Abstract
Understanding the metabolism of prostate cancer (PCa) is important for developing better diagnostic approaches and also for exploring new therapeutic targets. Magnetic resonance spectroscopy (MRS) techniques have been shown to be useful in the detection and quantification of metabolites. PCa illustrates metabolic phenotype, showing lower levels of citrate (Cit), a key metabolite of oxidative phosphorylation and alteration in several metabolic pathways to sustain tumor growth. Recently, dynamic nuclear polarization (DNP) studies have documented high rates of glycolysis (Warburg phenomenon) in PCa. High-throughput metabolic profiling strategies using MRS on variety of samples including intact tissues, biofluids like prostatic fluid, seminal fluid, blood plasma/sera, and urine have also played a vital role in understanding the abnormal metabolic activity of PCa patients. The enhanced analytical potential of these techniques in the detection and quantification of a large number of metabolites provides an in-depth understanding of metabolic rewiring associated with the tumorigenesis. Metabolomics analysis offers dual advantages of identification of diagnostic and predictive biomarkers as well as in understanding the altered metabolic pathways which can be targeted for inhibiting the cancer progression. This review briefly describes the potential applications of in vivo 1H MRS, high-resolution magic angle spinning spectroscopy (HRMAS) and in vitro MRS methods in understanding the metabolic changes of PCa and its usefulness in the management of PCa patients.
Keywords: Magnetic resonance spectroscopy (MRS), In vivo, In vitro MRS, HRMAS, Prostate cancer, Biomarker
Introduction
It is now well recognized that knowledge of cancer metabolism is essential to understand the process of carcinogenesis and development of therapeutic strategies. Cancer cells modify their metabolism to support continuous supply of various substrates for biosynthesis of membranes, genetic materials, and proteins required for their rapid proliferation. Prostate cancer (PCa) is a frequently diagnosed malignancy in elderly men worldwide (Greenlee et al. 2001). The prognosis of PCa varies among individuals; the disease course is aggressive which shows rapid progression to metastases, while it remains indolent in some patients for several years. Prostate-specific antigen (PSA) is used as a screening biomarker for PCa; however, its higher level is also reported in various other conditions like urinary retention, inflammation, and benign prostatic hyperplasia (BPH) (Catalona et al. 1991, 1994; Carter 2000). Trans-rectal ultrasound (TRUS)-guided biopsy serves as the “gold standard” for PCa diagnosis; however, it suffers from low specificity due to inaccurate sampling (Naughton et al. 1998; Rabbani et al. 1998). As the currently used clinical biomarkers lack enough specificity and sensitivity, there is an urgent need for biomarkers with more precision for better clinical management of PCa patients. Studies have shown connections between altered metabolic pathways and oncogenes that have a vital role in the development and progression of PCa (Wu et al. 2014; Zadra et al. 2013). Deeper understanding of the metabolic reprogramming through metabolomics has the potential to reveal novel metabolic signatures of PCa that could be utilized for the diagnosis, assessment of disease, aggressiveness, therapeutic targets, and resistance to therapy (Giunchi et al. 2019; Eidelman et al. 2017).
Nuclear magnetic resonance (NMR) spectroscopy is one of the most commonly used techniques to obtain an insight into metabolic profiles influenced by various pathological processes and understanding the tissue metabolism. In vivo MR spectroscopy (MRS) offers the capability of analyzing tissue biochemical levels from a specific region of interest (ROI) in a non-invasive manner. The ex vivo high-resolution magic angle spinning (HRMAS) MR spectroscopy is yet another method for metabolic characterization of intact tissue biopsy samples (Decelle and Cheng 2014; Fuss and Cheng 2016). The in vitro NMR spectroscopy can be applied to a wide variety of samples like tissue extracts, biofluids like blood plasma/sera, urine, prostatic fluids, seminal plasma etc. to understand the altered metabolic pathways in PCa (Fowler et al. 1992; Giskeødegård et al. 2015; Hahn et al. 1997; Jung et al. 2013; Kumar et al. 2014, 2016a, b). High-throughput metabolomics approaches based on these NMR techniques provides quantitative information on a large number of metabolites derived from both catabolic and anabolic processes in the cell (Lima et al. 2016; Gómez-Cebrián et al. 2019). Metabolomic analysis integrates the changes manifesting at the level of gene expression, protein expression, and metabolic abnormalities (Rysman et al. 2010; Lima et al. 2016; Gómez-Cebrián et al. 2019). The advantages of metabolic profiling have been recognized both in the determination of biomarkers of disease aggressiveness, diagnosis, prognosis, and identification of therapeutic targets based on altered metabolic pathways (Giunchi et al. 2019; Eidelman et al. 2017; Lima et al. 2016; Gómez-Cebrián et al. 2019).
This review briefly describes the potential of in vivo proton (1H) MRS, in vitro high-resolution 1H MRS, and HRMAS in the study of prostate cancer metabolism and its potential role in determining biomarkers which can be used in various aspects of PCa management like diagnosis and therapy.
MRS techniques to study PCa metabolism and altered metabolites
In vivo MRS techniques
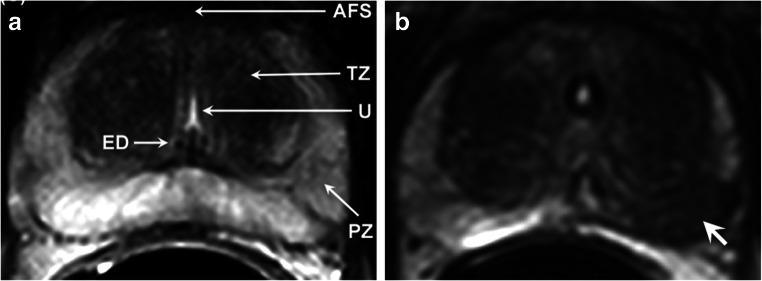
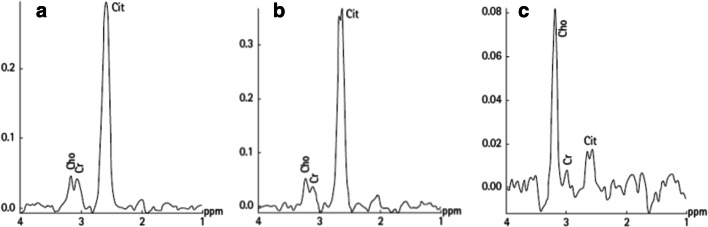
The advantage of in vivo MRS is that it detects metabolites in a non-invasive manner from a well-defined ROI. Being noninvasive, it has significant applications in longitudinal studies for treatment assessments and for evaluating the efficacy of various therapeutic regimens (Jagannathan 2014; Kumar et al. 2018; Tayari et al. 2017). The prostate tissue metabolism has been studied using the in vivo 1H multi-voxel spectroscopy technique, called MR spectroscopy imaging (MRSI) by various researchers (Jagannathan 2014; Kumar et al. 2008, 2018; Tayari et al. 2017; Kurhanewicz et al. 1993, 1996, 2002). For localized in vivo MRS studies, the cancer lesion is first localized on the MR image of the prostate by carrying out MR imaging in three orthogonal planes. These images are then used to identify the cancer location, localize it and perform MRS to obtain the metabolic information in vivo. Figures 1a and b show the T2-weighted MR images of prostate from a volunteer and a patient with PCa, respectively. The tumor is observed as a hypointense area (shown with arrow) in the peripheral zone (PZ) of the prostate, as shown in Fig. 1b. Proton MRSI spectrum obtained from a normal PZ area, from a patient with benign prostate hyperplasia (BPH) and from a prostate cancer, is shown in Fig. 2 a, b, and c, respectively (Nayyar et al. 2009). The in vivo 1H MR spectrum of the normal prostate shows resonances that are predominantly from citrate (Cit), creatine (Cr), choline-containing compounds (tCho), and polyamines. The two methylene groups are present in Cit which are magnetically equivalent and are strongly coupled. The signal due to Cit is observed at 2.6 ppm in the in vivo 1H MR spectrum of the normal prostate (Fig. 2a). Cho-containing compounds are observed as a singlet corresponding to tri-methyl groups of these compounds around 3.2 ppm (Fig. 2c). This composite peak arises with contributions from three Cho-containing compounds, namely, free Cho, glycerophosphocholine, and phosphocholine (Swanson et al. 2006; Swanson et al. 2008). These compounds are formed during the synthesis and catabolism of phospholipids. It is known that phospholipids are essential constituents of cellular membranes. Higher levels of these compounds are associated with the rapid proliferation of malignant cells in PCa. The peak due to methyl protons of creatine and phosphocreatine, assigned as tCr, possibly arises due to smooth muscle tissue present in the prostate (Kassen et al. 1996). Phosphocreatine and creatine compounds play a key role in transfer and storage of energy in cells (Wallimann et al. 1992). Spermine is a predominant polyamine observed in the 1H MR spectrum of prostate. The coupled spin system of ten methylene groups of spermine shows resonances at 1.81 ppm, 2.11 ppm, 3.13 ppm, 3.12 ppm, and 3.18 ppm, due to five sets of four magnetically equivalent protons (Willker et al. 1998); however, in in vivo MRSI, only a single resonance peak at 3.1 ppm could be observed and assigned to polyamines (PA) (mainly spermine) in several studies (Shukla-Dave et al. 2007; Klomp et al. 2011).
Fig. 1.
T2-weighted axial MR images of prostate a of a volunteer and b of a patient showing the prostate cancer (white arrows) (reproduced with permission from Elsevier from reference Kumar et al. 2018)
Fig. 2.
Representative proton in vivo MRSI spectrum obtained from a voxel positioned in the normal peripheral zone of a volunteer (a), from a BPH patient (b), and from a prostate cancer patient (c). Abbreviations used: Cho, Cho; Cr, creatine; Cit, Cit (reproduced with permission from John Wiley & Sons from Nayyar et al. 2009)
In general, the changes in various metabolites are expressed in terms of ratios of metabolites like [Cit/Cho], [(Cho + Cr)/Cit], [Cit/(Cho + Cr)], or [(Cho + Cr + PA)/Cit]. Significantly lower level of Cit while higher level of Cho have been observed in the spectrum acquired from malignant lesion in the peripheral zone (PZ) of the prostate (see Fig. 2c). Studies reported that higher [(Cho + Cr)/Cit] ratio in patients with PCa provide a high specificity for diagnosis of PCa. It was documented that in 98% of the PCa patients, the values of this parameter were found to be above by 3 standard deviations of the average value estimated in healthy PZ indicating its diagnostic potential (Kurhanewicz et al. 1996).
Dynamic nuclear polarization MRS
Recently, hyperpolarization using the dynamic nuclear polarization (DNP) technique has been used to acquire 13C spectra in vivo from PCa (Ardenkjaer-Larsen et al. 2003; Serrao and Brindle 2016). This method has been shown to enhance the nuclear polarization by more than 10,000-fold and therefore significantly increases the sensitivity of in vivo MRS (Gutte et al. 2015). A suitable 13C-labeled hyperpolarized substrate is injected intravenously into the body and its metabolic fate is then monitored using 13C MRS. The technique requires a special setup that includes the polarizer which should be placed close to the MRS scanner for polarization and subsequent use of the substrate. These polarized substrates have a short half-life; therefore, after injection of the hyperpolarized substrate, MRS must be carried out fast so that there is no significant loss of polarization. This is one of the major limiting factors of DNP MRS. 13C pyruvate is the most widely used substrate as it is a key metabolite of metabolic pathway glycolysis. The metabolic flux of 13C pyruvate to 13C alanine, 13C lactate, and 13C bicarbonate is monitored using in vivo 13C MRS. Nelson et al. (2013) reported the first application of hyperpolarized 13C MRS in human PCa and demonstrated the safety and feasibility of the procedure. Patients with PCa showed increased 13C lactate/13C pyruvate ratio in biopsy-proven PCa patients in comparison to the non-cancerous region within prostate. Julià-Sapé et al. (2019) reviewed the contribution of MRS(I) for in vivo evaluation of cancer metabolism following hyperpolarized substrates in various cancers (preclinical and in humans) including prostate, brain, and pancreas. Keshari et al. (2015) reported the feasibility of a multimodal approach combining FDGPET and hyperpolarized pyruvate 13C MRS to study changes in PCa metabolism and its response to nicotinamide phosphoribosyltransferase inhibition. Recently, Scroggins et al. (2018) evaluated the potential of 13C-hyperpolarized pyruvate MRSI to target the Warburg effect in PCa. Two human prostate cancer cell lines (DU145 and PC3) were grown as xenografts. Enhanced conversion of 13C pyruvate to 13C lactate was seen in DU145 xenograft tumors compared with PC3 xenograft tumors.
HRMAS NMR spectroscopy
High-resolution magic angle spinning (HRMAS) 1H MRS has shown its potential for providing valuable metabolic information from ex vivo intact tissue samples (Decelle and Cheng 2014; Fuss and Cheng 2016). Unlike in vivo MRS, a great number of metabolites can be identified and quantified by using HRMAS of tissue samples. Quantification of the levels of these metabolites have been found to be useful in understanding the altered metabolism in PCa and identification of cancer metabolic markers and can therefore be used as a clinical tool for PCa diagnosis and prognosis. The advantage of HRMAS is that it preserves tissue architecture and the same tissue specimens may be used for subsequent histopathology and molecular analysis. Several HRMAS studies have focused on improving diagnosis and monitoring treatment effects, understanding prognostication, and correlating the biomarkers with that obtained with the use of in vivo MRS. A strong positive correlation was reported between the Gleason score and [(tCho + creatine + spermine)/Cit] measured by both in vivo and ex vivo MRS (Selnaes et al. 2013). The role of metabolic profiling by HRMAS was documented in the monitoring of tumor aggressiveness and a significant correlation (r = 0.71) between metabolic profiles and the Gleason score was observed (Giskeødegård et al. 2013). An increase in the [(tCho + creatine + polyamines)/Cit] ratio was found to be clinically important indicator of malignancy with a sensitivity of 86.9% and a specificity of 85.2% to differentiate cancer from the normal tissues. The concentrations of two metabolites, spermine and Cit, were lower in high-grade cancer which differentiates them from low-grade cancer (Giskeødegård et al. 2013). Braadland et al. (2017) reported that ex vivo metabolic fingerprinting is useful in the identification of biomarkers that can predict cancer recurrence. This retrospective analysis suggested that longer recurrence-free survival was found in patients having higher spermine and Cit concentrations. Madhu et al. (2016) investigated the metabolic profile of cancerous tissue treated with degarelix (a novel gonadotrophin-releasing hormone blocker) and compared it with untreated patients and benign tissue by using HRMAS 1H NMR spectroscopy. They measured the absolute concentrations of several metabolites, including alanine, lactate, glutamine, glutamate, Cit, Cho compounds, creatine, taurine, myo-inositol, and polyamines. Untreated PCa patients showed elevated levels of lactate, alanine, and tCho compared with benign samples. The treatment with degarelix resulted in the lowering of lactate and tCho concentrations in biopsies from treated PCa patients. Vandergrift et al. (2018) compared the HRMAS measured metabolic profiles of benign and cancerous tissues. They documented the ability of metabolic profiling in identifying the tumor grade, stage, and in predicting recurrence of cancer. The metabolic profile of highly aggressive tumors showed elevated myo-inositol. It was suggested that myo-inositol is an endogenous tumor suppressor and can be used as a potential therapeutic target. The patients with less aggressive tumors can be identified using this biomarker and overtreatment can be avoided. The metabolic information was also suggested to be useful in stratifying the patients with more risk of recurrence (Vandergrift et al. 2018).
In vitro MR spectroscopy
In vitro high-resolution NMR spectroscopy based metabolomics studies have been the most powerful and informative tool to study cancer metabolism. Its advantages include performing analysis of different types of samples like tissue extracts, cell extracts, and body fluids like seminal fluid, prostatic fluid, blood, and urine. Also, the use of a high magnetic field spectrometer (above 400 MHz) in most studies offers the advantage of both high-resolution and high sensitivity. This enables identification of large number of metabolites and accurate estimation of their levels compared with other MR techniques. Further, it provides a more detailed analysis of altered metabolic pathways during oncogenesis.
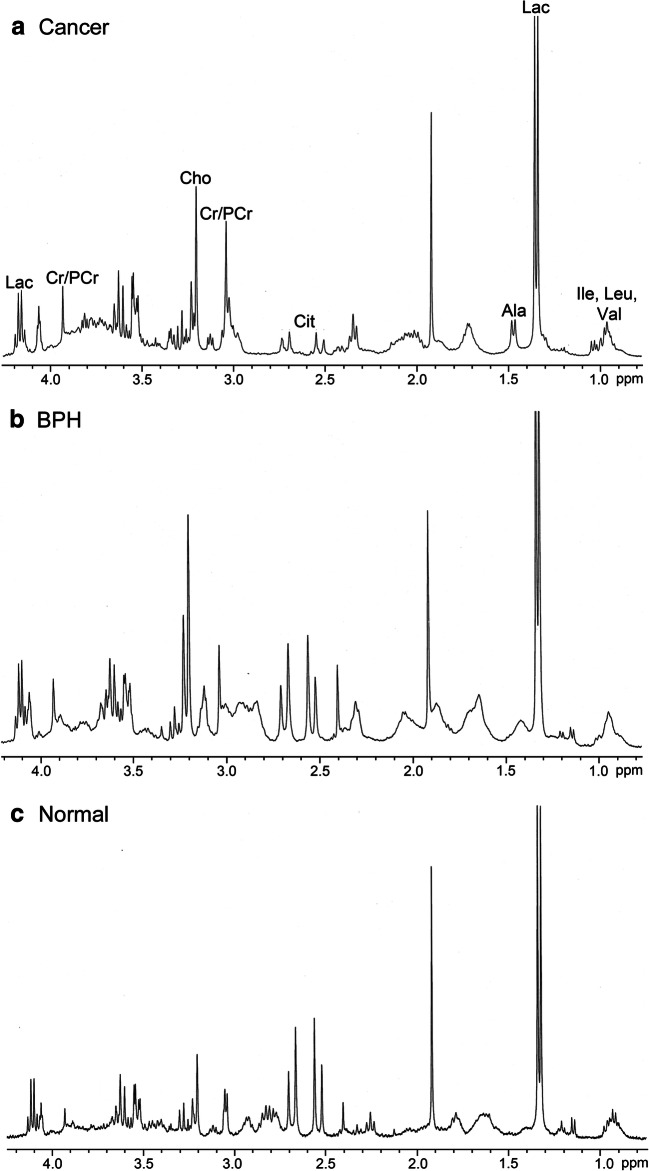
The proton NMR spectrum obtained from a malignant prostate tissue, BPH, and a normal tissue is shown in Fig. 3 (Kumar et al. 2014). A large number of metabolites can be identified and quantified using such a spectrum. Studies have explored the use of in vitro NMR studies in various aspects of clinical management of PCa, including detection, diagnosis and determination of aggressiveness. Fowler et al. (1992) reported 1H NMR spectroscopy of perchloric acid extracts of tissues obtained from patients with PCa and compared against those with BPH. Their results revealed significant differences in the metabolite ratios [(Cit + Cr + phosphocholine)/alanine] and [Cit/glutamate] between cancerous and BPH tissues (Fowler et al. 1992). Adenocarcinoma was characterized with lower Cit in comparison with BPH (Schiebler et al. 1993). The potential of spermine as a possible marker for diagnosis was assessed using in vitro NMR of prostate tissues (van der Graaf et al. 2000). The increase in sensitivity and specificity was obtained when lysine and lipids were combined with the analysis of Cit (Swindle et al. 2003) to distinguish PCa. A sensitivity of 100% and a specificity of 94% were obtained using Cho/Cr and lipid/lysine ratios in the detection of PCA (Swindle et al. 2003). By a pattern recognition approach on NMR spectroscopy data obtained from biopsy specimens from BPH, prostatic intraepithelial neoplasia (PIN) and PCa patients showed distinct MR spectral patterns with a sensitivity of 100% and a specificity of 97% (Swindle et al. 2008). Hahn et al. (1997) reported NMR based metabolomics approach to distinguish between malignant and benign prostate tissues. The multivariate analysis models of the NMR spectroscopy data classified the two groups (BPH and PCa) with 100% sensitivity, 95.5% specificity, and accuracy of 96.6% (Hahn et al. 1997). They also reported that resonances by Cit, glutamate, and taurine showed significant potential for diagnosis of PCa.
Fig. 3.
Representative example of the aliphatic region of the in vitro 1H NMR spectra acquired at 400 MHz from a cancer tissue, b benign prostatic hyperplasia (BPH) tiisue, and c normal prostate tissue. Ala, alanine; Cit, citrate; Cho, choline; Cr, creatine; Ile, isoleucine; Lac, lactate; Leu, leucine; PCr, phosphocreatine; Val, valine (reproduced with permission from John Wiley & Sons from Kumar et al. 2014)
Recently, studies have focused on the analysis of biofluids by 1H NMR spectroscopy. The metabolite levels in the biofluids are influenced by changes in the pathophysiology of an individual. Thus, metabolic profiling of biofluids has become an important strategy for identification of biomarkers and also in elucidating the molecular mechanisms of diseases. The metabolomics studies, carried out on PCa patients, focused on metabolic profiling of a variety of fluids, e.g., prostatic fluid, seminal fluid, sera/plasma, and urine. Averna et al. (2005) demonstrated the first use of high-resolution NMR spectroscopy of semen to diagnose PCa. Their study showed that measurement of Cit concentration by 1HNMR of seminal fluid potentially could be a new, rapid, and non-invasive method for screening of PCa. Kline et al. (2006) compared the concentration of Cit in the prostatic fluid and the semen of samples from both patients with PCa and normal controls. The average concentration of Cit was 2.7-fold higher in semen from controls (132.2 ± 30.1 mM) compared with those of PCa patients (48.0 ± 7.9 mM). They have also found similar results in expressed prostatic secretions; the concentration of Cit was found to be 221.4 ± 55.4 mM in healthy controls, which was significantly higher (p < 0.05) than that measured in prostatic fluid from PCa patients. Their analysis showed that the diagnostic performance of the measurement of Cit in both semen and prostatic fluid was better compared with conventional PSA test in detecting PCa. Serkova et al. (2008) also reported 1H NMR spectroscopy of prostatic secretions in patients with PCa (n = 52) and healthy controls (n = 26). Several metabolites like Cit, myo-inositol, alanine, lactate, phosphocholine, acetate, glutamine, spermine, and hydroxybutyrate were quantified. Their study also identified Cit, spermine, and myo-inositol as potential biomarkers for differentiating PCa patients using prostatic secretions. The decrease in the level of Cit in seminal fluid was found to be associated with the development of cancer of the prostate. Roberts et al. (2017) also reported that metabolomics of seminal plasma using NMR spectroscopy has potential for improving the diagnostic accuracy of PCa. However, there is still a need to validate the approach in a clinical cohort of high-risk subjects. This approach has differentiated patients with low and intermediate risk for developing PCa from those with benign disease indicating its potential utility in risk assessment. The samples from patients with high grade of PCa showed a high content of lipids/lipoproteins and lower levels of other metabolites.
Kumar et al. (2015) reviewed the applications of NMR-based metabolomics of prostate cancer. Bingol and Brüschweiler (2015) described the advances made in NMR-based metabolomics and how to combine the information obtained by NMR for elucidation of known and unknown compounds with high-resolution mass spectrometry. Pérez-Rambla et al. (2017) reported the metabolic profiling of urine from patients with PCa (n = 64) and compared those with BPH (n = 51). Univariate and multivariate approaches were used for comparison of metabolic profiles between the two groups. They found higher concentrations of several metabolites like glutamate, branched-chain amino acids (BCAA), and uridine in PCa patients. The concentrations of metabolites like dimethylglycine, glycine, 4-imidazole-acetate, and fumarate were lower in PCa patients compared with those with BPH. Kumar et al. (2016a) studied the metabolic profiling of filtered sera from patients with PCa and compared it with the sera obtained from patients with BPH and healthy controls using 1H NMR. The discriminant function analysis showed that metabolites like sarcosine, glycine, alanine, xanthine, creatine, and hypoxanthine have the potential to differentiate patients with abnormalities in prostate (PCa and BPH) from healthy controls. The classification accuracy by NMR was 86.2% in comparison with 68.1% by clinical laboratory method in differentiating BPH + PCa group from healthy controls. Several metabolites (sarcosine, alanine, glycine, creatinine, glycine, and Cit) were identified as potential biomarkers that differentiated patients with PCa from BPH. This analysis showed that NMR has a diagnostic accuracy for PCa of 88.3% in comparison with the standard clinical laboratory method (75.2%). Giskeødegård et al. (2015) analyzed the metabolic profile of blood plasma and serum obtained from patients with PCa (n = 29) and compared them with those with BPH (n = 21). This study applied three analytical techniques, mass spectrometry, gas chromatography, and NMR spectroscopy. This comprehensive metabolic analysis illustrated changes in lipid and amino acid metabolism in patients with PCa in comparison with BPH. They reported altered levels of fatty acids, phospholipids, and arginine as discriminating biomarkers for PCa. A sensitivity of 81.5% and specificity of 75.2% were obtained using this integrated approach based on results from three analytical platforms.
Altered metabolism in prostate Cancer
The study of prostate cancer metabolism is of great clinical interest to understand the mechanism of cancer progression and for identification of newer therapeutic targets (Giunchi et al. 2019). Metabolic rewiring alters the pathways in such a way so as to sustain supply of the necessary biochemicals and building blocks for rapid proliferation of cells for the progression of PCa. The MR spectroscopy studies described in the above sections have shown that a large number of metabolites can be detected and quantified using these methods. The alterations in these metabolic levels provide evidence for several changed metabolic pathways in PCa. Additionally, the knowledge of the metabolic differences between normal cells and cancer cells aid in the development of new methods of diagnosis and also for understanding the underlying biochemistry of tumor aggressiveness which can be used to obtain biomarkers of tumor aggressiveness. This information would be useful in the appropriate clinical management of PCa patients. The results on altered metabolites using various MR methods as discussed above have shown that numerous metabolic pathways including glycolysis, oxidative phosphorylation, amino acid, and lipid metabolism have been seen to be altered during carcinogenesis.
Consistently the most common and significant observation using various MR techniques has been the detection of lower levels of Cit metabolite in PCa patients in comparison with normal subjects or patients with BPH in various types of samples including prostate tissue, prostatic fluid, seminal fluid, and blood plasma samples. In normal healthy cells of the human body, Citrate oxidation is a key step in the Kreb’s cycle for aerobic respiration in which Cit is converted to isocitrate with the help of the enzyme aconitase (Dakubo et al. 2006). However, healthy prostate epithelial cells exhibit a distinct unique metabolic phenotype in comparison to other types of normal human cells. These cells accumulate large concentration of Cit, which is secreted as a part of the prostatic fluid, which is a component of semen from normal prostate gland (Costello and Franklin 2016). It is found that the enzyme m-aconitase which transforms the Cit to isocitrate is inhibited by the accumulation of zinc (Costello et al. 2005) in the normal prostate cells. Thus the Kreb’s cycle tend to be arrested beyond the generation of Cit in healthy prostate cells. The increased transport of zinc due to higher level of zinc transporter ZIP 1 in the normal prostatic epithelial cells leads to its accumulation (Costello et al. 2011).
In contrast, the malignant prostate cells exhibit a major change in metabolism. Malignant cells have zinc wasting phenotype, and therefore, there is no inhibition of the enzyme m-aconitase. Malignant prostate cells oxidize Cit and use the oxidative phosphorylation pathway to generate energy, and unlike other cancer cells, they have higher levels of aerobic glycolysis (Costello and Franklin 2016). The accumulation of zinc also leads to apoptotic death of mitochondria, and therefore, zinc wasting phenotype of malignant prostate cells is also beneficial in avoiding cell death in cancer (Feng et al. 2002).
Furthermore, DNP MRS studies have indicated that malignant cells of PCa exhibited the so called “Warburg effect” which has been reported in various other cancers (Gutte et al. 2015; Julià-Sapé et al. 2019; Scroggins et al. 2018). Higher levels of 13C lactate/13C pyruvate ratio have been reported in biopsy-proven PCa patients in comparison to non-cancerous region within the prostate (Nelson et al. 2013). Thus, both glycolysis and Kreb’s cycle pathways have been found to be altered in PCa for reasons related to enhanced energy requirements and supply of various substrates for rapid proliferation of cancer cells. Additionally, it has been reported that the interactions of the tumor stroma, comprising the myofibroblastic microenvironment and cancer-associated fibroblasts, also show the Warburg effect which is induced by prostate epithelial cancer cells. These fibroblasts also secrete pyruvate and lactate which is utilized by PCa cells for energy generation, and biosynthesis of various compounds needed for cell proliferation (Lucarelli et al. 2015; Pavlides et al. 2009; Fiaschi et al. 2012). NMR studies have also revealed enhanced levels of lactate and the amino acid alanine which also support the enhancement of the glycolytic pathway in PCa (Madhu et al. 2016; Kumar et al. 2016a).
The levels of several amino acids have also found to be altered in PCa. Significantly higher levels of alanine, glycine, lysine, glutamate, and glutamine have been reported in biopsied tissue of PCa patients as compared with BPH by HRMAS (Madhu et al. 2016). Sera samples analyzed by NMR also exhibited altered levels of several amino acids like alanine and glycine (Kumar et al. 2016a). Altered metabolism of glutamine and arginine has also been reported (Pan et al. 2015; Moncada et al. 2012). It was documented that higher levels of amino acids, glutamate, and glutamine in cancerous tissue might have been due to increase in glutaminolysis (DeBerardinis et al. 2007). Glutamine plays an important role in PCa metabolism by its involvement in several pathways in cells. It is transformed into glutamate by the enzyme glutaminase. Glutamate serves as an energy source by transforming into α-ketoglutarate, which is an intermediate of Krebs cycle thereby providing energy through oxidative phosphorylation. Thus, glutaminolysis is a mechanism that PCa utilize to produce ATP. Up-regulation of the glutaminase enzyme has been demonstrated in PCa (Pan et al. 2015; Moncada et al. 2012).
Further to this, there is evidence that glutamine plays an important role in de novo lipid synthesis in various cancers including PCa (Giunchi et al. 2019; Eidelman et al. 2017). Elevated uptake of the amino acid glutamine has also been reported in various cancers for de novo lipid synthesis for the formation of cell walls during the process of rapid proliferation. It has been suggested that through the process of reverse carboxylation, α-ketoglutarate can also be transformed into Cit under hypoxic condition which leads to lipid synthesis and tumor growth (Li and Zhang 2016). Thus, the glutaminolysis pathway may also be utilized to synthesis lipids from glutamine. Glutamine is converted into glutamate, which then enters into Kreb’s cycle by transforming into α-ketoglutarate. α-ketoglutarate is then utilized for lipogenesis through the process of reverse carboxylation.
The metabolism of the amino acid arginine was also found to be altered in the PCa. Arginine plays numerous roles in the growth of normal cells. Arginine can be transformed into glutamine and proline amino acids. It also has a unique role in the synthesis of nitric oxide, which has been shown to be an effector of cancer cells; however the exact role is not well understood (Qiu et al. 2015). It has been reported that a higher level of arginine is required for tumor growth (Feun et al. 2008). Metabolism based studies using cell lines also revealed altered levels of several amino acids, like glutamate, glutamine, leucine, isoleucine, valine, glycine, and alanine in cancer cells (Teahan et al. 2011). Furthermore, MR studies have reported that the synthesis of polyamines is a function of normal prostate cells (Shukla-Dave et al. 2007; Klomp et al. 2011). Lower levels of the polyamine, spermine has been found in prostate tissue of PCa patients. Additionally, the studies on seminal and prostatic fluid demonstrated lower levels of spermine in PCa patients (Lynch and Nicholson 1997; Serkova et al. 2008). It has been reported that polyamines are synthesized from the amino acid arginine (Blachier et al. 1995). Thus, lower level of this metabolite is indicative of altered arginine metabolism.
MR studies on blood plasma and serum have shown higher levels of the metabolite sarcosine in PCa patients and its level can be used to differentiate low-grade from high-grade PCa (Kumar et al. 2015). These results indicated altered biosynthesis of sarcosine in PCa; however, the role of this metabolite is unclear in carcinogenesis. Also, it is reported that the enzyme glycine N-methyltransferase plays a significant role in PCa metabolism. The enzyme N-methyltransferase catalyzes the transformation of glycine to sarcosine and participates both in the process of gluconeogenesis and metabolism of the amino acid methionine (Cernei et al. 2013). Data from NMR spectroscopy of blood plasma samples from PCa patients revealed reduced levels of glycine in PCa patients, which could have been due to the enhanced utilization of glycine for biosynthesis of sarcosine or in gluconeogenesis.
Metabolomics studies using NMR analysis of serum/plasma of patients with PCa demonstrated changes in membrane metabolism and fatty acids (Giskeødegård et al. 2015). Such alterations in fatty acid metabolism (lipid β-oxidation) provide the requisite energy for rapidly proliferating cancer cells and these lipids also serve as a key fuel source of PCa cells, particularly during early cancer progression (Giskeødegård et al. 2015). The malignant cells of the prostate also often use lipids synthesized from androgens through the expression of an androgen receptor (Heinlein and Chang 2004). It is further reported that PCa cells also synthesize fatty acids through de novo lipid synthesis which can then be used to generate energy. As discussed earlier, the enhanced glutaminolysis and reductive carboxylation of α-ketoglutarate are used to synthesize lipids. It has been documented that the lipid synthesizing phenotype is an important aspect in the progression of PCa (Giunchi et al. 2019; Eidelman et al. 2017). There is evidence that certain PCa cells overexpress markers like fatty acid synthase (FASN), steroyl CoA desaturase, and sterol regulatory element binding protein 1 (SREBP1), which indicate their capability of de novo synthesis of lipids (Deep and Schlaepfer 2016). Altered lipid metabolism has been shown essential for continued energy production and membrane synthesis. It is also important for post-translational modification of signaling molecules which play a vital role in malignant transformation and progression of tumors. Therefore, the understanding of the link between increased lipogenesis and malignant transformation in PCa has been an important area of research (Wu et al. 2014; Rysman et al. 2010).
Several metabolomics studies have revealed increased level of Cho in PCa tissue, indicating alterations in phospholipid membrane synthesis and hydrolysis (van Asten et al. 2008; García-Segura et al. 1999; Swindle et al. 2003). In vivo MRS studies of PCa tissue reported higher levels of membrane components like tCho (Jagannathan 2014; Kumar et al. 2008, 2018; Tayari et al. 2017; Kurhanewicz et al. 1993, 1996, 2002). Furthermore, increased levels of tCho and several other compounds containing Cho have been the most consistently correlated observation in cancer, by all MRS methods, including in vivo, ex vivo, and in vitro studies of tissues and cell extracts (Selnaes et al. 2013; Madhu et al. 2016). It has been reported that increased levels of PC, tCho, and altered levels of lipids are highly correlated with cancerous cells and are particularly associated with neoplastic transformation (Mori et al. 2016). These altered metabolic pathways also have connection with cancer invasion and metastasis (Mori et al. 2016).
NMR studies of the blood sera of PCa patients revealed increased levels of the metabolites creatinine and creatine compared with BPH patients (Kumar et al. 2016a, b). The augmented level of these metabolites is also indicative of increased energy requirements of PCa cells for rapid proliferation (Roberts et al. 2011). Studies have also indicated that glucose is also utilized through the pentose phosphate pathway to support various anabolic pathways essential for PCa growth (Tsouko et al. 2014). It has also been reported that advanced stage PCa is associated with metabolic syndrome. This clinical syndrome is characterized by intolerance to glucose, obesity, altered lipid levels in blood, and hypertension (Grundmark et al. 2010). Further to this point, diabetes and metabolic syndrome are associated with aggressiveness of PCa and poor prognosis (Lee et al. 2016). Thus, because of elevated proliferation rate and enhanced energy requirements, PCa cells derive energy through various pathways like glycolysis, oxidative phosphorylation as well as through lipids (Jung et al. 2013; Stenman et al. 2009).
Summary and future directions
In the past two decades, several research groups have evaluated the role of various MRS techniques in the study of metabolism of PCa and the utility of metabolic biomarkers in the clinical management of PCa, its detection, assessment, and in determining aggressiveness of PCa. In vivo MRS revealed unique metabolic features of malignant PCa cells characterized with lower Cit, polyamines, and high levels of tCho containing compounds indicating high proliferative activity. HRMAS 1H MRS has demonstrated that it is potential in providing valuable metabolic information from ex vivo intact tissue samples. Unlike in vivo MRS, a greater number of metabolites can be identified and quantified using HRMAS of tissue samples. High-throughput profiling approaches play a significant role in the delineation of abnormalities in metabolic pathways accompanying tumorigeneses and metastases. Metabolomics approaches involving simultaneous identification and quantification of all the metabolites involved in biosynthetic and catabolic processes in the cell have improved our understanding of altered metabolic pathways in PCa. The principal metabolic pathways altered in PCa cells include the citric acid cycle, oxidative phosphorylation, glycolysis, amino acid metabolism, lipid catabolism and biosynthesis. This analysis provided diagnostic and predictive biomarkers which may be used for clinical management of PCa. The NMR spectroscopy techniques have immense potential in the exploration of metabolic reprogramming of oncogenesis. However, most studies on PCa have focused only on determining biomarkers for diagnosis or assessment of aggressiveness. An integrated approach analyzing various types of samples like blood plasma, urine, seminal fluid, and prostatic fluid may help in determining newer biomarkers for enhanced diagnostic and prognostic potential and dysregulated metabolic pathways in PCa. These methodologies may help in making decisions in various situations of clinical dilemmas in PCa management.
Acknowledgments
The authors would like to thank their students and collaborators for many fruitful discussions, help and support. NRJ thanks the SERB, Department of Science and Technology, Government of India for the award of J. C. Bose Fellowship.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Uma Sharma, Email: umasharma69@gmail.com.
Naranamangalam R. Jagannathan, Email: jagan1954@hotmail.com
References
- Ardenkjaer-Larsen JH, Fridlund B, Gram A, Hansson G, Hansson L, Lerche MH, Servin R, Thaning M, Golman K. Increase in signal-to-noise ratio of > 10,000 times in liquid-state NMR. Proc Natl Acad Sci U S A. 2003;100:10158–10163. doi: 10.1073/pnas.1733835100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Averna TA, Kline EE, Smith AY, Sillerud LO. A decrease in 1H nuclear magnetic resonance spectroscopically determined Cit in human seminal fluid accompanies the development of prostate adenocarcinoma. J Urol. 2005;173:433–438. doi: 10.1097/01.ju.0000148949.72314.d7. [DOI] [PubMed] [Google Scholar]
- Bingol K, Brüschweiler R. Two elephants in the room: new hybrid nuclear magnetic resonance and mass spectrometry approaches for metabolomics. Curr Opin Clin Nutr Metab Care. 2015;18:471–477. doi: 10.1097/MCO.0000000000000206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blachier F, Selamnia M, Robert V, M'Rabet-Touil H, Duée PH. Metabolism of L-arginine through polyamine and nitric oxide synthase pathways in proliferative or differentiated human colon carcinoma cells. Biochim Biophys Acta. 1995;1268:255–262. doi: 10.1016/0167-4889(95)00083-5. [DOI] [PubMed] [Google Scholar]
- Braadland PR, Giskeødegård G, Sandsmark E, Bertilsson H, Euceda LR, Hansen AF, Guldvik IJ, Selnæs KM, Grytli HH, Katz B, Svindland A, Bathen TF, Eri LM, Nygård S, Berge V, Taskén KA, Tessem MB. Ex vivo metabolic fingerprinting identifies biomarkers predictive of prostate cancer recurrence following radical prostatectomy. Br J Cancer. 2017;117:1656–1664. doi: 10.1038/bjc.2017.346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter HB. A PSA threshold of 4.0 ng/mL for early detection of prostate cancer: the only rational approach for men 50 years old and older. Urology. 2000;55:796–799. doi: 10.1016/s0090-4295(00)00517-3. [DOI] [PubMed] [Google Scholar]
- Catalona WJ, Smith DS, Ratliff TL, Dodds KM, Coplen DE, Yuan JJ, Petros JA, Andriole GL. Measurement of prostate-specific antigen in serum as a screening test for prostate cancer. N Engl J Med. 1991;324:1156–1161. doi: 10.1056/NEJM199104253241702. [DOI] [PubMed] [Google Scholar]
- Catalona WJ, Richie JP, Ahmann FR, Hudson MA, Scardino PT, Flanigan RC, DeKernion JB, Ratliff TL, Kavoussi LR, Dalkin BL, Waters WB, MacFarlane MT, Southwick PC. Comparison of digital rectal examination and serum prostate specific antigen in the early detection of prostate cancer: results of a multicenter clinical trial of 6,630 men. J Urol. 1994;151:1283–1290. doi: 10.1016/s0022-5347(17)35233-3. [DOI] [PubMed] [Google Scholar]
- Cernei N, Heger Z, Gumulec J, Zitka O, Masarik M, Babula P, Eckschlager T, Stiborova M, Kizek R, Adam V. Sarcosine as a potential prostate cancer biomarker-a review. Int J Mol Sci. 2013;14:13893–13908. doi: 10.3390/ijms140713893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costello LC, Franklin RB. A comprehensive review of the role of zinc in normal prostate function and metabolism; and its implications in prostate cancer. Arch Biochem Biophys. 2016;611:100–112. doi: 10.1016/j.abb.2016.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costello LC, Franklin RB, Feng P. Mitochondrial function, zinc, and intermediary metabolism relationships in normal prostate and prostate cancer. Mitochondrion. 2005;5:143–153. doi: 10.1016/j.mito.2005.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costello LC, Franklin RB, Zou J, Feng P, Bok R, Swanson MG, Kurhanewicz J. Human prostate cancer ZIP1/zinc/citrate genetic/metabolic relationship in the TRAMP prostate cancer animal model. Cancer Biol Ther. 2011;12:1078–1084. doi: 10.4161/cbt.12.12.18367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dakubo GD, Parr RL, Costello LC, Franklin RB, Thayer RE. Altered metabolism and mitochondrial genome in prostate cancer. J Clin Pathol. 2006;59:10–16. doi: 10.1136/jcp.2005.027664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeBerardinis RJ, Mancuso A, Daikhin E, Nissim I, Yudkoff M, Wehrli S, Thompson CB (2007) Beyond aerobic glycolysis: Transformed cells can engage in glutamine metabolism that exceeds the requirement for protein and nucleotide synthesis. Proc Natl Acad Sci USA 104:19345–19350 [DOI] [PMC free article] [PubMed]
- Decelle EA, Cheng LL. High-resolution magic angle spinning 1H MRS in prostate cancer. NMR Biomed. 2014;27:90–99. doi: 10.1002/nbm.2944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deep G, Schlaepfer IR. Aberrant lipid metabolism promotes prostate cancer: role in cell survival under hypoxia and extracellular vesicles biogenesis. Int J Mol Sci. 2016;17(7):E1061. doi: 10.3390/ijms17071061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eidelman E, Twum-Ampofo J, Ansari J, Siddiqui MM. The metabolic phenotype of prostate cancer. Front Oncol. 2017;7:131. doi: 10.3389/fonc.2017.00131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng P, Li TL, Guan ZX, Franklin RB, Costello LC. Direct effect of zinc on mitochondrial apoptogenesis in prostate cells. Prostate. 2002;52:311–318. doi: 10.1002/pros.10128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feun L, You M, Wu CJ, Kuo MT, Wangpaichitr M, Spector S, Savaraj N. Arginine deprivation as a targeted therapy for cancer. Curr Pharm Des. 2008;14:1049–1057. doi: 10.2174/138161208784246199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiaschi T, Marini A, Giannoni E, Taddei ML, Gandellini P, De Donatis A, Lanciotti M, Serni S, Cirri P, Chiarugi P. Reciprocal metabolic reprogramming through lactate shuttle coordinately influences tumor-stroma interplay. Cancer Res. 2012;72:5130–5140. doi: 10.1158/0008-5472.CAN-12-1949. [DOI] [PubMed] [Google Scholar]
- Fowler AH, Pappas AA, Holder JC, Finkbeiner AE, Dalrymple GV, Mullins MS, Sprigg JR, Komoroski RA. Differentiation of human prostate cancer from benign hypertrophy by in vitro 1H NMR. Magn Reson Med. 1992;25:140–147. doi: 10.1002/mrm.1910250114. [DOI] [PubMed] [Google Scholar]
- Fuss TL, Cheng LL. Evaluation of cancer metabolomics using ex vivo high resolution magic angle spinning (HRMAS) magnetic resonance spectroscopy (MRS) Metabolites. 2016;6:11. doi: 10.3390/metabo6010011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- García-Segura JM, Sánchez-Chapado M, Ibarburen C, Viaño J, Angulo JC, González J, Rodríguez-Vallejo JM. In vivo proton magnetic resonance spectroscopy of diseased prostate: spectroscopic features of malignant versus benign pathology. Magn Reson Imaging. 1999;17:755–765. doi: 10.1016/s0730-725x(99)00006-5. [DOI] [PubMed] [Google Scholar]
- Giskeødegård GF, Bertilsson H, Selnæs KM, Wright AJ, Bathen TF, Viset T, Halgunset J, Angelsen A, Gribbestad IS, Tessem MB. Spermine and Cit as metabolic biomarkers for assessing prostate cancer aggressiveness. PLoS One. 2013;8:e62375. doi: 10.1371/journal.pone.0062375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giskeødegård GF, Hansen AF, Bertilsson H, Gonzalez SV, Kristiansen KA, Bruheim P, Mjøs SA, Angelsen A, Bathen TF, Tessem MB. Metabolic markers in blood can separate prostate cancer from benign prostatic hyperplasia. Br J Cancer. 2015;113:1712–1719. doi: 10.1038/bjc.2015.411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giunchi F, Fiorentino M, Loda M. The metabolic landscape of prostate cancer. Eur Urol Oncol. 2019;2:28–36. doi: 10.1016/j.euo.2018.06.010. [DOI] [PubMed] [Google Scholar]
- Gómez-Cebrián N, Rojas-Benedicto A, Albors-Vaquer A, López-Guerrero JA, Pineda-Lucena A, Puchades-Carrasco L. Metabolomics contributions to the discovery of prostate cancer biomarkers. Metabolites. 2019;9:48. doi: 10.3390/metabo9030048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenlee RT, Hill-Harmon MB, Murray T, Thun M. Cancer statistics. CA Cancer J Clin. 2001;51:15–36. doi: 10.3322/canjclin.51.1.15. [DOI] [PubMed] [Google Scholar]
- Grundmark B, Garmo H, Loda M, Busch C, Holmberg L, Zethelius B. The metabolic syndrome and the risk of prostate cancer under competing risks of death from other causes. Cancer Epidemiol Biomark Prev. 2010;19:2088–2096. doi: 10.1158/1055-9965.EPI-10-0112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutte H, Hansen AE, Johannesen HH, Clemmensen AE, Ardenkjær-Larsen JH, Nielsen CH, Kjær A. The use of dynamic nuclear polarization (13)C-pyruvate MRS in cancer. Am J Nucl Med Mol Imaging. 2015;5:548–560. [PMC free article] [PubMed] [Google Scholar]
- Hahn P, Smith IC, Leboldus L, Littman C, Somorjai RL, Bezabeh T. The classification of benign and malignant human prostate tissue by multivariate analysis of 1H magnetic resonance spectra. Cancer Res. 1997;57:3398–3401. [PubMed] [Google Scholar]
- Heinlein CA, Chang C. Androgen receptor in prostate cancer. Endocr Rev. 2004;25:276–308. doi: 10.1210/er.2002-0032. [DOI] [PubMed] [Google Scholar]
- Jagannathan NR. Prostate MR: current status, challenges and future directions. NMR Biomed. 2014;27:1–2. doi: 10.1002/nbm.3011. [DOI] [PubMed] [Google Scholar]
- Julià-Sapé M, Candiota AP, Arús C. Cancer metabolism in a snapshot: MRS(I) NMR Biomed. 2019;32:e4054. doi: 10.1002/nbm.4054. [DOI] [PubMed] [Google Scholar]
- Jung K, Reszka R, Kamlage B, Bethan B, Stephan C, Lein M, Kristiansen G. Tissue metabolite profiling identifies differentiating and prognostic biomarkers for prostate carcinoma. Int J Cancer. 2013;133:2914–2924. doi: 10.1002/ijc.28303. [DOI] [PubMed] [Google Scholar]
- Kassen A, Sutkowski DM, Ahn H, Sensibar JA, Kozlowski JM, Lee C. Stromal cells of the human prostate: initial isolation and characterization. Prostate. 1996;28:89–97. doi: 10.1002/(SICI)1097-0045(199602)28:2<89::AID-PROS3>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- Keshari KR, Wilson DM, Van Criekinge M, Sriram R, Koelsch BL, Wang ZJ, VanBrocklin HF, Peehl DM, O'Brien T, Sampath D, Carano RA, Kurhanewicz J. Metabolic response of prostate cancer to nicotinamide phophoribosyltransferase inhibition in a hyperpolarized MR/PET compatible bioreactor. Prostate. 2015;75:1601–1609. doi: 10.1002/pros.23036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kline EE, Treat EG, Averna TA, Davis MS, Smith AY, Sillerud LO. Citrate concentrations in human seminal fluid and expressed prostatic fluid determined via 1H nuclear magnetic resonance spectroscopy outperform prostate specific antigen in prostate cancer detection. J Urol. 2006;176:2274–2279. doi: 10.1016/j.juro.2006.07.054. [DOI] [PubMed] [Google Scholar]
- Klomp DW, Scheenen TW, Arteaga CS, van Asten J, Boer VO, Luijten PR. Detection of fully refocused polyamine spins in prostate cancer at 7 T. NMR Biomed. 2011;24:299–306. doi: 10.1002/nbm.1592. [DOI] [PubMed] [Google Scholar]
- Kumar R, Nayyar R, Kumar V, Gupta NP, Hemal AK, Jagannathan NR, Dattagupta S, Thulkar S. Potential of magnetic resonance spectroscopic imaging in predicting absence of prostate cancer in men with serum prostate-specific antigen between 4 and 10 ng/ml: a follow-up study. Urology. 2008;72:859–863. doi: 10.1016/j.urology.2008.01.014. [DOI] [PubMed] [Google Scholar]
- Kumar V, Dwivedi DK, Jagannathan NR (2014) High-resolution NMR spectroscopy of human body fluids and tissues in relation to prostate cancer. NMR Biomed 27:80–89 [DOI] [PubMed]
- Kumar D, Gupta A, Mandhani A, Sankhwar SN. Metabolomics-derived prostate cancer biomarkers: fact or fiction? J Proteome Res. 2015;14:1455–1464. doi: 10.1021/pr5011108. [DOI] [PubMed] [Google Scholar]
- Kumar D, Gupta A, Mandhani A, Sankhwar SN. NMR spectroscopy of filtered serum of prostate cancer: a new frontier in metabolomics. Prostate. 2016;76:1106–1119. doi: 10.1002/pros.23198. [DOI] [PubMed] [Google Scholar]
- Kumar D, Gupta A, Nath K. NMR-based metabolomics of prostate cancer: a protagonist in clinical diagnostics. Expert Rev Mol Diagn. 2016;16:651–661. doi: 10.1586/14737159.2016.1164037. [DOI] [PubMed] [Google Scholar]
- Kumar V, Bora GS, Kumar R, Jagannathan NR. Multiparametric (mp) MRI of prostate cancer. Prog Nucl Magn Reson Spectrosc. 2018;105:23–40. doi: 10.1016/j.pnmrs.2018.01.001. [DOI] [PubMed] [Google Scholar]
- Kurhanewicz J, Dahiya R, Macdonald JM, Chang LH, James TL, Narayan P (1993) Cit alterations in primary and metastatic human prostatic adenocarcinomas: 1H magnetic resonance spectroscopy and biochemical study. Magn Reson Med 29:149–157 [DOI] [PubMed]
- Kurhanewicz J, Vigneron DB, Hricak H, Narayan P, Carroll P, Nelson SJ (1996) Three-dimensional H-1 MR spectroscopic imaging of the in situ human prostate with high (0.24-0.7-cm3) spatial resolution. Radiology 198:795–805 [DOI] [PubMed]
- Kurhanewicz J, Swanson MG, Nelson SJ, Vigneron DB. Combined magnetic resonance imaging and spectroscopic imaging approach to molecular imaging of prostate cancer. J Magn Reson Imaging. 2002;16:451–463. doi: 10.1002/jmri.10172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J, Giovannucci E, Jeon JY. Diabetes and mortality in patients with prostate cancer: a meta-analysis. Springerplus. 2016;5:1548. doi: 10.1186/s40064-016-3233-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z, Zhang H. Reprogramming of glucose, fatty acid and amino acid metabolism for cancer progression. Cell Mol Life Sci. 2016;73:377–392. doi: 10.1007/s00018-015-2070-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lima AR, Bastos Mde L, Carvalho M, Guedes de Pinho P. Biomarker discovery in human prostate cancer: an update in metabolomics studies. Transl Oncol. 2016;9:357–370. doi: 10.1016/j.tranon.2016.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucarelli G, Rutigliano M, Galleggiante V, Giglio A, Palazzo S, Ferro M, Simone C, Bettocchi C, Battaglia M, Ditonno P. Metabolomic profiling for the identification of novel diagnostic markers in prostate cancer. Expert Rev Mol Diagn. 2015;15:1211–1224. doi: 10.1586/14737159.2015.1069711. [DOI] [PubMed] [Google Scholar]
- Lynch MJ, Nicholson JK. Proton MRS of human prostatic fluid: correlations between Cit, spermine, and myo-inositol levels and changes with disease. Prostate. 1997;30:248–255. doi: 10.1002/(sici)1097-0045(19970301)30:4<248::aid-pros4>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- Madhu B, Shaw GL, Warren AY, Neal DE, Griffiths JR. Response of Degarelix treatment in human prostate cancer monitored by HR-MAS 1H NMR spectroscopy. Metabolomics. 2016;12:120. doi: 10.1007/s11306-016-1055-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moncada S, Higgs EA, Colombo SL. Fulfilling the metabolic requirements for cell proliferation. Biochem J. 2012;446:1–7. doi: 10.1042/BJ20120427. [DOI] [PubMed] [Google Scholar]
- Mori N, Wildes F, Takagi T, Glunde K, Bhujwalla ZM. The tumor microenvironment modulates Cho and lipid metabolism. Front Oncol. 2016;6:262. doi: 10.3389/fonc.2016.00262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naughton CK, Smith DS, Humphrey PA, Catalona WJ, Keetch DW. Clinical and pathologic tumor characteristics of prostate cancer as a function of the number of biopsy cores: a retrospective study. Urology. 1998;52:808–813. doi: 10.1016/s0090-4295(98)00344-6. [DOI] [PubMed] [Google Scholar]
- Nayyar R, Kumar R, Kumar V, Jagannathan NR, Gupta NP, Hemal AK. Magnetic resonance spectroscopic imaging: current status in the management of prostate cancer. BJU Int. 2009;103:1614–1620. doi: 10.1111/j.1464-410X.2009.08446.x. [DOI] [PubMed] [Google Scholar]
- Nelson SJ, Kurhanewicz J, Vigneron DB, Larson PE, Harzstark AL, Ferrone M, van Criekinge M, Chang JW, Bok R, Park I, Reed G, Carvajal L, Small EJ, Munster P, Weinberg VK, Ardenkjaer-Larsen JH, Chen AP, Hurd RE, Odegardstuen LI, Robb FJ, Tropp J, Murray JA. Metabolic imaging of patients with prostate cancer using hyperpolarized [1-13C]pyruvate. Sci Transl Med. 2013;5(198):198ra108. doi: 10.1126/scitranslmed.3006070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan T, Gao L, Wu G, Shen G, Xie S, Wen H, Yang J, Zhou Y, Tu Z, Qian W. Elevated expression of glutaminase confers glucose utilization via glutaminolysis in prostate cancer. Biochem Biophys Res Commun. 2015;456:452–458. doi: 10.1016/j.bbrc.2014.11.105. [DOI] [PubMed] [Google Scholar]
- Pavlides S, Whitaker-Menezes D, Castello-Cros R, Flomenberg N, Witkiewicz AK, Frank PG, Casimiro MC, Wang C, Fortina P, Addya S, Pestell RG, Martinez-Outschoorn UE, Sotgia F, Lisanti MP. The reverse Warburg effect: aerobic glycolysis in cancer associated fibroblasts and the tumor stroma. Cell Cycle. 2009;8:3984–4001. doi: 10.4161/cc.8.23.10238. [DOI] [PubMed] [Google Scholar]
- Pérez-Rambla C, Puchades-Carrasco L, García-Flores M, Rubio-Briones J, López-Guerrero JA, Pineda-Lucena A. Non-invasive urinary metabolomic profiling discriminates prostate cancer from benign prostatic hyperplasia. Metabolomics. 2017;13:52. doi: 10.1007/s11306-017-1194-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu F, Huang J, Sui M. Targeting arginine metabolism pathway to treat arginine-dependent cancers. Cancer Lett. 2015;364:1–7. doi: 10.1016/j.canlet.2015.04.020. [DOI] [PubMed] [Google Scholar]
- Rabbani F, Stroumbakis N, Kava BR, Cookson MS, Fair WR. Incidence and clinical significance of false-negative sextant prostate biopsies. J Urol. 1998;159:1247–1250. [PubMed] [Google Scholar]
- Roberts MJ, Schirra HJ, Lavin MF, Gardiner RA. Metabolomics: a novel approach to early and noninvasive prostate cancer detection. Korean J Urol. 2011;52:79–89. doi: 10.4111/kju.2011.52.2.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts MJ, Richards RS, Chow CWK, Buck M, Yaxley J, Lavin MF, Schirra HJ, Gardiner RA. Seminal plasma enables selection and monitoring of active surveillance candidates using nuclear magnetic resonance-based metabolomics: a preliminary investigation. Prostate Int. 2017;5:149–157. doi: 10.1016/j.prnil.2017.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rysman E, Brusselmans K, Scheys K, Timmermans L, Derua R, Munck S, Van Veldhoven PP, Waltregny D, Daniëls VW, Machiels J, Vanderhoydonc F, Smans K, Waelkens E, Verhoeven G, Swinnen JV. De novo lipogenesis protects cancer cells from free radicals and chemotherapeutics by promoting membrane lipid saturation. Cancer Res. 2010;70:8117–8126. doi: 10.1158/0008-5472.CAN-09-3871. [DOI] [PubMed] [Google Scholar]
- Schiebler ML, Miyamoto KK, White M, Maygarden SJ, Mohler JL. In vitro high resolution 1H-spectroscopy of the human prostate: benign prostatic hyperplasia, normal peripheral zone and adenocarcinoma. Magn Reson Med. 1993;29:285–291. doi: 10.1002/mrm.1910290302. [DOI] [PubMed] [Google Scholar]
- Scroggins BT, Matsuo M, White AO, Saito K, Munasinghe JP, Sourbier C, Yamamoto K, Diaz V, Takakusagi Y, Ichikawa K, Mitchell JB, Krishna MC, Citrin DE. Hyperpolarized [1-(13)C]-pyruvate magnetic resonance spectroscopic imaging of prostate cancer in vivo predicts efficacy of targeting the Warburg effect. Clin Cancer Res. 2018;24:3137–3148. doi: 10.1158/1078-0432.CCR-17-1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selnaes KM, Gribbestad IS, Bertilsson H, Wright A, Angelsen A, Heerschap A, Tessem MB. Spatially matched in vivo and ex vivo MR metabolic profiles of prostate cancer -- investigation of a correlation with Gleason score. NMR Biomed. 2013;26:600–606. doi: 10.1002/nbm.2901. [DOI] [PubMed] [Google Scholar]
- Serkova NJ, Gamito EJ, Jones RH, O'Donnell C, Brown JL, Green S, Sullivan H, Hedlund T, Crawford ED. The metabolites Cit, myo-inositol, and spermine are potential age-independent markers of prostate cancer in human expressed prostatic secretions. Prostate. 2008;68:620–628. doi: 10.1002/pros.20727. [DOI] [PubMed] [Google Scholar]
- Serrao EM, Brindle KM. Potential clinical roles for metabolic imaging with hyperpolarized [1-(13)C]pyruvate. Front Oncol. 2016;6:59. doi: 10.3389/fonc.2016.00059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shukla-Dave A, Hricak H, Moskowitz C, Ishill N, Akin O, Kuroiwa K, Spector J, Kumar M, Reuter VE, Koutcher JA, Zakian KL. Detection of prostate cancer with MR spectroscopic imaging: an expanded paradigm incorporating polyamines. Radiology. 2007;245:499–506. doi: 10.1148/radiol.2452062201. [DOI] [PubMed] [Google Scholar]
- Stenman K, Hauksson JB, Gröbner G, Stattin P, Bergh A, Riklund K. Detection of polyunsaturated omega-6 fatty acid in human malignant prostate tissue by 1D and 2D high-resolution magic angle spinning NMR spectroscopy. MAGMA. 2009;22:327–331. doi: 10.1007/s10334-009-0187-x. [DOI] [PubMed] [Google Scholar]
- Swanson MG, Zektzer AS, Tabatabai ZL, Simko J, Jarso S, Keshari KR, Schmitt L, Carroll PR, Shinohara K, Vigneron DB, Kurhanewicz J. Quantitative analysis of prostate metabolites using 1H HR-MAS spectroscopy. Magn Reson Med. 2006;55:1257–1264. doi: 10.1002/mrm.20909. [DOI] [PubMed] [Google Scholar]
- Swanson MG, Keshari KR, Tabatabai ZL, Simko JP, Shinohara K, Carroll PR, Zektzer AS, Kurhanewicz J. Quantification of Cho- and ethanolamine-containing metabolites in human prostate tissues using 1H HR-MAS total correlation spectroscopy. Magn Reson Med. 2008;60:33–40. doi: 10.1002/mrm.21647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swindle P, McCredie S, Russell P, Himmelreich U, Khadra M, Lean C, Mountford C. Pathologic characterization of human prostate tissue with proton MR spectroscopy. Radiology. 2003;228:144–151. doi: 10.1148/radiol.2281011808. [DOI] [PubMed] [Google Scholar]
- Swindle P, Ramadan S, Stanwell P, McCredie S, Russell P, Mountford C. Proton magnetic resonance spectroscopy of the central, transition and peripheral zones of the prostate: assignments and correlation with histopathology. MAGMA. 2008;21:423–434. doi: 10.1007/s10334-008-0136-0. [DOI] [PubMed] [Google Scholar]
- Tayari N, Heerschap A, Scheenen TWJ, Kobus T. In vivo MR spectroscopic imaging of the prostate, from application to interpretation. Anal Biochem. 2017;529:158–170. doi: 10.1016/j.ab.2017.02.001. [DOI] [PubMed] [Google Scholar]
- Teahan O, Bevan CL, Waxman J, Keun HC. Metabolic signatures of malignant progression in prostate epithelial cells. Int J Biochem Cell Biol. 2011;43:1002–1009. doi: 10.1016/j.biocel.2010.07.003. [DOI] [PubMed] [Google Scholar]
- Tsouko E, Khan AS, White MA, Han JJ, Shi Y, Merchant FA, Sharpe MA, Xin L, Frigo DE. Regulation of the pentose phosphate pathway by an androgen receptor-mTOR-mediated mechanism and its role in prostate cancer cell growth. Oncogenesis. 2014;3:e103. doi: 10.1038/oncsis.2014.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Asten JJ, Cuijpers V, Hulsbergen-van de Kaa C, Soede-Huijbregts C, Witjes JA, Verhofstad A, Heerschap A. High resolution magic angle spinning NMR spectroscopy for metabolic assessment of cancer presence and Gleason score in human prostate needle biopsies. MAGMA. 2008;21:435–442. doi: 10.1007/s10334-008-0156-9. [DOI] [PubMed] [Google Scholar]
- van der Graaf M, Schipper RG, Oosterhof GO, Schalken JA, Verhofstad AA, Heerschap A. Proton MR spectroscopy of prostatic tissue focused on the detection of spermine, a possible biomarker of malignant behavior in prostate cancer. MAGMA. 2000;10:153–159. doi: 10.1007/BF02590640. [DOI] [PubMed] [Google Scholar]
- Vandergrift LA, Decelle EA, Kurth J, Wu S, Fuss TL, DeFeo EM, Halpern EF, Taupitz M, McDougal WS, Olumi AF, Wu CL, Cheng LL. Metabolomic prediction of human prostate cancer aggressiveness: magnetic resonance spectroscopy of histologically benign tissue. Sci Rep. 2018;8:4997. doi: 10.1038/s41598-018-23177-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallimann T, Wyss M, Brdiczka D, Nicolay K, Eppenberger HM. Intracellular compartmentation, structure and function of creatine kinase isoenzymes in tissues with high and fluctuating energy demands: the 'phosphocreatine circuit' for cellular energy homeostasis. Biochem J. 1992;281(Pt 1):21–40. doi: 10.1042/bj2810021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willker W, Flögel U, Leibfritz D (1998) A 1H/13C inverse 2D method for the analysis of the polyamines putrescine, spermidine and spermine in cell extracts and biofluids. NMR Biomed 11:47–54 [DOI] [PubMed]
- Wu X, Daniels G, Lee P, Monaco ME. Lipid metabolism in prostate cancer. Am J Clin Exp Urol. 2014;2:111–120. [PMC free article] [PubMed] [Google Scholar]
- Zadra G, Photopoulos C, Loda M. The fat side of prostate cancer. Biochim Biophys Acta. 2013;1831:1518–1532. doi: 10.1016/j.bbalip.2013.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]