Highlights
-
•
High hemodynamic response in the AI and SMA in the FoG when an APA was required.
-
•
Connectivity between the right and left insulae was correlated with severity of FoG.
-
•
Both groups showed different brain network organizations between SMA and bilateral AI.
-
•
SMA was found to be a hub in patients with FoG when an APA was required.
Keywords: Movement disorders, fMRI, Anterior insula, Supplementary motor area
Abstract
Specific impairments of anticipatory postural adjustment (APA) during step initiation have been reported in patients with Parkinson’s disease (PD) and freezing of gait (FoG). Although APA disruption has been associated with FoG, there is scarce knowledge about its neural correlates. We sought to better understand the neural networks involved with APA in patients with FoG by assessing the level of hemodynamic response of specific brain regions and the functional connectivity during the leg lifting task. In the current investigation, APAs of patients with PD, with and without (nFoG) freezing were assessed during a leg lifting task in an event-related, functional magnetic resonance imaging (er-fMRI) protocol. Results identified a high hemodynamic response in the right anterior insula (AI) and supplementary motor area (SMA) in the FoG group when an APA was required. The nFoG had stronger connectivity between the right and left insulae than the FoG group. The strength of this connectivity was negatively correlated with the severity of FoG. Both groups showed different brain network organizations comprising the SMA and the bilateral AI. The SMA was found to be a hub in patients with FoG when an APA was required for the task. Our findings suggest that both groups used compensatory mechanism comprising the insulae during APA. Neither group used the entire network comprised of the insulae and SMA to accomplish the task. The FoG group relied more on SMA as a hub than as part of a broader network to exchange information during the APA.
1. Introduction
Freezing of gait (FoG) in people with Parkinson’s disease (PD) is one of the most debilitating motor symptoms. FoG greatly impairs the ability to walk safely, resulting in a higher risk of falls, fall-related injuries, anxiety, and, consequently, a significant decrease in quality of life (Walton et al., 2015). One of the most common phenotypes of FoG is start hesitation, characterized by reduced, prolonged APA, and a delay in the voluntary step during step initiation (Schaafsma et al., 2003).
Two important systems are involved in step initiation: one system comprises cortical and subcortical levels, preparing the body in advance for movement by adjusting the muscle tone; the other system cortically modulates the step (Massion, 1992, Takakusaki, 2017). Given the challenge of shifting from an upright stance with both feet on the ground to a more unstable condition characterized by moving the body forward, these two systems must be coupled to elicit correct background muscle tone during the forward movement of the sway leg. When these systems are working properly, step initiation is characterized by an initial backward movement of the center of pressure toward the moving leg, then to the support leg, which is called anticipatory postural adjustment (APA). Once the center of body mass is adequately moved toward the support leg, the moving leg is released to take a step (Aruin, 2002).
Step initiation is thought to rely on circuits connecting subcortical areas (especially mesencephalic, subthalamic and cerebellar locomotor regions) with frontal areas including the supplementary motor area (SMA) (de Lima-Pardini et al., 2017a, de Lima-Pardini et al., 2017b, Jacobs et al., 2009a, Takakusaki, 2017). These cortical areas are thought to be responsible for a feedforward mode of control, projecting to the corticospinal tract (CST) (He et al., 1995), with substantial connections to subcortical locomotor regions through cortico-reticular tracts. The SMA modulates step initiation by sending information about the required postural muscle tone for a context-specific stepping pattern via cortico-reticular and reticulo-spinal tracts (Keizer and Kuypers, 1989, Takakusaki, 2017). In parallel, SMA together with the primary motor cortex contribute to the output from the CST eliciting voluntary motor commands to step.
Patients with FoG have impairments in the frontal cortex circuitry (Gallardo et al., 2018, Matsui et al., 2005), along with increased functional connectivity between SMA and areas known to be involved in step initiation, like cerebellar locomotor (CLR) and mesencephalic locomotor regions (MLR) (Fling et al., 2014a). These increases in connectivity were found to be positively correlated with FoG severity. Hemodynamic response of the frontal cortex, including SMA, increases during FoG (Maidan et al., 2016, Shine et al., 2013a, Vercruysse et al., 2014). Accordingly, a recent study showed that the APA amplitude increases during arrests of step initiation (Schlenstedt et al., 2018). We propose that increased APA amplitude may be due to increased SMA activity during FoG. Freezers were also shown to increase the activity of the anterior insula (AI) during FoG (Shine et al., 2013b). AI is a key area in the salience network (Seeley et al., 2007). Neuroimaging findings hint at a role for the AI in error awareness (Klein et al., 2007), encompassing emotional, cognitive, and autonomic processing. Therefore, besides the sensorimotor system, FoG seems to compromise brain areas known to be involved in other systems.
Patients with FoG show decreased structural and functional connectivity between the SMA and the subthalamic nucleus (STN), the hyperdirect pathway, responsible for modulation of response inhibition (Frank, 2006, Hanna-Pladdy et al., 2001). Besides, decreased connectivity in FoG between the dorsolateral prefrontal cortex (DLPFC), key for response inhibition, and the basal ganglia support the notion that FoG is associated with a decoupling between inhibitory control and movement (Shine et al., 2013b). Impairment of frontal networks related to inhibitory control may be related to deficits in APAs during self-initiated gait in FoG, including alterations in amplitude (Mancini et al., 2009, Schlenstedt et al., 2018), timing (Cohen et al., 2017) and number of APAs (Jacobs et al., 2009b) compared to those without FoG (nFoG). However, the neural correlates of APA alterations in patients with FoG have not been investigated yet.
Graph theory analysis has been used to investigate the organization of PD brain networks. This mathematical concept describes and quantifies the efficiency of a network (graph) based on the distance between the areas (nodes) and its number of connections (edges), which represents the energy cost for effective information processing (Bullmore and Sporns, 2012, Latora and Marchiori, 2001). These connections can be measured based on its global and local efficiency (network performance) and centrality (most important area in the network). It is known that PD patients have disrupted brain networks (Olde Dubbelink et al., 2014), which differ according to PD subtype (Zhang et al., 2014). However, there is scarce evidence on the efficiency of brain networks in patients with FoG. One study showed that PD patients with postural instability and gait difficulty (PIGD) have more disrupted hubs in the cerebellum (Ma et al., 2017). To our knowledge, only one study has investigated the FoG brain networks using graph theory (Maidan et al., 2019). They found that the dorsal attention network is affected in patients with FoG compared to healthy controls and PD patients without FoG. However, a specific network comprising the areas more consistently associated with movement initiation in patients with FoG has not been tested.
Our group published a protocol to assess neural control of APA in an MRI environment (de Lima-Pardini et al., 2017a, Lomond et al., 2013). Though the MRI limits the study of postural control given the required supine position and restricted movement of the body, our approach can be thought as an appropriate model for APA assessment due to similar lower limb anticipatory posture-movement coupling present during step initiation and in the proposed leg lifting task while lying inside the scanner. The task performed in the scanner is to lift the leg from the hip while in the supine position, requiring anticipatory weight shifting to the opposite side before the onset of the leg lift, in analogy with the transition from upright stance to gait. In contrast, when physical support is provided by placing pads under the knee, the APA is suppressed, similar to when hand support is given during step initiation (de Lima-Pardini et al., 2017b). This proof of concept study showed that the SMA shows high blood oxygen level-dependent (BOLD) in healthy young subjects when contrasting the conditions with versus without APA suppression (de Lima-Pardini et al., 2017a). Our protocol for assessing APA in fMRI could be an advantage to assess the pathophysiology of freezing of gait. It is well known that due to its transitory characteristic, FoG is difficult to provoke in a clinical setting (Mancini et al., 2019). Given that APAs in PD patients with FoG are consistently reduced relative to PD patients without FoG, brain data could be more reliably acquired during APA measurement compared with a situation in which motor arrests have to be elicited. In the present study, we used the same supine leg lifting protocol with fMRI to assess neural correlates of APAs in patients with PD, with and without FoG.
In this study, we sought to better understand the neural networks involved with APA in patients with FoG by assessing the level of hemodynamic response of specific brain regions and the functional connectivity during the leg lifting task. We hypothesized that during the task requiring an APA, patients with FoG would show more involvement of brain areas known to be associated with FoG compared to patients without FoG; that patients with FoG would show less global and local efficiency and less centrality than patients without FoG; and that worse inhibitory control, APA behaviour, and clinical measures from FoG patients would be correlated with the level of BOLD signal in areas known to be involved in FoG.
2. Methods
2.1. Participants
Thirty-eight right-handed, mid-stage patients with idiopathic PD (Hoehn and Yahr, 2001), diagnosed according to the UK Brain Bank criteria (Hughes, 1992) participated in this study. Inclusion criteria were: (1) a Hoehn & Yahr score of 3; (2) availability to engage in the task training, brain imaging, and neuropsychological assessments; (3) capacity to walk independently, without assistance devices; (4) ability to understand instructions to perform the experimental motor tasks; (5) absence of neurological diseases other than PD; (6) absence of musculoskeletal impairments possibly affecting performance of the experimental tasks. The exclusion criteria were as follows: (1) difficulty performing the experimental motor tasks (e.g. not lifting the leg following the specific command); (2) drop out of any assessment; (3) poor quality of the brain volumes acquired during the fMRI: head motion above 1 mm (Seto et al., 2001). Patients were classified as having FoG if they answered affirmatively the first question of the New FoG Questionnaire (NFOGQ) (Giladi et al., 2009, Nieuwboer et al., 2009) following the presentation of a video showing examples of individuals experiencing FoG. All the FoG patients scored 3 or 4 in the NFOGQ question: “How frequently do you experience episodes of freezing when initiating the first step?”, indicating moderate or severe FoG during step initiation. All evaluations were taken during the ON state of dopaminergic medications, within 2 h after the first levodopa dosage in the morning. All participants provided informed consent, and the experimental procedures were approved by the Institutional Review Board of the University of São Paulo. All experiments were performed following the Declaration of Helsinki.
2.2. Cognitive assessment
Cognitive evaluation was performed by a neuropsychologist to measure general cognitive function, global and inhibitory executive functions, functionality, and mood symptoms. Assessments included the Montreal Cognitive Assessment (MoCA) (Nasreddine et al., 2005), Clock Drawing test (Agrell and Dehlin, 2012), Frontal Assessment Battery (FAB) (Dubois et al., 2000), Phonemic Verbal Fluency (FAS) test (Borkowski et al., 1967), Hamilton Depression Rating Scale (HDRS) (Hamilton, 1960), Frontal Behavioral Inventory (FBI) (Kertesz et al., 1997), Stroop-III (Scarpina and Tagini, 2017), Pfeffer Questionnaire (Dutra et al., 2015), and Barrat Impulsiveness Scale (BIS) (Patton et al., 1995). To measure inhibitory control, we used three components from the regular FAS: verbal intrusion errors (FAS-INT) measures the failure to inhibit a pre-selected wrong answer; mean cluster size (FAS-MCS) is the production of words within semantic or phonemic subcategories; switching (FAS-switch) is the ability to shift between clusters (Troyer et al., 1997). The Stroop task, BIS, and FBI were also used to investigate inhibitory control (BIS-non-planning and FBI positive symptoms). The values obtained from FAB, BIS, FAS, FBI, and Stroop were transformed in z-scores correcting for the years of education.
2.3. Leg lifting task inside the scanner
Before performing the task in the scanner, participants were trained at the task under similar conditions as in the scanner until they demonstrated complete understanding of the task and consistent performance. Participants with inconsistent performance or poor understanding did not perform the task in the scanner. Participants performed the task of lifting the right leg in a supine position inside the MRI scanner under two conditions: with the moving leg supported (-APA) or unsupported (+APA). In the -APA condition, a pad was put below the knee of the moving leg to support the thigh; participants were asked to raise their lower leg by extending the knee. This condition reduced the need for a downward pressure APA with the opposite (left) leg. In the + APA condition, participants raised their leg from the hip without the knee support, which required anticipatory downward pressure with the opposite leg to stabilize the pelvis before leg lift. A touch sensor was placed on the right foot, which sent a signal to the force measurement system when the foot touched an adjustable brass bar placed 1 cm above (restricting the amplitude of the foot movement, to reduce the amount of head movement provoked).
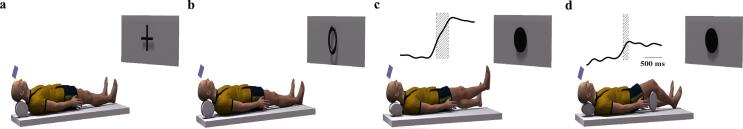
Visual commands were chosen over auditory for two main reasons: firstly, the acuity of the visual system could be corrected with glasses compatible with the MRI environment secondly, visual commands are not interfered by the loud noise of the scanner. Lying inside the scanner, participants saw three stimuli (cross, black circle, and white circle). Commands were presented on a mirror over subjects’ eyes, reflecting a screen 2.5 m away. The duration of each stimulus was randomized through Poisson probability distribution to improve the estimation of the hemodynamic response function (HRF) during brain volume acquisition (Hagberg et al., 2001). Duration of the first stimulus (cross - to relax) was 5.5–8.5 s (interstimulus interval). Duration of the prompt stimulus (first circle, black or white) was 1–3 s in steps of 0.5 s. The imperative stimulus to raise the leg (second circle, black or white) was presented for up to 5 s - the stimulus vanished at the onset of the leg lifting (signaled through a pressure sensor in the support base). Participants performed 30 trials of each condition of the leg lifting task in the MRI scanner. A light-coupled trigger was used to synchronizes the stimuli, the vertical forces applied by the right foot, and the movement of the left foot with brain volume acquisition. Head motion was prevented by using lateral pillows, pads, and tapes around the head. Also, velcro-connected bands were used to prevent trunk motion. Fig. 1 represents the experimental setup in the scanner with samples of APA curves obtained from the force measurement system in the conditions of unsupported (panel c) and supported (panel d) knee.
Fig. 1.
Experimental setup in the scanner. The sequence was to relax during the display of the cross (a); mentally prepare during the open or closed circle – open circle in this example (b); move the right leg during the open or closed circle – closed circle in the example, without support (+APA) (c) and with support (-APA) (d). The commands were displayed on a monitor and reflected on a mirror inside the scanner.
2.4. Motor performance variables
The APA onset was defined as the increase of the vertical force of the left foot by 2 standard deviations above the baseline mean (average of the previous 500 ms). APA amplitude was defined as the first peak of the vertical force after the APA onset. The amplitude was normalized by the average of 300 ms preceding APA onset (baseline). Leg lift onset was defined as the abrupt drop of the vertical force of the pressure sensor for the right moving leg. APA duration was defined as the time from the APA onset until the leg lift onset. Trials with multiple APAs were eliminated from the analysis, as they might be associated with actual motor arrests.
2.5. Image acquisition
Images were obtained in a 3.0 T MR system (Achieva 3.0 T, Philips – The Netherlands) with a 32-channel head coil (80 mT/m gradient maximum amplitude). BOLD-sensitive images were acquired using T2*- weighted gradient echo-planar imaging (EPI), SENSE acquisition: TR = 2.000 ms, TE = 30 ms, 40 slices, 3.0 mm slice thickness, 0.3 mm interslice gap, 3.0 mm isotropic voxels, 214 volumes (acquisition time: 6 m 58 s). Anatomical T1-weighted 3D images were used for reference and image registration (T1-FFE; TR = 7 ms, TE = 3.2 ms, 180 slices, FA = 8, 1 mm isotropic voxels).
2.6. Image processing for maps of brain activation
FSL software (www.fmrib.ox.ac.uk/ fsl/) was used to process the data and to obtain the BOLD (blood oxygen level-dependent) signal in both conditions. The following four stages were accomplished in analyzing the brain volumes:* (1) movement correction and calculation of mean displacement (MCFLIRT); (2) spatial smoothing (FWHM = 5 mm); (3) spatial normalization to standard space (affine, 12 DoF) (Jenkinson et al., 2002); (4) activation brain maps using a general linear model (GLM) implemented in FILM (FMRIB's Improved Linear Model) routines based on a semi-parametric estimation of residuals autocorrelation. The event of interest was the time from the onset of the first stimulus (circle for mental preparation) until 1 s after leg lifting onset. In the first-level analysis, a linear model was implemented to estimate BOLD signal in the event of interest compared to resting periods. In the second level, the contrast APA+ > APA- was acquired for each subject. In the third level, contrast maps comparing freezers and non-freezers were obtained using a mixed-effects model to include within-subject variances of parameter estimates. Age, disease duration, and levodopa equivalent daily dose were used as covariates for the group map analysis. Significance was set at 1% for the single-voxel level and at 5% (corrected for multiple comparisons using the Gaussian Random Field theory) at a mass-cluster level for group analyses. The beta of BOLD signal change from the regions of interest (ROIs) was extracted using the featquery processing routine from FSL. The whole brain analysis was thresholder using clusters determined by Z-score > 2.3 and a corrected cluster significance threshold of p < 0.05. The ROIs selected for this study are those known to be more consistently involved in the FoG and the APA control. The following coordinates in MNI were selected: bilateral cerebellar locomotor region (CLR): x = +−8, y = −52, z = −24, radius = 6 mm (Fling et al., 2014a), bilateral dorsolateral prefrontal cortex (DLPFC): x = + −42, y = 26, z = 28, radius = 8 mm (Shine et al., 2013a), bilateral primary motor area (M1): x = +−6, y = −31, z = 67, radius = 8 mm (Shine et al., 2013a), central supplementary motor area (SMA): x = 0, y = −11, z = 60, radius = 10 mm (Prodoehl et al., 2008), central mesencephalic locomotor region (MLR): x = 0, y = −29, z = −28, radius = 6 mm (Fling et al., 2014b) and bilateral anterior insula (AI): x = +−38, y = 21, z = −3, radius = 6 mm (Shine et al., 2013a), subthalamic nuclei (STN): x = +−12, y = −13, z = −8, radius = 6 mm (Prodoehl et al., 2008). The registration of the ROIs in the functional image was done on a single subject level.
2.7. Connectivity analysis
We computed ROI to ROI connectivity based on the result of the ROI analysis using the Matlab-based CONN toolbox (https://web.conn-toolbox.org/). Analysis followed the steps described elsewhere (Whitfield-Gabrieli and Nieto-Castanon, 2012). The conditions APA + and APA- were analyzed separately for the contrasts FoG > nFoG and nFoG > FoG. Age, disease duration, and levodopa equivalent daily dose were used as covariates. The functional volumes were slice-timing corrected, realigned, normalized, and smoothed. CONN implements the CompCor (component-based noise correction method) for temporal and spatial preprocessing to remove confounds in the BOLD signal such as physiological noise and head motion. A non-parametric two-sided multi-voxel pattern analysis (MVPA) omnibus test was used with p-uncorrected at the cluster level for p < 0.05. The strength of connectivity is displayed as effect size (average difference in Fisher-transformed correlation coefficients between the functional connectivity values for each pair of ROIs).
2.8. Graph theory analysis
A network is formed by the nodes or vertices and the connections between them. In this study, graph analysis was performed using CONN for the selected ROIs of the connectivity analysis comparing the global efficiency, local efficiency, clustering coefficient, and “betweenness centrality” between the groups. The correlation coefficient and z-score with False Discovery Rate (FDR) were corrected at p < 0.05 (two-sided). The threshold to detect the presence or absence of connections between pairs of selected ROIs was set as r = 0.15. Global efficiency is a way to assess the path length and efficiency of the graph or network. It is characterized by the inverse of the average shortest path between the nodes, so the shortest paths result in high efficiency of the network. The local efficiency of a node is the inverse of the shortest path connecting all its neighbors. Clustering coefficient is the probability that all neighbors relate to a specific node. High clustering would represent the robustness of a network, i.e. the resilience against random network damage. Betweenness centrality measures the number of times a node is crossed by the shortest paths in the graph; high values indicate that the node is a hub (Bassett and Bullmore, 2009, Rubinov and Sporns, 2010).
2.9. General statistical analysis
Unpaired Mann-Whitney U tests were used to compare the group averages of all the variables. The association between the brain and behavioral results was assessed through the Spearman test, controlled by disease duration, age, and levodopa dosage. Statistical significance was set at p < 0.05. The software package JASP (version 0.13.1) was used to run the analyses. Considering the addition of three covariates in brain analysis, and the small sample size, we decided not to correct for multiple comparisons, as this might lead to false negatives.
3. Results
Table 1 shows the demographics and clinical characteristics of both FoG and nFoG groups. Age, PD duration, and levodopa-equivalent daily dose were statistically different between the groups; FoG had longer disease duration and higher levodopa dose, and nFoG were older. The APA duration was non-significantly longer in the FoG group than the nFoG group. The two groups showed similar APA amplitude. There was a positive correlation between APA duration and APA amplitude only in the FoG group (r = 0.67, p = 0.008).
Table 1.
Mean (SD) values, and range values of demographics and motor behavior information for the FoG and nFoG groups with p-values from unpaired two-tailed Mann-Whitney U test.
| Variable | FoG | nFoG | P value |
|---|---|---|---|
| Number | 20 | 18 | – |
| Age (y) | 57.60 (11.48) 26–73 |
67.44 (6.02) 56–78 |
0.004 |
| Gender (F/M) | 6/14 | 5/13 | – |
| Disease duration (y) | 12.65 (5.63) | 6.47 (4.17) | <0.001 |
| 5–26 | 2–17 | ||
| UPDRS Part III | 33.50 (11.05) | 28.50 (10.48) | 0.192 |
| 17–54 | 9–47 | ||
| LEDD | 753.80 (488.20) | 437.50 (291.10) | 0.012 |
| 150–2400 | 50–1200 | ||
| NFOGQ | 18.95 (6.30) | 0 | – |
| 7–29 | |||
| APA duration (ms) | 253.00 (84.61) | 210.00 (58.93) | 0.133 |
| 126.16–421.87 | 143.75–356.91 | ||
| APA amplitude (normalized) | 0.35(0.08) | 0.33 (0.08) | 0.545 |
| 0.22–0.49 | 0.16–0.46 |
In bold: significant results (p < 0.05) of Mann-Whitney two-tailed. Abbreviations: UPDRS Part III (Unified Parkinson’s Disease Rating Sale Part III); H&Y (Hoehn & Yahr scale); LEDD (levodopa equivalent daily dose); NFOGQ (New Freezing of Gait Questionnaire); APA (anticipatory postural adjustment).
The following results are depicted as mean (M), standard deviation (SD), magnitude of the Mann-Whitney test (W), and r (magnitude of the correlation).
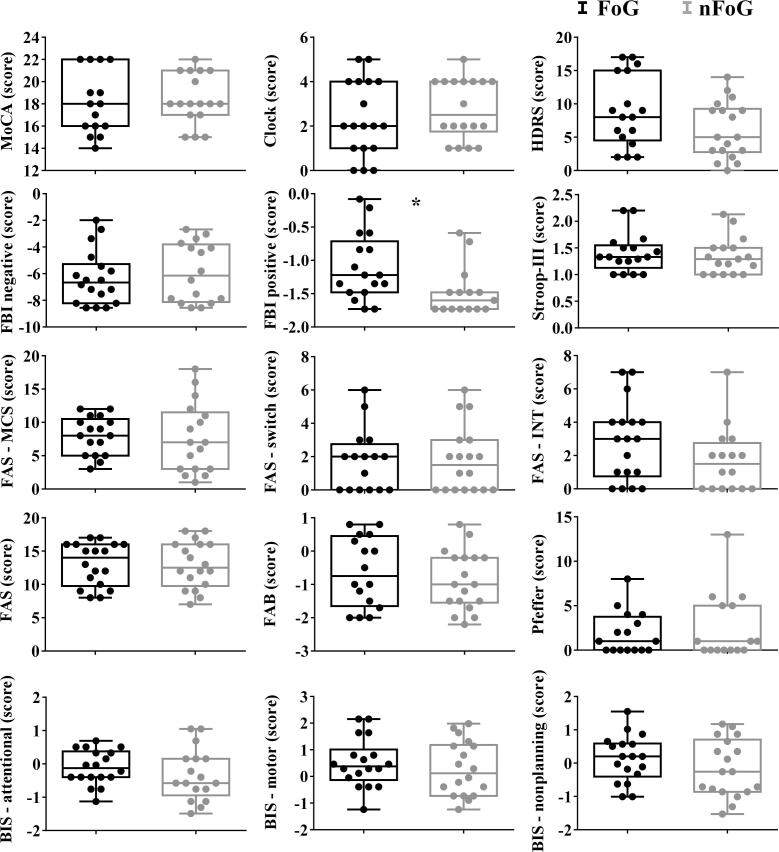
Comparison between the groups for the cognitive variables is represented in Fig. 2. Only the score of the positive symptoms of FBI showed significance due to higher values of impulsivity for FoG (M = -1.10, SD = 0.50) than for nFoG (M = -1.48, SD = 0.37), W = 193.50, p = 0.012. Note that other variables that measure inhibitory control were non-significantly worse in the FoG group than the nFoG group (FAS measures and BIS non-planning).
Fig. 2.
Boxplots and dispersion of individual scores obtained from the cognitive tests for both groups. The asterisk indicates p < 0.05. Abbreviations: MoCA – Montreal Cognitive Assessment, HDRS – Hamilton Depression Scale; FBI – Frontal Battery Inventory; BIS - Barrat Impulsiveness Scale.
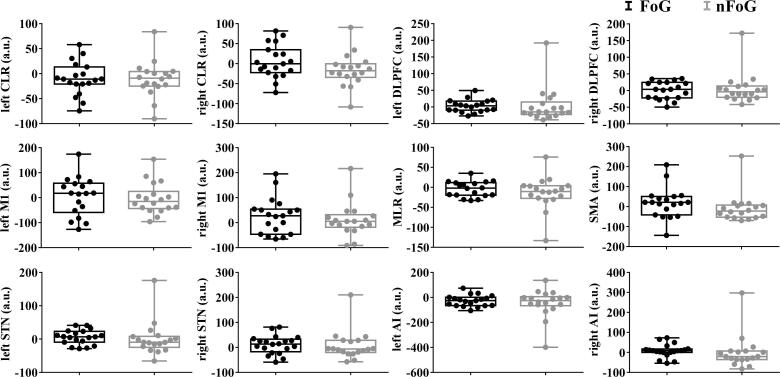
The whole brain analysis showed no differences of the BOLD signal between groups. Results of ROI analysis for the contrast (+APA > -APA) showed larger BOLD signal in the SMA for FoG (M = 18.39, SD = 76.18) than nFoG (M = -8.71, SD = 36.67). Regarding the analysis showed larger BOLD signal in the AIr for FoG (M = 7.70 SD = 32.51) than nFoG (M = -0.78, SD = 82.51) (Fig. 3). More details available in the supplementary material.
Fig. 3.
Boxplots and the dispersion of the individual values obtained from ROI analysis for both groups. Abbreviations: M1 – primary motor cortex, SMA – supplementary motor area, STN – subthalamic nucleus, DLPFC – dorsolateral prefrontal cortex, CLR – cerebellar locomotor region, MLR – mesencephalic locomotor region, AI – anterior insula, r – right, l – left.
ROI to ROI analysis detected a stronger connectivity between the right and left insulae for the nFoG group (M effect size = 0.70; SD = 0.16) compared with the FoG group (M effect size = 0.42; SD = 0.12), (T(33) = -2.18, p = 0.036) (Fig. 4).
Fig. 4.
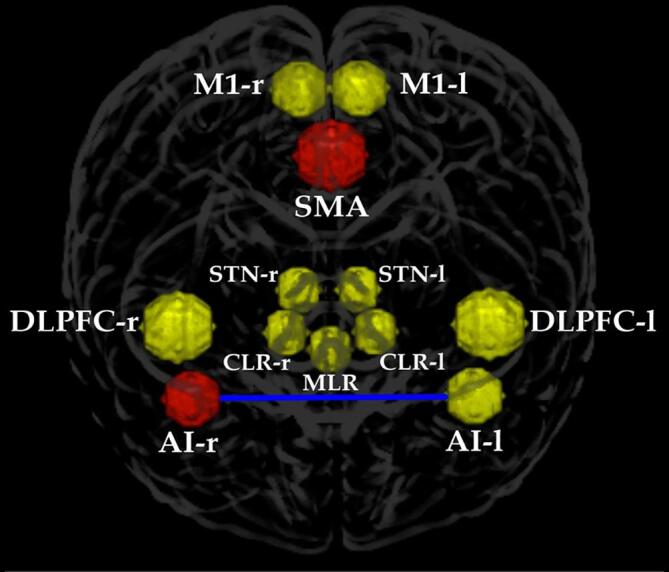
ROIs selected for this study. Yellow circles represent areas with no difference in hemodynamic response between the groups. Red circles are the areas that showed a higher level of BOLD signal for the FoG group. The blue line represents the stronger connectivity between the left and right insulae for the nFoG compared with the FoG group. Abbreviations: M1 – primary motor cortex, SMA – supplementary motor area, STN – subthalamic nucleus, DLPFC – dorsolateral prefrontal cortex, CLR – cerebellar locomotor region, MLR – mesencephalic locomotor region, AI – anterior insula, r – right, l – left. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
There was no significant difference between the groups for global efficiency. The nFoG group showed higher local efficiency in SMA (M = 0.91, SD = 0.19) than the FoG group (M = 0.89, SD = 0.17), T(29) = -3.68, p = 0.006, beta = -0.44). Also, nFoG showed higher values of cluster coefficient in SMA (M = 0.89, SD = 0.21), and left AI (M = 0.83, SD = 0.23) than the FoG group (MSMA = 0.83, SD = 0.21; MAIl = 0.77, SD = 0.20), SMA: T(29) = -3.97, p = 0.002, beta = -0.49, AIl: T(29) = -2.55, p = 0.03, beta = -0.49. The FoG group showed higher level of betweenness centrality of the SMA (M = 0.32, SD = 0.23) compared with the nFoG group (M = 0.12, SD = 0.03), T(33) = 3.25, p = 0.015, beta = 0.23.
Based on the effects found in the brain and behavioral results, correlation analysis included the following variables: NFOGQ score, UPDRS Part III, FBI positive score, APA duration, SMA beta, AIr beta, and strength of connectivity between left and right insulae. Table 2 displays the r and p values of these correlations for both groups. Only the correlation between the NFOGQ and strength of connectivity between the insulae was significant (r = -0.67, p = 0.018). The FoG group also showed a significant positive association between BOLD levels in the SMA and AIr (r = 0.54, p = 0.018). Our results also showed a non-significant negative correlation between the strength of the insulae connectivity and the positive symptoms of the FBI (r = -0.52, p = 0.08) in the FoG group.
Table 2.
Correlation between brain and behavior variables for both groups showing the r and p values.
| FBIpos |
APA time |
NFoGQ |
UPDRS Part III |
SMA |
AIr |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| FoG | nFoG | FoG | nFoG | FoG | nFoG | FoG | nFoG | FoG | nFoG | FoG | nFoG | ||||
| FBIpos | Spearman's r | — | — | ||||||||||||
| p-value | — | — | |||||||||||||
| APA time | Spearman's r | 0.02 | 0.28 | — | — | ||||||||||
| p-value | 0.944 | 0.340 | — | — | |||||||||||
| NFoFQ | Spearman's r | 0.12 | — | 0.05 | — | — | — | ||||||||
| p-value | 0.654 | — | 0.849 | — | — | — | |||||||||
| UPDRS Part III | Spearman's r | −0.22 | −0.12 | −0.15 | 0.13 | 0.12 | — | — | — | ||||||
| p-value | 0.403 | 0.682 | 0.547 | 0.624 | 0.622 | — | — | — | |||||||
| SMA | Spearman's r | −0.09 | −0.09 | 0.30 | 0.18 | 0.17 | — | −0.36 | 0.01 | — | — | ||||
| p-value | 0.740 | 0.762 | 0.225 | 0.505 | 0.479 | — | 0.128 | 0.961 | — | — | |||||
| AIr | Spearman's r | 0.19 | −0.03 | −0.06 | 0.05 | 0.35 | — | −0.15 | −0.19 | 0.54 | * | 0.259 | — | ||
| p-value | 0.475 | 0.920 | 0.809 | 0.856 | 0.131 | — | 0.527 | 0.441 | 0.018 | 0.298 | — | ||||
| CONN | Spearman's r | −0.52 | 0.41 | −0.09 | 0.07 | −0.67 | * | — | 0.02 | 0.44 | −0.20 | −0.42 | −0.42 | −0.03 | |
| p-value | 0.082 | 0.216 | 0.785 | 0.847 | 0.018 | — | 0.950 | 0.178 | 0.537 | 0.193 | 0.178 | 0.923 | |||
* p < 0.05.
4. Discussion
The present study is the first to investigate the brain network involved in the pathophysiology of APAs in PD patients with FoG. Our main results showed a greater hemodynamic response in the SMA and AIr for the FoG compared with the nFoG group when an APA was required. The FoG group also showed a significant positive association between BOLD levels in the SMA and AIr. Patients without FoG showed stronger connectivity between the insulae. In the group with FoG the strength of insulae connectivity was negatively associated with the severity of FoG. Regarding the efficiency of the network connecting the SMA with the bilateral insulae, patients with FoG showed higher betweenness centrality in SMA. On the other hand, the group without FoG showed higher local efficiency in the SMA and clustering in SMA and left AI.
We hypothesized that if FoG and APA pathophysiology are associated, a larger increase in BOLD signal in areas previously found to be related to motor arrests would be present in trials requiring an APA in the FoG group compared with the nFoG group (Maidan et al., 2016, Shine et al., 2013a, Shine et al., 2013b, Vercruysse et al., 2014). Our results showed that requiring an APA during the leg lifting task led to increased recruitment of the right AI and SMA in the FoG group. In previous studies without the occurrence of motor arrests, patients with FoG showed decreased activity of the prefrontal cortex and AI (Gallardo et al., 2018, Matsui et al., 2005, Shine et al., 2013a). In contrast, during motor arrests, higher activity of these areas has been found (Maidan et al., 2016, Shine et al., 2013a, Vercruysse et al., 2014). Despite the increased involvement of the right AI during APA in the FoG group, the group without FoG had stronger connectivity between the right and left insulae. This evidence points to a possible mechanism in the FoG group of increasing the demand for the right AI to compensate for decreased connectivity between the insulae. PD without FoG might successfully use the increased insulae connectivity to overcome the disrupted thalamo-cortico-basal ganglia circuitry during APA. Worth noting is the negative correlation between that connectivity and the severity of FoG. Those patients with stronger connectivity between the insulae showed less severity of FoG. Therefore, the insulae seem to have an important role in compensatory APA mechanisms in PD patients to overcome FoG.
The insulae are part of the salience network, with the right AI being thought to be especially important for the identification of stimuli related to movement inhibition in go/no-go tasks (Ghahremani et al., 2015, Swick et al., 2011). AI has been implicated in movement slowing after the identification of salient events (Cavanagh et al., 2010, Logan et al., 2014). FoG has been associated with impaired inhibitory control (Amboni et al., 2008, Cohen et al., 2014, Cohen et al., 2017, Jacobs et al., 2009b). Inhibitory control is related to specific facets of impulsive behavior, being more related with the willingness to withhold a response, rather than ability (Roberts et al., 2011). Some evidence supports that impulsivity is related to the lack of inhibitory control (Logan et al., 1997). Our results showed a non-significant negative correlation between the strength of the insulae connectivity and the positive symptoms of the FBI (r = −0.52, p = 0.08) in the FoG group, indicating that better control of impulsivity might be associated with stronger connectivity of the insulae.
Maidan et al (2019) found decreased global efficiency and increased local efficiency in the attention network of patients with FoG during resting state. The authors concluded that the decreased global efficiency demonstrates the lower capacity of FoG patients to transfer information across the entire network and that the increased local efficiency is a compensatory strategy. This interpretation may apply to our results; however, the patients in our study were performing a movement initiation task, whilst in Maidan’s they were in resting state. Therefore, the network efficiency in our study reflects the strategy that each pathological group (FoG and nFoG) used to accomplish the task. It is important to highlight that both groups showed similar APA amplitude and duration. However, FoG had a positive correlation between APA amplitude and duration. From this, we could suppose a compensatory strategy of prolonging the APA duration to increase APA amplitude. So, one could suppose that the APA behavior between the groups was different. Given that, the interpretation of the network efficiency must consider the specific strategy used by each group to accomplish the task. Maidan et al showed that, like healthy subjects, the nFoG group showed higher global efficiency than the FoG group in the attention network. Therefore, we expected to find increased values of global efficiency for the nFoG than for the FoG group. However, both groups in our study showed a similar level of global efficiency, which might mean that neither group is using the entire circuit during the task requiring an APA. This supposition is supported by the high clustering of the left AI and SMA in the nFoG group and the isolated efficiency of the SMA as a hub in the FoG group. Therefore, both groups clustered the network possibly as a compensatory strategy to accomplish the task.
One limitation of our study is that we measured brain function associated with APAs during a supine leg lifting task to better understand the basis for impaired APAs during step initiation in freezers. Clearly, in the supine position, gravity acts on the body differently compared to an upright stance. However, we found that this leg lifting task showed similar preparation-movement coupling behavior as seen before taking a step (de Lima-Pardini et al., 2017a). We got brain images with good quality and low levels of head movement due to the constraint of the range of motion of the leg and restraint of the head. Another limitation is having the subjects perform the tasks in the ON state. The main reason we assessed the patients ON was the great difficulty showed by some of them to perform the task while OFF (pilot studies). Thus, we kept the best dosage of the medication for each patient during the experiment. All our patients reported fewer and shorter freezing episodes under dopaminergic medication than when OFF, as previously found (Fietzek et al., 2013), but the episodes did not cease, as indicated by the high average NFOGQ scores in the usual ON-state. A third limitation of our study is the categorization of subjects with PD as freezers versus nonfreezers. Although validated and reliable (Nieuwboer et al., 2009), the NFOGQ for assessing FoG is subjective and consequently might not represent the real severity of the freezing. Future studies should implement biomechanical measurements and an adequate environment to provoke and assess the severity of FoG (Mancini et al., 2019). However, the subjective record from the patient and family is important and should be used together with the objective methods given the challenge of provoking FoG in a clinical setup. The conclusions of this study should be taken with caution due to the large variability of age, LEDD, and disease duration among the participants. These variables were used as covariates, which increases the power and accuracy of the results. However, the test could be contaminated by the differences among the expected values of the covariate measures between the groups. It is essential to highlight that despite the young age of three participants in the FoG group, none were diagnosed as genetic/juvenile PD. Also, although many participants in our sample were classified as having mild cognitive impairment by the classification of a study performed with elderly Brazilians (Cesar et al., 2019), all the included participants were able to complete the task as required. Moreover, we did not evaluate anxiety symptoms that are related to FoG (Witt et al., 2019) and could interfere on task performance. Despite these limitations, the performance in every trial was carefully monitored to confirm that they lifted the leg after the command and that the performance was consistent. As stated elsewhere, mild cognitive impairment is defined as the presence of cognitive decline with preserved functional abilities (Petersen, 2011), as seen in our sample. Finally, since our study is exploratory and we did not correct for multiple comparisons when comparing and correlating brain, cognitive and behavioral variables, additional dedicated studies are needed to confirm our results.
5. Conclusion
fMRI during a leg lifting task showed brain function specific to the APA requirement in PD patients with FoG compared to those without FoG. A greater level of the hemodynamic response in SMA and AIr found in patients with FoG is consistent with previous fMRI studies during motor arrests of simulated walking tasks, pointing to an important role of these areas in the pathophysiology of posture-gait coupling in PD. Our findings suggest that both groups used compensatory mechanism comprising the insulae during APA. Neither group used the entire network comprised of the insulae and SMA to accomplish the task. Our results suggest that the FoG group relied more on SMA as a hub than as part of a broader network to exchange information during the APA. In contrast, the nFoG group relied on SMA and the left insula to accomplish the task.
CRediT authorship contribution statement
Andrea C. de Lima-Pardini: Conceptualization, Methodology, Validation, Formal analysis, Investigation, Writing - original draft, Writing - review & editing, Visualization, Project administration, Funding acquisition. Daniel B. Coelho: Methodology, Formal analysis, Writing - review & editing, Visualization. Mariana P. Nucci: Methodology, Formal analysis, Writing - review & editing, Visualization. Catarina C. Boffino: Methodology, Writing - review & editing. Alana X. Batista: Methodology, Writing - review & editing. Raymundo M. de Azevedo Neto: Methodology, Writing - review & editing. Carla Silva-Batista: Methodology, Writing - review & editing. Egberto R. Barbosa: Methodology, Writing - review & editing. Rajal G. Cohen: Conceptualization, Methodology, Writing - review & editing. Fay B. Horak: Conceptualization, Methodology, Writing - review & editing. Luis A. Teixeira: Methodology, Writing - review & editing. Edson Amaro: Writing - review & editing, Project administration, Funding acquisition.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
Acknowledgments
The authors thank Antônio Cesário Monteiro da Cruz Jr for technical support; Carolina de Oliveira Souza, Janini Chen, Márcia Pereira Castro and Rachel Brant for helping in the recruitment and clinical assessment of the patients; Ellison Fernando Cardoso for the clinical assessment of the structural images; the patients for their willingness to participate.
Funding
This study was supported by Fundação de Amparo à Pesquisa do Estado de São Paulo (# 2013/15256-0) and Coordenação de Aperfeiçoamento de Pessoal de Nível Superior - Brasil.
Contributor Information
Andrea C. de Lima-Pardini, Email: acdl1@queensu.ca.
Daniel B. Coelho, Email: daniel.boari@ufabc.edu.br.
References
- Agrell, B., Dehlin, O., 2012. The clock-drawing test. 1998. Age Ageing 41 Suppl 3, 41-45. [DOI] [PubMed]
- Amboni M., Cozzolino A., Longo K., Picillo M., Barone P. Freezing of gait and executive functions in patients with Parkinson's disease. Mov. Disord. 2008;23:395–400. doi: 10.1002/mds.21850. [DOI] [PubMed] [Google Scholar]
- Aruin A.S. The organization of anticipatory postural adjustments. J Aut Cont. 2002;12:31–37. [Google Scholar]
- Bassett D.S., Bullmore E.T. Human brain networks in health and disease. Curr. Opin. Neurol. 2009;22:340–347. doi: 10.1097/WCO.0b013e32832d93dd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borkowski J.G., Benton A.L., Spreen O. Word fluency and brain damage. Neuropsychologia. 1967;5:135–140. [Google Scholar]
- Bullmore E., Sporns O. The economy of brain network organization. Nat. Rev. Neurosci. 2012;13:336–349. doi: 10.1038/nrn3214. [DOI] [PubMed] [Google Scholar]
- Cavanagh J.F., Frank M.J., Klein T.J., Allen J.J. Frontal theta links prediction errors to behavioral adaptation in reinforcement learning. Neuroimage. 2010;49:3198–3209. doi: 10.1016/j.neuroimage.2009.11.080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cesar K.G., Yassuda M.S., Porto F.H.G., Brucki S.M.D., Nitrini R. MoCA Test: normative and diagnostic accuracy data for seniors with heterogeneous educational levels in Brazil. Arq. Neuropsiquiatr. 2019;77:775–781. doi: 10.1590/0004-282X20190130. [DOI] [PubMed] [Google Scholar]
- Cohen R.G., Klein K.A., Nomura M., Fleming M., Mancini M., Giladi N., Nutt J.G., Horak F.B. Inhibition, executive function, and freezing of gait. J Parkinsons Dis. 2014;4:111–122. doi: 10.3233/JPD-130221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen R.G., Nutt J.G., Horak F.B. Recovery from Multiple APAs Delays Gait Initiation in Parkinson's Disease. Front. Hum. Neurosci. 2017;11:60. doi: 10.3389/fnhum.2017.00060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Lima-Pardini A.C., de Azevedo Neto R.M., Coelho D.B., Boffino C.C., Shergill S.S., de Oliveira Souza C., Brant R., Barbosa E.R., Cardoso E.F., Teixeira L.A., Cohen R.G., Horak F.B., Amaro E., Jr. An fMRI-compatible force measurement system for the evaluation of the neural correlates of step initiation. Sci. Rep. 2017;7:43088. doi: 10.1038/srep43088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Lima-Pardini A.C., Morais G.A.Z., Balardin J., Coelho D.B., Azzi N.M., Teixeira L.A., Sato J.R. Measuring cortical motor hemodynamics during assisted stepping–an fNIRS feasibility study of using a walker. Gait Posture. 2017;56:112–118. doi: 10.1016/j.gaitpost.2017.05.018. [DOI] [PubMed] [Google Scholar]
- Dubois B., Slachevsky A., Litvan I., Pillon B. The FAB: a Frontal Assessment Battery at bedside. Neurology. 2000;55:1621–1626. doi: 10.1212/wnl.55.11.1621. [DOI] [PubMed] [Google Scholar]
- Dutra M.C., Ribeiro R.D.S., Pinheiro S.B., de Melo G.F., Carvalho G.A. Accuracy and reliability of the Pfeffer Questionnaire for the Brazilian elderly population. Dement Neuropsychol. 2015;9:176–183. doi: 10.1590/1980-57642015DN92000012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fietzek U.M., Zwosta J., Schroeteler F.E., Ziegler K., Ceballos-Baumann A.O. Levodopa changes the severity of freezing in Parkinson's disease. Parkinsonism Relat Disord. 2013;19:894–896. doi: 10.1016/j.parkreldis.2013.04.004. [DOI] [PubMed] [Google Scholar]
- Fling B.W., Cohen R.G., Mancini M., Carpenter S.D., Fair D.A., Nutt J.G., Horak F.B. Functional reorganization of the locomotor network in Parkinson patients with freezing of gait. PLoS ONE. 2014;9 doi: 10.1371/journal.pone.0100291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fling B.W., Dutta G.G., Schlueter H., Cameron M.H., Horak F.B. Associations between Proprioceptive Neural Pathway Structural Connectivity and Balance in People with Multiple Sclerosis. Front. Hum. Neurosci. 2014;8:814. doi: 10.3389/fnhum.2014.00814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank M.J. Hold your horses: a dynamic computational role for the subthalamic nucleus in decision making. Neural. Netw. 2006;19:1120–1136. doi: 10.1016/j.neunet.2006.03.006. [DOI] [PubMed] [Google Scholar]
- Gallardo M.J., Cabello J.P., Corrales M.J., Torres-Donaire J., Bravo J.J., Talavera M.P., Leon A., Vaamonde-Gamo J. Freezing of gait in Parkinson's disease: functional neuroimaging studies of the frontal lobe. Neurol. Res. 2018;40:900–905. doi: 10.1080/01616412.2018.1484985. [DOI] [PubMed] [Google Scholar]
- Ghahremani A., Rastogi A., Lam S. The role of right anterior insula and salience processing in inhibitory control. J. Neurosci. 2015;35:3291–3292. doi: 10.1523/JNEUROSCI.5239-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giladi N., Tal J., Azulay T., Rascol O., Brooks D.J., Melamed E., Oertel W., Poewe W.H., Stocchi F., Tolosa E. Validation of the freezing of gait questionnaire in patients with Parkinson's disease. Mov. Disord. 2009;24:655–661. doi: 10.1002/mds.21745. [DOI] [PubMed] [Google Scholar]
- Hagberg G.E., Zito G., Patria F., Sanes J.N. Improved detection of event-related functional MRI signals using probability functions. Neuroimage. 2001;14:1193–1205. doi: 10.1006/nimg.2001.0880. [DOI] [PubMed] [Google Scholar]
- Hamilton M. A rating scale for depression. J. Neurol. Neurosurg. Psychiatry. 1960;23:56–62. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanna-Pladdy B., Heilman K.M., Foundas A.L. Cortical and subcortical contributions to ideomotor apraxia: analysis of task demands and error types. Brain. 2001;124:2513–2527. doi: 10.1093/brain/124.12.2513. [DOI] [PubMed] [Google Scholar]
- He S.Q., Dum R.P., Strick P.L. Topographic organization of corticospinal projections from the frontal lobe: motor areas on the medial surface of the hemisphere. J. Neurosci. 1995;15:3284–3306. doi: 10.1523/JNEUROSCI.15-05-03284.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoehn M.M., Yahr M.D. Parkinsonism: onset, progression, and mortality. 1967. Neurology. 2001;57:S11–26. [PubMed] [Google Scholar]
- Hughes A.J. J. Neurol. Neurosurg. Psychiatr. 1992;55:181–184. doi: 10.1136/jnnp.55.3.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs J.V., Lou J.S., Kraakevik J.A., Horak F.B. The supplementary motor area contributes to the timing of the anticipatory postural adjustment during step initiation in participants with and without Parkinson's disease. Neuroscience. 2009;164:877–885. doi: 10.1016/j.neuroscience.2009.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs J.V., Nutt J.G., Carlson-Kuhta P., Stephens M., Horak F.B. Knee trembling during freezing of gait represents multiple anticipatory postural adjustments. Exp. Neurol. 2009;215:334–341. doi: 10.1016/j.expneurol.2008.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkinson M., Bannister P., Brady M., Smith S. Improved optimization for the robust and accurate linear registration and motion correction of brain images. Neuroimage. 2002;17:825–841. doi: 10.1016/s1053-8119(02)91132-8. [DOI] [PubMed] [Google Scholar]
- Keizer K., Kuypers H.G. Distribution of corticospinal neurons with collaterals to the lower brain stem reticular formation in monkey (Macaca fascicularis) Exp. Brain Res. 1989;74:311–318. doi: 10.1007/BF00248864. [DOI] [PubMed] [Google Scholar]
- Kertesz A., Davidson W., Fox H. Frontal behavioral inventory: diagnostic criteria for frontal lobe dementia. Can. J. Neurol. Sci. 1997;24:29–36. doi: 10.1017/s0317167100021053. [DOI] [PubMed] [Google Scholar]
- Klein T.A., Endrass T., Kathmann N., Neumann J., von Cramon D.Y., Ullsperger M. Neural correlates of error awareness. Neuroimage. 2007;34:1774–1781. doi: 10.1016/j.neuroimage.2006.11.014. [DOI] [PubMed] [Google Scholar]
- Latora V., Marchiori M. Efficient behavior of small-world networks. Phys. Rev. Lett. 2001;87 doi: 10.1103/PhysRevLett.87.198701. [DOI] [PubMed] [Google Scholar]
- Logan G.D., Schachar R.J., Tannock R. Impulsivity and Inhibitory Control. Psychol. Sci. 1997;8:60–64. [Google Scholar]
- Logan G.D., Van Zandt T., Verbruggen F., Wagenmakers E.J. On the ability to inhibit thought and action: general and special theories of an act of control. Psychol. Rev. 2014;121:66–95. doi: 10.1037/a0035230. [DOI] [PubMed] [Google Scholar]
- Lomond K.V., Henry S.M., Jacobs J.V., Hitt J.R., Horak F.B., Cohen R.G., Schwartz D., Dumas J.A., Naylor M.R., Watts R., DeSarno M.J. Protocol to assess the neurophysiology associated with multi-segmental postural coordination. Physiol. Meas. 2013;34:N97–105. doi: 10.1088/0967-3334/34/10/N97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma L.Y., Chen X.D., He Y., Ma H.Z., Feng T. Disrupted Brain Network Hubs in Subtype-Specific Parkinson's Disease. Eur. Neurol. 2017;78:200–209. doi: 10.1159/000477902. [DOI] [PubMed] [Google Scholar]
- Maidan I., Jacob Y., Giladi N., Hausdorff J.M., Mirelman A. Altered organization of the dorsal attention network is associated with freezing of gait in Parkinson's disease. Parkinsonism Relat Disord. 2019;63:77–82. doi: 10.1016/j.parkreldis.2019.02.036. [DOI] [PubMed] [Google Scholar]
- Maidan I., Nieuwhof F., Bernad-Elazari H., Reelick M.F., Bloem B.R., Giladi N., Deutsch J.E., Hausdorff J.M., Claassen J.A., Mirelman A. The role of the frontal lobe in complex walking among patients with Parkinson's disease and healthy older adults: an fNIRS study. Neurorehabil. Neural Repair. 2016;30:963–971. doi: 10.1177/1545968316650426. [DOI] [PubMed] [Google Scholar]
- Mancini M., Bloem B.R., Horak F.B., Lewis S.J.G., Nieuwboer A., Nonnekes J. Clinical and methodological challenges for assessing freezing of gait: Future perspectives. Mov. Disord. 2019;34:783–790. doi: 10.1002/mds.27709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mancini M., Zampieri C., Carlson-Kuhta P., Chiari L., Horak F.B. Anticipatory postural adjustments prior to step initiation are hypometric in untreated Parkinson's disease: an accelerometer-based approach. Eur. J. Neurol. 2009;16:1028–1034. doi: 10.1111/j.1468-1331.2009.02641.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massion J. Movement, posture and equilibrium: interaction and coordination. Prog. Neurobiol. 1992;38:35–56. doi: 10.1016/0301-0082(92)90034-c. [DOI] [PubMed] [Google Scholar]
- Matsui H., Udaka F., Miyoshi T., Hara N., Tamaura A., Oda M., Kubori T., Nishinaka K., Kameyama M. Three-dimensional stereotactic surface projection study of freezing of gait and brain perfusion image in Parkinson's disease. Mov. Disord. 2005;20:1272–1277. doi: 10.1002/mds.20520. [DOI] [PubMed] [Google Scholar]
- Nasreddine Z.S., Phillips N.A., Bedirian V., Charbonneau S., Whitehead V., Collin I., Cummings J.L., Chertkow H. The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. J. Am. Geriatr. Soc. 2005;53:695–699. doi: 10.1111/j.1532-5415.2005.53221.x. [DOI] [PubMed] [Google Scholar]
- Nieuwboer A., Rochester L., Herman T., Vandenberghe W., Emil G.E., Thomaes T., Giladi N. Reliability of the new freezing of gait questionnaire: agreement between patients with Parkinson's disease and their carers. Gait Posture. 2009;30:459–463. doi: 10.1016/j.gaitpost.2009.07.108. [DOI] [PubMed] [Google Scholar]
- Olde Dubbelink K.T., Schoonheim M.M., Deijen J.B., Twisk J.W., Barkhof F., Berendse H.W. Functional connectivity and cognitive decline over 3 years in Parkinson disease. Neurology. 2014;83:2046–2053. doi: 10.1212/WNL.0000000000001020. [DOI] [PubMed] [Google Scholar]
- Patton J.H., Stanford M.S., Barratt E.S. Factor structure of the Barratt impulsiveness scale. J. Clin. Psychol. 1995;51:768–774. doi: 10.1002/1097-4679(199511)51:6<768::aid-jclp2270510607>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- Petersen R.C. Clinical practice. Mild cognitive impairment. N. Engl. J. Med. 2011;364:2227–2234. doi: 10.1056/NEJMcp0910237. [DOI] [PubMed] [Google Scholar]
- Prodoehl J., Yu H., Little D.M., Abraham I., Vaillancourt D.E. Region of interest template for the human basal ganglia: comparing EPI and standardized space approaches. Neuroimage. 2008;39:956–965. doi: 10.1016/j.neuroimage.2007.09.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts W., Fillmore M.T., Milich R. Linking impulsivity and inhibitory control using manual and oculomotor response inhibition tasks. Acta Psychol (Amst) 2011;138:419–428. doi: 10.1016/j.actpsy.2011.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubinov M., Sporns O. Complex network measures of brain connectivity: uses and interpretations. Neuroimage. 2010;52:1059–1069. doi: 10.1016/j.neuroimage.2009.10.003. [DOI] [PubMed] [Google Scholar]
- Scarpina F., Tagini S. The Stroop Color and Word Test. Front. Psychol. 2017;8:557. doi: 10.3389/fpsyg.2017.00557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaafsma J.D., Balash Y., Gurevich T., Bartels A.L., Hausdorff J.M., Giladi N. Characterization of freezing of gait subtypes and the response of each to levodopa in Parkinson's disease. Eur. J. Neurol. 2003;10:391–398. doi: 10.1046/j.1468-1331.2003.00611.x. [DOI] [PubMed] [Google Scholar]
- Schlenstedt C., Mancini M., Nutt J., Hiller A.P., Maetzler W., Deuschl G., Horak F. Are Hypometric Anticipatory Postural Adjustments Contributing to Freezing of Gait in Parkinson's Disease? Front. Aging Neurosci. 2018;10:36. doi: 10.3389/fnagi.2018.00036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeley W.W., Menon V., Schatzberg A.F., Keller J., Glover G.H., Kenna H., Reiss A.L., Greicius M.D. Dissociable intrinsic connectivity networks for salience processing and executive control. J. Neurosci. 2007;27:2349–2356. doi: 10.1523/JNEUROSCI.5587-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seto E., Sela G., McIlroy W.E., Black S.E., Staines W.R., Bronskill M.J., McIntosh A.R., Graham S.J. Quantifying head motion associated with motor tasks used in fMRI. Neuroimage. 2001;14:284–297. doi: 10.1006/nimg.2001.0829. [DOI] [PubMed] [Google Scholar]
- Shine J.M., Matar E., Ward P.B., Bolitho S.J., Gilat M., Pearson M., Naismith S.L., Lewis S.J. Exploring the cortical and subcortical functional magnetic resonance imaging changes associated with freezing in Parkinson's disease. Brain. 2013;136:1204–1215. doi: 10.1093/brain/awt049. [DOI] [PubMed] [Google Scholar]
- Shine J.M., Matar E., Ward P.B., Frank M.J., Moustafa A.A., Pearson M., Naismith S.L., Lewis S.J. Freezing of gait in Parkinson's disease is associated with functional decoupling between the cognitive control network and the basal ganglia. Brain. 2013;136:3671–3681. doi: 10.1093/brain/awt272. [DOI] [PubMed] [Google Scholar]
- Swick D., Ashley V., Turken U. Are the neural correlates of stopping and not going identical? Quantitative meta-analysis of two response inhibition tasks. Neuroimage. 2011;56:1655–1665. doi: 10.1016/j.neuroimage.2011.02.070. [DOI] [PubMed] [Google Scholar]
- Takakusaki K. Functional neuroanatomy for posture and gait control. J. Mov. Disord. 2017;10:1–17. doi: 10.14802/jmd.16062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Troyer A.K., Moscovitch M., Winocur G. Clustering and switching as two components of verbal fluency: evidence from younger and older healthy adults. Neuropsychology. 1997;11:138–146. doi: 10.1037//0894-4105.11.1.138. [DOI] [PubMed] [Google Scholar]
- Vercruysse S., Spildooren J., Heremans E., Wenderoth N., Swinnen S.P., Vandenberghe W., Nieuwboer A. The neural correlates of upper limb motor blocks in Parkinson's disease and their relation to freezing of gait. Cereb. Cortex. 2014;24:3154–3166. doi: 10.1093/cercor/bht170. [DOI] [PubMed] [Google Scholar]
- Walton C.C., Shine J.M., Hall J.M., O'Callaghan C., Mowszowski L., Gilat M., Szeto J.Y., Naismith S.L., Lewis S.J. The major impact of freezing of gait on quality of life in Parkinson's disease. J. Neurol. 2015;262:108–115. doi: 10.1007/s00415-014-7524-3. [DOI] [PubMed] [Google Scholar]
- Whitfield-Gabrieli S., Nieto-Castanon A. Conn: a functional connectivity toolbox for correlated and anticorrelated brain networks. Brain Connect. 2012;2:125–141. doi: 10.1089/brain.2012.0073. [DOI] [PubMed] [Google Scholar]
- Witt I., Ganjavi H., MacDonald P. Relationship between Freezing of Gait and Anxiety in Parkinson's Disease Patients: A Systemic Literature Review. Parkinsons Dis. 2019;2019:6836082. doi: 10.1155/2019/6836082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang D., Liu X., Chen J., Liu B. Distinguishing patients with Parkinson's disease subtypes from normal controls based on functional network regional efficiencies. PLoS ONE. 2014;9 doi: 10.1371/journal.pone.0115131. [DOI] [PMC free article] [PubMed] [Google Scholar]