ugalde et al. 2018; Zhang et al. 2018) and evolutionary analysis also suggested that these redox proteins were co-evolved with heme-binding domain proteins such as P450s (Harel et al. 2014). The latest study further confirmed that 2Fe–2S ferredoxins were expanded with the advent of photosynthesis (Campbell et al. 2019), indicating the availability of oxygen and thus evolution of P450s, making them the preferred choice for P450s as electron donors. Considering this evidence, it is assumed that the smaller ferredoxins such as 2Fe–2S redox proteins were favored by P450s compared with [3Fe–4S], [4Fe–4S], and a 7 Fe ferredoxin that contains a [3Fe–4S] and a [4Fe–4S] cluster.
Conclusions
Unravelling the factors governing the selectivity of ferredoxins to a particular P450 has enormous biotechnological potential, as it will help in designing a universal redox system that can supply electrons to any P450. However, because of the complexity of these interactions, limited progress has been achieved in understanding ferredoxins and P450 interactions. The factors governing P450s’ and ferredoxins’ interactions with respect to selectivity of each other are still far from clear. Functional and structural characterization of a large number of P450 and ferredoxin interactions from different bacterial species will provide more insight into this aspect. The current knowledge on interactions is quite basic and more efforts are needed to map the selectivity patterns of P450s and ferredoxins accurately in order to design universal electron transfer partners.
Acknowledgments
The authors want to thank Barbara Bradley, Pretoria, South Africa, for English language editing. The authors also thank the Proceedings of the National Academy of Sciences of the United States of America (PNAS, USA) for granting permission to use Figure 4 panel A, from the article: Sevrioukova IF, Li H, Zhang H, Peterson JA, Poulos TL (1999) Structure of a cytochrome P450–redox partner electron-transfer complex, Proceedings of the National Academy of Sciences 96:1863-1868.
Funding
Khajamohiddin Syed expresses sincere gratitude to the National Research Foundation (NRF), South Africa, for a research grant (Grant No. 114159). Zinhle Edith Chiliza thanks the NRF, South Africa, for a DST-NRF Innovation Master’s Scholarship for the year 2019 (Grant No. 117182).
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Disclaimer
The funders had no role in the design of the study; the collection, analyses, and interpretation of data; the writing of the manuscript; or the decision to publish the results.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Bernhardt R. Cytochromes P450 as versatile biocatalysts. J Biotechnol. 2006;124:128–145. doi: 10.1016/j.jbiotec.2006.01.026. [DOI] [PubMed] [Google Scholar]
- Campbell IJ, Bennett GN, Silberg JJ. Evolutionary relationships between low potential ferredoxin and flavodoxin electron carriers. Front Energy Res. 2019;7:79. doi: 10.3389/fenrg.2019.00079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fasan R. Tuning P450 enzymes as oxidation catalysts ACS. Catalysis. 2012;2:647–666. [Google Scholar]
- Fleming BD, Tian Y, Bell SG, Wong LL, Urlacher V, Hill HAO. Redox properties of cytochrome P450BM3 measured by direct methods. Eur J Biochem. 2003;270:4082–4088. doi: 10.1046/j.1432-1033.2003.03799.x. [DOI] [PubMed] [Google Scholar]
- Graham SE, Peterson JA. How similar are P450s and what can their differences teach us? Arch Biochem Biophys. 1999;369:24–29. doi: 10.1006/abbi.1999.1350. [DOI] [PubMed] [Google Scholar]
- Guengerich FP, Johnson WW. Kinetics of ferric cytochrome P450 reduction by NADPH− cytochrome P450 reductase: rapid reduction in the absence of substrate and variations among cytochrome P450 systems. Biochemistry. 1997;36:14741–14750. doi: 10.1021/bi9719399. [DOI] [PubMed] [Google Scholar]
- Guengerich FP, Munro AW. Unusual cytochrome p450 enzymes and reactions. J Biol Chem. 2013;288:17065–17073. doi: 10.1074/jbc.R113.462275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hannemann F, Bichet A, Ewen KM, Bernhardt R. Cytochrome P450 systems--biological variations of electron transport chains. Biochim Biophys Acta. 2007;1770:330–344. doi: 10.1016/j.bbagen.2006.07.017. [DOI] [PubMed] [Google Scholar]
- Harel A, Bromberg Y, Falkowski PG, Bhattacharya D. Evolutionary history of redox metal-binding domains across the tree of life. Proc Natl Acad Sci. 2014;111:7042–7047. doi: 10.1073/pnas.1403676111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiruma Y, et al. The structure of the cytochrome P450cam–putidaredoxin complex determined by paramagnetic NMR spectroscopy and crystallography. J Mol Biol. 2013;425:4353–4365. doi: 10.1016/j.jmb.2013.07.006. [DOI] [PubMed] [Google Scholar]
- Hlavica P. Mechanistic basis of electron transfer to cytochromes p450 by natural redox partners and artificial donor constructs. Adv Exp Med Biol. 2015;851:247–297. doi: 10.1007/978-3-319-16009-2_10. [DOI] [PubMed] [Google Scholar]
- Hlavica P, Schulze J, Lewis DF. Functional interaction of cytochrome P450 with its redox partners: a critical assessment and update of the topology of predicted contact regions. J Inorg Biochem. 2003;96:279–297. doi: 10.1016/s0162-0134(03)00152-1. [DOI] [PubMed] [Google Scholar]
- Isin EM, Guengerich FP. Complex reactions catalyzed by cytochrome P450 enzymes. Biochim Biophys Acta. 2007;1770:314–329. doi: 10.1016/j.bbagen.2006.07.003. [DOI] [PubMed] [Google Scholar]
- Klingenberg M. Pigments of rat liver microsomes. Arch Biochem Biophys. 1958;75:376–386. doi: 10.1016/0003-9861(58)90436-3. [DOI] [PubMed] [Google Scholar]
- Lamb DC, et al. On the occurrence of cytochrome P450 in viruses. Proc Natl Acad Sci. 2019;116:12343–12352. doi: 10.1073/pnas.1901080116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamb DC, Waterman MR. Unusual properties of the cytochrome P450 superfamily. Philos Transact R Soc B: Biol Sci. 2013;368:20120434. doi: 10.1098/rstb.2012.0434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le-Huu P, Heidt T, Claasen B, Laschat S, Urlacher VB. Chemo-, regio-, and stereoselective oxidation of the monocyclic diterpenoid β-cembrenediol by P450 BM3 ACS. Catalysis. 2015;5:1772–1780. [Google Scholar]
- Lewis DF, Hlavica P. Interactions between redox partners in various cytochrome P450 systems: functional and structural aspects. Biochim Biophys Acta (BBA)-Bioenerg. 2000;1460:353–374. doi: 10.1016/S0005-2728(00)00202-4. [DOI] [PubMed] [Google Scholar]
- Li S, Du L, Bernhardt R. Redox partners: function modulators of bacterial P450 Enzymes. Trends Microbiol. 2020;28:445–454. doi: 10.1016/j.tim.2020.02.012. [DOI] [PubMed] [Google Scholar]
- Liu J, et al. Metalloproteins containing cytochrome, iron–sulfur, or copper redox centers. Chem Rev. 2014;114:4366–4469. doi: 10.1021/cr400479b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miles CS, Ost TW, Noble MA, Munro AW, Chapman SK. Protein engineering of cytochromes P-450. Biochim Biophys Acta (BBA)-Protein Struct Molec Enzymol. 2000;1543:383–407. doi: 10.1016/S0167-4838(00)00236-3. [DOI] [PubMed] [Google Scholar]
- Munro AW, Lindsay JG, Coggins JR, Kelly SM, Price NC. NADPH oxidase activity of cytochrome P-450 BM3 and its constituent reductase domain. Biochim Biophys Acta (BBA)-Bioenerg. 1995;1231:255–264. doi: 10.1016/0005-2728(95)00083-U. [DOI] [PubMed] [Google Scholar]
- Nelson DR. Cytochrome P450 diversity in the tree of life. Biochim Biophys Acta, Proteins Proteomics. 2018;1866:141–154. doi: 10.1016/j.bbapap.2017.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortega Ugalde S, et al. Linking cytochrome P450 enzymes from Mycobacterium tuberculosis to their cognate ferredoxin partners. Appl Microbiol Biotechnol. 2018;102:9231–9242. doi: 10.1007/s00253-018-9299-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Page CC, Moser CC, Chen X, Dutton PL. Natural engineering principles of electron tunnelling in biological oxidation–reduction. Nature. 1999;402:47–52. doi: 10.1038/46972. [DOI] [PubMed] [Google Scholar]
- Page CC, Moser CC, Dutton PL. Mechanism for electron transfer within and between proteins. Curr Opin Chem Biol. 2003;7:551–556. doi: 10.1016/j.cbpa.2003.08.005. [DOI] [PubMed] [Google Scholar]
- Roncel M, Kirilovsky D, Guerrero F, Serrano A, Ortega JM. Photosynthetic cytochrome c550. Biochim Biophys Acta (BBA)-Bioenerg. 2012;1817:1152–1163. doi: 10.1016/j.bbabio.2012.01.008. [DOI] [PubMed] [Google Scholar]
- Sagadin T, Riehm J, Putkaradze N, Hutter MC, Bernhardt R. Novel approach to improve progesterone hydroxylation selectivity by CYP 106A2 via rational design of adrenodoxin binding. FEBS J. 2019;286:1240–1249. doi: 10.1111/febs.14722. [DOI] [PubMed] [Google Scholar]
- Sagadin T, Riehm JL, Milhim M, Hutter MC, Bernhardt R. Binding modes of CYP106A2 redox partners determine differences in progesterone hydroxylation product patterns. Commun Biol. 2018;1:1–9. doi: 10.1038/s42003-018-0104-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schilder J, Ubbink M. Formation of transient protein complexes. Curr Opin Struct Biol. 2013;23:911–918. doi: 10.1016/j.sbi.2013.07.009. [DOI] [PubMed] [Google Scholar]
- Sello MM, et al. Diversity and evolution of cytochrome P450 monooxygenases in Oomycetes. Sci Rep. 2015;5:11572. doi: 10.1038/srep11572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sevrioukova IF, Garcia C, Li H, Bhaskar B, Poulos TL. Crystal structure of putidaredoxin, the [2Fe-2S] component of the P450cam monooxygenase system from Pseudomonas putida. J Mol Biol. 2003;333:377–392. doi: 10.1016/j.jmb.2003.08.028. [DOI] [PubMed] [Google Scholar]
- Sevrioukova IF, Li H, Zhang H, Peterson JA, Poulos TL. Structure of a cytochrome P450–redox partner electron-transfer complex. Proc Natl Acad Sci. 1999;96:1863–1868. doi: 10.1073/pnas.96.5.1863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sevrioukova IF, Poulos TL. Structural biology of redox partner interactions in P450cam monooxygenase: a fresh look at an old system. Arch Biochem Biophys. 2011;507:66–74. doi: 10.1016/j.abb.2010.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sirim D, Widmann M, Wagner F, Pleiss J. Prediction and analysis of the modular structure of cytochrome P450 monooxygenases. BMC Struct Biol. 2010;10:34. doi: 10.1186/1472-6807-10-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sono M, Roach MP, Coulter ED, Dawson JH. Heme-containing oxygenases. Chem Rev. 1996;96:2841–2888. doi: 10.1021/cr9500500. [DOI] [PubMed] [Google Scholar]
- Strushkevich N, MacKenzie F, Cherkesova T, Grabovec I, Usanov S, Park HW. Structural basis for pregnenolone biosynthesis by the mitochondrial monooxygenase system. Proc Natl Acad Sci U S A. 2011;108:10139–10143. doi: 10.1073/pnas.1019441108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Syed K, Porollo A, Lam YW, Grimmett PE, Yadav JS. CYP63A2, a catalytically versatile fungal P450 monooxygenase capable of oxidizing higher-molecular-weight polycyclic aromatic hydrocarbons, alkylphenols, and alkanes. Appl Environ Microbiol. 2013;79:2692–2702. doi: 10.1128/AEM.03767-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tripathi S, Li H, Poulos TL. Structural basis for effector control and redox partner recognition in cytochrome P450. Science. 2013;340:1227–1230. doi: 10.1126/science.1235797. [DOI] [PubMed] [Google Scholar]
- Tripathi S, Li H, Poulos TL. Structural basis for effector control and redox partner recognition in cytochrome P450. Science. 2013;340:1227–1230. doi: 10.1126/science.1235797. [DOI] [PubMed] [Google Scholar]
- Wada A, Waterman MR. Identification by site-directed mutagenesis of two lysine residues in cholesterol side chain cleavage cytochrome P450 that are essential for adrenodoxin binding. J Biol Chem. 1992;267:22877–22882. [PubMed] [Google Scholar]
- White RE, Coon MJ. Oxygen activation by cytochrome P-450. Annu Rev Biochem. 1980;49:315–356. doi: 10.1146/annurev.bi.49.070180.001531. [DOI] [PubMed] [Google Scholar]
- Zhang L, et al. Structural insight into the electron transfer pathway of a self-sufficient P450 monooxygenase. Nat Commun. 2020;11:1–6. doi: 10.1038/s41467-020-16500-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W, et al. Mechanistic insights into interactions between bacterial class I P450 enzymes and redox partners ACS. Catalysis. 2018;8:9992–10003. [Google Scholar]