Abstract
Background
Peroxiredoxin 4 (Prdx4), a member of the Prdx family, can catalyze the reduction of reactive oxygen species. This study aims to explore whether Prdx4 can serve as an effective marker in follicular fluid (FF) for predicting in vitro fertilization/intracytoplasmic sperm injection (IVF/ICSI) cycle outcomes.
Methods
In this prospective study, all participants were recruited from the center of clinical reproductive medicine from 2017 September to 2018 December. Women with tubal or male factor infertility undergoing their first IVF/ICSI cycle were recruited (n=138). FF samples from each patient were collected on the day of oocyte retrieval. Prdx4 concentrations were measured, and the correlation between Prdx4 levels and IVF outcomes was analyzed.
Results
The results showed that pregnant women had higher levels of Prdx4 than nonpregnant women. Prdx4 was positively correlated with the oocyte fertilization rate (r =0.334; P=0.011) and good quality embryo rate (r =0.326; P=0.013). Furthermore, we found that the clinical pregnancy rate was positively correlated with Prdx4 levels in a concentration-dependent manner in the Prdx4 quartiles (<13.38, 13.83–16.93, 16.93–22.93, >22.93 ng/mL). The fertilization rates, clinical pregnancy rates and live pregnancy rates were all significantly higher in the highest Prdx4 quartile group than in the lowest quartile. Moreover, the results indicated that Prdx4 had an area under the receiver operating characteristic curve (AUC) of 0.754, corresponding to an optimal cutoff point of 22.30 ng/mL.
Conclusions
Our results provide evidence that higher expression of antioxidants, such as Prdx4, in the FF of IVF patients tends to indicate a higher likelihood of pregnancy through an oocyte quality mechanism.
Keywords: Follicular fluid (FF), oocyte quality, ovarian stimulation, oxidative stress, peroxiredoxin 4 (Prdx4)
Introduction
Approximately 9% of women of reproductive age worldwide suffer from infertility, and in vitro fertilization-embryo transfer (IVF-ET) technology offers the highest success rate for infertile patients (1). However, nearly 75% of IVF cycles do not result in a successful pregnancy, and more cycles are needed after IVF failure, which negatively impacts couples. Therefore, effective biomarkers for predicting the probability of pregnancy with IVF treatment are urgently needed. A positive outcome of an IVF procedure requires the successful occurrence of several events, such as oocyte maturation, ovulation and fertilization, especially oocyte quality, which plays a key role in the whole process. Follicular fluid (FF) is a major component of the biological environment that supports oocyte development and subsequent embryo development during IVF (2,3). The components of FF include various mediators, such as cytokines, growth factors, regulatory molecules, reactive oxygen species (ROS) and antioxidants (4). These mediators have direct effects on the quality and maturation ability of oocytes.
Peroxiredoxin 4 (Prdx4) is a member of the peroxiredoxin antioxidant enzyme family, whose members catalyze the reduction of ROS, such as H2O2 and various organic hydroperoxides, to form water and alcohols (5). In our previous studies, cellular Prdx4 was found to be expressed in the mouse cumulus-oocyte complex (COC) and human ovary (6). Moreover, we demonstrated that Prdx4 was expressed at lower levels in polycystic ovaries than in normal ovaries and that the expression of Prdx4 in the granulosa cells (GCs) of mature follicles was higher than that in the GCs of immature follicles (6). These observations suggested that cellular Prdx4 may play a role in follicular development. Many studies have identified Prdx4 as an antioxidant and suggested that the Prdx4 expression level might be an effective biomarker of oxidative stress (OS) in the body and that Prdx4 expression can predict the incidence of several diseases, such as cardiovascular disease and recurrent pregnancy loss (7-9). However, the role of Prdx4 in the female reproductive system, especially in females undergoing IVF, is still unknown.
The aim of this study was to investigate the relationship between Prdx4 levels in the FF of infertility patients and subsequent oocyte quality and IVF-ET outcomes, especially the clinical pregnancy rate. These results may provide clinically relevant information for predicting IVF outcomes and reveal the mechanism of Prdx4 activity in oocyte development. We present the following article in accordance with the STROBE reporting checklist (available at http://dx.doi.org/10.21037/atm-20-397).
Methods
This prospective clinical cohort study was conducted between 2017 September and 2018 December at the reproductive clinical center of The First Affiliated Hospital of Nanjing Medical University. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). This study was approved by the Institutional Ethics Committee of The First Affiliated Hospital of Nanjing Medical University (No. 2016-SRFA-038), and informed consent was obtained from all patients. A total of 138 infertility patients diagnosed with tubal or male factor infertility were recruited for this study. The exclusion criteria were as follows: ≥32 years of age; body mass index (BMI) ≥24 kg/m2; primary ovarian insufficiency; polycystic ovary syndrome; cycles with the dominant FF contaminated with blood during oocyte retrieval; cycles with the dominant FF not yielding oocytes; or any history of other gynecological or other endocrine diseases (thyroid disease, diabetes mellitus, or Cushing’s syndrome). The FF samples were obtained from patients who first underwent either IVF or intracytoplasmic sperm injection (ICSI).
COH, IVF-ET and follicular fluid collection
All participants were injected with the gonadotropin-releasing hormone (GnRH) agonist Decapeptyl/Triptorelin (Ferring Pharmaceuticals Co., Ltd, Germany) starting from the mid-luteal phase. Once pituitary downregulation was achieved after 2 weeks, subjects were injected with recombinant follicle-stimulating hormone (FSH) (Gonal F, Serono, Geneva, Switzerland). The sizes of the follicles were monitored periodically via ultrasound and serum estradiol assays. When there were three or more follicles with a mean diameter ≥17 mm, human chorionic gonadotropin (hCG) (Pregnyl, Organon, The Netherlands) was administered subcutaneously. Follicle size was estimated by ultrasound 36 h after the administration of hCG, and oocytes were aspirated. FF was carefully aspirated from follicles of optimal size (diameters in the range of 16–20 mm) in each patient during oocyte retrieval, and the FF of each follicle from the same patient was combined. The FF was aspirated without contamination with the flushing medium or culture medium. Oocytes were isolated, and FF samples from the same patient were pooled and centrifuged at 2,000 rpm for 10 min. Supernatants were stored at −70 °C until analysis. FF contaminated with culture medium or blood was discarded.
Assay of Prdx4 levels
Prdx4 levels in FF were assessed using an enzyme-linked immunosorbent assay (ELISA) (Abnova, Taipei, Taiwan) according to the manufacturer’s protocol as previously described (10).
Hydrogen peroxide (H2O2) assay and antioxidant activity
H2O2 concentrations in FF were measured using a colorimetric hydrogen peroxide kit (Ann Arbor, MI, USA) as previously described. The levels of glutathione (GSH), superoxide dismutase (SOD), catalase (CAT) and malondialdehyde (MDA) activity were measured as previously described. Glutathione peroxidase (GSH-Px) activity was measured using an Amplex Red Peroxidase Assay Kit (Molecular Probes, USA). Briefly, 25 µL of FF was dispensed in each well of a 96 well plate and mixed with 25 µL of reaction buffer (0.05 M sodium phosphate, pH 7.4). Then, 50 µL of a working solution of Amplex Red reagent (100 µm) and H2O2 (2 mm) was added into each microplate well, and samples were incubated at room temperature for 30 min and protected from light. Absorbances were measured with a 412 nm filter (Spectra Shell Microplate Reader, SLT Spectra).
Evaluation of parameters associated with oocyte quality
Fertilization was evaluated 16–18 h after insemination, and embryo cleavage was evaluated 24 h later. Embryo morphology was assessed on day 3 of in vitro culture, with consideration of the number of cells and percentage of fragmentation. The variables related to oocyte quality were as follows: (I) the fertilization rate, namely, the proportion of 2 pronuclei (PN) observed relative to the number of oocytes retrieved; (II) the cleavage rate, namely, the proportion of cleavage-stage embryos relative to the total number of 2PN; and (III) the proportion of good quality embryos, namely, embryos containing at least 7 cells and with less than or equal to 10% fragmentation on day 3 postinsemination relative to the total number of 2PN. One or two embryos were transferred 3 days after oocyte retrieval. The luteal phase was supported with 20 mg of oral dydrogesterone (Duphaston, Abbott, USA) and 400 mg of vaginal micronized progesterone (Utrogestan, Laboratoires Besins International, Paris, France) daily. Progesterone support was initiated on the day of oocyte retrieval and continued for 14 days; the treatment continued for another 8 weeks if a pregnancy was achieved. A clinical pregnancy was identified 4–5 weeks after oocyte retrieval by the presence of an intrauterine gestational sac and a pulsating fetal heartbeat. The live birth rate was defined as the number of deliveries that resulted in at least one live-born baby.
Statistical analysis
Data are presented as the mean ± standard error of the mean (SEM) for experimental studies and the mean ± standard deviation (SD) for demographic data. Data distribution was assessed for normality using the Shapiro-Wilk and Kolmogorov-Smirnov tests. Normally distributed data were analyzed with Student’s t-test. The χ2 test was used for qualitative data. Univariate analysis was performed using a nonparametric Spearman test. A receiver operating characteristic (ROC) curve was drawn. The sensitivity and specificity of the test at the set cut-off values were calculated, and the area under curve (AUC) was estimated. P<0.05 was considered statistically significant; P<0.01 was considered highly significant.
Results
Demographics and clinical data
Twenty-two patients who did not undergo ET, ten of whom did not have oocytes or embryos of ideal quality, were excluded, while twelve patients had their embryos frozen due to ovarian hyperstimulation syndrome or other reasons (Figure 1). To further determine the relationship between OS marker levels in FF and IVF outcomes, we divided the patients into the clinically pregnant and nonclinically pregnant groups. The main demographics and clinical parameters of the patients are summarized in Table 1.
Figure 1.
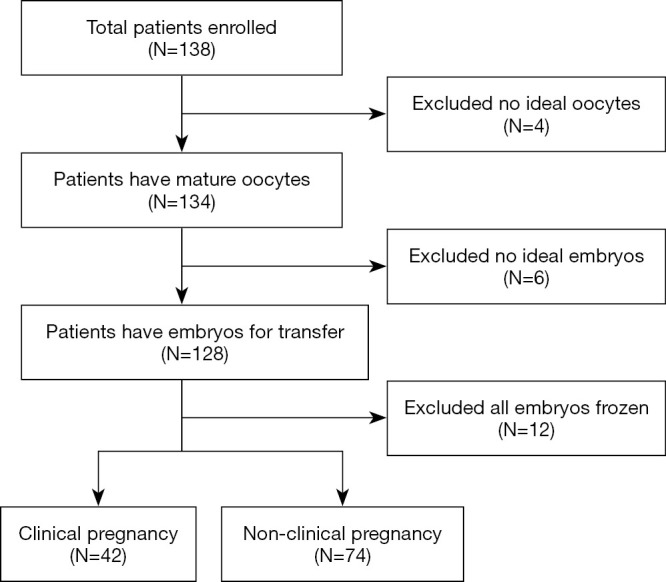
Flow chart of the study. The flow of analyses in this study and how exclusion criteria apply.
Table 1. Demographic and biochemical data of patients according to clinical pregnancy outcome.
| Variable | Pregnant (N=42) | Non-pregnant (N=74) | P value |
|---|---|---|---|
| Female age (year) | 27.71±3.68 | 28.53 ±3.09 | NS |
| Female BMI (kg/m2) | 21.25±2.51 | 22.06 ±2.30 | NS |
| Basal FSH (IU/L) | 6.95±1.61 | 7.13 ±2.15 | NS |
| AMH (ng/mL) | 5.36±2.78 | 5.89±3.34 | NS |
| Female smoking status (%) | |||
| Smoker | 11.90 | 13.51 | NS |
| Non-smoker | 88.10 | 86.49 | NS |
| Male age (year) | 29.32±3.55 | 30.23±3.20 | NS |
| Male BMI (kg/m2) | 23.37±3.12 | 24.12±3.89 | NS |
| Male smoking status (%) | |||
| Smoker | 57.14 | 47.30 | NS |
| Non-smoker | 42.86 | 52.70 | NS |
| Total Motile Sperm (million/mL) | 68.11±100.20 | 56.86±86.85 | NS |
| Gonadotropin days (days) | 10.11±3.32 | 10.84±3.85 | NS |
| Gonadotropin dose (IU) | 1,634.52±442.99 | 1,549.63±498.85 | NS |
| Mean size of retrieved follicles (mm) | 17.81±1.31 | 18.23±1.44 | NS |
| Number of oocytes retrieved | 11.59±5.76 | 9.53±3.91 | NS |
| Number of mature oocytes | 10.32±4.12 | 9.13±3.45 | NS |
| ICSI/IVF ratio | 42.86 | 44.59 | NS |
| Endometrium thickness on the day of embryo transfer (mm) | 11.01±2.24 | 10.82±2.16 | NS |
| Fertilization rate (%) | 84.20 | 82.81 | NS |
| Cleavage rate (%) | 97.66 | 95.23 | NS |
| Number of transfer embryos | 1.38±0.42 | 1.34±0.60 | NS |
| Good quality embryo rate (%) | 76.47 | 77.04 | NS |
Values are mean ± SD; *, statistically significant (P<0.05). NS, not significant.
Analysis of oxidative stress marker concentrations in pregnant versus nonpregnant patients
We studied the relationship between OS marker levels and IVF outcomes. There were no significant differences in the concentrations of ROS, such as H2O2, between the two groups. Additionally, no significant difference in the concentrations of GSH, a nonenzymatic antioxidant, was found between the two groups.
Interestingly, the levels of enzymatic antioxidants, such as GSH-Px, SOD and Prdx4, were all significantly higher in the clinically pregnant group (Table 2). The mean GSH-Px level in the pregnant group was 130.95±10.56 U/mL, while the mean GSH-Px level in the nonpregnant group was 100.83±5.40 U/mL (P=0.012). The mean SOD level in FF was 54.06±3.61 U/mL in the pregnant group and 43.87±1.76 U/mL in the nonpregnant group (P=0.011). Furthermore, the Prdx4 level was significantly higher in the pregnant group than in the nonpregnant group (22.26±1.65 vs. 16.43±0.97 ng/mL, P=0.003). However, neither the MDA level nor the CAT level was significantly different between the two groups.
Table 2. Oxidative stress marker concentrations in FF according to clinical pregnancy outcome.
| Variable | Pregnant (N=42) | Non-pregnant (N=74) | P value |
|---|---|---|---|
| H2O2 (μmol/L) | 21.39±5.30 | 23.61±2.89 | NS |
| GSH (mg/L) | 1.76±0.31 | 2.13±0.19 | NS |
| GSH-Px (U/mL) | 130.95±10.56 | 100.83±5.40 | 0.01* |
| MDA (mmol/L) | 3.90±0.30 | 3.87±0.16 | NS |
| CAT (U/mL) | 1.84±0.36 | 1.37±0.19 | NS |
| SOD (U/mL) | 54.06±3.61 | 43.87±1.76 | 0.01* |
| Prdx4 (ng/mL) | 22.26±1.65 | 16.43±0.97 | <0.01** |
Values are mean ± SEM; *, statistically significant (P<0.05); **, highly statistically significant (P<0.01), NS, not significant.
The association between OS marker levels and oocyte quality
Subsequently, we analyzed the association between GSH-Px, SOD or Prdx4 and oocyte quality in all participants (Table 3). We found that the SOD levels were positively correlated with the fertilization rate (r =0.307; P=0.020) and good quality embryo rate (r =0.306; P=0.021), but not with the number of oocytes retrieved (r =0.034; P=0.802) or the cleavage rate (r =0.154; P=0.253).
Table 3. The association between GSH-Px, SOD and Prdx4 levels and oocyte quality.
| Variable | GSH-Px | SOD | Prdx4 |
|---|---|---|---|
| Number of oocytes retrieved | r =−0.209; P=0.118 | r =0.034; P=0.802 | r =0.015; P=0.911 |
| Fertilization rate (%) | r =0.014; P=0.916 | r =0.307; P=0.020* | r =0.334; P=0.011* |
| Cleavage rate (%) | r =0.193; P=0.150 | r =0.154; P=0.253 | r =0.141; P=0.294 |
| Good quality embryo rate (%) | r =−0.042; P=0.754 | r =0.306; P=0.021* | r =0.326; P=0.013* |
Statistical analysis of the data was performed using the non-parametric Spearman test; *, statistically significant (P<0.05).
The level of Prdx4, a newly identified OS marker, was positively correlated with both the oocyte fertilization rate (r =0.334; P=0.011) and good quality embryo rate (r =0.326; P=0.013) in all patients. However, no significant association was found between the Prdx4 level and the number of oocytes retrieved or the cleavage rate.
Predictive ability of Prdx4 for clinical pregnancy
To further investigate the association between the Prdx4 level and oocyte quality, we divided all participants into four groups by quartering the levels of Prdx4 (<13.38, 13.83–16.93, 16.93–22.93, >22.93 ng/mL). Then, the clinical pregnancy rate was explored (Figure 2). The results showed that the pregnancy rate was positively correlated with the Prdx4 levels in a concentration-dependent manner in the four groups, with clinical pregnancy rates of 6.90%, 27.59%, 41.38% and 62.07% in the four groups, respectively.
Figure 2.
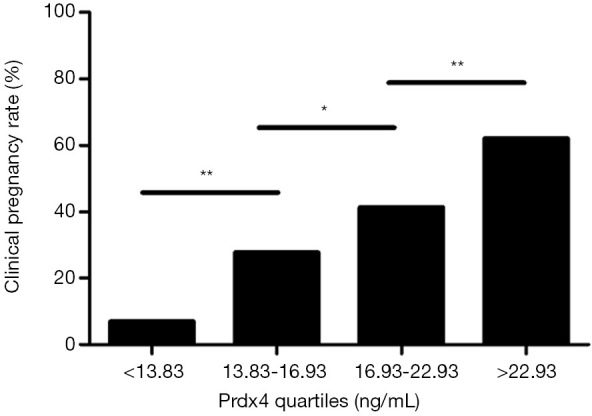
The clinical pregnancy rate was positively correlated with Prdx4 levels. All participants were divided into quartiles according to Prdx4 concentrations. The results showed that the clinical pregnancy rates were 6.9%, 27.59%, 41.38% and 62.07% in the four groups, respectively. *, statistically significant (P<0.05); **, highly statistically significant (P<0.01).
Then, the oocyte quality in these four groups was analyzed, and the results are shown in Table 4. The fertilization rates, clinical pregnancy rates and live pregnancy rates were all significantly higher in the highest Prdx4 quartile group than in the lowest quartile (P<0.01).
Table 4. Oocyte quality and pregnancy outcomes according to Prdx4 quartiles.
| Variable | Prdx4 quartiles (ng/mL) | P value | |||
|---|---|---|---|---|---|
| <13.38 | 13.83-16.93 | 16.93-22.93 | >22.93 | ||
| Retrieved oocyte number | 10.96±0.67 | 10.89±0.93 | 12.26±1.02 | 11.64±1.30 | NS |
| Fertilization rate (%) | 76.83 | 80.82 | 85.41 | 88.16 | <0.01** |
| Cleavage rate (%) | 97.34 | 98.14 | 97.53 | 98.26 | NS |
| Transferred embryo number | 1.33±0.08 | 1.35±0.07 | 1.38±0.06 | 1.32±0.07 | NS |
| Good quality embryo number per transferred cycle | 1.30±0.09 | 1.32±0.10 | 1.29±0.08 | 1.25±0.05 | NS |
| Clinical pregnancy rate (%) | 6.90 | 27.59 | 41.38 | 62.07 | <0.01** |
| Live pregnancy rate (%) | 6.90 | 24.14 | 37.93 | 58.62 | <0.01** |
Values are mean ± SEM; **, highly statistically significant (P<0.01). NS, not significant.
The predictive abilities of Prdx4 for clinical pregnancy were further analyzed with a ROC curve (Figure 3). Prdx4 showed a high accuracy for the prediction of clinical pregnancy with an AUC of 0.754 (95% CI: 0.659–0.849). The Prdx4 cutoff value for clinical pregnancy prediction was 22.30 ng/mL with a sensitivity of 65.0% and a specificity of 81.1%.
Figure 3.
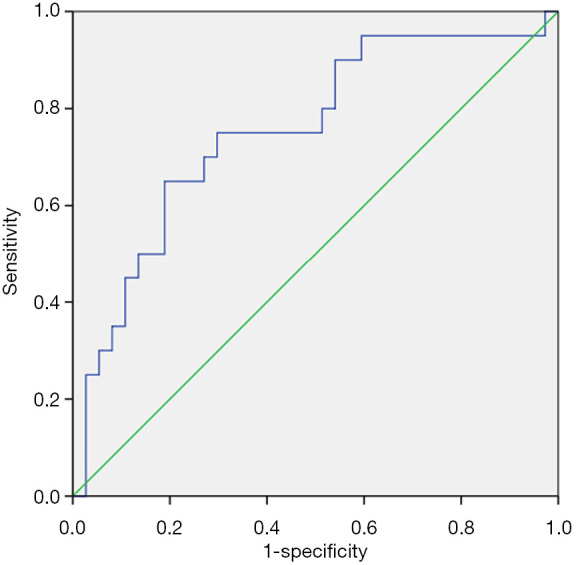
ROC curve for the Prdx4 levels in the prediction of clinical pregnancy.
Discussion
Since the identification of Prdx4 in human cells in 1997, several functions of Prdx4 have been reported, including its promotion of cell growth, involvement in cancer cell metastasis (11,12), and prevention of virus-induced oxidative damage in respiratory epithelial cells. Many studies have identified Prdx4 as an antioxidant, suggesting that the Prdx4 expression level might be an effective marker of OS in the body (13) and proposed that Prdx4 can predict the incidence of some diseases (14), such as cardiovascular disease (15) and recurrent pregnancy loss. Recent studies have shown that Prdx4 is independently associated with the mortality of type 2 diabetes patients (16,17). We have previously reported that Prdx4 is abundantly expressed in human ovarian GCs and can be associated with follicular development (6,18). FF is mainly secreted by GCs, and in this study, we found that the Prdx4 protein is abundantly expressed in the FF of IVF patients.
Many studies have reported the effects of a variety of ROS and antioxidants in FF on IVF outcomes, although the results are controversial (19-23). In this study, GSH-Px, SOD and Prdx4 were all strongly correlated with the clinical pregnancy outcome (Table 2). Oocyte quality is widely accepted to be a major factor associated with IVF outcomes. Our results strengthen the assumption that oocyte quality is associated with antioxidant levels in IVF patients. We analyzed the association between GSH-Px, SOD or Prdx4 and oocyte quality in the patients (Table 3). The results showed that SOD levels were positively correlated with fertilization (r =0.307; P=0.020) and good quality embryo (r =0.306; P=0.021) rates. Our results differ from those of Liu et al. (24), who reported several years ago that there is no correlation between SOD and oocyte maturity, embryo quality, fertilization, or cleavage-stage embryo rates. The current conflicting findings may be attributed to the different methodologies used and endpoint outcomes examined. Furthermore, the studies were observational, and confounding variables, such as age and patient recruitment standard, may have influenced the results.
Recent studies have indicated that the Prdx family is associated with female reproduction (25,26). The Prdx2 protein has been detected in human FF and found to be an important regulator in follicle atresia that functions by inhibiting GC apoptosis (25). The level of Prdx6 expression is increased in bovine oocytes and cumulus cells during the in vitro maturation (IVM) process. However, little is currently known about the function of Prdx4 in female reproduction. Accordingly, we first explored the relationship of Prdx4 expression with IVF outcomes. The results showed that Prdx4 levels were positively correlated with both the oocyte fertilization rate (r =0.334; P=0.011) and the good quality embryo rate (r =0.326; P=0.013). Furthermore, our study indicated that the pregnancy rate was positively correlated with Prdx4 levels in a concentration-dependent manner in the Prdx4 quartiles (<13.38, 13.83–16.93, 16.93–22.93, >22.93 ng/mL) (Figure 2). Increases in the fertilization rates, clinical pregnancy rates and live pregnancy rates with increasing Prdx4 was observed by quantiles (Table 4). Subsequently, the results indicated that Prdx4 had an area under the receiver operating characteristic curve (AUC) of 0.754 (95% CI: 0.659–0.849) (Figure 3). While the cutoff level of Prdx4 should be interpreted with caution, the cutoff value for clinical pregnancy prediction was 22.30 ng/mL with a sensitivity of 65.0% and a specificity of 81.1%. Based on the above results, we speculated that Prdx4, as an antioxidant, may be an effective biomarker for IVF patients to predict oocyte quality as well as subsequent pregnancy outcomes. However, the molecular mechanism is still unclear.
Previous studies have indicated that Prdx4 has two isoforms, a secreted type and an intracellular type. The former type can be secreted because the isoform lacks the N-terminal peptide, while the intracellular type is mainly localized to the endoplasmic reticulum. We suspected that Prdx4 expressed in the FF of IVF patients is mostly the secreted type. Endogenous oxygen radicals are generated during the oocyte development process. In response to the production of these harmful oxygen radicals, secretion of thiol-dependent peroxidases, such as Prdx4, will increase to facilitate their removal. Intracellular removal of hydrogen peroxides by Prdx4 and secretion of the enzyme are hypothesized to be proportionally regulated in response to the surrounding oxidative stress (17). Thus, Prdx4, as a part of the antioxidant defense system, can be regarded as a marker of oocyte damage and may therefore be indirectly linked to oxidative stress or even IVF outcomes. It is speculated that higher expression of antioxidants, such as Prdx4, in the follicular fluid of IVF patients tends to indicate better oocyte quality by decreasing oxidative stress (Figure 4).
Figure 4.
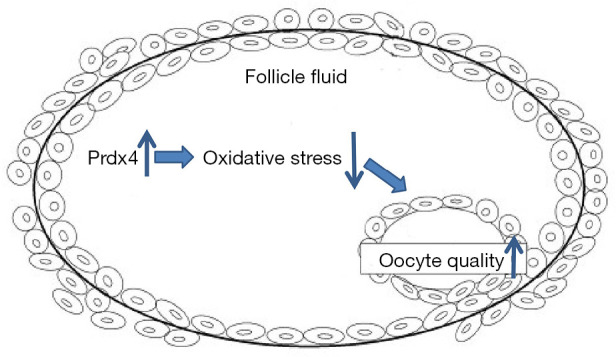
Schematic illustrating the possible roles of Prdx4 in follicular fluid. Upregulated expression of antioxidants, such as Prdx4, in the follicular fluid of IVF patients tends to improve oocyte quality by decreasing oxidative stress.
Previous studies have shown that during the oocyte IVM process, exogenous addition of antioxidants, such as β-cryptoxanthin, metformin, and CoQ10, can significantly reduce oxidative stress levels and improve oocyte quality and developmental potential (27,28). Based on these study results, we hypothesized that exogenous addition of Prdx4 protein into oocyte IVM culture medium may promote oocyte quality. In addition, when a patient shows a high level of Prdx4 in FF and she has extra oocytes for donation, we may suppose a better ongoing pregnancy outcome of the oocytes and might tend to prioritize the use of her oocytes.
Supplementary
The article’s supplementary files as
Acknowledgments
Funding: This work was supported by grants from the National Nature and Science Foundation of China (81701517), National Key Research and Development Program of China (2017YFC1001602, 2017YFC1001300), Jiangsu Province High Level Health Personnel Project (LGY2019066) and MerckSerono China Research Fund for Fertility Experts (CREATE-2016023).
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). This study was approved by the Institutional Ethics Committee of The First Affiliated Hospital of Nanjing Medical University (No. 2016-SRFA-038), and informed consent was obtained from all patients.
Footnotes
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at http://dx.doi.org/10.21037/atm-20-397
Data Sharing Statement: Available at http://dx.doi.org/10.21037/atm-20-397
Peer Review File: Available at http://dx.doi.org/10.21037/atm-20-397
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/atm-20-397). The authors have no conflicts of interest to declare.
References
- 1.Younis A, Clower C, Nelsen D, et al. The relationship between pregnancy and oxidative stress markers on patients undergoing ovarian stimulations. J Assist Reprod Genet 2012;29:1083-9. 10.1007/s10815-012-9831-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Paszkowski T, Traub AI, Robinson SY, et al. Selenium dependent glutathione peroxidase activity in human follicular fluid. Clin Chim Acta 1995;236:173-80. 10.1016/0009-8981(95)98130-9 [DOI] [PubMed] [Google Scholar]
- 3.Algriany O, Bevers M, Schoevers E, et al. Follicle size-dependent effects of sow follicular fluid on in vitro cumulus expansion, nuclear maturation and blastocyst formation of sow cumulus oocytes complexes. Theriogenology 2004;62:1483-97. 10.1016/j.theriogenology.2004.02.008 [DOI] [PubMed] [Google Scholar]
- 4.Yiyenoglu OB, Ugur MG, Ozcan HC, et al. Assessment of oxidative stress markers in recurrent pregnancy loss: a prospective study. Arch Gynecol Obstet 2014;289:1337-40. 10.1007/s00404-013-3113-4 [DOI] [PubMed] [Google Scholar]
- 5.Abbasi A, Corpeleijn E, Postmus D, et al. Peroxiredoxin 4, a novel circulating biomarker for oxidative stress and the risk of incident cardiovascular disease and all-cause mortality. J Am Heart Assoc 2012;1:e002956. 10.1161/JAHA.112.002956 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Meng Y, Qian Y, Gao L, et al. Downregulated expression of peroxiredoxin 4 in granulosa cells from polycystic ovary syndrome. PLoS One 2013;8:e76460. 10.1371/journal.pone.0076460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Iuchi Y, Okada F, Tsunoda S, et al. Peroxiredoxin 4 knockout results in elevated spermatogenic cell death via oxidative stress. Biochem J 2009;419:149-58. 10.1042/BJ20081526 [DOI] [PubMed] [Google Scholar]
- 8.Mehmeti I, Lortz S, Elsner M, et al. Peroxiredoxin 4 improves insulin biosynthesis and glucose-induced insulin secretion in insulin-secreting INS-1E cells. J Biol Chem 2014;289:26904-13. 10.1074/jbc.M114.568329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yi N, Xiao MB, Ni WK, et al. High expression of peroxiredoxin 4 affects the survival time of colorectal cancer patients, but is not an independent unfavorable prognostic factor. Mol Clin Oncol 2014;2:767-72. 10.3892/mco.2014.317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nawata A, Noguchi H, Mazaki Y, et al. Overexpression of peroxiredoxin 4 affects intestinal function in a dietary mouse model of nonalcoholic fatty liver disease. PLoS one 2016;11:e0152549. 10.1371/journal.pone.0152549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fujii J, Ikeda Y, Kurahashi T, et al. Physiological and pathological views of peroxiredoxin 4. Free Radic Biol Med 2015;83:373-9. 10.1016/j.freeradbiomed.2015.01.025 [DOI] [PubMed] [Google Scholar]
- 12.Kim TH, Song J, Alcantara Llaguno SR, et al. Suppression of peroxiredoxin 4 in glioblastoma cells increases apoptosis and reduces tumor growth. PLoS One 2012;7:e42818. 10.1371/journal.pone.0042818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Klichko VI, Orr WC, Radyuk SN. The role of peroxiredoxin 4 in inflammatory response and aging. Biochim Biophys Acta 2016;1862:265-73. 10.1016/j.bbadis.2015.12.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rafiei S, Tiedemann K, Tabaries S, et al. Peroxiredoxin 4: a novel secreted mediator of cancer induced osteoclastogenesis. Cancer Lett 2015;361:262-70. 10.1016/j.canlet.2015.03.012 [DOI] [PubMed] [Google Scholar]
- 15.Hwang JA, Song JS, Yu DY, et al. Peroxiredoxin 4 as an independent prognostic marker for survival in patients with early-stage lung squamous cell carcinoma. Int J Clin Exp Pathol 2015;8:6627-35. [PMC free article] [PubMed] [Google Scholar]
- 16.Ding Y, Yamada S, Wang KY, et al. Overexpression of peroxiredoxin 4 protects against high-dose streptozotocin-induced diabetes by suppressing oxidative stress and cytokines in transgenic mice. Antioxid Redox Signal 2010;13:1477-90. 10.1089/ars.2010.3137 [DOI] [PubMed] [Google Scholar]
- 17.Gerrits EG, Alkhalaf A, Landman GW, et al. Serum peroxiredoxin 4: a marker of oxidative stress associated with mortality in type 2 diabetes (ZODIAC-28). PLoS One 2014;9:e89719. 10.1371/journal.pone.0089719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Qian Y, Shao L, Yuan C, et al. Implication of differential peroxiredoxin 4 expression with age in ovaries of mouse and human for ovarian aging. Curr Mol Med 2016;16:243-51. 10.2174/1566524016666160225151647 [DOI] [PubMed] [Google Scholar]
- 19.Luddi A, Capaldo A, Focarelli R, et al. Antioxidants reduce oxidative stress in follicular fluid of aged women undergoing IVF. Reprod Biol Endocrinol 2016;14:57. 10.1186/s12958-016-0184-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rahiminejad ME, Moaddab A, Ganji M, et al. Oxidative stress biomarkers in endometrial secretions: a comparison between successful and unsuccessful in vitro fertilization cycles. J Reprod Immunol 2016;116:70-5. 10.1016/j.jri.2016.05.003 [DOI] [PubMed] [Google Scholar]
- 21.Combelles CM, Holick EA, Paolella LJ, et al. Profiling of superoxide dismutase isoenzymes in compartments of the developing bovine antral follicles. Reproduction 2010;139:871-81. 10.1530/REP-09-0390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pasqualotto FF, Sobreiro BP, Hallak J, et al. Sperm concentration and normal sperm morphology decrease and follicle-stimulating hormone level increases with age. BJU Int 2005;96:1087-91. 10.1111/j.1464-410X.2005.05806.x [DOI] [PubMed] [Google Scholar]
- 23.Yalcinkaya E, Cakiroglu Y, Doger E, et al. Effect of follicular fluid NO, MDA and GSH levels on in vitro fertilization outcomes. J Turk Ger Gynecol Assoc 2013;14:136-41. 10.5152/jtgga.2013.53323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liu F, He L, Liu Y, et al. The expression and role of oxidative stress markers in the serum and follicular fluid of patients with endometriosis. Clin Exp Obstet Gynecol 2013;40:372-6. [PubMed] [Google Scholar]
- 25.Yang S, Luo A, Hao X, et al. Peroxiredoxin 2 inhibits granulosa cell apoptosis during follicle atresia through the NFKB pathway in mice. Biol Reprod 2011;84:1182-9. 10.1095/biolreprod.110.087569 [DOI] [PubMed] [Google Scholar]
- 26.Park SJ, Kim JH, Lee DG, et al. Peroxiredoxin 2 deficiency accelerates age-related ovarian failure through the reactive oxygen species-mediated JNK pathway in mice. Free Radic Biol Med 2018;123:96-106. 10.1016/j.freeradbiomed.2018.05.059 [DOI] [PubMed] [Google Scholar]
- 27.Park YG, Lee SE, Son YJ, et al. Antioxidant β-cryptoxanthin enhances porcine oocyte maturation and subsequent embryo development in vitro. Reprod Fertil Dev 2018;30:1204-13. 10.1071/RD17444 [DOI] [PubMed] [Google Scholar]
- 28.Rodrigues-Cunha MC, Mesquita LG, Bressan F, et al. Effects of melatonin during IVM in defined medium on oocyte meiosis, oxidative stress, and subsequent embryo development. Theriogenology 2016;86:1685-94. 10.1016/j.theriogenology.2016.05.026 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The article’s supplementary files as


