Abstract
Background
Apple Cider Vinegar, (APCV) has been locally associated with a number of health benefits, including protection against oxidative stress and related ailments. It is on this background the present study assessed its protective effects against carbon tetrachloride (CCl4)-induced oxidative damage in kidneys of rats.
Methods
Twenty four adult rats of Wistar strain were randomly assigned to four groups (n = 6). While group I animals served as control; kidney oxidative damage was induced in groups II and III animals using a single intraperitoneal injection of CCl4 (100%, 1.73 mL/kg body weight, BW). Group II animals were left untreated and groups III and IV counterparts were administered APCV (1.56 mL/kg BW) once daily for a period of 7 days. Thereafter, the animals were fasted over night, and sacrificed by cervical dislocation, and samples (blood and kidney tissues) were collected for biochemical/histopathological examinations. Kidney function markers including urea, creatinine, sodium ion (Na+) and potassium ion (k+) were determined in the serum while thin sections of kidneys were processed for histopathological screening.
Results
Compared to the control animals, CCl4 administration caused kidney damage as evidenced by significant (P < 0.05) increase in the evaluated indices (urea, creatinine, Na+ and K+). Interestingly, treatment of CCl4-exposed rats with APCV markedly reversed the above alterations to near normal. Besides, APCV treatment ameliorated the histological derangements (hemorrhagic lesions) caused by CCl4 in the kidney of the experimental rats.
Conclusion
These observations apparently suggest that Apple cider vinegar has the therapeutic potential to protect against renal impairment and attendant malfunction.
Keywords: Apple cider vinegar, Kidney damage, Rats, Carbon tetrachloride, Oxidative stress
1. Introduction
The kidney is one of the extremely vital organs in the body obviously because of its indispensable metabolic roles (excretory and regulatory). Globally, kidney disease of any type is a severe and critical health challenge. With constant exposure to xenobiotics including nephrotoxins through modern day life style, the organ is susceptible to acute injury capable of compromising its physiological state and metabolic functions [1,2].
Carbon tetrachloride (CCl4) is a typical xenobiotic and potent nephrotoxic agent commonly used in experimental studies to assess the ability of a test compound to prevent or protect against tissue derangements [3]. Its toxicity is underlined by production of trichloromethyl radical (CCl3•) during its metabolic activation by cyochrome p450. Worse still, CCl3• is converted to a more hazardous radical known as trichloromethyl peroxylradical (CCl3O2•) under aerobic cellular condition [4]. Both radicals synergistically attack essential biomolecules in the kidney and other body tissues, and are responsible for kidney damage associated with CCl4, a phenomenon which may occur through altered intraglomerular hemodynamic, chronic inflammation, rhabdomyolysis, microangiopathy or tubular cell toxicity [1,2].
Conversely, plants and plant products are usually associated with protection against free radical-induced oxidative damage in the body systems, owing to their antioxidant constituents. In support of this claim, a number of studies have shown that compounds present in different parts of plants (leaf, stem, root, seed or fruit) can protect against oxidative damage stimulated by xenobiotics or free radicals [3,5].
Apple cider vinegar (APCV) is a plant product made from apple and acetic acid. It contains polyphenolic compounds with notable antioxidant properties. APCV is particularly rich in Gallic acid, Catechin, Epicatechin, Chlorogenic acid, Caffeic acid, and P-Coumaric (Budak et al., 2011). The apple-derived product is commonly consumed in various forms by individuals across different social and educational boundaries. Most times it is taken directly and other times used in salad dressings, making of marinades & vinaigrettes, food preservation, and chutneys, among other things. APCV is generally believed to boost the physiological state and function of vital organs in the body by acting as a detoxifying and purifying agent [6,7].
In this regard, this study assessed the protective role of apple cider vinegar in CCL4-induced oxidative kidney damage in Wistar rats. On the basis that there is a huge physiological and genetic similarity between rat and man, it is expected that findings from this study will have relevant applications to humans.
2. Materials and methods
2.1. Collection of apple cider vinegar
Apple cider vinegar (Brag) was purchased from a popular grocery shop in the city of Ibadan Nigeria, and preserved as instructed in manufacturer’s manual.
2.2. Animal management and administration
Twenty four rats of Wistar strain and body weight range of 180–200 g were used for the study. They were obtained from the Animal Unit of the Department of Physiology, University of Ibadan, Nigeria. The animals were handled humanely, kept in plastic suspended cages in a well ventilated and hygienic rat house, under suitable conditions of temperature and humidity. They were provided rat pellets and water ad libitum, and subjected to natural photoperiod of 12 h light/dark cycle. Sequel to a period of acclimatization (14 days), the animals were randomized into four groups (I-IV), a control group (I) and three experimental groups (II, III & IV), with each group containing six animals each (n = 6).
Group I animals served as control and were administered distilled water all through the study. Kidney oxidative damage was induced in groups II and III animals using a single intraperitoneal injection of CCl4 (100%, 1.73 mL/kg body weight, BW). Group II animals were left untreated and served as toxicant group while groups III and IV animals were treated with APCV (1.56 mL/kg BW) once daily for a period of 7 days. At the end of administration, the animals were fasted over night (12 h), and sacrificed by cervical dislocation.
2.3. Tissue preparation for biochemical analysis
Blood samples were collected from the retro orbital sinus of the eye by ocular puncture into non-heparinized bottles for serum analyses of kidney function markers including urea, creatinine, sodium ion (Na+) and potassium ion.(k+) using standard assay kits (Randox Lab Ltd. UK.). Serum was processed from whole blood using a table centrifuge at 3000 revolution per minute (r.p.m). Kidneys were also harvested and processed in 10% formalin solution for histological examination.
2.4. Histopathological processing and examination
Histopathological processing and examination of kidney tissues was carried out as described by [8].
2.5. Statistical analysis
Data analysis was performed using statistical software, Prism graphpad, version 6.4. The statistical significance of difference between groups was analyzed using the one-way analysis of variance (ANOVA), followed by independent-sample t-test. The level of significance was set at P < 0.05. The results were presented as the mean ± SD.
3. Results
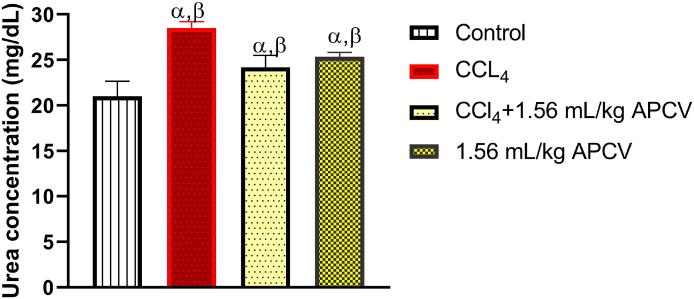
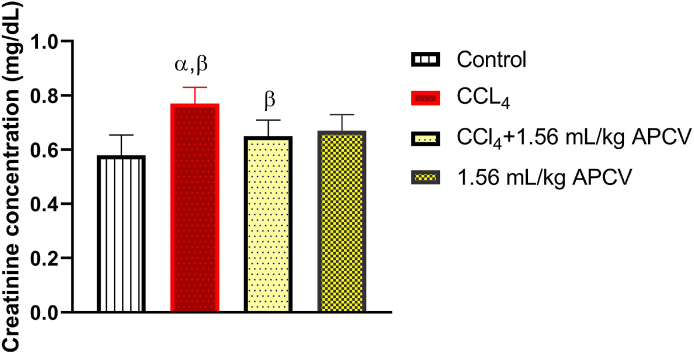
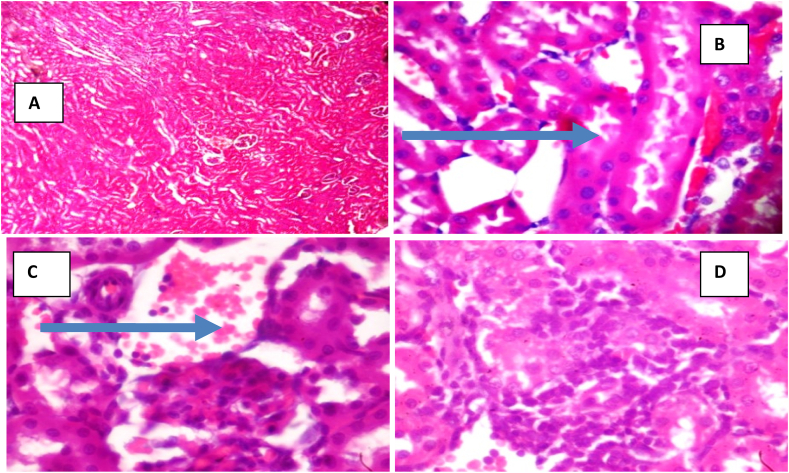
Fig. 1, Fig. 2 and Table 1 respectively show that CCl4 at the administered dose caused significant (p < 0.05) increases in the serum concentrations of urea, creatinine, Na+ and K+ whereas treatment of animals with APCV significantly decreased the serum concentrations of these parameters relative to the untreated group of animals (CCl4 group). The photomicrographs obtained from the histopathological examination of the kidney sections shows that CCl4 cause some degree of lesions in the organ and the damage was ameliorated by APCV treatment (Fig. 3).
Fig. 1.
The effect of Apple cider vinegar on urea concentration in CCl4-induced kidney damage
Values are expressed as mean ± SD of six rats (n = 6). α = Significant when compared to control β = Significant when compared to CCl4 group.
Fig. 2.
The effect of Apple cider vinegar on creatinine concentration in CCl4-induced kidney damage
Values are expressed as mean ± SD of six rats (n = 6). α = Significant when compared to control β = Significant when compared to CCl4 group.
Table 1.
The effect of Apple cider vinegar on serum concentrations of sodium ion (Na+) and potassium ion (K+) in CCl4-induced kidney damage.
| Treatment | Na+ (mmol/L) | K+(mmol/L) |
|---|---|---|
| Control | 136.0 ± 2.09 | 3.53 ± 0.22 |
| CCl4 alone | 139.7 ± 0.58∗ | 4.0 ± 0.10∗ |
| CCl4+ APCV | 137.0 ± 1.79∗∗ | 3.70 ± 1.78∗∗ |
| APCV alone | 137.0 ± 1.00 | 3.60 ± 0.14∗∗ |
Values are expressed as mean ± SD of six rats (n = 6). ∗ = Significant when compared to control ∗∗ = Significant when compared to CCl4 group.
Fig. 3.
Photomicrographs of representative kidney sections of the control and experimental groups
A: Photomicrographs of group I animals showing no visible lesions (x100). B: Photomicrographs of group II animals showing marked disseminated congestion/hemorrhagic lesion (blue arrows) (x400). C: Photomicrographs of group III animals showing congestion (blue arrows) (x400). D: Photomicrographs of group IV animals showing No visible lesion (x400). (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
4. Discussion
The metabolic and regulatory roles of the kidney are indispensable to life, and that arguably qualifies the organ as extremely vital in the body. However, modern day life style through constant exposure to xenobiotics including nephrotoxins makes it susceptible to oxidative derangement. It becomes important to rely on good diets, supplements or plant products that will not only protect the organ against oxidative stress but also keep it healthy. Apple cider vinegar as a plant product (made from apple and acetic acid) apparently possesses the potential to fulfill those requirements, based on its advertisement label. APCV is particularly rich in well known antioxidants such as Gallic acid, Epicatechin, Chlorogenic acid, and P-Coumaric (Budak et al., 2011), thus, capable of functioning as a detoxifying and purifying agent [6,7].
On the other hand, carbon tetrachloride (CCl4) is a typical xenobiotic and potent nephrotoxic agent commonly used in experimental studies [3], and the results obtained in this investigation validate its efficacy. In support of this, previous studies [4] have associated CCl4 metabolism with generation of free radicals (CCl3• and CCl3O2•), capable of altering the intraglomerular hemodynamic or instigating chronic inflammation, rhabdomyolysis, or tubular cell toxicity, leading to kidney damage [1,2]. It is on this background the present study was set up to assess the protective role of Apple cider vinegar in CCL4-induced oxidative damage in the kidneys of Wistar rats.
Serum levels of urea; creatinine, sodium ion (Na+) and potassium ion (K+) are well known clinical indicators of the physiological and functional state of the kidney. Each of these parameters has its normal physiological concentration range. A deviation from this value is taken as a sign of kidney morbidity. Our finding which is consistent with the report of [9] showed that CCl4 caused significant (p < 0.05) increases in the concentrations of the aforementioned indices (urea; creatinine, Na+ and K+) when compared to those of the control counterparts.
Serum urea or blood urea nitrogen (BUN) is a nitrogen-containing compound formed in the liver as end product of protein metabolism and ornithine cycle (also known as urea cycle). More than 80% of urea is excreted through the kidney; the gastrointestinal (GI) tract eliminates the remaining. The reference range for BUN is 10–20 mg/dL or 3.6–7.1 mmol/L (SI units) [10]. Like serum creatinine, the BUN concentration increases with reduction in the glomerular filtration rate (GFR), and vice versa (Dossetor, 1996). This means that serum urea is increased in conditions where renal clearance is decreased due to renal impairment. This explains the observation made in this study in which the serum urea of the animals exposed to CCl4 (a nephrotoxin) was significantly increased by a factor of 42.94% (28.34 mg/dL) compared to the control animals with estimated BUN value of 19.84 mg/dL. The ability of APCV to curtail the increase in BUN by a factor of 14.57% (24.18 mg/dL) as noted in this study is indicative of its potential to improve renal function in the test animals. Nonetheless, rise in BUN concentration can be due to other conditions which may not truly reflect renal functioning [11], hence, BUN is not an absolute kidney function indicator.
Creatinine, on the other hand, is a more critical index for assessing renal function. It is the by-product of creatine phosphate in muscle, and is produced at a constant rate by the body. In most cases, creatinine is cleared from the blood entirely by the kidney and it is the most commonly used endogenous marker for assessment of glomerular function [12]. Clinically, estimate of the glomerular filtration rate (GFR) remains the most practical means of assessing renal function. GFR is the rate (mm/min) at which substances are filtered or cleared from the blood through the kidney glomerulus. Serum creatinine level is elevated when there is a significant decline in the glomerular filtration rate [12]. The normal physiological range for serum creatinine though varies with age, gender and muscle mass, is usually 0.5–1.2 mg/dL or 44–106 μmol/L (SI units) [10]. In this investigation, the toxicant group (group II animals) showed significant increase in serum creatinine concentration (0.78 mg/dL) by a factor as much as 39.28% when compared to the control animals (0.56 mg/dL). Interestingly, treatment with APCV apparently enhanced the ability of the kidney to prevent creatinine accumulation in the blood by lowering the upsurge by 20.51%. This probably indicates that APCV protected rat kidneys against the damaging effects of CCl4. However, the mean values of serum creatinine estimated for both the control and experimental groups in this study were all below the upper reference boundary of 1.2 mg/dL. This may be adduced to the fact that almost half of kidney function must be lost before a marked rise in serum creatinine can be detected [13]. It implies that increase in serum creatinine is relatively gradual at the earliest stages of kidney damage, and 50% of GFR has to be compromised before creatinine baseline of about 0.6 mg/dL would increase to 1.3 mg/dL, and first be noted to be “abnormal” by most reference intervals (http://emedicine.medscape.com/article/238545). Thus, serum creatinine is a late marker of acute kidney injury.
One of the essential functions of the kidney is maintenance of the extracellular fluid volume and electrolyte balance. Serum sodium (Na+) and potassium (K+) are clinical parameters in assessing renal function vis-a-vis electrolyte, acid-base, and water balance. In individuals without any form of renal impairments, sodium accounts for approximately 95% of the osmolity of the extracellular compartment. The normal physiological range for serum sodium is between 135 and 145 mmol/L (US [14].The average daily intake of sodium in adults ranges from 90 to 250 mmol/day but the body requires only 1–2 mmol/day. The excess is excreted by the kidney, which carefully regulate the extracellular sodium level under hormonal influences [15]. Similarly, appropriate renal excretion or reabsorption of potassium is extremely important in maintaining potassium homeostasis. The normal potassium (K+) concentration is given as a serum potassium level between 3.5 and 5.0 mEq/L. As renal function declines, serum levels of sodium and potassium are elevated, and when the increase exacerbates beyond the upper reference boundaries for these ions, conditions respectively known as hypernatremia (serum sodium concentration exceeding 145 mmol/L) and hyperkalemia (serum potassium concentration exceeding 5.0 mEq/L) ensue [16].
Although the serum levels of sodium and potassium obtained in this study for all the groups were within the reference range, nonetheless, it is important to note that relative to the control group, the group II animals (untreated CCl4 exposed animals) recorded significant increases in both serum sodium and potassium, whereas the increases were lowered by co-administration of APCV in the treated group. This observation further supports the protective role of APCV against renal impairments.
Oxidative damage plays a key role in the pathogenesis of chemical-induced renal damage, and free radicals are obviously central in the mechanisms that lead to kidney toxicity. Interpretations of the results of the study thus far suggest that CCl4 to a large extent, inflicted renal impairments on the experimental rats and APCV ameliorated the damage caused by CCl4. This assertion is substantiated by the photomicrographs obtained from the histopathological examination of the kidney sections harvested from the control and experimental animals, which revealed that CCl4 induced notable lesions in kidneys of rats, and the damage was ameliorated by APCV treatment. The protective ability of APCV against renal impairments may be attributed to its array of polyphenolic compounds with notable antioxidant properties. APCV is particularly rich in Gallic acid, Epicatechin, Chlorogenic acid, and P-Coumaric among others (Budak et al., 2011). Besides, it is expedient to clearly state that administration of APCV to physiologically healthy rats did not cause any observable abnormality in the animals, attesting to the safety of the plant-based product when used at the recommended amounts.
5. Conclusion
Findings in this study suggest that apple cider vinegar to an extent protected experimental rats against CCl4-induced kidney toxicity. The commercially sold plant-base product (APCV) may provide some degree of therapeutic protection against acute renal impairments in humans.
CRediT authorship contribution statement
F.O. Asejeje: Conceptualization, Supervision, Writing - original draft, Methodology. O.M. Ighodaro: Methodology, Writing - review & editing. G.I. Asejeje: Conceptualization, Validation, Writing - review & editing. A.M. Adeosun: Methodology, Software, Formal analysis.
References
- 1.Schetz M., Dasta J., Goldstein S., Golper T. Drug-induced acute kidney njury. Curr Opin Crit Care. 2005;11(6):555–565. doi: 10.1097/01.ccx.0000184300.68383.95. [DOI] [PubMed] [Google Scholar]
- 2.Zager R.A. Pathogenetic mechanisms in nephrotoxic acute renal failure. Semin Nephrol. 1997;17(1):3–14. [PubMed] [Google Scholar]
- 3.Ighodaro O.M., Akinloye O.A. Sapium ellipticum (Hochst) Pax leaf extract: antioxidant potential in CCl4-induced oxidative stress model. Bull Fac Pharm Cairo Univ. 2018 Jun 1;56(1):54–59. [Google Scholar]
- 4.El-mohsen Ali S.A., Abdelaziz D.H.A. The protective effect of date seeds on nephrotoxicity sinduced by carbon tetrachloride in rats. Int J Pharmaceut Sci Rev Res. 2014;26(2):62–68. [Google Scholar]
- 5.Tatiya A.U., Surana S.J., Sutar M.P., Gamit N.H. Hepatoprotective effect of poly herbal formulation against varioushepatotoxic agents in rats. Pharmacogn Res. 2012;4:50–56. doi: 10.4103/0974-8490.91040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vijayakumar C. Evaluation of household sanitizers for reducing levels of Escherichia coli on iceberg lettuce. J Food Prot 2002. 2002;65:1646–1650. doi: 10.4315/0362-028x-65.10.1646. [DOI] [PubMed] [Google Scholar]
- 7.Hlebowicz J. Effect of apple cider vinegar on delayed gastric emptying in patients with type 1 diabetes mellitus: a pilot study. BMCGastroenterol2007. 2007;7:46. doi: 10.1186/1471-230X-7-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Arthur S.J., John B. Wolfe Medical Publishers, Ltd.; London: 1978. A colour Atlas of histopathological staining techniques; pp. 14–20. [Google Scholar]
- 9.Awodele O., Adeneye A.A., Aiyeola S.A., Benebo A.S. Modulatory effect of Mangifera indica against carbon tetrachloride induced kidney damage in rats. Interdiscipl Toxicol. 2015 Dec 1;8(4):175–183. doi: 10.1515/intox-2015-0027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pagana K.D., Pagana T.J., Pagana T.N. fourteenth ed. Elsevier; St. Louis, Mo: 2019. Mosby’s diagnostic & laboratory test reference. [Google Scholar]
- 11.Levey A.S. Measurement of renal function in chronic renal disease. Kidney Int. 1990 Jul;38(1):167–184. doi: 10.1038/ki.1990.182. [Medline] [DOI] [PubMed] [Google Scholar]
- 12.Lujambio I., Sottolano M., Luzardo L., Robaina S., Krul N., Thijs L. Estimation of glomerular filtration rate based on serum cystatin C versus creatinine in a Uruguayan population. Internet J Nephrol. 2014;2014:837106. doi: 10.1155/2014/837106. [Medline] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Laskin B.L., Nehus E., Goebel J., Furth S., Davies S.M., Jodele S. Estimated versus measured glomerular filtration rate in children before hematopoietic cell transplantation. Biol Blood Marrow Transplant. 2014 Jul 17 doi: 10.1016/j.bbmt.2014.07.008. [Medline] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Food and Drug Administration Blood serum chemistry - normal values. Invest Operat Manual. 2015 https://www.fda.gov/downloads/ICECI/Inspections/IOM/UCM135835.pdf Available at: [Google Scholar]
- 15.Rifai N., Horvath A.R., Wittwer C.T., editors. Tietz textbook of clinical chemistry and molecular diagnostics. sixth ed. Elsevier; New York, NY: 2018. [Google Scholar]
- 16.Muhsin S.A., Mount D.B. Diagnosis and treatment of hypernatremia. Best Pract Res Clin Endocrinol Metabol. 2016 Mar;30(2):189–203. doi: 10.1016/j.beem.2016.02.014. [Medline] [DOI] [PubMed] [Google Scholar]