Abstract
Zika virus is a member of the family of Flaviviridae, which is primarily spread to humans by mosquito bites. It has been linked to microcephaly in neonates, and as such, it poses a significant risk to human pregnancy. Zika virus infection is also implicated in other severe neurological disorders such as Guillain-Barre syndrome. There is currently no vaccine available to treat Zika virus disease, and as such, it represents a serious challenge to public health. Antigenic similarities between Zika and dengue can suggest artificially high infection rates of Zika within specific population groups. Here, we review recent literature and provide an update on the status of the Zika outbreak, including a description of available medical countermeasure options and current diagnosis methodology.
Keywords: Zika, Dengue, Flavivirus, Mosquitos, Microcephaly, Aedes aegypti
Introduction
Zika virus is able to infect humans and other mammals and was first discovered in the Uganda Zika Forest in 1947 (Dick 1952). Zika virus is a member of the family Flaviviridae and was first isolated from the blood of sentinel rhesus macaques in 1948 (Macnamara 1954). Zika antibody was first detected in humans in 1964 in Africa (Wikan and Smith 2016). It is challenging to detect Zika virus-specific antibodies because of their cross-reactivity to other related Flavivirus such as dengue and this is especially so in patients previously vaccinated against other flaviviruses (Cao-Lormeau et al. 2014). The first severe Zika outbreak was reported in Yap Island in 2007, where 73% of the population was infected with the Zika virus within 4 months (Zanluca et al. 2015). In 2013, there was a second major outbreak in French Polynesia (Malone et al. 2016). Since then the Zika virus has spread throughout the western hemisphere causing a massive epidemic in the continent of South America, with localised outbreaks in Argentina, Brazil, Venezuela, Paraguay, El Salvador, Columbia Southern United States, and Singapore (Culjat et al. 2016; Fontanet et al. 2016; Hoen et al. 2018; Kostyuchenko et al. 2016; Parola and Musso 2020). The list of countries with an elevated risk of Zika infections is shown in Table 1.
Table 1.
Zika virus: countries specific risk (WHO 2020)
| Angola | Bonaire | Cuba | French Guiana | Mexico | Grenadines |
|---|---|---|---|---|---|
| Anguilla | Brazil | Curacao | Gabon | Nigeria | Samoa |
| Antigua | Cambodia | Costa Rica | Grenada | Panama | Senegal |
| Argentina | Cameroon | Cote D’Ivoire | Guatemala | Papua New Guinea | Saint Martin |
| Aruba | Cape Verde | Cuba | Guyana | Paraguay | Turks and Caicos Islands |
| American Samoa | Cayman Islands | Curacao | Haiti | Philippines | Uganda |
| Angola | African Republic | Dominica | Honduras | Puerto Rico | Thailand |
| Bahamas | Texas | Dominican Republic | India | Saba | Tonga |
| Bangladesh | Colombia | Ecuador | Indonesia | Saint Barthelemy | Trinidad and Tobago |
| Barbados | Cape Verde | El Salvador | Jamaica | Saint Kitts and Nevis | Venezuela |
| Belize | Costa Rica | Ethiopia | Malaysia | Saint Lucia | Vietnam |
| Bolivia | Cote D’Ivoire | Fiji | Maldives | Saint Martin | Virgin Islands (British) |
Zika virus infection of pregnant mothers has been shown to produce neurological defects in the foetus at a case rate of 7% (Simpson 1964). Zika virus infection can additionally cause congenital syndrome (congenital disabilities) in infants, which includes a spectrum of defects (Campos et al. 2015). Recent studies suggested that the Zika virus directly causes neuronal tissue damage (Kikuti et al. 2018). However it is not presently understood why not all Zika virus infections produce brain abnormalities during embryonic development. Within the Flavivirus family, there are structural similarities between the Zika, and dengue viruses and the antibody raised against one of the viruses frequently cross-react with the other virus (Li et al. 2018). This cross-reactivity phenomenon has led to mistaken assessments of the degree of epidemic infection levels (Sirohi et al. 2016a).
It has been suggested that the binding of human dengue antibodies to the Zika virus does not neutralise the Zika virus, but it instead enhances Zika infection in cell clusters (Reina et al. 2020).
Composition and structure of the Zika virus
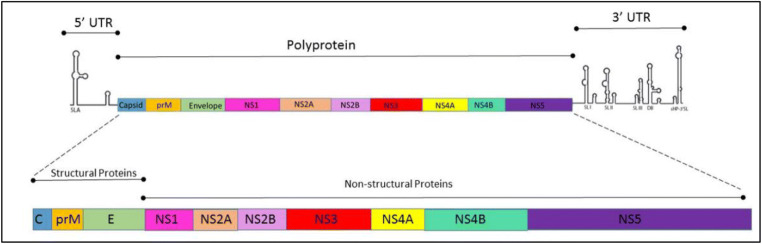
Zika virus genome is a single-stranded positive RNA with a single open reading frame located within two RNA-structural 5′ and 3′ untranslated regions (Bhargava et al. 2019). As described in the literature, the genome codes for three structural proteins and seven non-structural proteins involved in replication, assembly, and mediating the innate host responses to infection (Cox et al. 2015). Recent studies found that Zika virus surfaces share similar protein structure to other flaviviruses like DENV (Dengue viruses is a positive single-stranded RNA virus of family Flaviviridae) and WNV (West Nile virus is a single-stranded RNA virus of the family Flaviviridae) (Simpson 1964). This virus has 180 copies of protein E, which associates with protein M (Cai et al. 2017) to form the viral capsid. Protein E copies are assembled into an icosahedral symmetry which is formed by 90 dimers at a pH of 7.4 and anchored in the lipid bilayer (Zhang et al. 2013). According to Lin et al. (2018), protein E lies in parallel with the viral membrane (Figure 1).
Fig. 1.
Schematic representation of the Zika virus genome, 11 kb Zika virus genome, including the 5′ and 3′ untranslated region (Zhang et al. 2013).
Early filtration studies indicated that the size of Zika virus was in the range of 30–45 nm in diameter (Campos et al. 2015). Electron transmission microscopy (ETM) analysis reveals that virions are spherical and approximately 40–43 nm in diameter (Coelho et al. 2017). Zika particles can be inactivated at a pH of 6.2 by using potassium permanganate either at temperature 58 °C for 30 min or 60 °C for 15 min (Conte et al. 2017). The structure of both proteins (E and M) has been determined by the cryo-electron microscopy (cryoEM) (Faye et al. 2014; Rubin et al. 2016; Valente and Moraes 2019). The smaller M proteins reside underneath, the more abundant protein E binding to the cellular transmembrane domain (Cao-Lormeau and Musso 2014; Rathore et al. 2019). From X-ray crystallography, the structure of the NS1 protein was found to be similar to the corresponding protein in dengue but has a different distribution of electrical charge on its surface (Sirohi and Kuhn 2017). Zika virus surface composites are both positively and negatively charged central regions and negatively charged towards the distal ends (Ramharack and Soliman 2018; Wikan and Smith 2016).
The Zika virus genome is 10.7 kb and, aside from proteins E and M, encodes several non-structural proteins such as NS1, NS2A, NS2B, NS3, NS4A, NS4B, and NS5. Zika virus encoding genome and proteins is described in Table 2 (Marquez-Jurado et al. 2018).
Table 2.
Characterisation of structural and non-structural Zika virus proteins (Houa et al. 2017)
| Name | Size: Aa (kDa) | Cellular distribution | Subcellular location |
|---|---|---|---|
| C | 122 (14) | Cyto.& Nuc. | Golgi apparatus,LD,and nucleoli |
| PrM | 168 (19) | Cyto. | Endoplasmic reticulum, centrosome |
| Env | 504 (54) | Cyto. | Endoplasmic reticulum |
| NS1 | 352 (40) | Cyto.& Nuc. | Autophagosome |
| NS2a | 226 (24) | Cyto. | Endoplasmic reticulum |
| NS2b | 1.30 (14) | Cyto. | Golgi apparatus |
| NS3 | 617 (69) | Cyto. | Co-localize with Tubulin, centrosome |
| NS4a | 127 (14) | Cyto. | Golgi apparatus |
| NS4b | 251 (27) | Cyto. | Early endosome |
| NS5 | 903 (103) | Nuc. | Co-localize with SC35 |
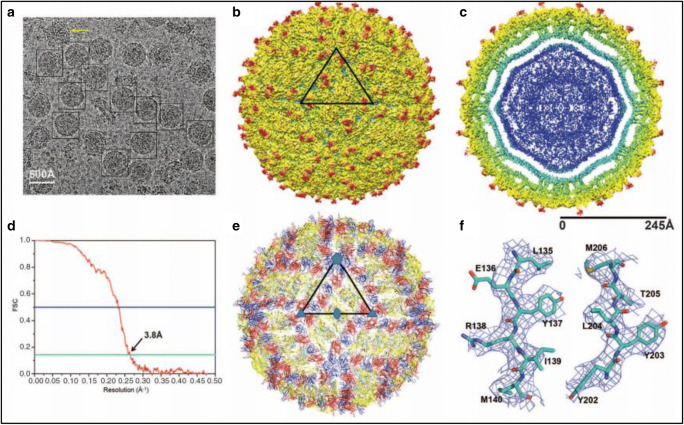
Both the structure of Zika virus and knowledge of its pathogenesis are essential for the rapid development of vaccines and therapeutics (Sirohi et al. 2016b) (Fig. 2)
Fig. 2.
The cyro-EM structure of Zika virus at 3.8 A° (Sirohi et al. 2016b). (A) Represents cryo-EM of frozen-hydrated Zika virus. (B) A surface shaded depth cued of Zika virus, (C) The radial density distribution colour coding of Zika virus. (D) Plot of Fourier shell correlation. (E) The backbone of E and M proteins. (F) Cryo-EM electron density of amino acids of E protein
Transmission and life cycle of Zika virus
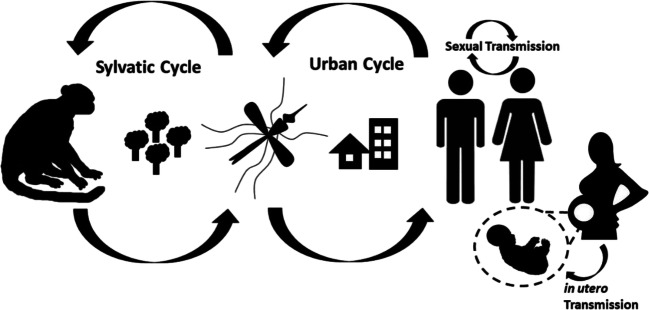
At the population level, Zika virus transmission involves both sylvatic and urban cycles and is facilitated by a variety of Aedes genera mosquito species (Gabaglia 2017). Zika virus is transmitted from the bite of infected female Aedes aegypti mosquito. After a human receives such a bite, the Zika virus spreads into the lymphatic system concentrating at the nodes (Simpson 1964). At these regions, the virus is amplified after which it then disseminates through the bloodstream to the peripheral tissue and visceral organs (Craig et al. 2016). The first symptoms following infection typically occur after 3 to 12 days (Kostyuchenko et al. 2016). Recent studies suggest that male-to-female sexual transmission constitutes a significant route of Zika virus infection, but female-to-male sexual transmission has not been observed (Zhang et al. 2013). It is not yet clear whether mosquitos or other mammals serve as an amplifying reservoir; however, Zika is maintained in nature in a sylvatic cycle transmission between non-human primates (NHP) and the forest-dwelling mosquitos (Peters and Stevenson 2019; Wikan and Smith 2016). Zika transmission through breastfeeding has not been demonstrated, but this may represent another significant infection route because milk has been shown to contain a high viral load (Javed et al. 2018). To prevent further spread of the Zika virus, it is critical to understand and reveal the amplifying reservoirs of the Zika virus (Figure 3).
Fig. 3.
Routes of Zika virus transmission (Basu and Tumban 2016)
Zika virus entry into the host cells requires a low-pH step to trigger fusion of the viral envelope with the endosome membrane. It will then release the nucleocapsid into the cytosol through the receptor protein of human cells. The role of the acidic pH for Zika virus infection of T98G cell is very well described in (Li et al. 2020). The acidification will result in a viral fusion with the endosomal membrane and releases the viral RNA into the cytoplasm. Entry to the cells is mostly mediated by the viral protein E. Proteolytic maturation results in highly complex and dynamic infection particles; this offers various possibilities to receptor interaction sites (Agrelli et al. 2019). However, the exact mode of entry is not yet understood. The virus spreads to the lymph and blood nodes, and it replicates in the cytoplasm (Li et al. 2020).
The Zika virus entry process depends on the protein pattern expressed in viral particle lattice, and it appears to rely on the degree of proteolytic maturation of the particles (Perera-Lecoin et al. 2013). Cell types also play a significant role in this process, and the cellular receptors such as T cell and receptor tyrosine kinases and those receptor families can recognise lipids in the viral membrane and mediate Zika virus entry (Hamel et al. 2015); (Perera-Lecoin et al. 2013). The ability of Zika virus to infect human cells has been extensively described in cell culture studies (Hamel et al. 2015). In the process of receptor binding and recognition, multiple molecules are used in combination of infectious entries (Kaufmann and Rossmann 2012). The Zika virus can produce ensemble structures with different virions circulating in an organism able to exhibit different tissue tropisms. Zika virus may use apoptotic mimicry as an entry mechanism, which further enables it to increase the range of Zika virus susceptible cells (Agrelli et al. 2019). Thus, the degree of viral particle maturations and the amount of proteins M, E, and NS1 exposed at the capsid surface help to regulate the Zika virus entry mechanism (Agrelli et al. 2019).
Symptoms
Some of the most detailed case notes accompanying Zika virus infection are due to reports associated with the disease of scientists and medics who have contracted the virus during either laboratory or fieldwork (Simpson 1964). The majority of those who have been infected by the Zika virus have minimal symptoms during a mild and short-lived 2–7-day illness (Zorrilla et al. 2016). As mentioned, Zika virus infection in a woman during pregnancy causes congenital brain abnormalities in the foetus, which includes microcephaly (Faye et al. 2014). Typically, the majority of patients do not get seriously ill, and therefore, their infection occurs without hospital admission (Campos et al. 2015). Once infected, the affected person is more likely to be protected from future infections (Rubin et al. 2016).
Microcephaly
Perhaps the most alarming aspect of recent outbreaks has been the high prevalence of congenital malformations, including foetal microcephaly, in babies born to infected mothers (Coelho et al. 2017). Zika virus-induced neurodevelopmental disorder results in abnormally formed brain and head (Sirohi et al. 2016a). Zika virus has been shown to attenuate the growth of human neural progenitor cells (Simpson 1964). It is not yet clear what type of exposure and whether symptomatic display during infection necessarily indicates a higher risk to the developing foetus (Sirohi et al. 2016a). In addition, the other malformations reported in foetus and newborn babies with congenital Zika virus infections include brain atrophy, arthrogryposis, and foetal abnormalities (Kikuti et al. 2018).
Diagnosis and test for Zika virus
Zika virus can be detected from human blood within the first 10 days of infection with viral loads peaking at the onset of symptoms based on the nucleic acid amplification test (Gabaglia 2017). Zika virus diagnosis typically requires the presence of fever with arthralgia or with arthritis, and no associated purulent conjunctivitis (Malone et al. 2016). The first stage test involves a conjunction of symptoms with the identification of anti-Zika virus IgM antibodies (Sumita et al. 2016). Secondary confirmation requires a PCR-based laboratory test to detect the presence of Zika virus RNA (Sumita et al. 2016). As mentioned, it is difficult to distinguish between Zika-infected patients and patients previously infected with, or vaccinated against, other related Flavivirus (Zhang et al. 2013).
Conclusion
Currently, there is no anti-Zika virus vaccine available, and important challenges remain for developing this clinical vaccine (Zhang et al. 2013). However, typically the Zika virus infection is mild, and the standard treatment for the majority of non-seriously ill patients involves the provision of supporting nurse care for symptom relief (Simpson 1964). With this study, I note the following points. Zika virus outbreak has been declared as a public health threat in many countries (Fontanet et al. 2016; Zanluca et al. 2015). Countries with a significant risk level of Zika virus infection are presented in Table 1 (as judged by the WHO (2020)). As the virus is currently endemic to several areas (Barnard et al. 2018), the risk of its further international spread is likely. Locations with suitable mosquito vectors facilitate virus transmission.
Zika virus vectors, epidemiology, and pathology remain uncertain. Diagnosis remains subjective, and laboratory diagnostics are not widely available. The commercial test of the Zika virus is limited to these, including molecular assays and lateral flow tests (Sumita et al. 2016). In some cases, similar tests/assays for chikungunya and dengue viruses are used, thereby reducing specificity and increasing the number of false-positive detections (Mardekian and Roberts 2015). According to the World Health Organization (WHO), the prevention measures of vector control are a current priority (Campos et al. 2015). As discussed, current diagnosis methods are not optimal. There is a need for cheap, fast, and reliable testing methods for Zika virus diagnosis. It is hoped that this review can act as a quick primer of recent research (and recent outbreaks) related to the Zika virus.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Agrelli A, de Moura RR, Crovella S, Brandao LAC. Zika virus entry mechanisms in human cells. Infect Genet Evol. 2019;69:22–29. doi: 10.1016/j.meegid.2019.01.018. [DOI] [PubMed] [Google Scholar]
- Barnard TR, Rajah MM, Sagan SM (2018) Contemporary Zika virus isolates induce more dsRNA and produce more negative-strand intermediate in human astrocytoma cells. Viruses 10 [DOI] [PMC free article] [PubMed]
- Basu R, Tumban E. Zika virus on a spreading spree: what we now know that was unknown in the 1950’s. Virol J. 2016;13:165. doi: 10.1186/s12985-016-0623-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhargava S, Patel T, Gaikwad R, Patil UK, Gayen S. Identification of structural requirements and prediction of inhibitory activity of natural flavonoids against Zika virus through molecular docking and Monte Carlo based QSAR simulation. Nat Prod Res. 2019;33:851–857. doi: 10.1080/14786419.2017.1413574. [DOI] [PubMed] [Google Scholar]
- Cai L, Sun Y, Song Y, Xu L, Bei Z, Zhang D, Dou Y, Wang H. Viral polymerase inhibitors T-705 and T-1105 are potential inhibitors of Zika virus replication. Arch Virol. 2017;162:2847–2853. doi: 10.1007/s00705-017-3436-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campos GS, Bandeira AC, Sardi SI. Zika virus outbreak, Bahia, Brazil. Emerg Infect Dis. 2015;21:1885–1886. doi: 10.3201/eid2110.150847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao-Lormeau VM, Musso D. Emerging arboviruses in the Pacific. Lancet. 2014;384:1571–1572. doi: 10.1016/S0140-6736(14)61977-2. [DOI] [PubMed] [Google Scholar]
- Cao-Lormeau VM, Roche C, Teissier A, Robin E, Berry AL, Mallet HP, Sall AA, Musso D. Zika virus, French Polynesia, South Pacific, 2013. Emerg Infect Dis. 2014;20:1085–1086. doi: 10.3201/eid2006.140138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coelho SVA, Neris RLS, Papa MP, Schnellrath LC, Meuren LM, Tschoeke DA, Leomil L, Vercoza BRF, Miranda M, Thompson FL, da Poian AT, Souza TML, Carneiro FA, Damaso CR, Assuncao-Miranda I, de Arruda LB. Development of standard methods for Zika virus propagation, titration, and purification. J Virol Methods. 2017;246:65–74. doi: 10.1016/j.jviromet.2017.04.011. [DOI] [PubMed] [Google Scholar]
- Conte FP, Martins RS, Cajaraville A, Nascimento HJ, Jurgilas PB, de Lima SMB, Missailidis S, Arissawa M. Production of monoclonal antibody that recognizes Zika virus and other flaviviruses in serum-free conditions. Monoclon Antib Immunodiagn Immunother. 2017;36:264–271. doi: 10.1089/mab.2017.0029. [DOI] [PubMed] [Google Scholar]
- Cox BD, Stanton RA, Schinazi RF. Predicting Zika virus structural biology: challenges and opportunities for intervention. Antivir Chem Chemother. 2015;24:118–126. doi: 10.1177/2040206616653873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig AT, Paterson BJ, Durrheim DN. Commentary: Zika virus: the latest newcomer. Front Microbiol. 2016;7:1028. doi: 10.3389/fmicb.2016.01028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Culjat M, Darling SE, Nerurkar VR, Ching N, Kumar M, Min SK, Wong R, Grant L, Melish ME. Clinical and imaging findings in an infant with Zika embryopathy. Clin Infect Dis. 2016;63:805–811. doi: 10.1093/cid/ciw324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dick GW. Zika virus. II. Pathogenicity and physical properties. Trans R Soc Trop Med Hyg. 1952;46:521–534. doi: 10.1016/0035-9203(52)90043-6. [DOI] [PubMed] [Google Scholar]
- Faye O, Freire CC, Iamarino A, Faye O, de Oliveira JV, Diallo M, Zanotto PM, Sall AA. Molecular evolution of Zika virus during its emergence in the 20(th) century. PLoS Negl Trop Dis. 2014;8:e2636. doi: 10.1371/journal.pntd.0002636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fontanet A, Cao-Lormeau VM, Dub T, Mallet HP, Ghawche F. Association between Guillain-Barre syndrome and Zika virus infection – Authors’ reply. Lancet. 2016;387:2600. doi: 10.1016/S0140-6736(16)30773-5. [DOI] [PubMed] [Google Scholar]
- Gabaglia CR. Zika virus and diagnostics. Curr Opin Pediatr. 2017;29:107–113. doi: 10.1097/MOP.0000000000000446. [DOI] [PubMed] [Google Scholar]
- Hamel R, Dejarnac O, Wichit S, Ekchariyawat P, Neyret A, Luplertlop N, Perera-Lecoin M, Surasombatpattana P, Talignani L, Thomas F, Cao-Lormeau VM, Choumet V, Briant L, Despres P, Amara A, Yssel H, Misse D. Biology of Zika virus infection in human skin cells. J Virol. 2015;89:8880–8896. doi: 10.1128/JVI.00354-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoen B, Schaub B, Funk AL, Ardillon V, Boullard M, Cabie A, Callier C, Carles G, Cassadou S, Cesaire R, Douine M, Herrmann-Storck C, Kadhel P, Laouenan C, Madec Y, Monthieux A, Nacher M, Najioullah F, Rousset D, Ryan C, Schepers K, Stegmann-Planchard S, Tressieres B, Volumenie JL, Yassinguezo S, Janky E, Fontanet A. Pregnancy outcomes after ZIKV infection in French territories in the Americas. N Engl J Med. 2018;378:985–994. doi: 10.1056/NEJMoa1709481. [DOI] [PubMed] [Google Scholar]
- Houa W, Cruz-Cosme R, Armstrong N, Obwolo LA, Wen F, Hu W, Luo M-H, Tang Q. Molecular cloning and characterization of the genes encoding the proteins of Zika virus. Gene. 2017;628:116–128. doi: 10.1016/j.gene.2017.07.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Javed F, Manzoor KN, Ali M, Haq IU, Khan AA, Zaib A, Manzoor S. Zika virus: what we need to know? J Basic Microbiol. 2018;58:3–16. doi: 10.1002/jobm.201700398. [DOI] [PubMed] [Google Scholar]
- Kaufmann B, Rossmann MG. Molecular mechanisms involved in the early steps of Flavivirus cell entry. Microbs Infect. 2012;13:1–9. doi: 10.1016/j.micinf.2010.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kikuti M, Cardoso CW, Prates APB, Paploski IAD, Kitron U, Reis MG, Mochida GH, Ribeiro GS (2018) Congenital brain abnormalities during a Zika virus epidemic in Salvador, Brazil, April 2015 to July 2016. Euro Surveill:23 [DOI] [PMC free article] [PubMed]
- Kostyuchenko VA, Lim EX, Zhang S, Fibriansah G, Ng TS, Ooi JS, Shi J, Lok SM. Structure of the thermally stable Zika virus. Nature. 2016;533:425–428. doi: 10.1038/nature17994. [DOI] [PubMed] [Google Scholar]
- Li M, Santpere G, Imamura Kawasawa Y, Evgrafov OV, Gulden FO, Pochareddy S, Sunkin SM, Li Z, Shin Y, Zhu Y, Sousa AMM, Werling DM, Kitchen RR, Kang HJ, Pletikos M, Choi J, Muchnik S, Xu X, Wang D, Lorente-Galdos B, Liu S, Giusti-Rodriguez P, Won H, de Leeuw CA, Pardinas AF, Brainspan C, Psych EC, Psych EDS, Hu M, Jin F, Li Y, Owen MJ, O'Donovan MC, Walters JTR, Posthuma D, Reimers MA, Levitt P, Weinberger DR, Hyde TM, Kleinman JE, Geschwind DH, Hawrylycz MJ, State MW, Sanders SJ, Sullivan PF, Gerstein MB, Lein ES, Knowles JA, Sestan N (2018) Integrative functional genomic analysis of human brain development and neuropsychiatric risks. Science 362 [DOI] [PMC free article] [PubMed]
- Li M, Zhang D, Li C, Zheng Z, Fu M, Ni F, Liu Y, Du T, Wang H, Griffin GE, Zhang M, Hu Q. Characterization of Zika virus endocytic pathways in human glioblastoma cells. Front Microbiol. 2020;11:242. doi: 10.3389/fmicb.2020.00242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin HH, Yip BS, Huang LM, Wu SC. Zika virus structural biology and progress in vaccine development. Biotechnol Adv. 2018;36:47–53. doi: 10.1016/j.biotechadv.2017.09.004. [DOI] [PubMed] [Google Scholar]
- Macnamara FN. Zika virus: a report on three cases of human infection during an epidemic of jaundice in Nigeria. Trans R Soc Trop Med Hyg. 1954;48:139–145. doi: 10.1016/0035-9203(54)90006-1. [DOI] [PubMed] [Google Scholar]
- Malone RW, Homan J, Callahan MV, Glasspool-Malone J, Damodaran L, Schneider ADB, Zimler R, Talton J, Cobb RR, Ruzic I, Smith-Gagen J, Janies D, Wilson J, Zika Response Working Group Zika virus: medical countermeasure development challenges. PLoS Negl Trop Dis. 2016;10:e0004530. doi: 10.1371/journal.pntd.0004530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mardekian SK, Roberts AL. Diagnostic options and challenges for dengue and chikungunya viruses. Biomed Res Int. 2015;2015:834371. doi: 10.1155/2015/834371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marquez-Jurado S, Nogales A, Avila-Perez G, Iborra FJ, Martinez-Sobrido L, Almazan F (2018) An alanine-to-valine substitution in the residue 175 of Zika virus NS2A protein affects viral RNA synthesis and attenuates the virus in vivo. Viruses 10 [DOI] [PMC free article] [PubMed]
- Parola P, Musso D (2020) Zika, dengue, chikungunya and yellow fever infections in Europe? - Winter is over, warm days are coming - so hedge your bets. Travel Med Infect Dis:101614 [DOI] [PubMed]
- Perera-Lecoin M, Meertens L, Carnec X, Amara A. Flavivirus entry receptors: an update. Viruses. 2013;6:69–88. doi: 10.3390/v6010069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters R, Stevenson M. Zika virus diagnosis: challenges and solutions. Clin Microbiol Infect. 2019;25:142–146. doi: 10.1016/j.cmi.2018.12.002. [DOI] [PubMed] [Google Scholar]
- Ramharack P, Soliman MES. Zika virus NS5 protein potential inhibitors: an enhanced in silico approach in drug discovery. J Biomol Struct Dyn. 2018;36:1118–1133. doi: 10.1080/07391102.2017.1313175. [DOI] [PubMed] [Google Scholar]
- Rathore APS, Saron WAA, Lim T, Jahan N, St John AL. Maternal immunity and antibodies to dengue virus promote infection and Zika virus-induced microcephaly in fetuses. Sci Adv. 2019;5:eaav3208. doi: 10.1126/sciadv.aav3208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reina J, Lopez de Bilbao C, Riera M. Prevalence of influenza B Yamagata lineage in adults in the 2017-2018 flu season. Med Clin (Barc) 2020;154:29–30. doi: 10.1016/j.medcli.2018.09.003. [DOI] [PubMed] [Google Scholar]
- Rubin EJ, Greene MF, Baden LR. Zika Virus and Microcephaly. N Engl J Med. 2016;374:984–985. doi: 10.1056/NEJMe1601862. [DOI] [PubMed] [Google Scholar]
- Simpson DI. Zika Virus Infection in Man. Trans R Soc Trop Med Hyg. 1964;58:335–338. [PubMed] [Google Scholar]
- Sirohi D, Kuhn RJ. Zika virus structure, maturation, and receptors. J Infect Dis. 2017;216:S935–S944. doi: 10.1093/infdis/jix515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sirohi D, Chen Z, Sun L, Klose T, Pierson TC, Rossmann MG, Kuhn RJ. The 3.8 A resolution cryo-EM structure of Zika virus. Science. 2016;352:467–470. doi: 10.1126/science.aaf5316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sirohi D, Chen Z, Sun L, Klose T, Pierson TC, Rossmann MG & Kuhn RJ (2016b) The 3.8 Å resolution cryo-EM structure of Zika virus EVOLUTIONARY GENETICS, 352, 467 [DOI] [PMC free article] [PubMed]
- Sumita LM, Rodrigues JP, Ferreira NE, Felix AC, Souza NC, Machado CM, Junior HF. Detection of human anti-Zika virus IgG by ELISA using an antigen from in vitro infected vero cells: preliminary results. Rev Inst Med Trop Sao Paulo. 2016;58:89. doi: 10.1590/S1678-9946201658089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valente AP, Moraes AH. Zika virus proteins at an atomic scale: how does structural biology help us to understand and develop vaccines and drugs against Zika virus infection? J Venom Anim Toxins Incl Trop Dis. 2019;25:e20190013. doi: 10.1590/1678-9199-JVATITD-2019-0013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO (2020) Zika Virus [Online]. Available: https://www.who.int/news-room/fact-sheets/detail/zika-virus. Accessed 29 Feb 2020
- Wikan N, Smith DR. Zika virus: history of a newly emerging arbovirus. Lancet Infect Dis. 2016;16:e119–e126. doi: 10.1016/S1473-3099(16)30010-X. [DOI] [PubMed] [Google Scholar]
- Zanluca C, Melo VC, Mosimann AL, Santos GI, Santos CN, Luz K. First report of autochthonous transmission of Zika virus in Brazil. Mem Inst Oswaldo Cruz. 2015;110:569–572. doi: 10.1590/0074-02760150192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Sheng J, PLEVKA P, Kuhn RJ, Diamond MS, Rossmann MG. Dengue structure differs at the temperatures of its human and mosquito hosts. Proc Natl Acad Sci U S A. 2013;110:6795–6799. doi: 10.1073/pnas.1304300110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zorrilla CD, Mosquera AM, Rabionet S, Rivera-Vinas J (2016) HIV and Zika in pregnancy: parallel stories and new challenges. Obstet Gynecol Int J 5 [DOI] [PMC free article] [PubMed]