Abstract
Preeclampsia (PE) is a pregnancy-specific complication that seriously threatens the health and safety of mothers and infants. The etiology of PE has not been fully elucidated, and no effective treatments are currently available. A pregnant woman with PE often has to make a tough choice on either endangering her own health to give a birth or being forced to terminate her pregnancy. It is recommended by the International Federation of Gynecology and Obstetrics that the combination of maternal high-risk factors and biomarkers could form a good strategy for predicting the risk of PE. Such a combination may also enable more effective monitoring and early clinical intervention in high-risk populations to reduce the risk of PE. Therefore, biomarkers validated by extensive clinical research may be formally applied for clinical PE risk prediction. In this review, we summarized data from clinical research on potential biomarkers and classified them according to the current four major hypotheses, namely placental or trophoblast ischemia and hypoxia, vascular endothelial injury, oxidative stress, and immune dysregulation. Additionally, we also discussed the underlying mechanisms by which these potential biomarkers may be involved in the pathogenesis of PE. Finally, we propose that multiple biomarkers reflecting different aspects of the disease pathogenesis should be used in combination to detect the high-risk PE population in support of clinically targeted intervention and prevention of PE. It is expected that tests made of more sensitive and reliable PE biomarkers based on the aforementioned major hypotheses could potentially improve the accuracy of PE prediction in the future.
Keywords: Preeclampsia, Biomarker, Predict, Hypothesis
Introduction
Preeclampsia (PE) mainly occurs after 20 weeks of pregnancy and manifests as hypertension, proteinuria, and tissue edema, with systemic multiple organ damage. It is a pregnancy-specific complication with an incidence of 3–8 % and is one of the main causes of maternal and neonatal mortality [1].
The etiology of PE has not yet been fully elucidated, and there are no clinically effective treatments other than the termination of pregnancy. It is well established that people with a high risk of PE could take low-dose aspirin during early pregnancy to effectively reduce the risk of PE. The International Federation of Gynecology and Obstetrics (FIGO) recommends that the maternal risk factors, such as maternal age, obesity, and previous or family history of PE, combined with biomarkers can better predict the risk of PE and may prompt increased monitoring and early clinical intervention of PE in high-risk populations to reduce the risk of PE [2]. Therefore, biomarkers validated by extensive clinical research may be formally applied to clinical PE risk prediction, which is of great clinical significance.
Currently, the pathogenesis of PE is dominated by five classic hypotheses: placental or trophoblast ischemia and hypoxia, vascular endothelial injury, oxidative stress, genetics, and immune dysregulation. A family history of PE is one of the established risk factors for PE, indicating a genetic predisposition to the disease. Some epidemiological and genetic studies have also confirmed the trend of familial development of PE, but the underlying mechanism remains elusive due to complex ethnic, geographical, and other factors [3]. Since there are few clinical studies on PE gene markers, except for several clinical studies that report the possible relationship between gene polymorphism and PE [[4], [5], [6]]. We mainly summarize the potential PE biomarkers based on the mechanisms involved in the other four leading hypotheses.
Clinical research status of biomarkers for PE
All searches were conducted in English in PubMed by December 2019 to obtain articles related to potential predictive markers for PE. General search terms included ‘pre-eclampsia’ and similar terms, such as ‘preeclampsia’ and ‘PE’, and other terms included ‘biomarker’, ‘marker’, ‘screen’, and ‘predict’. Given that our goal was to investigate the clinical research status of these biomarkers, we also searched for (‘clinical case-control study’ OR ‘clinical cohort study’) AND (‘serum’ OR ‘plasma’). We then summarized the potential PE biomarkers based on the mechanisms involved in the major hypotheses (Table 1 and Fig. 1).
Table 1.
Summary of current clinical studies of potential biomarkers for PE based on major hypotheses.
| Biomarkers | Subjects | Sample | Limitation | Refs. |
|---|---|---|---|---|
| 1. The placental or trophoblast ischemia and hypoxia hypothesis | ||||
| PAPP-A | 42 PE; 410 controls |
Serum (11–14 weeks of gestation) | The sample size was small, especially cases with PE. | [9] |
| AT1-AA | 30 PE; 30 gestational age-matched controls; |
Serum (three stages: <27; 28–33; >34 weeks of gestation; prospective study) | The sample size was small; Low sensitivity. | [12] |
| PP-13 | 47 PE; 290 controls |
Serum (9–12 weeks of gestation) | Inconsistent test reagents. | [15] |
| 42 PE; 410 controls |
Serum (11–14 weeks of gestation) | [9] | ||
| HGF | 52 PE; 104 controls |
Plasma (15–20 weeks of gestation) | The study population was single; The detection time was relatively late. | [19] |
| HIF-1 | 23 early-onset PE; 23 late-onset PE; 23 controls |
Serum (≥20 weeks of gestation; cross-sectional study) | A few cases were classified as early-onset PE or late-onset PE for the research. | [21] |
| miR-210 | 20 PE; 56 controls |
Serum (15–20 weeks of gestation) | The sample size was small; Low specificity. | [24] |
| 2. The oxidative stress hypothesis | ||||
| ROS | 80 PE; 80 controls |
Plasma (29–41 weeks of gestation) | Low specificity. | [27] |
| HbF | 86 PE; 347 controls |
Serum (mean 13.7 weeks of gestation) | The cohort had a higher level of risk factors for PE than a normal population. | [29] |
| A1M | 86 PE; 347 controls |
Serum (mean 13.7 weeks of gestation) | The sample size was small; Low specificity. | [29] |
| 41 PE; 50 controls |
Serum (30–39 weeks of gestation) | [31] | ||
| Hcy | 103 mild PE; 44 severe PE; 4418 controls |
Serum (11–13 weeks of gestation) | Samples were not collected repeatedly. | [33] |
| 3. The immune dysregulation hypothesis | ||||
| NLR | 49 mild PE; 15 severe PE; 376 controls |
Venous blood(16–18 weeks of gestation) | The sample size of the patients was small; Low sensitivity. | [38] |
| TH | 9 PE; 77 controls |
Serum (5–16 weeks of gestation) | The cohort had a higher level of risk factors for PE than a normal population; The number of patients with PE is limited. | [43] |
| CRP | 26 mild PE; 33 severe PE; 50 controls |
Serum (34–38 weeks of gestation) | The study was cross-sectional; The sample size of the patients was small. | [45] |
| NGAL | 128 PE; 183 controls |
Serum (10–14 weeks of gestation) | A relatively small number of participants; Low specificity. | [47] |
| 4. The vascular endothelial injury hypothesis | ||||
| TG | 139 mild PE; 143 severe PE; 37 controls |
Plasma (27–30 weeks of gestation) | This parameter is variable and affected by multiple influences, duplicate and continuous monitoring is required; Low specificity. | [50] |
| 53 early-onset PE; 18 late-onset PE; 2086 controls |
Plasma (at 18 weeks of gestation) | [51] | ||
| PlGF | 60 mild PE; 60 severe PE; 30 controls |
Serum (26–28 weeks of gestation) | High specificity and sensitivity when combined with other parameters. | [53] |
| 62 PE; 1560 controls |
Plasma (6–15 and 20–25 weeks of gestation) | [54] | ||
| VEGF | 23 non-severe PE; 19 severe PE; 42 controls |
Placenta (immediately after the delivery) | A study with a case-control model in a different population is needed. | [56] |
| 40 late-onset PE; 40 controls |
Serum (32–38 weeks of gestation) | [57] | ||
| sFlt-1 | 15 early-onset PE; 19 late-onset PE; 144 controls |
Plasma (19–25, 27–31 and 34–38 weeks of gestation) | A few cases were classified as early-onset PE or late-onset PE for research; High specificity and sensitivity when combined with other parameters. | [59] |
| 62 PE; 1560 controls |
Plasma (6–15 and 20–25 weeks of gestation) | [54] | ||
| sEng | 62 PE; 1560 controls |
Plasma (6–15 and 20–25 weeks of gestation) | The cohort had a higher level of risk factors for PE than a normal population; The detection time is relatively late. | [54] |
| 54 PE; 28 controls |
Serum (after clinical manifestations) | [61] | ||
| CC | 52 PE; 52 controls |
Serum (24–36 weeks of gestation) | The study was cross-sectional; Low specificity. | [65] |
Controls: the normotensive pregnant women who didn’t develop any pregnancy complications.
Abbreviations: PAPP-A, Pregnancy-associated plasma protein-A; AT1-AA, Angiotensin II type 1 receptor autoantibody; PP-13, Placental protein-13; HGF, Hepatocyte growth factor; HIF-1, Hypoxia-inducible factor-1; miR-210, microRNA 210; ROS, Reactive oxygen species; HbF, Fetal hemoglobin; A1M, α1 microglobulin; Hcy, Homocysteine; TH, Helper T cell; CRP, C-reactive protein; NGAL, Neutrophil gelatinase-associated lipocalin; NLR, Neutrophil/lymphocyte ratio; TG, Triglyceride; PlGF, Placental growth factor; VEGF, vascular endothelial growth factor; sFlt-1, soluble tyrosine kinase-like receptor-1; sEng, soluble endothelin; CC, Cystatin C.
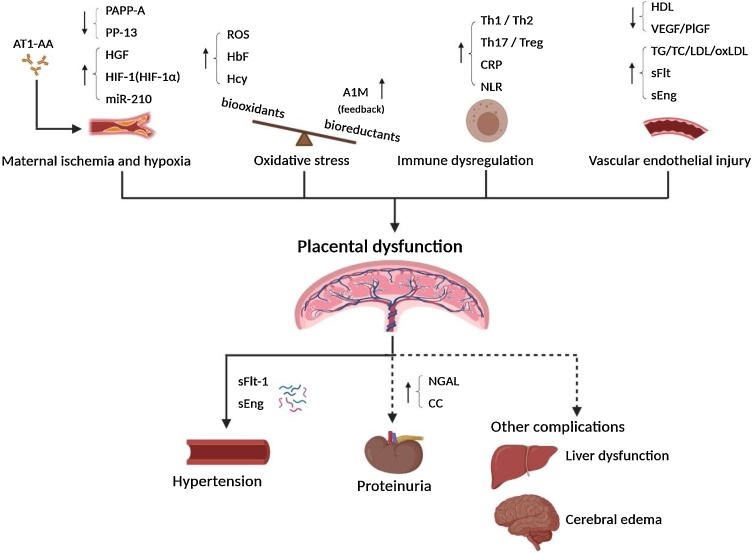
Fig. 1.
The interaction of biomarkers in the pathogenesis of PE.
The changes of various biomarkers related to the onset of PE and their possible role in the pathogenesis of PE. The dashed lines in the figure indicate that PE symptoms do not always occur. All figures in this review article were created by BioRender.com.
To obtain more information about potential biomarkers, we performed a more specific PubMed search, including the general search terms and the name of individual biomarkers, such as [(‘pre-eclampsia’ OR ‘preeclampsia’ OR ‘PE’) AND (‘biomarker’ OR ‘marker’ OR ‘screen’ OR ‘predict’) AND (‘clinical case-control study’ OR ‘clinical cohort study’) AND (‘serum’ OR ‘plasma’) AND (‘Pregnancy-associated plasma protein-A’ OR ‘PAPP-A’)], and the clinical research status of these biomarkers is shown in Table 2.
Table 2.
Current clinical research status of potential biomarkers of PE.
| Biomarkers | Clinical research status to predict PE |
Other related diseases | Comments | ||
|---|---|---|---|---|---|
| >30 studies | 5-30 studies | <5 studies | |||
| 1.The placental or trophoblast ischemia and hypoxia hypothesis | |||||
| PAPP-A | √ | Down syndrome; IUGR; Placental abruption; Preterm delivery; Fatal death | The specificity and sensitivity of PE prediction are expected to be improved by combining PAPP-A with other indicators such as UTPI. | ||
| AT1-AA | √ | Gestational hypertension | Indicators of kidney injury may help to distinguish PE from gestational hypertension. | ||
| PP-13 | √ | IUGR; Preterm delivery | The prediction effect is better when PP-13 is combined with other indicators, such as UTPI. | ||
| HGF | √ | Cancer; Heart disease; Hypertension; Bowel inflammation | The effectiveness of HGF in predicting the risk of PE in early pregnancy needs to be confirmed by more extensive clinical studies. | ||
| HIF-1 | √ | Cancer; Anemia; RA; Stroke; CADs | Longitudinal studies are needed to confirm the effectiveness of HIF-1 in predicting the risk of PE. | ||
| miR-210 | √ | Myocardial infarction; Adrenocortical carcinoma; Breast cancer | More extensive clinical studies are needed to conduct to confirm the effectiveness of miR-210 in predicting the risk of PE. | ||
| 2.The oxidative stress hypothesis | |||||
| ROS | √ | Cancer; CADs; Stroke; Heart disease | Prospective studies are needed to confirm the effectiveness of ROS in predicting the risk of PE. | ||
| HbF | √ | Thalassemia; Sickle cell disease; Gestational diabetes; Leukemia | The ratio of HbF to total hemoglobin combined with α1 microglobulin is a better predictor of PE than the HbF level alone. | ||
| A1M | √ | Cerebral hemorrhage; Chronic leg ulcers | The effectiveness of A1M in predicting the risk of PE in early pregnancy needs to be confirmed by more extensive clinical studies. | ||
| Hcy | √ | Heart disease; Stroke; Alzheimer’s disease; Osteoporosis | The prediction effect is better when Hcy is combined with other indicators, such as UTPI and sEng. | ||
| 3.The immune dysregulation hypothesis | |||||
| NLR | √ | RA | More longitudinal cohort studies are needed to accurately determine the best timing and cutoff values of NLR that may be used in the clinic. | ||
| TH | √ | Hypersensitivity; Autoimmune diseases | The effectiveness of TH in predicting the risk of PE in early pregnancy needs to be confirmed by more extensive clinical studies. | ||
| CRP | √ | CADs; Stroke; Heart disease; Hypertension; Diabetes; Metabolic syndrome | CRP is more likely to be a diagnostic marker of PE than a predictive marker. | ||
| NGAL | √ | AKI; Chronic kidney disease | The prediction effect is better when NGAL is combined with other markers, such as PlGF. | ||
| 4.The vascular endothelial injury hypothesis | |||||
| TG | √ | CADs; Stroke; Heart disease; Hypertriglyceridemia; Metabolic syndrome | The prediction effect is better when TG is combined with other markers, such as UTPI and PlGF. | ||
| PlGF | √ | Atherosclerosis | The sFlt-1/PlGF ratio is more accurate as a predictive marker for PE than using either alone. | ||
| VEGF | √ | RA; Central nervous system injuries; Angiosarcoma; Diabetic retinopathy; Breast cancer | The prediction effect is better when VEGF is combined with other markers, such as UTPI and PlGF. | ||
| sFlt-1 | √ | IUGR | The prediction effect is better when sFlt-1 is combined with other markers, such as PI or PlGF. | ||
| sEng | √ | CADs; Hypertension; Heart disease; Type II diabetes | The PlGF/sEng ratio is more sensitive and specific to predict PE risk than using either separately. | ||
| CC | √ | CADs; Alzheimer’s disease; AKI; Chronic kidney disease | The effectiveness of CC in predicting the risk of PE in early pregnancy needs to be confirmed by more prospective clinical studies. | ||
Abbreviations: PAPP-A, Pregnancy-associated plasma protein-A; AT1-AA, Angiotensin II type 1 receptor autoantibody; PP-13, Placental protein-13; HGF, Hepatocyte growth factor; HIF-1, Hypoxia-inducible factor-1; miR-210, microRNA 210; ROS, Reactive oxygen species; HbF, Fetal hemoglobin; A1M, α1 microglobulin; Hcy, Homocysteine; TH, Helper T cell; CRP, C-reactive protein; NGAL, Neutrophil gelatinase-associated lipocalin; NLR, Neutrophil/lymphocyte ratio; TG, Triglyceride; PlGF, Placental growth factor; VEGF, vascular endothelial growth factor; sFlt-1, soluble tyrosine kinase-like receptor-1; sEng, soluble endothelin; CC, Cystatin C; IUGR, Intrauterine growth restriction; RA, Rheumatoid arthritis; CADs, Cardiovascular diseases; AKI, Acute kidney injury; UTPI, uterine artery pulsatility index.
Four major hypotheses and related biomarkers
Placental or trophoblast ischemia and hypoxia hypothesis and related biomarkers
The placental or trophoblast ischemia and hypoxia hypothesis indicates that there is a placental trophoblast defect that invades the uterine myometrium, resulting in impaired uterine spiral artery remodeling, increased blood flow resistance, decreased placental blood flow, and insufficient placental contraction, which may induce PE. Notably, the insufficient infiltration of placental trophoblasts may be caused by endocrine dysfunction [7].
Pregnancy-associated plasma protein-A (PAPP-A)
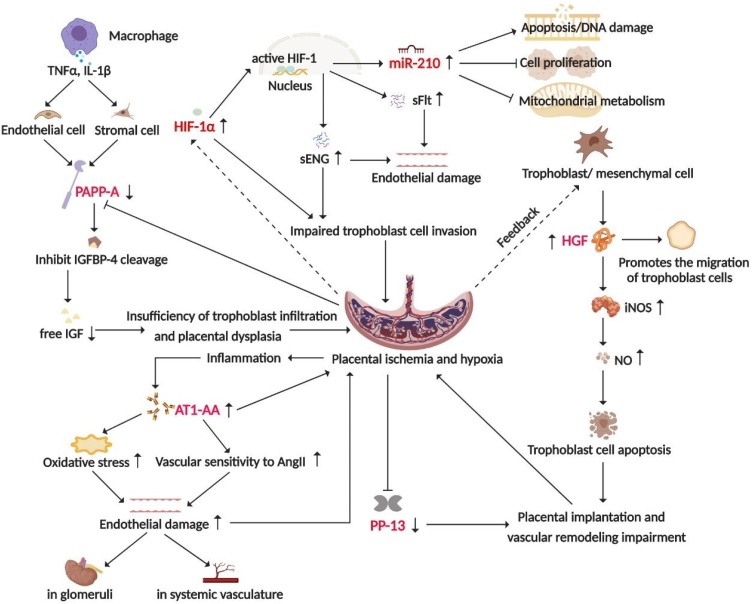
PAPP-A is a protease that targets insulin-like growth factor-binding proteins (IGFPBs) and can positively regulate the role of insulin-like growth factors (IGFs). Decreased levels of PAPP-A cause IGFs to be bound by IGFPB-4, which leads to reduced free IGF levels and insufficient trophoblast infiltration and placental dysplasia. In turn, placental ischemia and hypoxia affect PAPP-A secretion from the placenta. Such a vicious cycle eventually leads to the occurrence of PE [8] (Fig. 2). A prospective cohort study showed that for a false-positive rate (FPR) of 20 %, the predictive sensitivity of PAPP-A to all PE is 58 %, and the sensitivity is only 21 % at a 5 % FPR [9]. As a promising biomarker, the specificity and sensitivity of PE prediction are expected to be improved by combining PAPP-A with other indicators such as the uterine artery pulsatility index (UTPI).
Fig. 2.
The placental or trophoblast ischemia and hypoxia hypothesis that explains the pathogenesis of PE.
The placenta of PE patients exhibits ischemia and hypoxia, which reduces the expression of PAPP-A and PP-13, and increases the expression of AT1-AA, HIF-1α, and miR-210. The abnormal expression of these proteins induces endothelial damage, inadequate trophoblastic cell invasion, and abnormal placental implantation, further exacerbating placental ischemia and hypoxia. As a negative feedback regulation, trophoblast cells secrete more HGF to promote trophoblast migration and infiltration. The dashed lines in the figure indicate that these biomarkers still need to be verified by more large-scale clinical longitudinal studies as indicators to predict the risk of PE.
Angiotensin II type 1 receptor autoantibody (AT1-AA)
AT1-AA is initially detected in PE patients. However, its possible role in the pathogenesis of PE remains unclear, which may be related to hypoxia-ischemia caused by insufficient placental implantation and inflammation due to vascular endothelial damage. Moreover, AT1-AA may exacerbate oxidative stress and increase vascular sensitivity to Angiotensin II (AngII), resulting in extensive endothelial damage [10,11] (Fig. 2). Herse et al. found that AT1-AA was detected in 70 % of PE patients, while 80 % of controls were negative for AT1-AA [12]. Elevated AT1-AA in serum is significantly related to the occurrence of PE, indicating that AT1-AA has the potential to predict PE risk [13].
Placental protein-13 (PP-13)
PP-13 belongs to the galectin superfamily, it is highly expressed in the placenta and plays an important role in placental implantation and vascular remodeling [14] (Fig. 2). Chafetz et al. found that the PP13 levels were significantly decreased in PE patients at 9–12 weeks of pregnancy. When the maternal PP13 levels were used to predict PE in early pregnancy, the sensitivity was 79 % at a 90 % specificity rate [15]. Odibo et al. reported that when FPR was set to 20 %, PP13 had a sensitivity of 79 % to predict early-onset PE and only 49 % to predict all PE [9]. Studies have shown that PP-13 levels in early pregnancy have become an effective means of predicting the occurrence of PE, especially when it is combined with other indicators, such as UTPI [14,16].
Hepatocyte growth factor (HGF)
HGF is an acidic protein that promotes the migration and reproduction of epithelial cells. It is mainly secreted by trophoblast and chorionic mesenchymal cells. In vitro experiments have demonstrated that the infiltration capacity of trophoblast cells is increased 4-fold after HGF stimulation [17]. HGF can also stimulate the expression of inducible nitric oxide synthase (iNOS), regulate the apoptosis of human trophoblast cells through NO, and induce PE [18] (Fig. 2). A retrospective study showed that women with PE had significantly higher HGF concentrations in plasma than normotensive women, and the risk of subsequent PE in women with HGF concentrations above 702.5 ng/ml was 3.2-fold higher than the risk in those below 702.5 ng/ml [19]. However, the effectiveness of HGF in predicting the risk of PE in early pregnancy needs to be confirmed by more extensive clinical studies.
Hypoxia-inducible factor-1 (HIF-1)
HIF-1 is the main regulator of the cellular response to hypoxia stress and the core of maintaining oxygen homeostasis. It is a heterodimer transcription factor composed of two subunits, HIF-1α and HIF-1β. HIF-1α is sensitive to oxygen, and it is quickly inactivated and degraded under normoxic conditions, while under hypoxic conditions, HIF-1α degradation is suppressed. When HIF-1α binds to HIF-1β, active HIF-1 is formed, which is transferred to the nucleus and regulates the expression of various genes, such as sFlT-1 and sENG [20] (Fig. 2). Significant upregulation of HIF-1α was observed in the sera of PE patients, especially in patients with early-onset PE [21]. This indicates that HIF-1α may be a potential biomarker for predicting the risk of PE, which still needs further clinical validation.
MicroRNA 210 (miR-210)
MiR-210 is an important hypoxic sensor produced after HIF-1 activation, and it may mediate apoptosis of trophoblast cells through the mitochondrial metabolic pathway, which may participate in the development of PE [22,23] (Fig. 2). Studies have found that the miR-210 levels in the sera of PE patients increased significantly, which was detected months before clinical diagnosis [24]. With the development of second-generation sequencing technology, miR-210 may become a potential biomarker for predicting the risk of PE after more extensive clinical research has been conducted.
Oxidative stress hypothesis and related biomarkers
There is a balance between oxidation and antioxidation in normotensive pregnant women. When this balance is broken and causes oxidative stress, this imbalance will activate or destroy endothelial cells and participate in the occurrence of PE. Excessive systemic inflammation in PE patients would enhance the local oxidative stress response, leading to reduced antioxidants and increased lipid peroxides, forming a vicious cycle [25].
Reactive oxygen species (ROS)
ROS is a general term for oxygen-containing compounds with high biological activity produced during aerobic metabolism in cells. Medium and high concentrations of ROS may induce apoptosis of pancreatic β cells, vascular endothelial cells, or trophoblast cells and then cause corresponding diseases, such as PE [26] (Fig. 3). It is generally believed that the ROS levels in PE patients are significantly higher than those in normotensive pregnant women [27]. Recently, with the emergence of various methods for detecting intracellular ROS products, ROS is likely to become promising markers for predicting the risk of PE, but prospective studies are required to confirm this hypothesis.
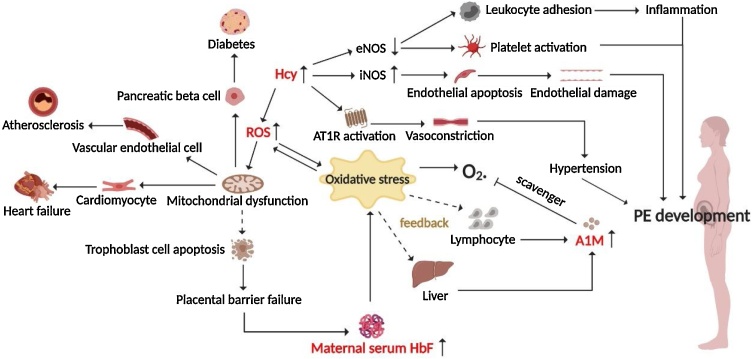
Fig. 3.
The oxidative stress hypothesis explaining the pathogenesis of PE.
Elevated Hcy level in PE patients may lead to endothelial damage and systemic inflammation through a variety of pathways, and participate in the pathogenesis of PE. Moreover, Hcy also promotes the rise of ROS levels, induces the apoptosis of trophoblast cells, and destroys the placental barrier, which increases the level of HbF in maternal peripheral blood and further exacerbates oxidative stress. To resist the damage caused by oxidative stress, the liver or lymphocytes release more A1M to clear free oxygen radicals from the body. The dashed lines in the figure indicate that these biomarkers still need to be verified by more large-scale clinical longitudinal studies as indicators to predict the risk of PE.
Fetal hemoglobin (HbF)
HbF is synthesized in pregnancy and rarely occurs in the peripheral blood of nonpregnant women. The placental barrier is damaged under pathological conditions such as PE, leading HbF to enter the maternal peripheral blood, cause maternal systemic oxidative stress, and participate in oxidative damage to the placental tissue, thereby further destroying the placental barrier [28] (Fig. 3). Some studies found that the serum HbF level of pregnant women who would develop PE later was significantly increased; moreover, the ratio of HbF to total hemoglobin combined with α1 microglobulin is a better predictor of PE than the HbF level alone [29], but the prospect of this application remains to be confirmed by large-scale prospective studies.
α1 microglobulin (A1M)
A1M is mainly synthesized in the liver and lymphocytes, which can help cells resist damage induced by heme, HbF, and oxidative stress. A1M is a reductase and scavenger for free heme and oxygen free radicals [30] (Fig. 3). A1M levels in the sera of PE patients are increased compared with that in normotensive pregnant women [31]. It is believed that the combination of A1M with HbF and/or heme has good predictability for PE. For example, A1M, HbF, and heme were combined with uterine artery Doppler and demonstrated a predictive sensitivity of 60 % and a specificity of 95 % for PE [29].
Homocysteine (Hcy)
Hcy is an important intermediate as methionine is metabolized into cysteine, and its level decreases during normal pregnancy. Elevated plasma Hcy may enhance iNOS activity and reduce endothelial nitric-oxide synthase (eNOS) activity, which leads to endothelial cell apoptosis, activates platelets, and increases leukocyte adhesion. Hcy may also activate angiotensin II type 1 receptor (AT1R) to aggravate endothelial damage and promote vasoconstriction [32] (Fig. 3). Hcy is positively correlated with PE severity in the third trimester of pregnancy [33]. Studies have found that high levels of plasma Hcy are easily oxidized, producing a large amount of ROS and resulting in endothelial damage, which may lead to pregnancy hypertension [34] (Fig. 3). Therefore, Hcy is expected to serve as a potential indicator for predicting the risk of PE.
Immune dysregulation hypothesis and related biomarkers
The immune dysregulation hypothesis suggests that the change in the autoimmune response may cause abnormal placental implantation in the first trimester of pregnancy and subsequent insufficient placental perfusion, resulting in ischemia, shedding of the trophotropic artery and the production of multiple cytokines, leading to extensive vascular endothelial damage and the clinical manifestations of PE [35].
Neutrophils
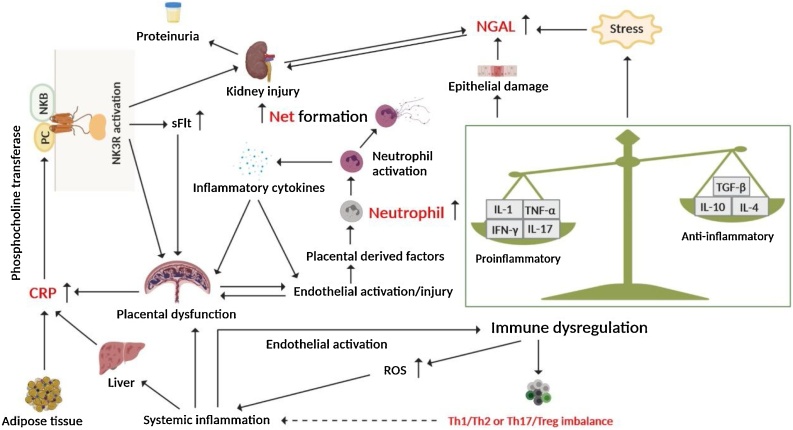
Neutrophils are the most abundant white blood cells in the human body. Activated neutrophils produce an extracellular fibrous grid consisting mainly of chromatin and granular proteins, known as the neutrophil extracellular trap (Net). Placental derived factors (IL-8 and placental microfragments) induce a large number of Nets in the placenta of PE patients, suggesting that Nets may be involved in the pathogenesis of PE [36]. In addition, PE promotes neutrophil transplacental migration and endothelial activation, which triggers Net formation [37]. Neutrophil/lymphocyte ratio (NLR) is a newly emerged systemic inflammatory biomarker. The activation of inflammatory pathways in PE is manifested by an increase in neutrophil-dominated white blood cell counts, resulting in increased NLR. Moreover, neutrophils may release a variety of inflammatory cytokines to activate inflammatory cells and immune responses, leading to oxidative stress and endothelial injury, thereby promoting the development of PE [38,39]. To date, there have been several studies on the use of NLR for PE prediction, and overall, NLR has a promising application prospect as a biomarker for clinical prediction and disease severity assessment of PE due to its convenience. However, more longitudinal cohort studies are still needed in this area to accurately determine the best timing and cutoff values for clinical use [38,40,41].
Helper T cells (TH)
THs can recognize antigen fragments presented by MHC class II molecules of antigen-presenting cells and activate other direct immune response cells. Th1 and Th17 cells mainly secrete proinflammatory cytokines, which can prevent pregnancy infection, inhibit trophoblast invasion, and stimulate their apoptosis. Th2 and Treg cells mainly secrete anti-inflammatory cytokines, which play an important role in pregnancy immune tolerance. These cytokines are in a dynamic equilibrium state during normal pregnancy, and their abnormal expression may induce immune dysregulation, leading to diseases such as PE [42] (Fig. 4). Several studies have documented that Th1 or Th17 cell-secreted proinflammatory cytokines, such as IFN-γ, are significantly increased and that Th2 or Treg cell-secreted anti-inflammatory cytokines, such as IL-4, are significantly reduced, which is related to the pathogenesis of PE [42,43]. This suggests the detection of these cytokines may help to predict the risk of PE. However, the predictive effect remains to be verified by more clinical studies.
Fig. 4.
The immune dysregulation hypothesis elucidating the pathogenesis of PE.
Abnormal cytokine release in PE patients increases CRP and NGAL expression, leading to placental or renal damage. Placenta-derived factors induce the activation of neutrophils, which in turn produce more inflammatory cytokines to activate the immune response and promote the development of PE. In addition, the activation of neutrophils will also generate a large number of Nets. The dashed line in the figure indicates that this biomarker still needs to be verified by more large-scale clinical longitudinal studies as an indicator to predict the risk of PE.
C-reactive protein (CRP)
CRP is an acute-phase protein produced by the liver or placental cells in response to inflammatory stimuli. It is converted to neurokinin B (NKB) by phosphocholine transferase and activates neurokinin 3 receptor (NK3R), resulting in placental or kidney damage and promoting the secretion of sFlt, which is involved in the pathogenesis of PE [44] (Fig. 4). It was found that serum CRP levels in PE patients were significantly higher than those in normotensive pregnant women and were positively correlated with the severity of the disease [45], suggesting that serum CRP is related to the onset of PE; however, it is more likely to be a diagnostic marker of PE than a predictive marker.
Neutrophil gelatinase-associated lipocalin (NGAL)
NGAL, also known as lipocalin-2 or ferritin, is a member of the lipocalin superfamily and is produced in response to stress. NGAL may cause acute kidney injury and is one of the most effective biomarkers of acute kidney injury [46] (Fig. 4). The level of NGAL in the sera of PE patients was significantly increased, and it was related to the severity of PE. Furthermore, the serum NGAL levels were positively correlated with creatinine, uric acid, and 24 -h proteinuria, suggesting that NGAL is related to renal injury caused by PE [47]. The combination of NGAL with other markers (such as PlGF) can be used to predict the risk of PE.
Vascular endothelial injury hypothesis and related biomarkers
The vascular endothelial injury hypothesis suggests that PE mainly undergoes the following two stages: decreased trophoblast cell infiltration capacity leads to insufficient infiltration of the myometrium, shallow placental implantation, impaired uterine spiral artery remodeling, ischemia, and hypoxia. Then, various cytokines secreted by the placenta enter the maternal circulation, causing extensive vascular endothelial damage. Systemic vascular endothelial injury in pregnant women may damage multiple systems and organs to varying degrees, and with the further development of the disease, PE symptoms, such as proteinuria and hypertension, appear [48].
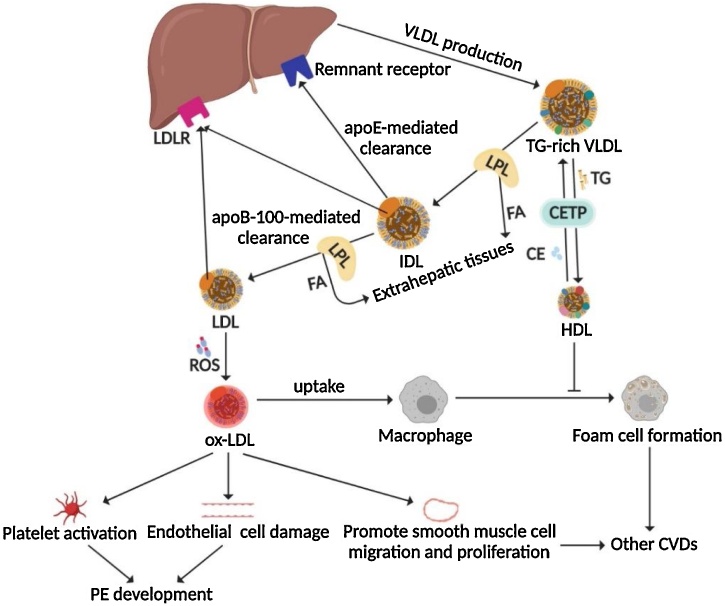
Triglyceride (TG)
TG is the most abundant lipid in the human body. It is well known that dyslipidemia, that is, abnormally elevated levels of TC, LDL, and ox-LDL, especially elevated TG levels and decreased HDL levels, is one of the common risk factors for PE. Elevated serum TG will increase the proportion of low-density LDL particles, and the accumulation of TG-rich residual lipoproteins is prone to form ox-LDL under oxidative stress environments, leading to endothelial dysfunction and systemic inflammatory responses, which are the main clinical features of PE [49] (Fig. 5 and Fig. 6). It was found that the maternal TG level in the first trimester of pregnancy was significantly related to the occurrence of PE [50]. In particular, high TG levels in early pregnancy are associated with the risk of early-onset rather than late-onset PE [51]. Closely monitoring the maternal TG level in early pregnancy is conducive to the early prediction and prevention of PE, especially when it is combined with other markers (such as UTPI and PlGF).
Fig. 5.
The schematic diagram of dyslipidemia involvement with PE and other CVDs.
The metabolic process of transforming from TG-rich VLDL produced by the liver to other lipoproteins, and the possible mechanisms involved in the development of PE and other CVDs.
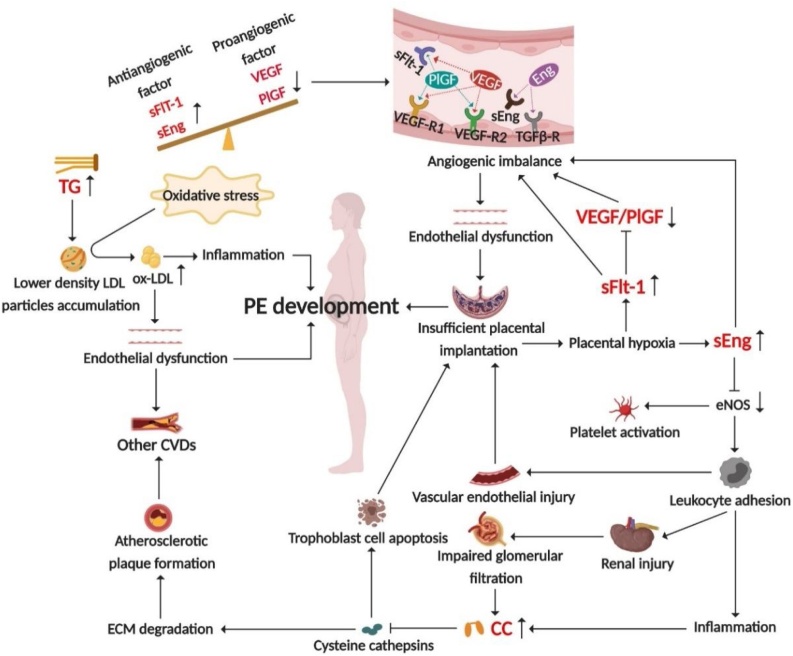
Fig. 6.
The vascular endothelial injury hypothesis illustrating the pathogenesis of PE.
The expression of antiangiogenic factors such as sFlt-1 and sEng increased in PE patients, while the expression of proangiogenic factors such as VEGF decreased, which is manifested as an imbalance of angiogenesis and extensive endothelial injury, resulting in insufficient placental implantation, impaired glomerular filtration, and increased CC level. TG levels in PE patients also elevated, and they were prone to form ox-LDL under oxidative stress, leading to endothelial dysfunction and systemic inflammation, and participating in the pathogenesis of PE.
Placental growth factor (PlGF)
PlGF is mainly secreted by trophoblast cells and vascular endothelial cells. PlGF not only promotes the proliferation and differentiation of trophoblast cells through autocrine signaling but also regulates placental neovascularization and physiological remodeling of the placenta in the form of paracrine signaling [52] (Fig. 6). Studies have found that the serum level of PlGF in patients with PE is significantly lower than the level in normotensive pregnant women and is positively correlated with the severity of PE [53]. However, it still needs to be combined with other maternal indicators to show better predictive value; for example, the sFlt-1/PlGF ratio is more accurate as a predictive marker for PE than using either alone [54,55].
Vascular endothelial growth factor (VEGF)
VEGF is a mitogenic factor for vascular endothelial cells and can effectively promote endothelial cell proliferation and angiogenesis (Fig. 6). It has been demonstrated that the VEGF levels in the placenta and serum of PE patients are significantly lower than those of normotensive pregnant women [56,57]. Therefore, the detection of the VEGF levels may help predict the risk and severity of PE.
Soluble fms-like tyrosine kinase -1 (sFlt-1)
sFlt-1 is produced by splicing the extracellular domain of Flt-1 and is secretable. It can compete with Flt-1 and inhibit the functions of VEGF and PlGF, and the increase in the sFlt-1 levels in PE patients is associated with a decrease in free VEGF and P1GF [58] (Fig. 6). Elevated serum sFlt-1 levels in PE patients could be detected before the onset of the disease, and early-onset (≤32 weeks) PE patients have serum sFlt-1 levels that were 43 times higher than normotensive pregnant women. However, the serum level of sFlt-1 in late-onset (>35 weeks) PE patients increased by only three-fold, so sFlt-1 is considered to have a better predictive value for early-onset PE than for late-onset PE [59].
Soluble endothelin (sEng)
sEng is a homodimeric membrane glycoprotein that can inhibit eNOS activity by binding to TGF-β, can activate platelets, and increase leukocyte adhesion, further causing extensive endothelial damage and participating in the pathogenesis of PE [60] (Fig. 6). Studies have demonstrated that the serum sEng level in PE patients is significantly elevated and is positively correlated with the severity of the disease, while the PlGF/sEng ratio is more sensitive and specific to predict PE risk than using either separately [54,61].
Cystatin C (CC)
CC is a natural cysteine cathepsin inhibitor and is a sensitive endogenous biomarker that reflects changes in the glomerular filtration rate and the degree of glomerular endotheliosis [62,63]. Cysteine cathepsins inhibited by CC may promote trophoblast apoptosis and degrade the extracellular matrix (ECM) to promote the formation of atherosclerotic plaques [64] (Fig. 6). Studies have found that the serum CC level in pregnant women who developed PE in the future increased significantly during the first trimester of pregnancy and increased with the aggravation of PE, which is linearly correlated with the degree of glomerular endothelial hyperplasia [65]. Zhang et al. found that the combination of NGAL and CC can effectively predict PE risk during the first trimester of pregnancy [47]. This suggests that serum CC is a potential biomarker to predict the occurrence of PE.
Summary and outlook
With the development of biotechnology, many PE biomarkers with a potential for the clinical application have been continuously discovered. They are of great clinical significance for identifying high-risk PE populations in early pregnancy and obtaining clinical intervention opportunities as soon as possible. However, many studies on PE biomarkers have been limited to small sample sizes, cross-sectional studies, and differing diagnostic criteria for the disease. Therefore more large-scale longitudinal studies are still needed. The current biochemical indicators of clinical prenatal examination mainly include blood lipids, thyroid function, coagulation function, and hepatorenal function. Therefore, as we mentioned before, biomarkers such as TG can be detected in routine prenatal examinations, which may have good clinical application prospects [66]. Certain biomarkers recommended by FIGO, such as PlGF and PAPP-A, have better predictive effects on PE, so they are suitable for the development of test kits for clinical applications. Additionally, by summarizing the biomarkers involved in these major hypotheses and related mechanisms, we have recognized that PE is heterogeneous and complex. Moreover, indicators related to the single hypothesis mechanism cannot accurately predict the risk of PE in most cases. Therefore, an in-depth study of the biomarkers involved in the pathogenesis of PE may prompt us to add multiple biomarkers that reflect different aspects of disease pathogenesis to combined tests to classify the high-risk PE population in more detail, which is conducive to clinically targeted intervention and prevention of PE. Looking forward, the discovery of novel PE biomarkers based on these major hypotheses will be the main strategy for predicting the risk of PE.
Declaration of Competing Interest
The authors report no declarations of interest.
Acknowledgments
This work was supported by the national natural science foundation of china (project approval number: 81573162) and the national new drug creation program of china (project approval number: 2018ZX09201017-004).
Contributor Information
Li-Kun Gong, Email: lkgong@cdser.simm.ac.cn.
Bing-Shun Wang, Email: wangbingshun@sjtu.edu.cn.
References
- 1.Fardiazar Z., Ramin M., Madarek E.O., Atashkhouei S., Torab R., Goldust M. Complications in premature labor between severe preeclampsia and normal pregnancies. Pak J Biol Sci. 2013;16:446–450. doi: 10.3923/pjbs.2013.446.450. [DOI] [PubMed] [Google Scholar]
- 2.Poon L.C., Shennan A., Hyett J.A. The International Federation of Gynecology and Obstetrics (FIGO) initiative on pre‐eclampsia: a pragmatic guide for first‐trimester screening and prevention. Int J Gynecol Obstet. 2019:1–33. doi: 10.1002/ijgo.12802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Thakoordeen S., Moodley J., Naicker T. Candidate gene, genome-wide association and bioinformatic studies in pre-eclampsia: a review. Curr Hypertens Rep. 2018;20:91. doi: 10.1007/s11906-018-0891-x. [DOI] [PubMed] [Google Scholar]
- 4.Pegoraro R.J., Hira B., Rom L., Moodley J. Plasminogen activator inhibitor type 1 (PAI1) and platelet glycoprotein IIIa (PGIIIa) polymorphisms in Black South Africans with pre-eclampsia. Acta Obstet Gynecol Scand. 2003;82:313–317. doi: 10.1034/j.1600-0412.2003.00085.x. [DOI] [PubMed] [Google Scholar]
- 5.Pegoraro R.J., Chikosi A., Rom L., Roberts C., Moodley J. Methylenetetrahydrofolate reductase gene polymorphisms in black South Africans and the association with preeclampsia. Acta Obstet Gynecol Scand. 2004;83:449–454. doi: 10.1111/j.0001-6349.2004.0355.x. [DOI] [PubMed] [Google Scholar]
- 6.Roberts C.B., Rom L., Moodley J., Pegoraro R.J. Hypertension-related gene polymorphisms in pre-eclampsia, eclampsia and gestational hypertension in Black South African women. J Hypertens. 2004;22:945–948. doi: 10.1097/00004872-200405000-00016. [DOI] [PubMed] [Google Scholar]
- 7.Palei A.C., Spradley F.T., Warrington J.P., George E.M., Granger J.P. Pathophysiology of hypertension in pre-eclampsia: a lesson in integrative physiology. Acta Physiol (Oxf) 2013;208:224–233. doi: 10.1111/apha.12106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Christians J.K., Beristain A.G. ADAM12 and PAPP-A: candidate regulators of trophoblast invasion and first trimester markers of healthy trophoblasts. Cell Adhes Migr. 2016;10:147–153. doi: 10.1080/19336918.2015.1083668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Odibo A.O., Zhong Y., Goetzinger K.R. First-trimester placental protein 13, PAPP-A, uterine artery Doppler and maternal characteristics in the prediction of pre-eclampsia. Placenta. 2011;32:598–602. doi: 10.1016/j.placenta.2011.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brewer J., Liu R., Lu Y. Endothelin-1, oxidative stress, and endogenous angiotensin II: mechanisms of angiotensin II type I receptor autoantibody-enhanced renal and blood pressure response during pregnancy. Hypertension. 2013;62:886–892. doi: 10.1161/HYPERTENSIONAHA.113.01648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cunningham M.W., Jr., Williams J.M., Amaral L. Agonistic autoantibodies to the angiotensin II type 1 receptor enhance angiotensin II-induced renal vascular sensitivity and reduce renal function during pregnancy. Hypertension. 2016;68:1308–1313. doi: 10.1161/HYPERTENSIONAHA.116.07971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Herse F., Verlohren S., Wenzel K. Prevalence of agonistic autoantibodies against the angiotensin II type 1 receptor and soluble fms-like tyrosine kinase 1 in a gestational age-matched case study. Hypertension. 2009;53:393–398. doi: 10.1161/HYPERTENSIONAHA.108.124115. [DOI] [PubMed] [Google Scholar]
- 13.Buttrup Larsen S., Wallukat G., Schimke I. Functional autoantibodies against Endothelin-1 receptor type A and Angiotensin II receptor type 1 in patients with preeclampsia. Pregnancy Hypertens. 2018;14:189–194. doi: 10.1016/j.preghy.2018.10.002. [DOI] [PubMed] [Google Scholar]
- 14.Gadde R., Cd D., Sheela S.R. Placental protein 13: an important biological protein in preeclampsia. J Circ Biomark. 2018;7 doi: 10.1177/1849454418786159. 1849454418786159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chafetz I., Kuhnreich I., Sammar M. First-trimester placental protein 13 screening for preeclampsia and intrauterine growth restriction. Am J Obstet Gynecol. 2007;197:35. doi: 10.1016/j.ajog.2007.02.025. e1-7. [DOI] [PubMed] [Google Scholar]
- 16.Seravalli V., Grimpel Y.I., Meiri H., Blitzer M., Baschat A.A. Relationship between first-trimester serum placental protein-13 and maternal characteristics, placental Doppler studies and pregnancy outcome. J Perinat Med. 2016;44:543–549. doi: 10.1515/jpm-2015-0324. [DOI] [PubMed] [Google Scholar]
- 17.Ma L., Li G., Cao G. dNK cells facilitate the interaction between trophoblastic and endothelial cells via VEGF-C and HGF. Immunol Cell Biol. 2017;95:695–704. doi: 10.1038/icb.2017.45. [DOI] [PubMed] [Google Scholar]
- 18.Haider S., Meinhardt G., Saleh L. Self-renewing trophoblast organoids recapitulate the developmental program of the early human placenta. Stem Cell Rep. 2018;11:537–551. doi: 10.1016/j.stemcr.2018.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kim S.Y., Park S.Y., Kim M.J. Maternal plasma hepatocyte growth factor concentrations in women who subsequently developed preeclampsia. J Genet Med. 2012;9:78–83. [Google Scholar]
- 20.Zhang Y., Zhao H.-J., Xia X.-R. Hypoxia-induced and HIF1α-VEGF-mediated tight junction dysfunction in choriocarcinoma cells: implications for preeclampsia. Clin Chim Acta. 2019;489:203–211. doi: 10.1016/j.cca.2017.12.010. [DOI] [PubMed] [Google Scholar]
- 21.Sriyanti R., Mose J.C., Masrul M., Suharti N. The difference in maternal serum hypoxia-inducible factors-1alpha levels between early onset and late-onset preeclampsia. Open Access Maced J Med Sci. 2019;7:2133–2137. doi: 10.3889/oamjms.2019.601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li J., Wu G., Cao Y., Hou Z. Roles of miR-210 in the pathogenesis of pre-eclampsia. Arch Med Sci. 2019;15:183–190. doi: 10.5114/aoms.2018.73129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lv Y., Lu C., Ji X. Roles of microRNAs in preeclampsia. J Cell Physiol. 2019;234:1052–1061. doi: 10.1002/jcp.27291. [DOI] [PubMed] [Google Scholar]
- 24.Anton L., Olarerin-George A.O., Schwartz N. miR-210 inhibits trophoblast invasion and is a serum biomarker for preeclampsia. Am J Pathol. 2013;183:1437–1445. doi: 10.1016/j.ajpath.2013.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Raijmakers M.T., Dechend R., Poston L. Oxidative stress and preeclampsia: rationale for antioxidant clinical trials. Hypertension. 2004;44:374–380. doi: 10.1161/01.HYP.0000141085.98320.01. [DOI] [PubMed] [Google Scholar]
- 26.Yang S., Lian G. ROS and diseases: role in metabolism and energy supply. Mol Cell Biochem. 2019 doi: 10.1007/s11010-019-03667-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mihu D., Sabau L., Ciortea R. Preeclampsia and the imbalance between reactive oxygen species and antioxidants. Ginecoeu. 2014;10:6–8. [Google Scholar]
- 28.Gilani S.I., Anderson U.D., Jayachandran M. Urinary extracellular vesicles of podocyte origin and renal injury in preeclampsia. J Am Soc Nephrol. 2017;28:3363–3372. doi: 10.1681/ASN.2016111202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Anderson U.D., Gram M., Ranstam J., Thilaganathan B., Kerstrom B., Hansson S.R. Fetal hemoglobin, alpha1-microglobulin and hemopexin are potential predictive first trimester biomarkers for preeclampsia. Pregnancy Hypertens. 2016;6:103–109. doi: 10.1016/j.preghy.2016.02.003. [DOI] [PubMed] [Google Scholar]
- 30.Romantsik O., Agyemang A.A., Sveinsdottir S. The heme and radical scavenger alpha1-microglobulin (A1M) confers early protection of the immature brain following preterm intraventricular hemorrhage. J Neuroinflamm. 2019;16:122. doi: 10.1186/s12974-019-1486-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hsu T.Y., Hsieh T.T., Yang K.D. Proteomic profiling reveals alpha1-antitrypsin, alpha1-microglobulin, and clusterin as preeclampsia-related serum proteins in pregnant women. Taiwan J Obstet Gynecol. 2015;54:499–504. doi: 10.1016/j.tjog.2014.01.007. [DOI] [PubMed] [Google Scholar]
- 32.Liu S., Sun Z., Chu P. EGCG protects against homocysteine-induced human umbilical vein endothelial cells apoptosis by modulating mitochondrial-dependent apoptotic signaling and PI3K/Akt/eNOS signaling pathways. Apoptosis. 2017;22:672–680. doi: 10.1007/s10495-017-1360-8. [DOI] [PubMed] [Google Scholar]
- 33.Sun F., Qian W., Zhang C., Fan J.X., Huang H.F. Correlation of maternal serum homocysteine in the first trimester with the development of gestational hypertension and preeclampsia. Med Sci Monit. 2017;23:5396–5401. doi: 10.12659/MSM.905055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dovinová I., Hrabárová E., Jansen E. ADMA, homocysteine and redox status improvement affected by 7-nitroindazole in spontaneously hypertensive rats. Biomed Pharmacother. 2018;106:1478–1483. doi: 10.1016/j.biopha.2018.07.096. [DOI] [PubMed] [Google Scholar]
- 35.Geldenhuys J., Rossouw T.M., Lombaard H.A., Ehlers M.M., Kock M.M. Disruption in the regulation of immune responses in the placental subtype of preeclampsia. Front Immunol. 2018;9:1659. doi: 10.3389/fimmu.2018.01659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gupta A.K., Hasler P., Holzgreve W., Gebhardt S., Hahn S. Induction of neutrophil extracellular DNA lattices by placental microparticles and IL-8 and their presence in preeclampsia. Hum Immunol. 2005;66:1146–1154. doi: 10.1016/j.humimm.2005.11.003. [DOI] [PubMed] [Google Scholar]
- 37.Gupta A.K., Joshi M.B., Philippova M. Activated endothelial cells induce neutrophil extracellular traps and are susceptible to NETosis-mediated cell death. FEBS Lett. 2010;584:3193–3197. doi: 10.1016/j.febslet.2010.06.006. [DOI] [PubMed] [Google Scholar]
- 38.Panwar M., Kumari A., Hp A., Arora R., Singh V., Bansiwal R. Raised neutrophil lymphocyte ratio and serum beta hCG level in early second trimester of pregnancy as predictors for development and severity of preeclampsia. Drug Discov Ther. 2019;13:34–37. doi: 10.5582/ddt.2019.01006. [DOI] [PubMed] [Google Scholar]
- 39.Kang Q., Li W., Yu N. Predictive role of neutrophil-to-lymphocyte ratio in preeclampsia: a meta-analysis including 3982 patients. Pregnancy Hypertens. 2020;20:111–118. doi: 10.1016/j.preghy.2020.03.009. [DOI] [PubMed] [Google Scholar]
- 40.Oylumlu M., Ozler A., Yildiz A. New inflammatory markers in pre-eclampsia: echocardiographic epicardial fat thickness and neutrophil to lymphocyte ratio. Clin Exp Hypertens. 2014;36:503–507. doi: 10.3109/10641963.2013.863324. [DOI] [PubMed] [Google Scholar]
- 41.Gogoi P., Sinha P., Gupta B., Firmal P., Rajaram S. Neutrophil-to-lymphocyte ratio and platelet indices in pre-eclampsia. Int J Gynaecol Obstet. 2019;144:16–20. doi: 10.1002/ijgo.12701. [DOI] [PubMed] [Google Scholar]
- 42.Bellos I., Karageorgiou V., Kapnias D., Karamanli K.E., Siristatidis C. The role of interleukins in preeclampsia: a comprehensive review. Am J Reprod Immunol. 2018;80 doi: 10.1111/aji.13055. [DOI] [PubMed] [Google Scholar]
- 43.Salazar Garcia M.D., Mobley Y., Henson J. Early pregnancy immune biomarkers in peripheral blood may predict preeclampsia. J Reprod Immunol. 2018;125:25–31. doi: 10.1016/j.jri.2017.10.048. [DOI] [PubMed] [Google Scholar]
- 44.Lowry P., Woods R. The placenta controls the physiology of pregnancy by increasing the half-life in blood and receptor activity of its secreted peptide hormones. J Mol Endocrinol. 2018;60:R23–R30. doi: 10.1530/JME-17-0275. [DOI] [PubMed] [Google Scholar]
- 45.Jannesari R., Kazemi E. Level of high sensitive C-reactive protein and procalcitonin in pregnant women with mild and severe preeclampsia. Adv Biomed Res. 2017;6:140. doi: 10.4103/2277-9175.218032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lei L., Li L.P., Zeng Z. Value of urinary KIM-1 and NGAL combined with serum Cys C for predicting acute kidney injury secondary to decompensated cirrhosis. Sci Rep. 2018;8:7962. doi: 10.1038/s41598-018-26226-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhang H.-B., Fan J.-M., Zhu L.-L., Yuan X.-H., Shen X.-W. Combination of NGAL and cystatin C for prediction of preeclampsia at 10-14 weeks of gestation. Clin Lab. 2019;65 doi: 10.7754/Clin.Lab.2018.180831. [DOI] [PubMed] [Google Scholar]
- 48.Szpera-Gozdziewicz A., Breborowicz G.H. Endothelial dysfunction in the pathogenesis of pre-eclampsia. Front Biosci. 2014;19:734–746. doi: 10.2741/4240. [DOI] [PubMed] [Google Scholar]
- 49.Adank M.C., Benschop L., Peterbroers K.R. Is maternal lipid profile in early pregnancy associated with pregnancy complications and blood pressure in pregnancy and long term postpartum? Am J Obstet Gynecol. 2019;221:150. doi: 10.1016/j.ajog.2019.03.025. e1- e13. [DOI] [PubMed] [Google Scholar]
- 50.Sun L.-J., Xu G.-F., Lv M., Zhou H., Huang H.-F., Luo Q. Predictive value of maternal serum biomarkers for preeclampsia and birth weight: a case-control study in Chinese pregnant women. J Womens Health (Larchmt) 2018;27:1519–1524. doi: 10.1089/jwh.2017.6793. [DOI] [PubMed] [Google Scholar]
- 51.Clausen Torun, Djurovic Srdjan, Henriksen T. Dyslipidemia in early second trimester is mainly a feature of women with early onset pre-eclampsia. Br J Obstet Gynaecol. 2001;108:1081–1087. doi: 10.1111/j.1471-0528.2001.00247.x. [DOI] [PubMed] [Google Scholar]
- 52.Chau K., Hennessy A., Makris A. Placental growth factor and pre-eclampsia. J Hum Hypertens. 2017;31:782–786. doi: 10.1038/jhh.2017.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ghosh S.K., Raheja S., Tuli A., Raghunandan C., Agarwal S. Serum PLGF as a potential biomarker for predicting the onset of preeclampsia. Arch Gynecol Obstet. 2012;285:417–422. doi: 10.1007/s00404-011-1960-4. [DOI] [PubMed] [Google Scholar]
- 54.Kusanovic J.P., Romero R., Chaiworapongsa T. A prospective cohort study of the value of maternal plasma concentrations of angiogenic and anti-angiogenic factors in early pregnancy and midtrimester in the identification of patients destined to develop preeclampsia. J Matern Fetal Neonatal Med. 2009;22:1021–1038. doi: 10.3109/14767050902994754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zeisler H., Llurba E., Chantraine F. Predictive value of the sFlt-1:PlGF ratio in women with suspected preeclampsia. N Engl J Med. 2016;374:13–22. doi: 10.1056/NEJMoa1414838. [DOI] [PubMed] [Google Scholar]
- 56.Agarwal R., Kumari N., Kar R. Evaluation of placental VEGFA mRNA expression in preeclampsia: a case control study. J Obstet Gynaecol India. 2019;69:142–148. doi: 10.1007/s13224-018-1128-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Cim N., Kurdoglu M., Ege S., Yoruk I., Yaman G., Yildizhan R. An analysis on the roles of angiogenesis-related factors including serum vitamin D, soluble endoglin (sEng), soluble fms-like tyrosine kinase 1 (sFlt1), and vascular endothelial growth factor (VEGF) in the diagnosis and severity of late-onset preeclampsia. J Matern Fetal Neonatal Med. 2017;30:1602–1607. doi: 10.1080/14767058.2016.1219986. [DOI] [PubMed] [Google Scholar]
- 58.Nakada Y., Onoue K., Nakano T. AST-120, an oral carbon absorbent, protects against the progression of atherosclerosis in a mouse chronic renal failure model by preserving sFlt-1 expression levels. Sci Rep. 2019;9:15571. doi: 10.1038/s41598-019-51292-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ohkuchi A., Hirashima C., Suzuki H. Evaluation of a new and automated electrochemiluminescence immunoassay for plasma sFlt-1 and PlGF levels in women with preeclampsia. Hypertens Res. 2010;33:422–427. doi: 10.1038/hr.2010.15. [DOI] [PubMed] [Google Scholar]
- 60.Chang X., Yao J., He Q., Liu M., Duan T., Wang K. Exosomes from women with preeclampsia induced vascular dysfunction by delivering sFlt (Soluble fms-like tyrosine kinase)-1 and sEng (Soluble endoglin) to endothelial cells. Hypertension. 2018;72:1381–1390. doi: 10.1161/HYPERTENSIONAHA.118.11706. [DOI] [PubMed] [Google Scholar]
- 61.Zhang L., Li X., Zhou C., You Z., Zhang J., Cao G. The diagnosis values of serum STAT4 and sEng in preeclampsia. J Clin Lab Anal. 2019:e23073. doi: 10.1002/jcla.23073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Strevens H., Wide-Swensson D., Grubb A. Serum cystatin C reflects glomerular endotheliosis in normal, hypertensive and pre-eclamptic pregnancies. BJOG: Int J Obstet Gynaecol. 2003;110:825–830. [PubMed] [Google Scholar]
- 63.Lee H. Cystatin C in pregnant women is not a simple kidney filtration marker. Kidney Res Clin Pract. 2018;37:313–314. doi: 10.23876/j.krcp.18.0146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.van der Laan S.W., Fall T., Soumare A. Cystatin C and cardiovascular disease: a Mendelian randomization study. J Am Coll Cardiol. 2016;68:934–945. doi: 10.1016/j.jacc.2016.05.092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Niraula A., Lamsal M., Baral N. Cystatin-C as a marker for renal impairment in preeclampsia. J Biomark. 2017;2017 doi: 10.1155/2017/7406959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.He Yun-ting, Xiao-jin Wang, Bing-shun Wang. Advances and prospect in first trimester prediction for pre-eclampsia (in Chinese) J Int Obst Gynecol. 2020 (in press) [Google Scholar]