Abstract
The high-order structure of mitotic chromosomes remains to be fully elucidated. How nucleosomes compact at various structural levels into a condensed mitotic chromosome is unclear. Cryogenic preservation and imaging have been applied for over three decades, keeping biological structures close to the native in vivo state. Despite being extensively utilized, this field is still wide open for mitotic chromosome research. In this review, we focus specifically on cryogenic efforts for determining the mitotic nanoscale chromatin structures. We describe vitrification methods, current status, and applications of advanced cryo-microscopy including future tools required for resolving the native architecture of these fascinating structures that hold the instructions to life.
Keywords: Mitotic, Chromosome, Chromatin, Nanoscale, Cryo, Freezing, Microscopy
Introduction
Chromosomes were first discovered over a century ago with microscopy having been applied for decades to image these fascinating structures that hold the instructions for life. The basic building block of mitotic chromosomes is chromatin, the DNA-protein complex composed of discrete nucleosomes, which can be thought of as the “atoms” of the chromosome structure. To date, our understanding of how these nucleosomes are organized into highly compact chromosomes so-called the ‘high-order structure’ is largely unknown (Yusuf et al. 2019). Nucleosome compaction and de-compaction play an essential role in packaging DNA during vital biological processes including transcription, gene expression, DNA replication, faithful separation, and repair (Dixon et al. 2016; Ramachandran and Henikoff 2016). The most compact state of chromatin occurs in the mitotic state of the cell cycle, in which chromosomes condense before cell division and then segregate into daughter cells after significant conformational changes (Liang et al. 2015). This mechanism involves the formation of chromatin loops that tether the axial structure of the chromosome (Batty and Gerlich 2019). For understanding how chromatin is organized into mitotic chromosomes, chromosome conformation capture that includes Hi-C (Hsieh et al. 2016; Naumova et al. 2013) and direct imaging (Flors and Earnshaw 2011; Lakadamyali and Cosma 2015) has been applied.
A range of imaging technologies have been applied for nanoscale elucidation of chromatin at various compaction states ranging from the 2-nm-thick DNA fiber to the compact mitotic chromosome (Flors and Earnshaw 2011). Advanced microscopy using X-rays revealed the nucleosome’s crystal structure consisting of DNA (145–147 bp) wrapped approximately 1.65 times around eight histone proteins (core histone octamer composed of H2A, H2B, H3, and H4) (Davey et al. 2002; Luger et al. 1997). This nucleosome-histone octamer exhibits a 11 nm diameter bead on a string-like structure observed by electron microscopy (EM) in vitro, (Olins and Olins 1974). The tetra nucleosome structure has also been resolved using X-rays (Schalch et al. 2005), EM (Ekundayo et al. 2017), and cryo-EM (Song et al. 2014). Further, this 11-nm bead on a string-like structure gives rise to the controversial high-order 30-nm structure that is the most debated topic in the field (Krietenstein and Rando 2020; Yusuf et al. 2019) with many models proposed (Bendandi et al. 2020; Ohno et al. 2018; Yusuf et al. 2019). Cryo-EM has shown an interdigitated one-start solenoid model displaying 30-nm fibers (Robinson et al. 2006) whereas other cryo studies do not favor this structure (Studer et al. 2008). Apart from nucleosomes being the building blocks, the overall mitotic chromosome compaction is dependent on numerous proteins with over 158 been identified (Ohta et al. 2010), which include the non-histone scaffold proteins such as cohesion, condensin, and topoisomerases (Beseda et al. 2020). The various stages of chromatin structure condensation are partly understood with several factors implicated (Antonin and Neumann 2016; Beseda et al. 2020; Swedlow and Hirano 2003; Woodcock and Ghosh 2010).
Imaging chromatin structures close to the native state at nanoscopic resolution is vital for understanding the architecture of these compact and complex structures. In this review, we summarize our current knowledge of higher-order mitotic chromosome structure from chemically preserved samples. We then focus on the need of cryo technology for mitotic chromatin nanoscale structure determination. We highlight vitrification methods including cryo microscopy studies used for unraveling the high-order structure of intact mitotic chromosomes. Further, we discuss current limitations and the future need for cryo technology for elucidating the high-order hierarchy of these fascinating structures.
Lessons from fixed chromosome samples
To unravel the nanoscale chromatin structure, advanced microscopy has been applied, revolutionizing our understanding of chromosome structure (Lakadamyali and Cosma 2015). Even though useful structural information was provided, these studies heavily relied on preserving/fixing samples using harsh chemicals, mainly aldehydes, dehydration steps, and staining. Such harsh conditions influence the native sample configuration and hence what is observed is a reproducible artifact not the actual native state (Al-Amoudi et al. 2004; Li et al. 2017). These chemically preserved chromosome samples have been imaged using different ultra-high-resolution microscopes that are mentioned below and summarized in Table 1.
Table 1.
Comparison of microscopy studies that used chemical preservation for investigating chromatin structure
| Microscope | Species | Cell type | Isolated chromosomes or intact cells | Staining | Findings | Publication |
|---|---|---|---|---|---|---|
| STED | Human | HeLa | Intact cells | mEGFP-CAP-H2 | Condensin II subunit CAP-H2 protein enriched around longitudinal chromatid axis | Walther and Ellenberg (2018) |
| SPDM (SMLM) | Human | HeLa | Isolated | Hoechst 33342 | Density maps displaying a mean localization accuracy around 14 nm | Szczurek et al. (2014) |
| PLM (SMLM) | Human | HeLa | Isolated | Auto fluorescence | Nucleotide density variation in chromatids and fragile site like features | Dong et al. (2016) |
| SIM | Human | HeLa | Isolated | anti-Topo Iiα, antihistone H3, anti-hCAP-E, anti-KIF4A | Two main lateral strands with a twisted axial distribution of scaffold proteins (FIB included) | Poonperm et al. (2015) |
| PALM | Drosophila | Embryo | Cell | H2AvD-EGFP | 70-nm filamentous blocks composed of stripes of 35-nm sub-filaments | Matsuda et al. (2010) |
| SEM | Human | B lymphocyte | Isolated | Platinum-based dye | Globule chromatin size between 15 and 30 nm, with characteristic diameter of around 20 nm | Shemilt et al. (2014) |
| SBFSEM | Human | B lymphocyte | Isolated | Platinum blue | Internal structural cavities seen using MAA samples only | Yusuf et al. (2014) |
| SBFSEM | Human | B lymphocyte | Inside cells | Platinum blue | Porous network structure on chromosome arms with 50 nm resolution in 3D | Chen et al. (2017) |
| FIBSEM | Barley | Seeds –Hordeum vulgare | Isolated | Platinum blue immunogold labeled for phosphorylated histone H3 |
Parallel fibrils seen at centromere and extended cavities on chromosome arms Strong labeling in the pericentric regions with a signal at the centromere |
Schroeder-Reiter et al. (2009) |
| TEM | Human | Primary human small airway epithelial and U2OS cells | Intact cells | DRAQ5 followed by osmium tetroxide | Chromatin forms flexible chains with diameters between 5 and 24 nm | Ou et al. (2017) |
| TEM | Human | HeLa | Isolated | Ionic liquid anti-CAP-E, Fluoronanogold | CAP-E observed at central axis in each chromatid and diffused in arms displaying helical structure | Phengchat et al. (2019) |
| AFM | AFM | Lymphocytes B-ALL-1 | Isolated | Giemsa and metal staining | Structure of chromatid arm not uniform. Ridges and grooves seen that correspond to G-positive and G-negative bands | Ushiki and Hoshi (2008) |
| X-ray | Human | B lymphocyte | Isolated | Platinum blue | Internal fibrous ultrastructures observed | Yan et al. (2016) |
| X-ray | Human | HeLa | Isolated | - | Chromosome axial structure determined using both 2D and 3D | Nishino et al. (2009) |
NIH3T3 immortalized mouse embryonic fibroblast cell line, SMLM single-molecule localization microscopy, STED stimulated emission depletion, SPDM spectral position determination microscopy, PLM photon localization microscopy, SIM structured illumination microscopy, PALM photoactivated localization microscopy, SEM scanning electron microscopy, SBFSEM 3D serial block face SEM, FIBSEM focused ion beam SEM, TEM transmission electron microscopy, AFM atomic force microscopy, HeLa human epithelial cells, U2OS human osteosarcoma cells, B-ALL-1 B cell acute lymphoblastic leukemia
Super resolution microscopy (SRM), an advanced fluorescence imaging method, provides high resolution (~ 10 to 30-nm) (Galbraith and Galbraith 2011; Schermelleh et al. 2019; Xu and Liu 2019) and has been extensively used for interphase chromatin structures (Fang et al. 2018; Otterstrom et al. 2019). SRM to lesser extent has been explored for unraveling mitotic chromatin structures. Stimulated emission depletion (STED) SRM on fixed HeLa cells showed sub-chromosomal localization of immuno-stained condensin II subunit CAP-H2 protein along the length of mitotic chromosomes being enriched mainly around the longitudinal chromatid axis (Walther and Ellenberg 2018). Three-dimensional structured illumination microscopy (3D-SIM) together with focused ion beam (FIB) milling EM done on HeLa cells showed distribution of chromosome scaffold proteins condensins and topoisomerase IIα having two main lateral strands twisted around each other within the chromatid axis (Poonperm et al. 2015).
Single-molecule localization microscopy (SMLM) using a modified spectral position determination microscopy (SPDM) approach displayed a mean localization accuracy around 14 nm from high-quality DNA density maps of mitotic HeLa chromosomes after staining with Hoechst 33342 (Szczurek et al. 2014). DNA single-molecule photo-activated localization microscopy (PALM) showed sub 20-nm structure resolution in unstained HeLa chromosomes with nucleotide density variations and fine features on chromatids (Dong et al. 2016). PALM revealed ∼ 70-nm structures composed of 35-nm stripes on Drosophila mitotic chromosomes after labeling with H2AvD-EGFP, a histone H2A variant (Matsuda et al. 2010).
EM is a contrast imaging method that provides detail at high resolution (Kourkoutis et al. 2012). Scanning electron microscopy (SEM) has provided surface information on B lymphocyte chromosomes showing chromatin fibers ranging between 25 and 35-nm diameters (Shemilt et al. 2014). Often, a nucleoplasm layer is seen on the surface of the chromosomes that hinder high-resolution chromatin substructure determination (Shemilt et al. 2014). This has been overcome by applying 3D serial block face SEM (SBFSEM) that embeds the sample into resin followed by automated diamond knife sectioning providing or resulting in nanoscale resolution of B lymphocyte chromosomes that were isolated (Yusuf et al. 2014) and within a prophase nucleus (Chen et al. 2017). This study showed porous network structures on chromosomes with sister chromatids having conserved diameters of around 765-nm (Chen et al. 2017). An alternative method to SBFSEM is focused ion beam SEM (FIBSEM) that uses an ion beam for sectioning and has provided structural information of barley chromosomes (Schroeder-Reiter et al. 2009). Centromeres displayed parallel fibrils whereas both chromosome arms (p and q) showed extended cavities also known as chromomeres (Schroeder-Reiter et al. 2009).
Transmission electron microscopy (TEM) provides angstrom resolution but is not suitable for studying intact mitotic chromosomes due to their thickness (Yusuf et al. 2019). ChromEM tomography (ChromEMT) a multitilt EM tomography and a labeling method showed irregular disordered chains of nucleosomes with 5 and 24-nm diameters, indicating that the 10-nm fiber is heterogeneous (Ou et al. 2017). A helical structure composed of chromatin loops was detected when the CAP-E protein, a condensin subunit, was labeled with gold nanoparticles after the chromosome was isolated by FIB and imaged using ET (Phengchat et al. 2019).
Atomic force microscopy (AFM) provides atomic resolution with surface topology information and has been applied for determining chromosomal detailed structures and reviewed in detail elsewhere (Kalle and Strappe 2012). Giemsa (G) banded chromosomes displayed ridges and grooves corresponding to heterochromatin (dark) and euchromatin (light) bands, respectively. This technique revealed highly twisted chromatin fiber loops with stronger compaction in the ridged regions than grooved. Additionally, granular and/or fibrous 50–60-nm structures were also seen on the surface (Ushiki and Hoshi 2008).
Mitotic chromosomes have also been imaged at nanoscale resolution using X-rays that can penetrate the sample with no sectioning needed (Yan et al. 2016) as they have a shorter wavelength (Cervantes 2016). A pioneering hard X-ray diffraction study displayed 30-nm and 120-nm structures in 2D and 3D, respectively, of unstained intact mitotic chromosomes (Nishino et al. 2009).
Cryo preservation and imaging of mitotic chromosomes
Imaging biological samples near-native state is achieved after vitrifying the biological sample in amorphous ice, known as cryopreservation, cryofixation, or cryo-immobilization. This allows instant fixation of all molecules present in the sample that remain at the set position displaying a true representation of the sample at the given point of freezing (temperature below − 140 °C with freezing that should occur rapidly within microseconds at high rates (~ 104 °C/s or higher). Ice crystal growth is prevented in this procedure allowing macromolecule immobilization and allowing the specimen to be in an amorphous (close to native) state (Lučić et al. 2013).
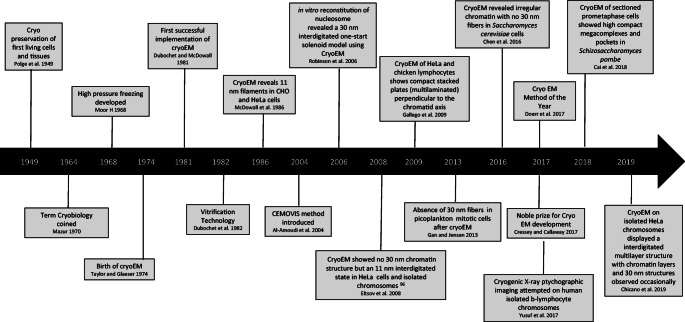
The cryo-electron tomography (cryo-ET) known as the 2016 “Method of the Year” (Doerr 2017) was awarded the 2017 Nobel Prize for cryo-EM development including determination of high-resolution biomolecule structures in solution (Cressey and Callaway 2017). Cryo-ET provides 3D position of the sample inside cells at ∼ 4-nm resolution (Gan and Jensen 2012). Both cryo-EM and cryo-ET have enhanced our understanding in resolving atomic structures of the nucleosome (Chua et al. 2016; Zhou et al. 2019a) and interphase chromatin organization, (Eltsov et al. 2008; Fussner et al. 2012; Zhou et al. 2019b), respectively. The number of microscopy studies on mitotic nanoscale structure under cryo conditions has been limited (Fig. 1).
Fig. 1.
Timeline. Key events in the development of cryo preservation methods and imaging of mitotic nanoscale chromatin structures
Cryo-EM/ET cannot be applied directly for mitotic chromosome imaging as this technology requires the thickness of the sample to be below 1 μM (Henderson et al. 2007). Therefore, the cryoelectron microscopy of vitreous section (CEMOVIS) method (Al-Amoudi et al. 2004) has proven critical for mitotic chromosomes where thicker biological samples after freezing can be cryosectioned using cryo-ultramicrotomy. The CEMOVIS method after 100–150-nm sections has been applied directly on unstained Chinese hamster ovary (CHO) and HeLa cells with 11-nm chromatin filaments (McDowall et al. 1986). A total of 30-nm fibers were not seen in 40-nm cryosections of chromosomes within mitotic HeLa cells, instead highly disordered and interdigitated structures were visualized (Eltsov et al. 2008). Compact stacked multilaminated plates were seen after applying Cryo-EM on HeLa and chicken lymphocyte cells that orientated perpendicular to the chromatid axis (Gállego et al. 2009). A recent 3D cryoTEM study that used HeLa cells showed that frozen hydrated DNA is densely packed, forming stacked sheets of chromatin, is planar, and forms multilaminar plates that are stabilized by interactions between nucleosomes. Having a 13-nm thickness between the two layers (single layer 7.5-nm) implicated that nucleosomes in the layers interdigitate. Together with small angle X-ray scattering (SAXS) data, a chromosome model was proposed composed of stacked chromatin layers positioned perpendicular to the axis of the chromosome (Chicano et al. 2019).
Mitotic chromatin in frozen-hydrated Schizosaccharomyces pombe displayed megacomplexes and pockets, showing more compaction at the oligo-nucleosome than the di-nucleosome level compared with interphase chromatin (Cai et al. 2018). Mitotic chromatin organization showed no evidence of 30-nm fibers in budding yeast Saccharomyces cerevisiae (Chen et al. 2016) and picoplankton (Gan et al. 2013). An X-ray cryo-ptychography experiment has been attempted on human chromosomes but required more work in optimizing the setup before any concrete conclusion could be made (Yusuf et al. 2017).
What next? Is there a need for Cryo?
Even though cryo imaging has proven useful for numerous chromatin-based studies including complexes, it has not been fully exploited for intact mitotic chromosome investigation. Freezing of chromosomes can be achieved using the different vitrification methods that include plunge freezing into liquid ethane or propane (samples below 1 μm), slam freezing (samples up to 10 μm thickness), and high pressure freezing (thicker volume samples above 200 μm). However, chemical preservation or freeze substitution (uses organic solvents (acetone or methanol) at low temperatures (approx. − 78° to − 90 °C and placed into resin after staining and sectioned at room temperature before imaging)) will be the only option if the microscope of choice does not have cryo capability for sample cooling during imaging. These procedures are technically challenging, time consuming, can cause artifacts, and are costly. Current high-throughput structure determination has been prevented due to several limitations that include (i) thickness of the compact mitotic chromosomes; (ii) challenging cryo sample preparation steps, handling, and preservation; (iii) powerful nanometer cryo imaging microscopes with sufficient resolution; and (iv) computational tools for image acquisition and detailed processing.
The number of studies done on mitotic chromosomes has not been fully exploited using cryo technology. However, we are now witnessing an increase in the number of studies (see Fig. 1) using advanced cryo technologies that recapitulate the close to native state of mitotic chromatin structure. The CEMOVIS method (Al-Amoudi et al. 2004) is so far the widely explored on mitotic chromosomes (see timeline). No interspecies structural variation was observed for the presence of the 30-nm chromatin structure apart from one study that occasionally showed this on HeLa chromosomes (Chicano et al. 2019). HeLa is a cancer cell line (Mirabelli et al. 2019) and has been widely used for determining human nanoscale chromatin structures in both chemical preserved (Table 1) and cryo studies (Fig. 1). HeLa cells are extremely complex and heterogeneous and display abnormal karyotypes (Macville et al. 1999) that can add to the variability reported in current imaging studies and may not represent the ‘true picture’.
We are now witnessing various imaging approaches that are enhancing our knowledge in cryo imaging of biological samples but are yet to be explored for mitotic chromosomes. SBFSEM does not yet have a cryogenic stage for the instrument; therefore, the samples have to be imaged at room temperature after resin embedding (Yusuf et al. 2014). Therefore, freeze substitution of chromosomes after high pressure freezing and SBFSEM would be a positive way forward (Yusuf et al. 2016). Alternatively, cryo-FIB that has full cryo capability (Schertel et al. 2013) would be useful providing close to native state imaging and is yet to be experimented directly on vitrified chromosomes. Another potential method that needs exploring is stochastic optical reconstruction microscopy (STORM) SRM under cryogenic conditions as this allowed 12-nm resolution to be achieved on bacterial cells using a low-cost super-hemispherical solid immersion lens (superSIL) (Bateman et al. 2019; Wang et al. 2019).
A combination of microcopy approaches is also being used and looks promising (Schertel et al. 2013). 3D correlative light-electron microscopy (CLEM) performed using light and SBFSEM has been performed to understand the role of Ki-67 in metaphase chromosomes at ultra-structural resolution (Booth et al. 2016). This correlative technology using with 3D SIM, SMLM, and FIB-SEM has been applied on mammalian cells after combining vitreous freezing and is known as cryoCLEM (Yusuf et al. 2016). This SRM combined with FIBSEM may serve useful for DNA/protein structural interactions on mitotic chromosomes.
As cryo preservation only allows a snapshot of a biological process at a single time point, therefore it is key to trace dynamic chromatin movement in vivo. SRM using PALM and tracking of live cells has shown ∼ 140 nm and ∼ 200 nm mitotic chromatin domain that were suggested to be retained throughout the cell cycle (Nozaki et al. 2017). Furthermore, new microfluidic-based technology allows direct correlation of live imaging and room temperature electron microscopy with millisecond time resolution after the sample is cryofixed (Fuest et al. 2018). Recently, this technology has been combined with cryo-FIB to prepare frozen hydrated electron transparent sections for cryo-ET (Fuest et al. 2019). This powerful 4D high-resolution space-time correlative light and electron microscopy (st-CLEM) method is useful but needs to be explored on mitotic chromosomes. The use of DNA painting that allows blinking after binding of short dye-labeled (‘imager’) oligonucleotides to their complementary target (‘docking’) strands is serving useful for chromosome nanoscale SRM imaging (Nir et al. 2018; Schnitzbauer et al. 2017).
The future of chromosome imaging without doubt is moving towards full cryogenic settings that will be crucial for answering fundamental biological questions in the chromosome field. We must consider mitotic chromatin complexity from current studies (Fig. 1; Table 1) considering variations in different organisms, developmental stages including pluripotency and epigenetic states, cell types (undifferentiated vs differentiated), cell cycle stages (interphase vs metaphase), chromosome types ((sub)metacentric/acrocentric), compaction states (g-bands), e.g., heterochromatin vs euchromatin and telomeres/centromeres. Furthermore, cryo imaging has been performed both on isolated chromosomes and on chromosomes inside a cell after performing the CEMOVIS/cryo-EM method (Fig. 1) indicating no major structural variability seen so far and this would have to be carefully considered for future studies. Powerful and affordable 3D cryo-microscopes with nanoscale resolution together with faster image processing tools, correct sample, and labeling choice will be essential for unraveling nucleosome-nucleosome with other protein/DNA interactions. Overall, this would assist in identifying disease-specific signatures relating to genome disorganization especially in cancer where chromosomal aberrations take place.
Funding
The Aga Khan University and generous donors provided financial support. Others include the Brookhaven National Laboratory supported by the U.S. Department of Energy, Office of Science, Office of Basic Energy Sciences, under Contract No. DE-SC0012704. This work was partially supported by the UK BBSRC (BB/H022597/1) under a “Professorial Fellowship for imaging chromosomes by coherent X-ray diffraction”.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
The original online version of this article was revised: In the originally published article, the name of the 4th author was incorrectly presented as El-Naser Lalani. The correct name is El-Nasir Lalani, which is also given above.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Change history
10/13/2020
A correction to this paper has been published: <ExternalRef><RefSource>https://doi.org/10.1007/s12551-020-00767-5</RefSource><RefTarget Address="10.1007/s12551-020-00767-5" TargetType="DOI"/></ExternalRef>.
References
- Al-Amoudi A, Chang JJ, Leforestier A, McDowall A, Salamin LM, Norlen LP, ..., Dubochet J (2004) Cryo-electron microscopy of vitreous sections. EMBO J, 23(18):3583–3588 [DOI] [PMC free article] [PubMed]
- Antonin W, Neumann H. Chromosome condensation and decondensation during mitosis. Curr Opin Cell Biol. 2016;40:15–22. doi: 10.1016/j.ceb.2016.01.013. [DOI] [PubMed] [Google Scholar]
- Bateman BC, Zanetti-Domingues LC, Moores AN, Needham SR, Rolfe DJ, Wang L, Clarke DT, Martin-Fernandez ML (2019) Super-resolution microscopy at cryogenic temperatures using solid immersion lenses. Bio-protocol 9(22):e3426. 10.21769/BioProtoc.3426 [DOI] [PMC free article] [PubMed]
- Batty P, Gerlich DW. Mitotic chromosome mechanics: how cells segregate their genome. Trends Cell Biol. 2019;29(9):717–726. doi: 10.1016/j.tcb.2019.05.007. [DOI] [PubMed] [Google Scholar]
- Bendandi A, Dante S, Zia SR, Diaspro A, Rocchia W. Chromatin compaction multiscale modeling: a complex synergy between theory, simulation, and experiment. Front Mol Biosci. 2020;7:15. doi: 10.3389/fmolb.2020.00015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beseda T, Cápal P, Kubalová I, Schubert V, Doležel J, Šimková H. Mitotic chromosome organization: general rules meet species-specific variability. Comput Struct Biotechnol J. 2020;18:1311–1319. doi: 10.1016/j.csbj.2020.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Booth DG, Beckett AJ, Molina O, Samejima I, Masumoto H, Kouprina N, ..., Earnshaw WC (2016) 3D-CLEM reveals that a major portion of mitotic chromosomes is not chromatin. Mol Cell, 64(4):790–802 [DOI] [PMC free article] [PubMed]
- Cai S, Chen C, Tan ZY, Huang Y, Shi J, Gan L. Cryo-ET reveals the macromolecular reorganization of S. pombe mitotic chromosomes in vivo. Proc Natl Acad Sci. 2018;115(43):10977–10982. doi: 10.1073/pnas.1720476115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cervantes GA (2016) Technical fundamentals of radiology and CT. IOP Publishing
- Chen C, Lim HH, Shi J, Tamura S, Maeshima K, Surana U, Gan L. Budding yeast chromatin is dispersed in a crowded nucleoplasm in vivo. Mol Biol Cell. 2016;27(21):3357–3368. doi: 10.1091/mbc.E16-07-0506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen B, Yusuf M, Hashimoto T, Estandarte AK, Thompson G, Robinson I. Three-dimensional positioning and structure of chromosomes in a human prophase nucleus. Sci Adv. 2017;3(7):e1602231. doi: 10.1126/sciadv.1602231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chicano A, Crosas E, Otón J, Melero R, Engel BD, Daban JR. Frozen-hydrated chromatin from metaphase chromosomes has an interdigitated multilayer structure. EMBO J. 2019;38(7):e99769. doi: 10.15252/embj.201899769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chua EY, Vogirala VK, Inian O, Wong AS, Nordenskiöld L, Plitzko JM, ..., Sandin S (2016) 3.9 Å structure of the nucleosome core particle determined by phase-plate cryo-EM. Nucleic Acids Res 44(17):8013–8019 [DOI] [PMC free article] [PubMed]
- Cressey D, Callaway E. Cryo-electron microscopy wins chemistry Nobel. Nat News. 2017;550(7675):167. doi: 10.1038/nature.2017.22738. [DOI] [PubMed] [Google Scholar]
- Davey CA, Sargent DF, Luger K, Maeder AW, Richmond TJ. Solvent mediated interactions in the structure of the nucleosome core particle at 1.9 Å resolution. J Mol Biol. 2002;319(5):1097–1113. doi: 10.1016/S0022-2836(02)00386-8. [DOI] [PubMed] [Google Scholar]
- Dixon JR, Gorkin DU, Ren B. Chromatin domains: the unit of chromosome organization. Mol Cell. 2016;62(5):668–680. doi: 10.1016/j.molcel.2016.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doerr A. Cryo-electron tomography. Nat Methods. 2017;14(1):34–34. [Google Scholar]
- Dong B, Almassalha LM, Stypula-Cyrus Y, Urban BE, Chandler JE, Nguyen TQ, Sun C, Zhang HF, Backman V (2016) Superresolution intrinsic fluorescence imaging of chromatin utilizing native, unmodified nucleic acids for contrast. Proc Natl Acad Sci 113(35):9716–9721 [DOI] [PMC free article] [PubMed]
- Dubochet J, McDowall AW. Vitrification of pure water for electron microscopy. J Microsc. 1981;124(3):3–4. [Google Scholar]
- Dubochet J, Lepault J, Freeman RBJA, Berriman JA, Homo JC. Electron microscopy of frozen water and aqueous solutions. J Microsc. 1982;128(3):219–237. [Google Scholar]
- Ekundayo B, Richmond TJ, Schalch T. Capturing structural heterogeneity in chromatin fibers. J Mol Biol. 2017;429(20):3031–3042. doi: 10.1016/j.jmb.2017.09.002. [DOI] [PubMed] [Google Scholar]
- Eltsov M, MacLellan KM, Maeshima K, Frangakis AS, Dubochet J. Analysis of cryo-electron microscopy images does not support the existence of 30-nm chromatin fibers in mitotic chromosomes in situ. Proc Natl Acad Sci. 2008;105(50):19732–19737. doi: 10.1073/pnas.0810057105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang K, Chen X, Li X, Shen Y, Sun J, Czajkowsky DM, Shao Z. Super-resolution imaging of individual human subchromosomal regions in situ reveals nanoscopic building blocks of higher-order structure. ACS Nano. 2018;12(5):4909–4918. doi: 10.1021/acsnano.8b01963. [DOI] [PubMed] [Google Scholar]
- Flors C, Earnshaw WC. Super-resolution fluorescence microscopy as a tool to study the nanoscale organization of chromosomes. Curr Opin Chem Biol. 2011;15(6):838–844. doi: 10.1016/j.cbpa.2011.10.004. [DOI] [PubMed] [Google Scholar]
- Fuest M, Nocera GM, Modena MM, Riedel D, Mejia YX, Burg TP. Cryofixation during live-imaging enables millisecond time-correlated light and electron microscopy. J Microsc. 2018;272(2):87–95. doi: 10.1111/jmi.12747. [DOI] [PubMed] [Google Scholar]
- Fuest M, Schaffer M, Nocera GM, Galilea-Kleinsteuber RI, Messling JE, Heymann M, ..., Burg TP (2019) In situ microfluidic cryofixation for cryo focused ion beam milling and cryo electron tomography. Sci Rep 9(1):1–10 [DOI] [PMC free article] [PubMed]
- Fussner E, Strauss M, Djuric U, Li R, Ahmed K, Hart M, ..., Bazett-Jones DP (2012) Open and closed domains in the mouse genome are configured as 10-nm chromatin fibres. EMBO Rep 13(11):992–996 [DOI] [PMC free article] [PubMed]
- Galbraith CG, Galbraith JA. Super-resolution microscopy at a glance. J Cell Sci. 2011;124(10):1607–1611. doi: 10.1242/jcs.080085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gállego I, Castro-Hartmann P, Caravaca JM, Caño S, Daban JR. Dense chromatin plates in metaphase chromosomes. Eur Biophys J. 2009;38(4):503. doi: 10.1007/s00249-008-0401-1. [DOI] [PubMed] [Google Scholar]
- Gan L, Jensen GJ. Electron tomography of cells. Q Rev Biophys. 2012;45(1):27–56. doi: 10.1017/S0033583511000102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gan L, Ladinsky MS, Jensen GJ. Chromatin in a marine picoeukaryote is a disordered assemblage of nucleosomes. Chromosoma. 2013;122(5):377–386. doi: 10.1007/s00412-013-0423-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson GP, Gan L, Jensen GJ. 3-D ultrastructure of O. tauri: electron cryotomography of an entire eukaryotic cell. PLoS One. 2007;2(8):e749. doi: 10.1371/journal.pone.0000749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsieh THS, Fudenberg G, Goloborodko A, Rando OJ. Micro-C XL: assaying chromosome conformation from the nucleosome to the entire genome. Nat Methods. 2016;13(12):1009–1011. doi: 10.1038/nmeth.4025. [DOI] [PubMed] [Google Scholar]
- Kalle W, Strappe P. Atomic force microscopy on chromosomes, chromatin and DNA: a review. Micron. 2012;43(12):1224–1231. doi: 10.1016/j.micron.2012.04.004. [DOI] [PubMed] [Google Scholar]
- Kourkoutis LF, Plitzko JM, Baumeister W. Electron microscopy of biological materials at the nanometer scale. Annu Rev Mater Res. 2012;42:33–58. [Google Scholar]
- Krietenstein N, Rando OJ. Mesoscale organization of the chromatin fiber. Curr Opin Genet Dev. 2020;61:32–36. doi: 10.1016/j.gde.2020.02.022. [DOI] [PubMed] [Google Scholar]
- Lakadamyali M, Cosma MP. Advanced microscopy methods for visualizing chromatin structure. FEBS Lett. 2015;589(20):3023–3030. doi: 10.1016/j.febslet.2015.04.012. [DOI] [PubMed] [Google Scholar]
- Li Y, Almassalha LM, Chandler JE, Zhou X, Stypula-Cyrus YE, Hujsak KA, ..., Dravid VP (2017) The effects of chemical fixation on the cellular nanostructure. Exp Cell Res 358(2):253–259 [DOI] [PMC free article] [PubMed]
- Liang Z, Zickler D, Prentiss M, Chang FS, Witz G, Maeshima K, Kleckner N. Chromosomes progress to metaphase in multiple discrete steps via global compaction/expansion cycles. Cell. 2015;161(5):1124–1137. doi: 10.1016/j.cell.2015.04.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lučić V, Rigort A, Baumeister W. Cryo-electron tomography: the challenge of doing structural biology in situ. J Cell Biol. 2013;202(3):407–419. doi: 10.1083/jcb.201304193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luger K, Mäder AW, Richmond RK, Sargent DF, Richmond TJ. Crystal structure of the nucleosome core particle at 2.8 Å resolution. Nature. 1997;389(6648):251–260. doi: 10.1038/38444. [DOI] [PubMed] [Google Scholar]
- Macville M, Schröck E, Padilla-Nash H, Keck C, Ghadimi BM, Zimonjic D, ..., Ried T (1999) Comprehensive and definitive molecular cytogenetic characterization of HeLa cells by spectral karyotyping. Cancer Res 59(1):141–150 [PubMed]
- Matsuda A, Shao L, Boulanger J, Kervrann C, Carlton PM, Kner P, Sedat JW. Condensed mitotic chromosome structure at nanometer resolution using PALM and EGFP-histones. PLoS One. 2010;5(9):e12768. doi: 10.1371/journal.pone.0012768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazur P. Cryobiology: the freezing of biological systems. Science. 1970;168(3934):939–949. doi: 10.1126/science.168.3934.939. [DOI] [PubMed] [Google Scholar]
- McDowall AW, Smith JM, Dubochet J. Cryo-electron microscopy of vitrified chromosomes in situ. EMBO J. 1986;5(6):1395–1402. doi: 10.1002/j.1460-2075.1986.tb04373.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirabelli P, Coppola L, Salvatore M. Cancer cell lines are useful model systems for medical research. Cancers. 2019;11(8):1098. doi: 10.3390/cancers11081098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moor H (1968) Snap freezing under high pressure: a new fixation technique for freeze-etching. In Proceedings of the 4th European Regional Conference on Electron Microscopy, Rome, 1968 (Vol. 2, pp. 445-446)
- Naumova N, Imakaev M, Fudenberg G, Zhan Y, Lajoie BR, Mirny LA, Dekker J. Organization of the mitotic chromosome. Science. 2013;342(6161):948–953. doi: 10.1126/science.1236083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nir G, Farabella I, Estrada CP, Ebeling CG, Beliveau BJ, Sasaki HM, ..., Erceg J (2018) Walking along chromosomes with super-resolution imaging, contact maps, and integrative modeling. PLoS Genet 14(12):e1007872 [DOI] [PMC free article] [PubMed]
- Nishino Y, Takahashi Y, Imamoto N, Ishikawa T, Maeshima K. Three-dimensional visualization of a human chromosome using coherent X-ray diffraction. Phys Rev Lett. 2009;102(1):018101. doi: 10.1103/PhysRevLett.102.018101. [DOI] [PubMed] [Google Scholar]
- Nozaki T, Imai R, Tanbo M, Nagashima R, Tamura S, Tani T, ..., Wendt KS (2017) Dynamic organization of chromatin domains revealed by super-resolution live-cell imaging. Mol Cell 67(2):282–293 [DOI] [PubMed]
- Ohno M, Priest DG, Taniguchi Y. Nucleosome-level 3D organization of the genome. Biochem Soc Trans. 2018;46(3):491–501. doi: 10.1042/BST20170388. [DOI] [PubMed] [Google Scholar]
- Ohta S, Bukowski-Wills JC, Sanchez-Pulido L, de Lima Alves F, Wood L, Chen ZA, ..., Fukagawa T (2010) The protein composition of mitotic chromosomes determined using multiclassifier combinatorial proteomics. Cell 142(5):810–821 [DOI] [PMC free article] [PubMed]
- Olins AL, Olins DE. Spheroid chromatin units (ν bodies) Science. 1974;183(4122):330–332. doi: 10.1126/science.183.4122.330. [DOI] [PubMed] [Google Scholar]
- Otterstrom J, Castells-Garcia A, Vicario C, Gomez-Garcia PA, Cosma MP, Lakadamyali M. Super-resolution microscopy reveals how histone tail acetylation affects DNA compaction within nucleosomes in vivo. Nucleic Acids Res. 2019;47(16):8470–8484. doi: 10.1093/nar/gkz593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ou HD, Phan S, Deerinck TJ, Thor A, Ellisman MH, O’shea CC (2017) ChromEMT: visualizing 3D chromatin structure and compaction in interphase and mitotic cells. Science 357(6349) [DOI] [PMC free article] [PubMed]
- Phengchat R, Hayashida M, Ohmido N, Homeniuk D, Fukui K. 3D observation of chromosome scaffold structure using a 360° electron tomography sample holder. Micron. 2019;126:102736. doi: 10.1016/j.micron.2019.102736. [DOI] [PubMed] [Google Scholar]
- Polge C, Smith AU, Parkes AS. Revival of spermatozoa after vitrification and dehydration at low temperatures. Nature. 1949;164(4172):666–666. doi: 10.1038/164666a0. [DOI] [PubMed] [Google Scholar]
- Poonperm R, Takata H, Hamano T, Matsuda A, Uchiyama S, Hiraoka Y, Fukui K. Chromosome scaffold is a double-stranded assembly of scaffold proteins. Sci Rep. 2015;5(1):1–10. doi: 10.1038/srep11916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramachandran S, Henikoff S. Nucleosome dynamics during chromatin remodeling in vivo. Nucleus. 2016;7(1):20–26. doi: 10.1080/19491034.2016.1149666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson PJ, Fairall L, Huynh VA, Rhodes D. EM measurements define the dimensions of the “30-nm” chromatin fiber: evidence for a compact, interdigitated structure. Proc Natl Acad Sci. 2006;103(17):6506–6511. doi: 10.1073/pnas.0601212103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schalch T, Duda S, Sargent DF, Richmond TJ. X-ray structure of a tetranucleosome and its implications for the chromatin fibre. Nature. 2005;436(7047):138–141. doi: 10.1038/nature03686. [DOI] [PubMed] [Google Scholar]
- Schermelleh L, Ferrand A, Huser T, Eggeling C, Sauer M, Biehlmaier O, Drummen GP. Super-resolution microscopy demystified. Nat Cell Biol. 2019;21(1):72–84. doi: 10.1038/s41556-018-0251-8. [DOI] [PubMed] [Google Scholar]
- Schertel A, Snaidero N, Han HM, Ruhwedel T, Laue M, Grabenbauer M, Möbius W. Cryo FIB-SEM: volume imaging of cellular ultrastructure in native frozen specimens. J Struct Biol. 2013;184(2):355–360. doi: 10.1016/j.jsb.2013.09.024. [DOI] [PubMed] [Google Scholar]
- Schnitzbauer J, Strauss MT, Schlichthaerle T, Schueder F, Jungmann R. Super-resolution microscopy with DNA-PAINT. Nat Protoc. 2017;12(6):1198. doi: 10.1038/nprot.2017.024. [DOI] [PubMed] [Google Scholar]
- Schroeder-Reiter E, Pérez-Willard F, Zeile U, Wanner G. Focused ion beam (FIB) combined with high resolution scanning electron microscopy: a promising tool for 3D analysis of chromosome architecture. J Struct Biol. 2009;165(2):97–106. doi: 10.1016/j.jsb.2008.10.002. [DOI] [PubMed] [Google Scholar]
- Shemilt LA, Estandarte AKC, Yusuf M, Robinson IK. Scanning electron microscope studies of human metaphase chromosomes. Philos Trans R Soc A Math Phys Eng Sci. 2014;372(2010):20130144. doi: 10.1098/rsta.2013.0144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song F, Chen P, Sun D, Wang M, Dong L, Liang D, et al. Cryo-EM study of the chromatin fiber reveals a double helix twisted by tetranucleosomal units. Science. 2014;344(6182):376–380. doi: 10.1126/science.1251413. [DOI] [PubMed] [Google Scholar]
- Studer D, Humbel BM, Chiquet M. Electron microscopy of high pressure frozen samples: bridging the gap between cellular ultrastructure and atomic resolution. Histochem Cell Biol. 2008;130(5):877–889. doi: 10.1007/s00418-008-0500-1. [DOI] [PubMed] [Google Scholar]
- Swedlow JR, Hirano T. The making of the mitotic chromosome: modern insights into classical questions. Mol Cell. 2003;11(3):557–569. doi: 10.1016/s1097-2765(03)00103-5. [DOI] [PubMed] [Google Scholar]
- Szczurek AT, Prakash K, Lee HK, Żurek-Biesiada DJ, Best G, Hagmann M, Birk U. Single molecule localization microscopy of the distribution of chromatin using Hoechst and DAPI fluorescent probes. Nucleus. 2014;5(4):331–340. doi: 10.4161/nucl.29564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor KA, Glaeser RM. Electron diffraction of frozen, hydrated protein crystals. Science. 1974;186(4168):1036–1037. doi: 10.1126/science.186.4168.1036. [DOI] [PubMed] [Google Scholar]
- Ushiki T, Hoshi O. Atomic force microscopy for imaging human metaphase chromosomes. Chromosom Res. 2008;16(3):383. doi: 10.1007/s10577-008-1241-7. [DOI] [PubMed] [Google Scholar]
- Walther N, Ellenberg J (2018) Quantitative live and super-resolution microscopy of mitotic chromosomes. In Methods in cell biology (Vol. 145, pp. 65-90). Academic Press [DOI] [PubMed]
- Wang L, Bateman B, Zanetti-Domingues LC, Moores AN, Astbury S, Spindloe C, ..., Rolfe DJ (2019) Solid immersion microscopy images cells under cryogenic conditions with 12 nm resolution. Commun Biol 2(1):1–11 [DOI] [PMC free article] [PubMed]
- Woodcock CL, Ghosh RP. Chromatin higher-order structure and dynamics. Cold Spring Harb Perspect Biol. 2010;2(5):a000596. doi: 10.1101/cshperspect.a000596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J, Liu Y. A guide to visualizing the spatial epigenome with super-resolution microscopy. FEBS J. 2019;286(16):3095–3109. doi: 10.1111/febs.14938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan H, Nazaretski E, Lauer K, Huang X, Wagner U, Rau C, ..., Bouet N (2016) Multimodality hard-x-ray imaging of a chromosome with nanoscale spatial resolution. Sci Rep 6:20112 [DOI] [PMC free article] [PubMed]
- Yusuf M, Chen B, Hashimoto T, Estandarte AK, Thompson G, Robinson I. Staining and embedding of human chromosomes for 3-D serial block-face scanning electron microscopy. Biotechniques. 2014;57(6):302–307. doi: 10.2144/000114236. [DOI] [PubMed] [Google Scholar]
- Yusuf M, Chen B, Robinson I (2016) Future prospects of 3d human chromosome imaging by serial block face scanning electron microscopy. Single Cell Biol 5(2)
- Yusuf M, Zhang F, Chen B, Bhartiya A, Cunnea K, Wagner U, ..., Robinson IK (2017) Procedures for cryogenic X-ray ptychographic imaging of biological samples. IUCrJ 4(2):147–151 [DOI] [PMC free article] [PubMed]
- Yusuf M, Kaneyoshi K, Fukui K, Robinson I. Use of 3D imaging for providing insights into high-order structure of mitotic chromosomes. Chromosoma. 2019;128(1):7–13. doi: 10.1007/s00412-018-0678-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou BR, Yadav KS, Borgnia M, Hong J, Cao B, Olins AL, ..., Zhang P (2019a) Atomic resolution cryo-EM structure of a native-like CENP-A nucleosome aided by an antibody fragment. Nat Commun 10(1):1–7. [DOI] [PMC free article] [PubMed]
- Zhou Z, Li K, Yan R, Yu G, Gilpin CJ, Jiang W, Irudayaraj JM. The transition structure of chromatin fibers at the nanoscale probed by cryogenic electron tomography. Nanoscale. 2019;11(29):13783–13789. doi: 10.1039/c9nr02042j. [DOI] [PMC free article] [PubMed] [Google Scholar]