Highlights
-
•
Duration of joint inflammatory pain behaviour is prolonged in juvenile rats compared to adult rats.
-
•
Spinal neuron hyperexcitability mirror pain behaviours of both juvenile and adult rats.
-
•
Persistent upregulation of interleukin-6 uniquely observed in spinal cord of juvenile rats.
-
•
Inhibition of spinal interleukin-6 activity rescued pain and normalised spinal neuron activity.
Keywords: Chronic pain, Inflammation, Sensory behaviour, Electrophysiology, Interleukin-6, Juvenile arthritis
Abstract
Pain is the most debilitating symptom in juvenile idiopathic arthritis. As pain correlates poorly to the extent of joint pathology, therapies that control joint inflammation are often inadequate as analgesics. We test the hypothesis that juvenile joint inflammation leads to sensitisation of nociceptive circuits in the central nervous system, which is maintained by cytokine expression in the spinal cord. Here, transient joint inflammation was induced in postnatal day (P)21 and P40 male Sprague-Dawley rats with a single intra-articular ankle injection of complete Freund’s adjuvant. Hindpaw mechanical pain sensitivity was assessed using von Frey hair and weight bearing tests. Spinal neuron activity was measured using in vivo extracellular recording and immunohistochemistry. Joint and spinal dorsal horn TNFα, IL1β and IL6 protein expression was quantified using western blotting. We observed greater mechanical hyperalgesia following joint inflammation in P21 compared to P40 rats, despite comparable duration of swelling and joint inflammatory cytokine levels. This is mirrored by spinal neuron hypersensitivity, which also outlasted the duration of active joint inflammation. The cytokine profile in the spinal cord differed at the two ages: prolonged upregulation of spinal IL6 was observed in P21, but not P40 rats. Finally, spinal application of anti-IL-6 antibody (30 ng) reduced the mechanical hyperalgesia and neuronal activation. Our results indicate that persistent upregulation of pro-inflammatory cytokines in the spinal dorsal horn is associated with neuronal sensitisation and mechanical hyperalgesia in juvenile rats, beyond the progress of joint pathology. In addition, we provide proof of concept that spinal IL6 is a key target for treating persistent pain in JIA.
1. Introduction
Juvenile idiopathic arthritis is a heterogeneous group of musculoskeletal diseases affecting around 0.1% of children in the UK (Manners and Bower, 2002). While the treatment of JIA has been revolutionised by the introduction of biologics (Learoyd et al., 2019, Lomholt et al., 2013, Shiff, 2017), pain remains a major problem (Cornelissen, 2014, de Lalouvière et al., 2014, Schaible, 2009). Electronic diary entries of JIA patients showed virtually no pain-free periods, and 86% of patients suffered from high levels of pain at least once a month (Shiff, 2017, Cornelissen, 2014). In addition, pain may not reflect disease activity (Lomholt et al., 2013, Leegaard, 2013, Consolaro and Ravelli, 2013) as JIA patients often have generalised hypersensitivity (not restricted to the affected joints) to light pressure, touch, noxious cold and heat stimulations when compared to healthy children (Shiff, 2017, Cornelissen, 2014). An analysis of pain severity in JIA reveals that one in 10 patients have concerning pain trajectories in the 24 months post diagnosis, but that initial active joint counts does not always reflect these trajectories (Shiff, 2017). Rodent models of JIA display a similar dissociation of joint inflammation and pain behaviour providing a basis for isolating biological mechanisms, independent of social or clinical factors (Learoyd et al., 2019).
The underlying mechanisms of joint inflammatory pain are complex (Schaible, 2009). Clinical and experimental studies have shown that joint inflammation enhances the production and release of pro-inflammatory cytokines (e.g. tumour necrosis factor alpha (TNFα), interleukin (IL) 1 and 6) by the immune system (de Jager, 2006, Prelog, 2008). These cytokines can activate peripheral nociceptors and cause pain (Choy and Panayi, 2001, Sommer and Kress, 2004). Prolonged activation of peripheral nociceptors can sensitise the pain pathways of the central nervous system (CNS) (Watkins et al., 1995), and induce adaptive changes that lead to hyperalgesia (Schaible, 2010). Importantly, pain sensitisation can also arise from increased cytokine levels within the CNS itself, due to activation of neuroimmune pathways (Guo, 2007, Raghavendra et al., 2004). Thus local pro-inflammatory cytokines within spinal cord pain circuits can directly excite neurons such that pain is maintained beyond the disease process itself, a phenomenon known as central sensitisation (Guo, 2007, Raghavendra et al., 2004, Zhang, 2011). In patients where their pain persists beyond the period of active joint inflammation, neuroinflammation may be better correlated with pain than peripheral inflammation. Cytokine induced central sensitisation is therefore an important mechanism for the maintenance of chronic arthritic pain (de Lalouvière et al., 2014).
Much of our mechanistic understanding of joint inflammatory pain derives from studies using adult animal models. However, as there are important age-related differences in pain processing, the results are not directly applicable to JIA. Furthermore, the normal maturation of pain processing is influenced by abnormal sensory experience (Beggs, 2012) and there is evidence that early life injury can ‘prime’ nociceptive circuits leading to increased pain upon re-injury in adulthood (Walker, 2009, Walker, 2003, Walker et al., 2009). There are significant differences in neuroimmune activation and cytokine regulation following peripheral nerve injury between young and adult animals (McKelvey, 2015, Vega-Avelaira et al., 2009, Vega-Avelaira et al., 2007, Moss, 2007, Costigan, 2009), upon injury the cytokine profile within the spinal dorsal horn switches from an anti-inflammatory responses during early infancy to pro-inflammatory around adolescence, characterized by significant increases in TNFα and Brain-derived neurotropic factor (BDNF) (McKelvey, 2015). The changes in neuroimmune responses directly affect their pain behaviours, indicating important functional developmental regulation of spinal neuroimmune pathways (Moriarty, 2019).
There is a clear clinical need for better understanding of mechanisms underlying JIA pain (de Lalouvière et al., 2014). In this study, we investigated the mechanisms underlying joint inflammatory pain using a model of monoarthritis in juvenile and peri-pubertal rodents. We test the hypothesis that central modulation of joint pain differs between juvenile and adult animals, and that joint inflammation triggers a distinct age-related pattern of cytokine expression in the spinal cord which explains the chronic pain of juvenile arthritis.
2. Materials and methods
2.1. Animals
Male Sprague-Dawley weaned juvenile (postnatal day (P)21) and peri-pubertal (P40) rats were obtained from the Biological Services Unit, University College London, and Charles River Laboratories (Boucherville, QC, Canada). All animals were maintained on a 12-h light/dark cycle at constant ambient temperature with access to food and water ad libitum. All animals were group-housed in 4 with littermates in conventional cages, with wood shavings and sawdust as bedding. All experiments were approved by the University College London Animal Care Committee or the University of Calgary Animal Care Committee, adhered to the United Kingdom Animal (Scientific Procedures) Act 1986 and Canadian Council on Animal Care guidelines. Reporting is based on the ARRIVE Guidelines for Reporting Animal Research developed by the National Centre for Replacement, Refinement and Reduction of Animals in Research, London, United Kingdom (Kilkenny, 2010).
2.2. Induction of monoarthritis in ankle joints
Microinjection of Complete Freund’s Adjuvant (CFA) (Sigma-Aldrich, UK) into the intra-articular space was used to produce arthritic inflammation of the left ankle joint under isoflurane anaesthesia (5% in 100% medical O2 for induction, 2–3% for injection). Juvenile (P21) and adult (P40) animals received 10 μL and 20 μL of CFA mixture (1 part CFA to 1 part mineral oil) respectively, age-matched controls received saline. A 30-gauge needle attached to a 100 µL Hamilton Syringe was inserted into the left ankle joint capsule from the posterior lateral aspect and advanced until a palpable release of pressure. Following injection, the needle remained in the joint for at least 30 s to ensure that the injectate remained in the joint space.
2.3. Sensory behaviour tests
In CFA or saline injected P21 and P40 rats, mechanical withdrawal threshold on the ipsilateral and contralateral paw, weight bearing and joint diameter of the ipsilateral ankle were measured before injections (baseline), and up to 24 days post-injections. Calibrated von Frey hairs (vFh) were applied to the ipsilateral and contralateral hindpaws, and mechanical thresholds were determined using the up–down method (Chaplan, 1994). Hindlimb weight bearing was assessed using an incapacitance meter (Churchill Electronic Services), which measures the weight supported by each hindlimb independently. Ipsilateral ankle joint diameter was measured using a pair of calibrated callipers.
2.4. Intrathecal injection
Intrathecal injections were performed for 3 consecutive days (days 7 to 9) after intra-articular injection of CFA in a subset of P21 animals. Recombinant rat anti-IL6 antibody (MAB406, R&D Systems) was reconstituted in sterile saline and diluted to the following concentrations: 3 ng/10 µL, 10 ng/10 µL and 30 ng/10 µL. Under brief isoflurane anaesthesia (3%), a 1 mL insulin syringe was inserted between the L5 and L6 spinal vertebrae and 10 µL of the desired concentration of anti-IL6 was administered. Control animals received intrathecal injections of sterile saline (10 µL). Intrathecal injections were given near the end of the light cycle of animals, after the completion of sensory behaviour tests.
2.5. In vivo electrophysiology
Electrophysiology was performed at 3 days or 14 days after P21 or P40 intra-articular injection of CFA or saline. Rats were anesthetized with isoflurane (1.8% in medical oxygen, Univentor unit 400, Royem Scientific), tracheotomised and artificially ventilated using a small animal ventilator (model 687, Harvard Apparatus, MA, USA). Animals were mounted onto a stereotaxic frame (Kopf Instruments, Tujunga, CA, USA). A laminectomy was performed to expose the lumbar spinal cord.
To isolate individual neurones in the spinal cord dorsal horn, a 6 µm tipped glass-coated carbon fibre microelectrode (Kation Scientific, Minneapolis, USA) was lowered through the cord with an in vivo Micromanipulator unit (Scientifica, UK) while stroking the plantar surface of the hindpaw as a search stimulus for dorsal horn wide dynamic range (WDR) cells in lamina IV-VI. Once a cell (single unit) was identified, spontaneous firing (baseline neuronal activity in the absence of peripheral stimulation) was recorded for 1 min. Then, cutaneous receptive fields to brush and pinch stimulation were mapped and the number of spikes per stimulus to brush,pinch and vFh stimulation of the receptive field were recorded. Brush and pinch stimuli were applied for 0.5 s and 3 s respectively. The range of vFh weight used for P21 animals was 2 g to 26 g, and 2 g to 60 g for older animals. For pinch, recordings continued for 10 s after the stimulus to include after-discharge. Stimulus evoked potentials were digitalised using PowerLab 4/30 interface and isolated using the Chart 5 software spike histogram plug-in (AD Instruments Ltd, Oxford, UK).
2.6. cFos immunohistochemistry
P21 rats received intra-articular injections of CFA and intrathecal injections of either saline (control) or anti-IL6 (30 ng/10 µL) as described. 3 days after the last intrathecal injection, a brief pinch was applied to the left, inflamed ankle joint using calibrated forceps. Within two hours after ankle pinch, animals underwent transcardial perfusion of phosphate-buffered saline (PBS) and 4% paraformaldehyde (PFA), the lumbar spinal cords were removed, post-fixed overnight at 4 °C before cryopreserving in 30% sucrose azide. 40 µm thick coronal spinal cord sections were cut and collected on a freezing cryostat. Free-floating sections were incubated overnight at room temperature in rabbit anti-cFos (1.500, ab190289, Abcam) and AlexaFluor 647 conjugated anti-Isolectin 4 (IB4, I32450, Invitrogen), followed by donkey anti-rabbit AlexaFluor 488 (1:500, A-21206, Invitrogen).
Images were obtained using a Nikon A1R multiphoton microscope, quantification of cFos immunopositive cells was performed using ImageJ (NIH). The superficial and deep regions were delineated with positive IB4 immunoreactivity, indicating termination of primary afferents in the superficial lamina of the dorsal horn (sDH). The rest of the dorsal portion of the spinal dorsal horn, up to the central canal was counted as the deep dorsal horn (dDH). 4 sections of the lumbar spinal cord were imaged and quantified from a total of 5 saline and 4 anti-IL6 treated animals. The data presented were total counts of cFos immunopositive cells either within the sDH or dDH, each datapoint represent cFos count of each spinal cord slice.
2.7. Cytokine protein analysis
Rats were sacrificed at day 3 and day 14 after P21 or P40 intra-articular CFA or saline injections. The ipsilateral and contralateral ankle joints and dorsal horn quadrants of L3–L5 spinal cord segments were collected. Proteins were extracted in 250 μL lysis buffer (NP-40 1%, Tris-HCl 20 mM, pH 7.2, NaCl 100 mM, EDTA 1 mM, and 1% of protease inhibitor and PMSF; all from Sigma, UK) by homogenisation using FastPrep biopulverisor system (MP Biomeidcals, UK), incubated for 2 h on ice and centrifuged at 12,000g to remove the debris. Samples (20 μg of proteins per well) were run on 10% Bis-Tris gels (Biorad Laboratories, UK) by electrophoresis and transferred onto a Polyvinylidene fluoride (PVDF) membrane (Biorad Laboratories, UK). Membranes were then blocked in i-Block (0.2% in Tris-buffered saline; Applied Biosystems, UK) and incubated with primary antibodies against Interleukin-6 (IL6), Interleukin 1-beta (IL1β),Tumour necrosis factor-alpha (TNFα) and Glyceraldehyde 3-phosphate dehydrogenase (GAPDH) (MAB406, MAB401, MAB4101, AF5718, R&D Systems, UK; 1:500 in 0.2% i-block solution). Primary antibodies were detected using horseradish peroxidase (HRP) conjugated secondary antibodies (Santa Cruz Biotechnology, CA, USA). HRP activity was visualized using Chemi Doc XRS (Biorad). Signal intensity was measured using ImageJ software (NIH). Signals for IL6, IL1β and TNFα were normalized to GAPDH. Positive control experiments conducted with purified IL6 (506-IL-CF, R&D Systems), IL1β (501-RL-CF, R&D Systems) and TNFα (510-RT-CF, R&D Systems) protein showed dose-dependent increases in HRP signals (Supplementary Fig. 1).
2.8. Statistical data analysis
Statistical analyses and graphing were performed using GraphPad Prism 8 (GraphPad software, La Jolla, CA, USA) and SPSS 23. P < 0.05 was considered statistically significant. Data are represented as means ± standard error of mean (SEM).
Normality tests were applied, and parametric tests were used where appropriate. Mechanical withdrawal threshold data was log2 transformed and, along with all other behavioural data, was analysed by repeated measures two-way ANOVA followed by Bonferroni post-hoc analysis. Electrophysiological and immunoprecipitation data were analysed by three-way ANOVA followed by Bonferroni post-hoc analysis.
3. Results
3.1. Intra-articular injection of complete Freund’s adjuvant induced comparable transient joint inflammation between juvenile (P21) and peri-pubertal (P40) rats
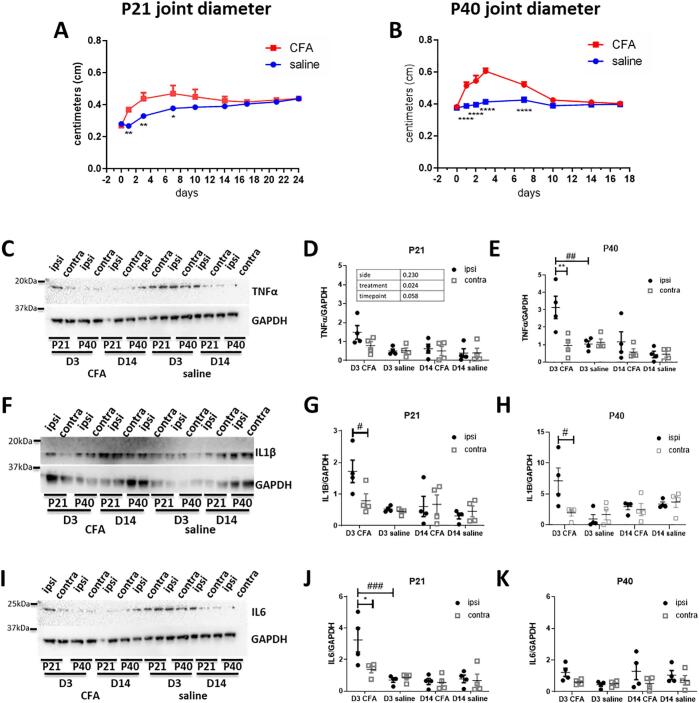
To investigate age related differences in changes within the joint itself following transient joint inflammation, we first measured ipsilateral joint diameter at baseline and up to 24 days after the intra-articular injection of complete Freund’s adjuvant (CFA, 1 part CFA to 1 part mineral oil, 10 µL and 20 µL at P21 and P40 respectively). Fig. 1A-B show that joint diameters ipsilateral to CFA injections were significantly larger compared to saline injections in both juvenile (P21, F(1, 6) = 7.08, P = 0.04, repeated measures two-way ANOVA) and peri-pubertal rats (P40, F (1, 10) = 79.49, P = 0.000005, repeated measures two-way ANOVA). Since the animals are at different stages of growth, direct comparison of increases in joint size is not possible but Fig. 1A -B show that the progress of joint swelling was comparable at the two ages. Joint swelling peaked at 3 days post injection and returned to normal 10 days after injection in both P21 (saline: 0.33 ± 0.012 vs CFA: 0.438 ± 0.038, p = 0.0042, Bonferroni multiple comparisons) and P40 (saline: 0.413 ± 0.012 vs. CFA: 0.605 ± 0.017, p = 0.000059, Bonferroni multiple comparisons) rats.
Fig. 1.
The time course of ankle joint inflammation in juvenile (P21) and peri-pubertal (P40) rats following CFA injections. (A, B) Ipsilateral joint diameter, (Djouhri, 2006) at baseline and up to 24 days after CFA injections (n = 4–6 for all groups). Significant and comparable swelling was observed in both P21 (A, p < 0.05, effect of treatment, two-way ANOVA) and P40 rats (B, p < 0.0001, effect of treatment, two-way ANOVA), peaking at day 3 and returning to normal by day 10. (C-K) Western blot analysis of pro-inflammatory cytokines in the ankle joints of P21 and P40 rats at day 3 and at day 14 after CFA injections. Expression of TNFα, IL1β and IL6 were normalised to GAPDH. (n = 4 per age and timepoint). (C) Representative western blot image of TNFα expression in the ipsilateral and contralateral joints of P21 and P40 rats, at day 3 and 14 after CFA and saline intra-articular injections. (D, E) Injection of CFA into the left ankle joints significantly increased the expression of TNFα in P21 (D, p < 0.05, effect of treatment, three-way ANOVA) and P40 rats (E, p < 0.01, effect of treatment, three-way ANOVA) at day 3, returning to normal by day 14. (F) Representative western blot image of IL1β expression. (G, H) Similarly, the expression of IL1β increased after CFA injections in the ipsilateral ankle joints of both P21 (G, p < 0.01, effect of treatment, three-way ANOVA) and P40 rats (H, p < 0.05, effect of treatment, three-way ANOVA) at day 3 returning to normal by day 14. (I) Representative western blot image of IL6 expression. (J) The expression of IL6 significantly increased in the ipsilateral ankle after CFA injection in P21 rats (p < 0.01, effect of treatment, three-way ANOVA) returning to normal by day 3. (K) No changes in the expression of IL6 in the joints after CFA injection in P40 rats. Data are presented as mean ± SEM. (A, B) *p < 0.05, **p < 0.01, ****p < 0.0001 are compared with saline. (C–H) *p < 0.05, **p < 0.01 are compared between ipsilateral and contralateral ankle; ##p < 0.01, ###p < 0.001 are compared with saline controls.
To examine the inflammatory processes within the joints we measured TNFα, IL1β and IL6 at day 3 and 14 after CFA or saline injections in P21 and P40 rats (de Jager, 2006), as they are pro-inflammatory cytokines present in arthritic joints (Marinova-Mutafchieva, 1997), and targets of routinely prescribed biological treatments.
The overall expression of TNFα was increased in CFA injected animals compared to saline controls (P21: F(1, 6) = 5.828, P = 0.024; P40: F(1, 10) = 7.693, P = 0.011, three-way ANOVA. Fig. 1C-E). The increase in TNFα occurred at day 3 but not at day 14 after CFA injection in P40 animals, expression of TNFα was significantly higher in the ipsilateral ankle joint compared to both the contralateral ankle and the saline injected ankles (Fig. 1E).
The expression of IL1β followed a similar pattern to that observed for TNFα (P21: F (1, 6) = 10.381, P = 0.004; P40: F (1, 10) = 5.046, P = 0.034, three-way ANOVA. Fig. 1F-H). A significant increase in IL1β was found in CFA injected ankle joints in both P21 and P40 rats, at day 3 for both age groups, and was significantly higher in the ipsilateral compared to contralateral ankle joint.
The expression of IL6 was significantly higher in the ankles of CFA injected in P21 rats only (F (1, 6) = 7.769, P = 0.01, three-way ANOVA. Fig. 1I-K), and this increase was only observed at day 3 after injection. Furthermore, the expression of IL6 was enhanced in the ipsilateral ankle compared to the contralateral and saline injected ankle at day 3 after injections.
3.2. Ankle joint inflammatory pain hypersensitivity is more persistent in juvenile compared to peri-pubertal rats
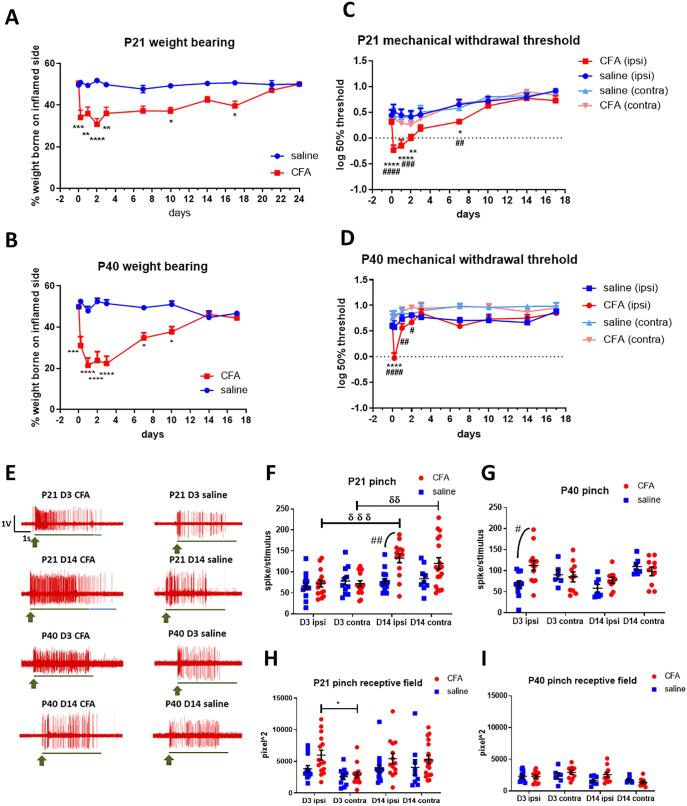
We next compared pain sensitivity following joint inflammation in P21 and P40 rats. Mechanical pain was assessed by weight bearing, measured at baseline (before injection) and up to 24 days after a single intra-articular injection of CFA into the left ankle. Data presented in Fig. 2A & B shows the percentage of weight borne on the injected hindlimb as a percentage of total body weight, which in naive, non-injured animals is around 50%. CFA inflammation of the left ankle joints induced significant weight bearing deficits on the ipsilateral hindlimb of both P21 (F (1, 132) = 80.4, P = 0.00023, repeated measures two-way ANOVA. Fig. 2A) and P40 rats (F (1, 63) = 97.72, P < 0.0001, repeated measures two-way ANOVA. Fig. 2B). In P21 rats weight bearing deficits maintained for over 2 weeks and did not return to normality until 21 days post injection (Fig. 2A). In P40 rats, this deficit was detectable at 4 h and peaked within the first 3 days, followed by a steady return to normality at 14 days post injection (Fig. 2B).
Fig. 2.
Mechanical pain hypersensitivity and spinal dorsal horn neuron excitability (A) Significant weight bearing deficits were observed after CFA injection in P21 rats (p < 0.001, effect of treatment, two-way ANOVA). This deficit was detectable at 4 h, peaked within the first 3 days and continued for up to 3 weeks. (B) Significant weight bearing deficits were also observed after CFA injection in P40 rats (p < 0.001, effect of treatment, two-way ANOVA). The weight bearing deficit returned to normal by day 10 after CFA injection in P40 but only after day 21 in P21 rats. (C) Hindpaw mechanical withdrawal threshold significantly decreased after CFA injection in P21 rats (p < 0.001, effect of treatment, two-way ANOVA). This decrease was observed in the ipsilateral paw only, detectable at 4 h, peaked within the first 3 days and returned to baseline 1 week after CFA injection. (D) A comparable decrease in hindpaw mechanical withdrawal threshold was observed after CFA injection in P40 rats (p < 0.0001, effect of treatment, two-way ANOVA). N = 4–6 rats per group. *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001 are compared with saline (A, B) and saline ipsilateral hindpaw (C, D). #p < 0.05, ##p < 0.01, ####p < 0.0001 are compared with CFA contralateral hindpaw (C, D). (E) Representative traces of single unit extracellular recordings, showing pinch evoked responses in the spinal dorsal horn neurons of P21 and P40 rats, day (D) 3 and 14 after CFA or saline intra-articular injections. Green arrow depicts start of pinch stimulation, green line indicates duration of pinch stimulation, blue line indicates duration of pinch after discharges. Scale bar: x-axis = duration of stimulation (s), y-axis = amplitude of responses (V) (F) Pinch evoked firing significantly increased in CFA injected P21 rats compared to saline controls (p < 0.01, effect of treatment, three-way ANOVA) at day 14 in the ipsilateral and contralateral dorsal horn. (G) In P40, pinch evoked firing increased after CFA injection in the ipsilateral dorsal horn only at day 3. (H) Injection of CFA into the left ankle joint induced an expansion of receptive field in the ipsilateral cord in P21 rats (p < 0.01, effect of treatment, three-way ANOVA) at day 3. By day 14, expansion of receptive field in both ipsilateral and contralateral neurons were observed. No significant changes in the size of receptive fields were observed in P40 rats following CFA injection. *p < 0.05 are compared between ipsilateral and contralateral dorsal horn, #p < 0.05, ##p < 0.01 are compared with saline controls, δδ p < 0.01, δδδ p < 0.0001 are compared between day 3 and day 14. 24–32 cells from 5 to 6 P21 animals per treatment and timepoint; 14–26 cells from 4 to 6 P40 animals per treatment and timepoint.
Mechanical allodynia in the hindpaws ipsilateral and contralateral to the inflamed ankle was also measured (Fig. 2C-D). Consistent with previous reports (Shan, 2007, Shan, 2007, Sun, 2008), joint inflammation resulted in a significant decrease in mechanical withdrawal threshold in the ipsilateral paw in both juvenile (F (3, 30) = 9.543, P = 0.00013, repeated measures two-way ANOVA) and peri-pubertal rats (F (1, 63) = 97.72, P = 0.000001, repeated measures two-way ANOVA). In P21 rats, mechanical thresholds undergo a normal developmental increase throughout the test period not seen in peri-pubertal animals, despite this, a decrease in mechanical withdrawal threshold was detected at 4 h after CFA injections, and had fully resolved by 10 days (Fig. 2C). Recovery from CFA induced mechanical allodynia was faster in P40 rats, a decrease in mechanical withdrawal threshold was observed at 4 h after CFA injections, and resolved after a week (Fig. 2D).
3.3. Central sensitisation of spinal nociceptive neurons following monoarthritis is longer lasting and more extensive in P21 compared to P40 rats
The data above suggested that the prolonged pain following joint inflammation at P21 is not attributable to differences in the local joint inflammatory process itself. Pain sensitivity is critically linked to hyperexcitability in spinal neurons, known as central sensitisation (Samad, 2001, Woolf and Salter, 2000), so we compared the effect of monoarthritis upon the excitability of spinal neurons using in vivo single unit electrophysiology, at the two ages.
Ankle joint inflammation caused a small, generalised increase in spontaneous firing of spinal cord dorsal horn neurons at both ages (P21: F (1, 102) = 9.143, P = 0.0032; P40: F (1, 74) = 6.838, P = 0.0108, three-way ANOVA) with no differences between ipsilateral and contralateral dorsal horn. At P21, there was greater variability in post arthritis spontaneous activity (mean ± SEM, spikes/min. After CFA, D3 ipsi: 3.667 ± 1.827, D14 ipsi: 3.143 ± 0.954; after saline, D3 ipsi: 1.385 ± 0.5, D14 ipsi: 0.667 ± 0.294, Supplementary Fig. 2A & B).
Next, the number of spikes evoked by cutaneous noxious pinch stimulation of the hindpaw (Fig. 2E-G) and the size of pinch receptive field (Fig. 2H-I) were measured in dorsal horn WDR neurons (Stanfa et al., 1992). At P21, joint inflammation significantly increased pinch evoked firing (F (1, 102) = 10.07, P = 0.00198, three-way ANOVA) at day 14 after CFA injection compared to animals who received saline injections. Pinch evoked firing of both ipsilateral and contralateral dorsal horn neurons was higher at day 14 compared to day 3 post CFA injection. At day 3 after CFA injection, an expansion of receptive field was observed in the ipsilateral dorsal horn (ipsi: 5994.441 ± 790.062 vs. contra: 2872.078 ± 403.940, P = 0.0269, Bonferroni multiple comparisons). Joint inflammation also significantly increased the number of spikes in the post-pinch after-discharge in P21 rats (P = 0.00003, Supplementary Fig. 2C) at day 3, compared to saline controls, with no difference between ipsilateral and contralateral neurons.
Ankle joint inflammation also increased dorsal horn neuron pinch evoked firing activity at P40, but the effects were transient compared to P21. Ipsilateral responses to pinch increased at day 3 only compared to saline controls and no changes were observed at 14 days (Fig. 2G). Furthermore, joint inflammation did not significantly alter hindpaw receptive field area or pinch evoked after-discharges (Fig. 2I and Supplementary Fig. 2D).
vFh evoked firing was measured to assess overall excitability of ipsilateral spinal dorsal horn neurons (Supplementary Fig. 3), and showed that hypersensitivity to mechanical stimulation was longer lasting in P21 compared to P40. In P21, vFh evoked firing was increased in CFA injected animals compared to saline, at day 3 (F(1, 282) = 8.272, P = 0.0045, two-way ANOVA, Supplementary Fig. 3A) and day 14 post-injection (F(1, 248) = 11.54, P = 0.00079, Supplementary Fig. 3B). At both timepoints, the increase in vFh evoked firing was greater when suprathreshold vFh was applied (day 3: vFh 15 g, P = 0.037; vFh 26 g, P = 0.041; day 14: vFh 60 g, P = 0.0044). In P40, vFh evoked firing was increased in CFA injected animals at day 3 (F(1, 160) = 2.442, P = 0.021) but not at day 14 post injection.
Low-threshold innocuous brush evoked activity was observed in dorsal horn neurons of P21 or P40 rats at 3- and 14-days following ankle joint inflammation (Supplementary Fig. 2E to H). No significant differences were detectable, indicating that spinal processing of tactile stimulation following joint inflammation is unaffected regardless of age, under these recording conditions.
3.4. The pattern and time course of pro-inflammatory cytokine expression in the spinal cord dorsal horn following joint inflammation differs between P21 and P40 rats
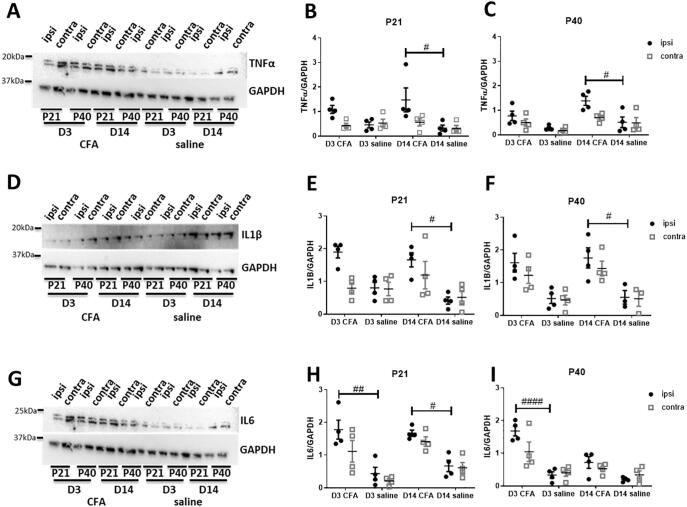
To study the molecular basis of spinal neuron sensitisation, the expression of pro-inflammatory cytokines, TNFα, IL1β and IL6, in the ipsilateral and contralateral spinal cord dorsal horn were investigated using western blots at day 3 and 14 after ankle joint injections.
Fig. 3A-C show that the expression of dorsal horn TNFα was significantly increased following injection of CFA into the ankle joints of both P21 (F (1, 6) = 10.321, P = 0.004, three-way ANOVA. Fig. 3B) and P40 rats (F (1, 10) = 18.419, P = 0.00025, three-way ANOVA. Fig. 3C). Levels of TNFα was significant higher in the ipsilateral dorsal horn at day 14 after CFA injections compared to saline controls.
Fig. 3.
The expression of pro-inflammatory cytokines at day 3 and day 14 after CFA injections in the spinal cord were measured in juvenile (P21) and peri-pubertal (P40) rats. Expression of TNFα, IL1β and IL6 were normalised to GAPDH (n = 4 per age and timepoint). (A) Representative western blot image of TNFα expression in the ipsilateral and contralateral spinal dorsal horns of P21 and P40 rats, at day 3 and 14 after CFA or saline intra-articular injections. (B, C) The expression of TNFα was significantly increased following CFA injections in the ipsilateral dorsal horn of P21 (p < 0.01, effect of treatment, three-way ANOVA) and P40 rats (p < 0.0001, effect of treatment, three-way ANOVA) at both day 3 and 14 in P21 rats (B), but only on day 14 in P40 rats (C). (D) Representative western blot image of IL1β expression. (E, F) Similarly, expression of IL1β increased following CFA injection in P21 and P40 rats (p < 0.0001 for both ages, effect of treatment, three-way ANOVA). (E) In P21 rats IL1β increased in the ipsilateral dorsal horn at day 3, but in both ipsilateral and contralateral by day 14. (F) In P40 rats, the expression of IL1β was increased in both the ipsilateral and contralateral dorsal horn at day 3 and 14 after CFA injection. (G) Representative western blot image of IL6 expression. (H, I) The expression of IL6 was significantly increased following CFA injection in both P21 and P40 rats (p < 0.0001 for both ages, effect of treatment, three-way ANOVA) in both the ipsilateral and contralateral dorsal horn. (H) In P21 rats an increase in IL6 was found on both day 3 and 14, whereas in P40 (I), the increase was only evident on day 3. Data are presented as mean ± SEM. #p < 0.05, ##p < 0.01, ####p < 0.0001 are compared with saline controls.
The expression of dorsal horn IL1β followed a similar course to that of TNFα, as joint inflammation significantly increased the level of IL1β detected in the dorsal horn of both P21 and P40 rats (P21: F(1, 6) = 23.327, P = 0.00006, P40: F (1, 10) = 35.067, P = 0.0000058, three-way ANOVA. Fig. 3D-F). At both ages, the expression of IL1β 14 days after CFA injections was higher in the ipsilateral dorsal horn compared to saline controls.
The expression of IL6 in the spinal cord dorsal horn was also increased by joint inflammation (P21: F(1, 6) = 51.286, P = 0.0000024, P40: F (1, 10) = 39.054, P = 0.000018, three-way ANOVA, Fig. 3G-I). In P40 rats, the expression of IL6 was higher in the ipsilateral dorsal horn of CFA injected animals compared to saline controls at day 3, but not at 2 weeks. In contrast, time post CFA injection was not a factor in P21 rats: the expression of IL6 was higher in the ipsilateral dorsal horn of CFA injected animals at both day 3 and day 14 when compared to saline controls.
3.5. A functional role for spinal IL6 in prolonged joint inflammatory pain behaviours in juvenile rats
The data above shows that P21 rats display prolonged pain and spinal neuron activity compared to P40 rats after intra-articular injection of CFA. The expression of IL6 in the spinal dorsal horn were prolonged in P21 but not P40 rats. This suggests that spinally released IL6 has a role in maintaining ‘late-phase’ pain in juvenile rats.
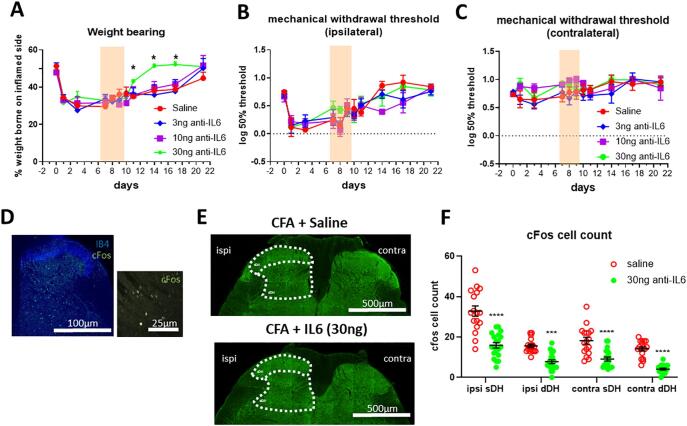
To test this, anti-IL6 antibody (3, 10 and 30 ng in 10 μL per injection, one dose per animal) was intrathecally injected 7, 8 and 9 days after intra-articular injection of CFA. 30 ng of anti-IL6 accelerated the recovery of weight bearing following P21 ankle monoarthritis and reversed weight bearing deficits on days 11, 14 and 17 (Bonferroni multiple comparisons, P = 0.014, P = 0.02 and P = 0.011 respectively) (Fig. 4A), but did not have an effect on von Frey hair mechanical sensitivity in either the ipsilateral or the contralateral hindpaw (Fig. 4B-C).
Fig. 4.
The effect of spinal IL6 blockade on mechanical pain hypersensitivity in juvenile (P21) rats following CFA injection into the ankle at baseline. Anti-IL6 antibody (3, 10 and 30 ng in 10 μL per injection) was administered intrathecally on day 7, 8 and 9. Sensory behavioural tests were performed one day after each injection. (n = 4 for saline, n = 5 for each dose of anti-IL6. (A) Anti-IL6 alleviated weight bearing deficits (p < 0.05, interaction between treatment and time, two-way ANOVA), the weight borne on the inflamed side increased on day 11, 14 and 17 after 3 injections of anti-IL6 (30 ng). (B, C) Anti-IL6 did not alter the mechanical sensitivity in the hindpaw. (D) 10x Image of IB4 and cFos immunoreactivity in the spinal cord (left panel), 60x image of cFos immunoreactivity (right panel). (E) Representative image of cFos immunoreactivity in the sDH and dDH of CFA injected animals, after either intrathecal injections of saline or 30 ng of anti-IL6 antibody (N = 5/group). (F) Analysis of cFos immunoreactivity revealed significant decrease in cFos immunopositive cells after anti-IL6 (P < 0.0001). ipsi = ipsilateral, contra = contralateral, sDH = superficial dorsal horn, dDH = deep dorsal horn. Data are presented as mean ± SEM. *p < 0.05, ***p < 0.001 and ****p < 0.0001 are compared with saline controls.
To test whether upregulation of IL6 is responsible for sensitisation of spinal neurons, cFos immunoreactivity was used to measure dorsal horn recent neuronal activity after intrathecal anti-IL6 (30 ng) treatment (Fig. 4D-E). Fig. 4F shows the expected increase in cFos expressing dorsal horn neurons, 9 days following ankle joint inflammation in P21 rats treated with intrathecal saline. After anti-IL6, the number of cFos expressing neurons in P21 rats was significantly reduced (F (1, 140) = 139.2, P < 0.0001, two-way ANOVA). The decrease in cFos immunopositive cells was observed in the ipsilateral and contralateral superficial dorsal horn (ipsi: saline vs. 30 ng anti-IL6, 32.824 ± 2.54 vs. 15.85 ± 1.324, P < 0.0001; contra: saline vs. 20 ng anti-IL6, 18.176 ± 1.715 vs. 9.1 ± 0.992, P = 0.0000115; Bonferroni multiple comparisons), where nociceptive primary afferent terminates, as well as the ipsilateral and contralateral deep dorsal horn (ipsi: saline vs. 30 ng anti-IL6, 15.588 ± 0.845 vs. 7.75 ± 0.92, P = 0.00018; contra: saline vs. 30 ng anti-IL6, 14.118 ± 0.988 vs. 4.1 ± 0.452, P = 0.0000012, Bonferroni multiple comparisons).
4. Discussion
The results support the hypothesis that joint inflammation triggers an age-related pattern of cytokine expression in the spinal cord which is responsible central sensitization and chronic pain in juvenile arthritis. We report pain behaviour is longer lasting in juvenile rats despite comparable joint inflammatory processes to peri-pubertal rats. This prolonged pain is accompanied by more widespread central sensitisation of spinal dorsal horn nociceptive neurons. We show that the profile and time-course of pro-inflammatory cytokine expression in the spinal cord following joint inflammation differs at the two ages, most notably in IL-6. In addition, we show a potential role of spinal IL-6 in pain behaviour of juvenile arthritic rats as intrathecal blockade of IL6 reduced weight bearing deficits and dorsal horn neuronal cFos expression.
Activation of peripheral immune cells and release of cytokines within the joint capsules (de Jager, 2006) is important for the healing process, but cytokines can also activate peripheral nociceptors and cause pain. Here we observed an increase in the expression of pro-inflammatory cytokines in the joint tissue, reflecting the duration of joint swelling that was similar at both ages. High levels of TNFα, IL1β and IL6 were found on day 3 after the induction of monoarthritis in the ankle joints of both juvenile and peri-pubertal rats, which coincided with the peak of active swelling and pain behaviours. There were some age-related differences in joint cytokines at day 3, our findings indicate greater TNFα increase in peri-pubertals and greater IL6 increase in juveniles, and suggest age-dependent cytokine modulation of pain and inflammatory processing. Nonetheless, expression of TNFα, IL1β and IL6 in the joints resolved by day 14 and are unlikely to explain the difference in prolonged pain behaviour observed in juvenile animals.
Using in vivo electrophysiology to record the activity of spinal dorsal horn neurons, we observed hyperexcitability following induction of ankle joint monoarthritis, as measured by increased spontaneous firing, responses to suprathreshold vFh, pinch evoked firing and expanded cutaneous receptive fields. These are all features of central sensitization of nociceptive circuits associated with inflammatory pain (Woolf and King, 1990, Li et al., 1999). Importantly we discovered a difference in the time course of monoarthritis induced neuronal hyperexcitability between juvenile and peri-pubertal rats, which was longer lasting and more widespread, involving both ipsilateral and contralateral neurons in juvenile rats.
Of note, baseline neuronal hyperexcitability in the spinal pain circuit is a feature in juvenile animals, due to delayed maturation of segmental and descending inhibitory pathways (Hathway, 2009). This was reflected in the steady increase in mechanical sensory thresholds observed in our P21 behavioural studies. However, dorsal horn and behavioural responses to surgical incision (Walker et al., 2009, Ririe et al., 2008) or to non-arthritic carrageenan inflammation at P21 are not significantly greater than in adults (Torsney and Fitzgerald, 2002). Therefore, the spinal neuronal hyperexcitability observed in this study is a direct consequence of joint inflammation rather than developmental regulations.
Joint inflammation and subsequent activation of nociceptors leads to the activation of microglia within the spinal cord (Shan, 2007). These microglia and their production of cytokines within the spinal nociceptive circuits can induce hyperexcitability of neurons through the increase of excitatory postsynaptic currents and the decrease of inhibitory postsynaptic currents (Kawasaki, 2008). Over time this can lead to a sensitisation of spinal neurons and the chronification of pain (Choy and Panayi, 2001, Schaible, 2010). The enhanced dorsal horn hypersensitivity and prolonged pain behaviour following P21 ankle joint monoarthritis could be explained by a difference in microglia activation and cytokine release at this age. Microglia proliferate in the CNS until P20-30 before reaching a stable state at P40 (Mosser, 2017). In addition, microglial activation and cytokine release following peripheral nerve injury are known to be developmentally regulated (Beggs, 2012, Vega-Avelaira et al., 2009) with evidence that the neuroimmune response profile in the dorsal horn following nerve injury switches from anti- to pro-inflammatory after P21 in rats and mice (McKelvey, 2015).
Here we found high levels of TNFα, IL1β and IL6 in the spinal cord, at both 3 and 14 days after the induction of monoarthritis, which mirrored the time course of neuronal hyperexcitability in the spinal cord and joint inflammatory pain behaviours, strongly supporting a role of spinal cord cytokines in the generation of pain (Vazquez, 2012). Increases in the expression of pro-inflammatory cytokines were observed in both the ipsilateral and contralateral dorsal horn, consistent with the bilateral increase in spinal cord neuronal hyperexcitability, beyond the affected joint. Our western blot experiments showed multiple bands of target cytokine expression, suggesting the involvement of both cleaved and precursor forms of TNFα, IL1β and IL6. Global hyperalgesia is a particular feature of pain in patients suffering from JIA (Cornelissen, 2014, Hogeweg, 1995) and the spread of cytokine release and central sensitisation along the spinal cord could be an explanation for this.
The increase in IL6 was notably stronger and longer lasting in juvenile rats compared to peri-pubertal rats, which suggests that spinally released IL6 plays an important role in maintaining juvenile joint inflammatory pain. When a specific IL6 antibody was administered intrathecally, daily from 7 to 9 days after the induction of monoarthritis, established weight bearing pain behaviours were alleviated in juvenile rats. The effect of spinal IL6 inhibition observed in this study was only partially dose dependent. Our observation in the concomitant reduction in cFos (marker of recent neuronal activity) immunoreactivity suggests that the alleviation in pain behaviours were in part mechanistically related to a decrease in spinal neuronal activity. Previous reports have shown that injections into the lumbar intrathecal space of Sprague Dawley rats remained on the spinal cord with minimal leakage to the dorsal root ganglia (DRG) (Chang, 2016). However, intrathecal injection of the anti-IL6 antibody could also affect cytokine production and release in the DRG by either resident or infiltrating immune cells (Ledeboer, 2007). Future studies on the subpopulation of spinal neurons impacted by joint inflammation, and the immune responses at the level of the DRG would add to the understanding of pain behaviours in juvenile joint inflammatory pain (Koch et al., 2018).
The role of IL-6 within juvenile arthritis requires further investigation but we provide a proof that inhibition of spinally release cytokine is a potential analgesic target, for juvenile arthritis patients whose disease is well controlled but still suffer from high levels of pain. Current JIA biologic treatments do not cross the blood brain barrier and therefore have limited actions on the activity of central nociceptive circuits (Robinson et al., 2001). Future therapeutic strategies should consider the prolonged time course of central sensitisation in JIA and target CNS neuroimmune activity and central IL-6 release to relieve pain.
Our study provides a preclinical understanding of juvenile joint inflammatory pain, which was previously lacking. We demonstrate in a rodent model that age-related mechanisms underlie joint inflammatory pain. Juvenile arthritic joint pain is more prolonged and associated with greater central sensitization due to a long-lasting release of IL-6 into the spinal cord.
Author contributions
CHTK and MF conceived and designed the project. CHTK, AEL and JCP performed the experiments. CHTK, AEL, JCP, TT and MF analysed the data. TT and MF supervised the experiments. CHTK, TT and MF wrote the manuscript.
Funding
This work is supported by a Versus Arthritis (formerly Arthritis Research UK) award 21,322 to MF, and a project grant from the Canadian Institute of health (CIHR) MOP133523 to TT. CHTK is supported by CIHR postgraduate fellowship.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.bbi.2020.08.004.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
Images of western blots. (A) Specificity of antibodies used for western blot. Positive control experiments showed dose-dependent increases in the expression of IL6, TNFα and IL1β (0.5 µg, 1 µg and 2 µg), at the corresponding molecular weights (IL6: 22kDa, TNFα: 17 kDa, IL1β: 17kDa).
The effect of CFA injections into the left ankle joints on brush evoked activity in juvenile (P21) and peri-pubertal (P40) rats. (A, B) CFA significantly increased spontaneous firing, in P21 (A, p < 0.01, effect of treatment, three-way ANOVA) and P40 rats (B, p < 0.05, effect of treatment, three-way ANOVA) in both the ipsilateral and contralateral dorsal horn. (A) The increase in spontaneous firing was observed at day 3 and day 14 in P21 animals, but only at day 3 in P40 (B). (C) In P21 rats injection of CFA into the left ankle significantly increased the number of after-discharges in spinal neurons (p < 0.0001, effect of treatment, three-way ANOVA) in both the ipsilateral and contralateral dorsal horn, at both day 3 and day 14. (D) In P40, there was no significant change in after-discharges. (E, F) Brush evoked firing in P21 and P40 rats respectively. Monoarthritis did not change brush evoked firing activity, at either day 3 or day 14. (G. H) The size of receptive fields was not altered in inflamed P21 and P40 rats. Data are presented as mean ± SEM. # p < 0.05 comparison between CFA and saline injected animals. N = 24-32 cells from 5-6 P21 animals per treatment and timepoint; N = 14-26 cells from N = 4-6 P40 animals per treatment and timepoint.
The effect of CFA injections into the left ankle joints on vFh evoked activity in ipsilateral spinal dorsal horn neurons of juvenile (P21) and peri-pubertal (P40) rats. (A) CFA significantly increased vFh evoked firing at day 3 and 14 (B) in P21 rats (day 3: p < 0.01, day 14: p < 0.001, effect of treatment, two-way ANOVA). (C) CFA significantly increase vFh evoked firing at day 3 in P40 rats but not at 14 (D) (day 3: p < 0.05, effect of treatment, two-way ANOVA). Data are presented as mean ± SEM. *p<0.05, **p < 0.01 comparison between CFA and saline injected animals. N = 13-18 cells from 5 P21 animals per treatment and timepoint. N = 7-12 cells from 4 P40 animals per treatment and timepoint.
References
- Beggs S. Priming of adult pain responses by neonatal pain experience: maintenance by central neuroimmune activity. Brain. 2012;135(Pt 2):404–417. doi: 10.1093/brain/awr288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang M.-F. Effective gene expression in the rat dorsal root ganglia with a non-viral vector delivered via spinal nerve injection. Sci. Rep. 2016;6(1):35612. doi: 10.1038/srep35612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaplan S. Quantitative assessment of tactile allodynia in the rat paw. J. Neurosci. Methods. 1994;53(1):55–63. doi: 10.1016/0165-0270(94)90144-9. [DOI] [PubMed] [Google Scholar]
- Choy E.H.S., Panayi G.S. Cytokine Pathways and Joint Inflammation in Rheumatoid Arthritis. N. Engl. J. Med. 2001;344(12):907–916. doi: 10.1056/NEJM200103223441207. [DOI] [PubMed] [Google Scholar]
- Consolaro A., Ravelli A. Paediatric rheumatology: Juvenile idiopathic arthritis—are biologic agents effective for pain? Nat. Rev. Rheumatol. 2013;9(8):447–448. doi: 10.1038/nrrheum.2013.108. [DOI] [PubMed] [Google Scholar]
- Cornelissen L. Pain hypersensitivity in juvenile idiopathic arthritis: a quantitative sensory testing study. Pediat. Rheumatol. 2014;12(1):39. doi: 10.1186/1546-0096-12-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costigan M. T-Cell Infiltration and Signaling in the Adult Dorsal Spinal Cord Is a Major Contributor to Neuropathic Pain-Like Hypersensitivity. J. Neurosci. 2009;29(46):14415. doi: 10.1523/JNEUROSCI.4569-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Jager W. Blood and synovial fluid cytokine signatures in patients with juvenile idiopathic arthritis; a cross-sectional study. Ann. Rheum. Dis. 2006 doi: 10.1136/ard.2006.061853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Lalouvière L.L.H., Ioannou Y., Fitzgerald M. Neural mechanisms underlying the pain of juvenile idiopathic arthritis. Nat. Rev. Rheumatol. 2014;10(4):205–211. doi: 10.1038/nrrheum.2014.4. [DOI] [PubMed] [Google Scholar]
- Djouhri L. Spontaneous pain, both neuropathic and inflammatory, is related to frequency of spontaneous firing in intact C-fiber nociceptors. J. Neurosci. 2006;26(4):1281–1292. doi: 10.1523/JNEUROSCI.3388-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo W. Glial–cytokine–neuronal interactions underlying the mechanisms of persistent pain. The J. Neurosci. 2007;27(22):6006–6018. doi: 10.1523/JNEUROSCI.0176-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hathway G.J. The changing balance of brainstem–spinal cord modulation of pain processing over the first weeks of rat postnatal life. J. Physiol. 2009;587(12):2927–2935. doi: 10.1113/jphysiol.2008.168013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogeweg J.A. The pain threshold in juvenile chronic arthritis. Br. J. Rheumatol. 1995;34(1):61–67. doi: 10.1093/rheumatology/34.1.61. [DOI] [PubMed] [Google Scholar]
- Kawasaki Y. Cytokine mechanisms of central sensitization: distinct and overlapping role of interleukin-1beta, interleukin-6, and tumor necrosis factor-alpha in regulating synaptic and neuronal activity in the superficial spinal cord. J. Neurosci. 2008;28(20):5189–5194. doi: 10.1523/JNEUROSCI.3338-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilkenny C. Animal research: reporting in vivo experiments: the ARRIVE guidelines. J. Gene Med. 2010;12(7):561–563. doi: 10.1002/jgm.1473. [DOI] [PubMed] [Google Scholar]
- Koch S.C., Acton D., Goulding M. Spinal Circuits for Touch, Pain, and Itch. Annu. Rev. Physiol. 2018;80(1):189–217. doi: 10.1146/annurev-physiol-022516-034303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Learoyd A.E., Sen D., Fitzgerald M. The pain trajectory of juvenile idiopathic arthritis (JIA): translating from adolescent patient report to behavioural sensitivity in a juvenile animal model. Pediat. Rheumatol. 2019;17(1):60. doi: 10.1186/s12969-019-0360-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ledeboer A. Intrathecal interleukin-10 gene therapy attenuates paclitaxel-induced mechanical allodynia and proinflammatory cytokine expression in dorsal root ganglia in rats. Brain Behav. Immun. 2007;21(5):686–698. doi: 10.1016/j.bbi.2006.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leegaard A. Decreased pain threshold in juvenile idiopathic arthritis: a cross-sectional study. J. Rheumatol. 2013;40(7):1212–1217. doi: 10.3899/jrheum.120793. [DOI] [PubMed] [Google Scholar]
- Li J., Simone D.A., Larson A.A. Windup leads to characteristics of central sensitization. Pain. 1999;79(1):75–82. doi: 10.1016/S0304-3959(98)00154-7. [DOI] [PubMed] [Google Scholar]
- Lomholt J.J., Thastum M., Herlin T. Pain experience in children with juvenile idiopathic arthritis treated with anti-TNF agents compared to non-biologic standard treatment. Pediatr. Rheumatol. Online J. 2013;11(1):21. doi: 10.1186/1546-0096-11-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manners P.J., Bower C. Worldwide prevalence of juvenile arthritis why does it vary so much? J. Rheumatol. 2002;29(7):1520–1530. [PubMed] [Google Scholar]
- Marinova-Mutafchieva L. Dynamics of proinflammatory cytokine expression in the joints of mice with collagen-induced arthritis (CIA) Clin. Exp. Immunol. 1997;107(3):507–512. doi: 10.1046/j.1365-2249.1997.2901181.x. [DOI] [PubMed] [Google Scholar]
- McKelvey R. Neuropathic Pain Is Constitutively Suppressed in Early Life by Anti-Inflammatory Neuroimmune Regulation. J. Neurosci. 2015;35(2):457–466. doi: 10.1523/JNEUROSCI.2315-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moriarty O. Priming of Adult Incision Response by Early-Life Injury: Neonatal Microglial Inhibition Has Persistent But Sexually Dimorphic Effects in Adult Rats. J. Neurosci. 2019;39(16):3081. doi: 10.1523/JNEUROSCI.1786-18.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moss A. Spinal microglia and neuropathic pain in young rats. Pain. 2007;128(3):215–224. doi: 10.1016/j.pain.2006.09.018. [DOI] [PubMed] [Google Scholar]
- Mosser C.A. Microglia in CNS development: Shaping the brain for the future. Prog. Neurobiol. 2017;149–150:1–20. doi: 10.1016/j.pneurobio.2017.01.002. [DOI] [PubMed] [Google Scholar]
- Prelog M. Premature aging of the immune system in children with juvenile idiopathic arthritis. Arthritis Rheum. 2008;58(7):2153–2162. doi: 10.1002/art.23599. [DOI] [PubMed] [Google Scholar]
- Raghavendra V., Tanga F.Y., DeLeo J.A. Complete Freunds adjuvant-induced peripheral inflammation evokes glial activation and proinflammatory cytokine expression in the CNS. Eur. J. Neurosci. 2004;20(2):467–473. doi: 10.1111/j.1460-9568.2004.03514.x. [DOI] [PubMed] [Google Scholar]
- Ririe D.G., Bremner L.R., Fitzgerald M. Comparison of the immediate effects of surgical incision on dorsal horn neuronal receptive field size and responses during postnatal development. Anesthesiology. 2008;109(4):698–706. doi: 10.1097/ALN.0b013e3181870a32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson W.H., Genovese M.C., Moreland L.W. Demyelinating and neurologic events reported in association with tumor necrosis factor alpha antagonism: by what mechanisms could tumor necrosis factor alpha antagonists improve rheumatoid arthritis but exacerbate multiple sclerosis? Arthritis Rheum. 2001;44(9):1977–1983. doi: 10.1002/1529-0131(200109)44:9<1977::AID-ART345>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- Samad T.A. Interleukin-1[beta]-mediated induction of Cox-2 in the CNS contributes to inflammatory pain hypersensitivity. Nature. 2001;410(6827):471–475. doi: 10.1038/35068566. [DOI] [PubMed] [Google Scholar]
- Schaible H.-G. Joint pain. Exp. Brain Res. 2009;196(1):153–162. doi: 10.1007/s00221-009-1782-9. [DOI] [PubMed] [Google Scholar]
- Schaible H.G. The role of proinflammatory cytokines in the generation and maintenance of joint pain. Ann. N. Y. Acad. Sci. 2010;1193(1):60–69. doi: 10.1111/j.1749-6632.2009.05301.x. [DOI] [PubMed] [Google Scholar]
- Shan S. New evidence for the involvement of spinal fractalkine receptor in pain facilitation and spinal glial activation in rat model of monoarthritis. Pain. 2007;129(1–2):64–75. doi: 10.1016/j.pain.2006.09.035. [DOI] [PubMed] [Google Scholar]
- Shan S. Is functional state of spinal microglia involved in the anti-allodynic and anti-hyperalgesic effects of electroacupuncture in rat model of monoarthritis? Neurobiol. Dis. 2007;26(3):558–568. doi: 10.1016/j.nbd.2007.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiff N.J. Trajectories of pain severity in juvenile idiopathic arthritis: results from the Research in Arthritis in Canadian Children Emphasizing Outcomes cohort. Pain. 2017 doi: 10.1097/j.pain.0000000000001064. [DOI] [PubMed] [Google Scholar]
- Sommer C., Kress M. Recent findings on how proinflammatory cytokines cause pain: peripheral mechanisms in inflammatory and neuropathic hyperalgesia. Neurosci. Lett. 2004;361(1–3):184–187. doi: 10.1016/j.neulet.2003.12.007. [DOI] [PubMed] [Google Scholar]
- Stanfa L.C., Sullivan A.F., Dickenson A.H. Alterations in neuronal excitability and the potency of spinal mu, delta and kappa opioids after carrageenan-induced inflammation. Pain. 1992;50(3):345–354. doi: 10.1016/0304-3959(92)90040-I. [DOI] [PubMed] [Google Scholar]
- Sun S. Evidence for suppression of electroacupuncture on spinal glial activation and behavioral hypersensitivity in a rat model of monoarthritis. Brain Res. Bull. 2008;75(1):83–93. doi: 10.1016/j.brainresbull.2007.07.027. [DOI] [PubMed] [Google Scholar]
- Torsney C., Fitzgerald M. Age-dependent effects of peripheral inflammation on the electrophysiological properties of neonatal rat dorsal horn neurons. J. Neurophysiol. 2002;87(3):1311–1317. doi: 10.1152/jn.00462.2001. [DOI] [PubMed] [Google Scholar]
- Vazquez E. Spinal interleukin-6 is an amplifier of arthritic pain in the rat. Arthritis Rheum. 2012;64(7):2233–2242. doi: 10.1002/art.34384. [DOI] [PubMed] [Google Scholar]
- Vega-Avelaira D., Moss A., Fitzgerald M. Age-related changes in the spinal cord microglial and astrocytic response profile to nerve injury. Brain Behav. Immun. 2007;21(5):617–623. doi: 10.1016/j.bbi.2006.10.007. [DOI] [PubMed] [Google Scholar]
- Vega-Avelaira D., Géranton S.M., Fitzgerald M. Differential regulation of immune responses and macrophage/neuron interactions in the dorsal root ganglion in young and adult rats following nerve injury. Molecular Pain. 2009;5(1):70. doi: 10.1186/1744-8069-5-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker S.M. Neonatal inflammation and primary afferent terminal plasticity in the rat dorsal horn. Pain. 2003;105(1):185–195. doi: 10.1016/s0304-3959(03)00201-x. [DOI] [PubMed] [Google Scholar]
- Walker S.M. Long-term impact of neonatal intensive care and surgery on somatosensory perception in children born extremely preterm. Pain. 2009;141(1–2):79–87. doi: 10.1016/j.pain.2008.10.012. [DOI] [PubMed] [Google Scholar]
- Walker S.M., Tochiki K.K., Fitzgerald M. Hindpaw incision in early life increases the hyperalgesic response to repeat surgical injury: Critical period and dependence on initial afferent activity. PAIN®. 2009;147(1–3):99–106. doi: 10.1016/j.pain.2009.08.017. [DOI] [PubMed] [Google Scholar]
- Watkins L.R., Maier S.F., Goehler L.E. Immune activation: the role of pro-inflammatory cytokines in inflammation, illness responses and pathological pain states. Pain. 1995;63(3):289–302. doi: 10.1016/0304-3959(95)00186-7. [DOI] [PubMed] [Google Scholar]
- Woolf C.J., King A.E. Dynamic alterations in the cutaneous mechanoreceptive fields of dorsal horn neurons in the rat spinal cord. J. Neurosci. 1990;10(8):2717. doi: 10.1523/JNEUROSCI.10-08-02717.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolf C.J., Salter M.W. Neuronal Plasticity: Increasing the Gain in Pain. Science. 2000;288(5472):1765–1768. doi: 10.1126/science.288.5472.1765. [DOI] [PubMed] [Google Scholar]
- Zhang L. TNF-alpha contributes to spinal cord synaptic plasticity and inflammatory pain: distinct role of TNF receptor subtypes 1 and 2. PAIN®. 2011;152(2):419–427. doi: 10.1016/j.pain.2010.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Images of western blots. (A) Specificity of antibodies used for western blot. Positive control experiments showed dose-dependent increases in the expression of IL6, TNFα and IL1β (0.5 µg, 1 µg and 2 µg), at the corresponding molecular weights (IL6: 22kDa, TNFα: 17 kDa, IL1β: 17kDa).
The effect of CFA injections into the left ankle joints on brush evoked activity in juvenile (P21) and peri-pubertal (P40) rats. (A, B) CFA significantly increased spontaneous firing, in P21 (A, p < 0.01, effect of treatment, three-way ANOVA) and P40 rats (B, p < 0.05, effect of treatment, three-way ANOVA) in both the ipsilateral and contralateral dorsal horn. (A) The increase in spontaneous firing was observed at day 3 and day 14 in P21 animals, but only at day 3 in P40 (B). (C) In P21 rats injection of CFA into the left ankle significantly increased the number of after-discharges in spinal neurons (p < 0.0001, effect of treatment, three-way ANOVA) in both the ipsilateral and contralateral dorsal horn, at both day 3 and day 14. (D) In P40, there was no significant change in after-discharges. (E, F) Brush evoked firing in P21 and P40 rats respectively. Monoarthritis did not change brush evoked firing activity, at either day 3 or day 14. (G. H) The size of receptive fields was not altered in inflamed P21 and P40 rats. Data are presented as mean ± SEM. # p < 0.05 comparison between CFA and saline injected animals. N = 24-32 cells from 5-6 P21 animals per treatment and timepoint; N = 14-26 cells from N = 4-6 P40 animals per treatment and timepoint.
The effect of CFA injections into the left ankle joints on vFh evoked activity in ipsilateral spinal dorsal horn neurons of juvenile (P21) and peri-pubertal (P40) rats. (A) CFA significantly increased vFh evoked firing at day 3 and 14 (B) in P21 rats (day 3: p < 0.01, day 14: p < 0.001, effect of treatment, two-way ANOVA). (C) CFA significantly increase vFh evoked firing at day 3 in P40 rats but not at 14 (D) (day 3: p < 0.05, effect of treatment, two-way ANOVA). Data are presented as mean ± SEM. *p<0.05, **p < 0.01 comparison between CFA and saline injected animals. N = 13-18 cells from 5 P21 animals per treatment and timepoint. N = 7-12 cells from 4 P40 animals per treatment and timepoint.