Abstract
Background
Indoleamine 2,3-dioxygenase (IDO), a limiting enzyme in the IDO/kynurenine (Kyn) pathway, converts tryptophan (Trp) into Kyn, and plays a significant role in immune suppression and tumor immune evasion. This study aimed to investigate the association between IDO activity and clinical outcomes in non-small cell lung cancer (NSCLC) patients who underwent radiotherapy (RT).
Methods
Serum Kyn and Trp levels were measured in 104 NSCLC patients by high-performance liquid chromatography at baseline, and the following RT. The correlation between IDO activity, as computed by Kyn: Trp ratios and survival was estimated using Kaplan-Meier curves. Cox proportional hazard models are used in the univariate and multivariate analyses.
Results
Both the Kyn levels and Kyn:Trp ratios were reduced after RT at a biologically equivalent dose (BED) of <70 Gy, while these increased at a BED of ≥70 Gy. Post/pre-Kyn levels were positively correlated with an objective response. Patients with a higher Kyn:Trp ratio pre-RT had the worse median progression-free survival (mPFS, 13.5 vs. 24.5 months, P=0.049). Higher post/pre-Kyn:Trp ratios were correlated with improved median overall survival (mOS, 23.8 months vs. not reached, P=0.032). On the multivariate analysis, pre-RT Kyn:Trp and post/pre-Kyn:Trp ratios remained as independent predictive factors for PFS and OS, respectively.
Conclusions
It was proved that RT could alter IDO-mediated immune activity and establish strong correlations between IDO activity and survival outcomes in NSCLC patients treated with RT. These present findings suggest that the profiling of IDO activity might allow for the prompt adjustment of RT doses and better predict patient response to RT.
Keywords: Indoleamine 2,3-dioxygenase activity (IDO activity); radiotherapy-induced immune response; non-small cell lung cancer (NSCLC); prognosis
Introduction
The morbidity and mortality rate of lung cancer is still the highest among all cancers, regardless of the number of therapeutic advances (1). The increase in tumor immunotherapy in recent years has revolutionized treatment strategies for lung cancer patients. However, merely approximately 20–40% of patients show an effective response to immunotherapy (2). Radiation therapy may activate a patient’s antitumor immune response in various potential ways by reshaping the patient’s immune and tumor microenvironment. Studies have shown that radiotherapy (RT) combined with immunotherapy, can generate synergistic effects to enhance the antitumor immune response. The results of the landmark clinical trial represented by the PACIFIC study (3) unveiled the prelude of RT combined with immunotherapy in non-small cell lung cancer (NSCLC), suggesting that the optimized combination therapy mode eliminates metastasis and significantly prolongs overall survival (OS). At present, multiple immune regulatory targets are being investigated, including indoleamine 2,3-dioxygenase (IDO).
IDO is a crucial enzyme in the conversion of tryptophan (Trp) to kynurenine (Kyn) outside the liver. Since Kyn is the primary metabolite of Trp metabolism, the plasma Kyn:Trp ratio is used to monitor IDO activity (4).
Previous studies have shown that IDO plays a role in immune checkpoints in cancer and chronic inflammatory diseases (5). Lung cancer cells often over-express IDO mRNA (6). Clinical studies have shown that elevated IDO activity is associated with poor prognosis in patients with lung cancer (7,8). Furthermore, it has been reported that augmented Trp catabolism and, consequently, high serum Kyn concentrations are associated with more advanced tumors, chemotherapy resistance, and decreased survival in NSCLC (7,9). The high expression of IDO in tumor cells and antigen-presenting cells can reduce Trp levels in the tumor microenvironment, and increase the level of urine, which regulates reactive oxygen species, general control, nonderepressible-2 kinase, and other molecules. Consequently, this leads to the inhibition of CD8+ T effector cells and natural killer cells, and the activation of CD4+ T regulatory cells and myeloid-derived suppressor cells (10-12), which assist tumor cells in escaping immunosurveillance.
However, it is still unclear how IDO-mediated immune activity changes after RT in NSCLC patients and whether these changes influence patient survival. In the present study, it was hypothesized that IDO activity could be altered by RT, which would affect survival in patients with NSCLC. The IDO-mediated immune activity was evaluated by quantifying the molecules associated with IDO checkpoints before and after RT. Furthermore, the association between the changes in IDO activity and patient survival was explored under different radiation doses.
We present the following article in accordance with the REMARK reporting checklist (available at http://dx.doi.org/10.21037/atm-20-5634).
Methods
Patient and treatment
All procedures performed in this study involving human participants were in accordance with the Declaration of Helsinki (as revised in 2013). The Ethics Review Committee approved this retrospective study of Zhejiang Cancer Hospital. All patients provided written informed consent before therapy.
The study population formed of 104 NSCLC patients who were pathologically or cytologically diagnosed with NSCLC between April 2014 and November 2017. All patients received conventional radiotherapy (CRT) or stereotactic body radiation therapy (SBRT). The gross tumor volume (GTV) images include the primary tumor and metastatic lymph nodes. For the patients receiving CRT, the clinical target volume (CTV) was 6–8 mm outward for the corresponding GTV, according to the pathological tumor type [squamous: 6 mm, adenocarcinoma: 8 mm; repairing correctly according to the structure of the organ at risk (OAR)]. The planning target volume (PTV) resulted from the expansion of the GTV by 4 mm. For the patients receiving SBRT, ITV was contoured following GTV and edited at the lung window 4D-CT base to contain the tumor’s full movement. A 0.5 cm expansion defined the PTV from the ITV. The dose prescription was set at 100%, 99%, and 95% of the composite GTV, ITV, and PTV, respectively, receiving the prescribed dose. CRT was delivered at the total dose was 60–70 Gy/30–35 fractions/6–7 weeks. SBRT was delivered at the total dose was 48–64 Gy/6–8 fractions/2–3 weeks.
Follow-up and sample collection
Patients were evaluated weekly during the RT and were routinely followed up approximately every three months for the first year, every six months for the second year, and annually after that. Clinical data were collected, including age, gender, smoking history, histology, clinical-stage, Eastern Cooperative Oncology Group (ECOG), performance status (PS), biologically effective dose (BED), and chemotherapy regimens. The follow-up included blood routine, contrast-enhanced computed tomography (CE-CT) of the chest and abdomen, ultrasonography of the neck and clavicular lymph nodes, comprehensive metabolic panel, and lung tumor biomarkers. The review of distant metastases included a brain magnetic resonance imaging (MRI) and bone scan, which was reviewed annually. Also, 5 mL of peripheral venous blood samples were collected from patients before RT, and at one week after RT. The serum was collected and stored at −80 °C.
Measurement of serum Trp and Kyn
Serum Trp and Kyn levels were measured using high-performance liquid chromatography (HPLC) by the Dionex UltiMate 3000 HPLC system (Dionex, Sunnyvale, CA, USA). A Thermo Hypersil Gold (100×2.1 mm, 1.9 µm) was used with a flow rate of 0.2 mL·min−1. The injection volume was 20 µL, and the column temperature was supported Cat 25 °C. Then, 0.5 mL of serum samples were deproteinized using equal volumes of 5% perchloric acid solution, vortex-mixed and centrifuged at 10,000 rpm for then minutes. Afterward, 20 µL of the clean upper layer was injected into the chromatographic system. After that, serum Kyn was detected on a UV channel at 360 nm, and Trp was detected on a UV channel at 278 nm. Each sample was analyzed in triplicate, and the mean concentrations of these samples were recorded. For quality control purposes, triplicate testing verifies the analytic coefficients of variation to be less than 5%.
Statistical analysis
The OS was defined as the time from the first day of RT to the date of death or last follow-up. Progression-free survival (PFS) was defined as the time from the first day of RT to the date of cancer progression, death, or last follow-up. The Kaplan-Meier log-ranking test was applied to compare the survival difference between groups. Variables were incorporated into the final multivariate analysis according to clinical relevance and statistical significance on the univariate analysis (cutoff, P<0.15). The multivariate Cox proportional hazard model was used to estimate the hazard ratio (HR) with a 95% confidence interval (95% CI). The IDO checkpoint-associated molecules from different time points are compared using an unpaired Student’s t-test. All statistical analyses were performed using IBM SPSS Statistics 23.0 (IBM, Inc., NY, USA). The scattered plot figures for the IDO dynamic changes were generated using GraphPad Prism 8 (GraphPad Software Inc., San Diego, CA, USA).
Results
Study population and clinical outcomes
The 104 NSCLC patients in the present study (86 males and 18 females) had a median age of 66 years old (range, 42–88 years old). The median follow-up period for all patients was 20.8 months (range, 1.4–55.1 months). All 104 patients (100%) received RT, while 53 patients (51%) received platinum-based chemotherapy. Table 1 presents patient demographics and clinical features. The median OS and PFS were 33.7 (95% CI, 20.2–47.2) and 18.9 (95% CI, 12.4–25.3) months, respectively. Peripheral blood samples were collected from all patients before RT. Peripheral blood samples after RT were collected from 84 patients.
Table 1. Univariate cox regression analysis for progression-free survival and overall survival for the clinicopathologic features of 104 patients.
| Clinical factors | Patients (n) | PFS | OS | |||
| HR (95% CI) | P value | HR (95% CI) | P value | |||
| Gender | 0.053 | |||||
| Male | 86 | 1.00 | 1.00 | |||
| Female | 18 | 0.64 (0.30–1.34) | 0.231 | 0.31 (0.10–1.02) | ||
| Age (years) | 0.436 | |||||
| <65 | 43 | 1.00 | 1.00 | |||
| ≥65 | 61 | 0.88 (0.53–1.47) | 0.625 | 0.79 (0.43–1.44) | ||
| Smoking (pack-year) | 0.084 | |||||
| <30 | 33 | 1.00 | 1.00 | |||
| ≥30 | 71 | 0.99 (0.58–1.70) | 0.980 | 1.864 (0.92–3.78) | ||
| ECOG | 0.197 | |||||
| 0 | 46 | 1.00 | 1.00 | |||
| 1 | 58 | 1.29 (0.779–2.139) | 0.323 | 1.49 (0.81–2.73) | ||
| Location type | 0.423 | |||||
| Central type | 31 | 1.00 | 1.00 | |||
| Peripheral type | 73 | 0.667 (0.395–1.126) | 0.129 | 0.77 (0.41–1.45) | ||
| Histology | ||||||
| Squamous cell carcinoma | 51 | 1.00 | 0.458 | 1.00 | 0.021 | |
| Adenocarcinoma | 43 | 1.388 (0.571–3.372) | 0.413 | 0.45 (0.23–0.87) | 0.018 | |
| No specific type | 10 | 1.051 (0.425–2.598) | 0.260 | 0.35 (0.12–1.02) | 0.055 | |
| Stage | 0.002 | |||||
| I–II | 42 | 1.00 | 1.00 | |||
| III–IV | 62 | 2.406 (1.380–4.195) | 0.002 | 3.102 (1.52–6.33) | ||
| BED | 0.032 | |||||
| <70 | 16 | 1.00 | 1.00 | |||
| ≥70 | 88 | 0.594 (0.308–1.144) | 0.119 | 0.45 (0.21–0.93) | ||
| Chemotherapy | 0.019 | |||||
| No | 51 | 1.00 | 1.00 | |||
| Yes | 53 | 1.62 (0.97–2.72) | 0.065 | 2.12 (1.13–3.96) | ||
PFS, progression-free survival; OS, overall survival; HR, hazard ratio; CI, confidence interval; ECOG, Eastern Cooperative Oncology Group; BED, Biological effective dose.
Dynamics of Kyn levels and Kyn:Trp ratios at different radiation doses
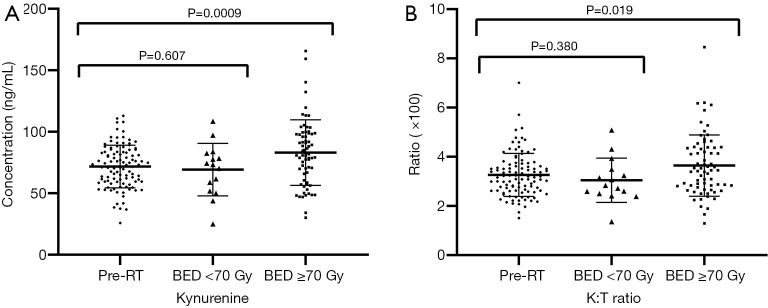
Serum Kyn levels (Figure 1A) and Kyn:Trp ratios (Figure 1B) were measured at pre-RT, post-RT with BED <70 Gy, and post-RT with BED ≥70 Gy. There were no significant changes in serum Kyn concentrations or Kyn:Trp ratios observed between pre-RT and post-RT with BED <70 Gy, and both parameters significantly increased following RT with BED ≥70 Gy.
Figure 1.
Dynamic changes of IDO-associated molecular activity. (A) The plot reveals the individual and mean activity levels for kynurenine. (B) The Kyn:Trp ratio at pre-RT and post-RT under different BED. Bars: median, standard deviation. The P values were calculated using the unpaired Student’s t-tests. IDO, indoleamine 2,3-dioxygenase; Trp, tryptophan; Kyn, kynurenine; RT, radiotherapy; BED, biologically equivalent doses.
IDO biomarkers and survival outcomes
On the univariate analysis, multiple clinicopathologic features revealed significant correlations with PFS and OS (Table 1). Specifically, an earlier stage (P=0.002) was associated with better PFS (Table 1). Adenocarcinoma histology (P=0.018), earlier stage (P=0.002), higher BED (P=0.032), and chemotherapy (P=0.019) revealed significant correlations with improved OS (Table 1).
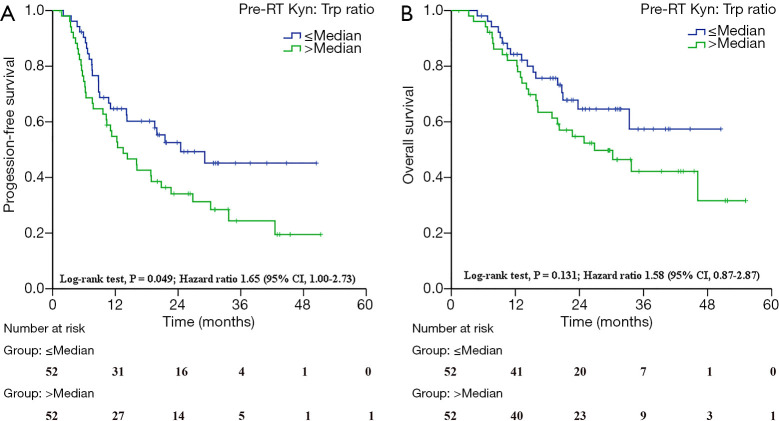
Next, the association between IDO activity and patient survival outcomes was carefully analyzed. With the median as the cutoff, patients with high post/pre-RT Kyn levels had increased objective response rates (ORRs; 64.29% vs. 40.48%, P=0.029; Table 2). Similarly, these high post/pre-RT Kyn:Trp ratios correlated with better OS (HR =2.02, 95% CI, 1.06–3.84; log-ranking P=0.032; Table 3). Furthermore, the high Kyn:Trp ratios at pre-RT correlated with shortened PFS (HR =1.65, 95% CI, 1.00–2.73; log-ranking P=0.049; Figure 2A, Table 3), and the trend towards worse OS (HR =1.58, 95% CI, 0.87–2.87; log-rank P=0.131; Figure 2B, Table 3). Serum Kyn levels at pre-RT had no significant associations with OS (P=0.287) or PFS (P=0.200) (Table 3). However, neither the post-RT serum Kyn levels nor the Kyn:Trp ratios predicted the changes in OS or PFS.
Table 2. Association between IDO activity and ORR.
| ORR | Post/Pre Kyn | χ | P value | |
|---|---|---|---|---|
| 1a | 2a | |||
| Ob | 25 | 15 | 4.773 | 0.029 |
| 1b | 17 | 27 | ||
1a, Post/Pre Kyn ≤ median; 2a, Post/Pre Kyn > median pre-RT; Ob, stable disease or progression; 1b, partial response or complete response. IDO, indoleamine 2,3-dioxygenase; ORR, objective response rate; Kyn, kynurenine.
Table 3. Associations between IDO activity and changes with progression-free survival and overall survival of NSCLC.
| Timepoints | IDO activities | Patients (n) | PFS | OS | |||||
|---|---|---|---|---|---|---|---|---|---|
| Progression n (%) | mPFS (months) | P value | Death n (%) | mOS (months) | P value | ||||
| Pre-RT (n=104) | Kynurenine | 0.200 | 0.287 | ||||||
| ≤ Median | 52 | 25 (48.1) | 26.8 | 18 (34.6) | 30.3 | ||||
| > Median | 52 | 37 (71.2) | 14.3 | 26 (50.0) | 33.7 | ||||
| Kyn:Trp ratio | 0.049 | 0.131 | |||||||
| ≤ Median | 52 | 25 (48.1) | 24.5 | 17 (32.7) | Not reached | ||||
| > Median | 52 | 37 (71.2) | 13.5 | 27 (51.9) | 26.8 | ||||
| Post-RT (n=84) | Kynurenine | 0.500 | 0.598 | ||||||
| ≤ Median | 42 | 21 (50.0) | 18.9 | 16 (38.1) | Not reached | ||||
| > Median | 42 | 29 (69.0) | 19.5 | 22 (52.4) | 30.2 | ||||
| Kyn:Trp ratio | 0.693 | 0.401 | |||||||
| ≤ Median | 42 | 24 (57.1) | 16.1 | 20 (47.6) | 23.7 | ||||
| > Median | 42 | 26 (61.9) | 19.5 | 18 (42.9) | Not reached | ||||
| Post/Pre (n=84) | Kynurenine | 0.879 | 0.414 | ||||||
| ≤ Median | 42 | 25 (59.5) | 16.1 | 21 (50.0) | 30.2 | ||||
| > Median | 42 | 25 (59.5) | 19.9 | 17 (40.5) | Not reached | ||||
| Kyn:Trp ratio | 0.084 | 0.032 | |||||||
| ≤ Median | 42 | 28 (66.7) | 12.3 | 24 (57.1) | 23.8 | ||||
| > Median | 42 | 22 (52.4) | 29.1 | 14 (33.3) | Not reached | ||||
IDO, indoleamine 2,3-dioxygenase; NSCLC, non-small cell lung cancer; PFS, progression-free survival; OS, overall survival; mPFS, median progression-free survival; mOS, median overall survival; Kyn, kynurenine; Trp, tryptophan; RT, radiotherapy.
Figure 2.
Baseline IDO-mediated immune activity and treatment outcomes. (A) The pre-RT Kyn:Trp ratio for progression-free survival. (B) The pre-RT Kyn:Trp ratio for overall survival. IDO, indoleamine 2,3-dioxygenase; RT, radiotherapy; Trp, tryptophan; Kyn, kynurenine; CI, confidence interval.
On the multivariate analysis, taking into consideration the clinicopathologic features, it was found that higher pre-RT Kyn:Trp ratios (HR =1.74, 95% CI, 1.00–3.03, log-ranking P=0.049; Table 4) were associated with shortened PFS, while a higher post/pre-RT Kyn:Trp ratio (HR =0.48, 95% CI, 0.24–0.99, P=0.045; Table 4) was an independent prognostic factor for better OS.
Table 4. Multivariate cox regression analysis for progression-free survival and overall survival with the clinicopathologic features.
| Clinical factors | PFS (n=104) | OS (n=84) | |||
|---|---|---|---|---|---|
| HR (95% CI) | P value | HR (95% CI) | P value | ||
| Gender | 0.038 | ||||
| Male | 1.00 | ||||
| Female | 0.11 (0.01–0.88) | ||||
| Smoking (pack-year) | 0.477 | 0.891 | |||
| <30 | 1.00 | 1.00 | |||
| ≥30 | 0.80 (0.43–1.48) | 0.93 (0.35–2.48) | |||
| Location type | 0.610 | ||||
| Central type | 1.00 | ||||
| Peripheral type | 1.19 (0.62–2.28) | ||||
| Histology | |||||
| Squamous cell carcinoma | 1.00 | 0.736 | 1.00 | 0.290 | |
| Adenocarcinoma | 0.97 (0.51–1.83) | 0.918 | 0.52 (0.23–1.20) | 0.125 | |
| No specific type | 0.68 (0.25–1.87) | 0.452 | 0.59 (0.14–2.46) | 0.458 | |
| Stage | 0.009 | 0.025 | |||
| I–II | 1.00 | 1.00 | |||
| III–IV | 2.68 (1.28–5.62) | 3.78 (1.18–12.09) | |||
| BED | 0.559 | 0.352 | |||
| <70 | 1.00 | 1.00 | |||
| ≥70 | 0.79 (0.43–1.48) | 0.67(0.28–1.57) | |||
| Chemotherapy | 0.745 | 0.282 | |||
| No | 1.00 | 1.00 | |||
| Yes | 0.88 (0.41–1.89) | 0.56(0.19–1.62) | |||
| Pre Kyn:Trp ratio | 0.049 | ||||
| ≤ Median | 1.00 | ||||
| > Median | 1.74 (1.00–3.03) | ||||
| Post/Pre Kyn:Trp ratio | 0.045 | ||||
| ≤ Median | 1.00 | ||||
| > Median | 0.48 (0.24–0.99) | ||||
PFS, progression-free survival; OS, overall survival; HR, hazard ratio; CI, confidence interval; BED, biologically effective dose; Kyn, kynurenine; Trp, tryptophan.
Discussion
In the present study, the following was revealed: (I) IDO activity, as represented by the Kyn:Trp ratio, did not significantly change, albeit a slight decrease, after RT with BED <70 Gy, but this significantly increased at BED ≥70 Gy; (II) IDO activity at baseline significantly correlated with PFS; (III) post/pre-Kyn levels significantly correlated with ORR; (IV) post/pre-Kyn:Trp ratios significantly correlated with OS. These results support the present initial hypothesis that RT can alter IDO-mediated immune activity.
The present data revealed that the effect of RT on IDO immune activity was dose-dependent. These results were in line with the findings reported by Wang et al., which revealed that Kyn:Trp ratio and Kyn concentration reduced in the initial phase of RT (lower doses), while both increased after high doses of RT (13). However, the underlying mechanism is still unclear. It was speculated that low doses of RT were not sufficient to produce maximal immune activation. Nevertheless, high doses of RT could potentially damage normal tissues and the immune system, and thereby counteract the benefits of RT (14). Indeed, no significant change in serum Kyn concentrations or Kyn:Trp ratios were observed between pre-RT and post-RT with BED <70 Gy, while both parameters significantly increased following RT with BED ≥70 Gy. Therefore, finding the best RT dose that activates antitumor immunity, without causing excessive immune depletion, would be necessary. Further studies are needed to explore potential mechanisms and perfect the RT dose with maximal immune response. IDO activity could serve as a marker to check RT-induced immune activity and allow for the prompt adjustment of RT doses.
Consistent with previous studies that reported the immune status of IDO as a potential predictor of chemotherapy or immunotherapy response (9,15), it was found that the low activity of IDO, that is, the low Kyn:Trp ratio (favorable baseline), was significantly associated with better PFS, and this was in agreement with the results reported by Wang et al. (13). The present study demonstrated that IDO activity at baseline is an important and independent prognostic biomarker for NSCLC patients treated with RT. Also, the present study unveiled the relationship between the dynamics of IDO activity and patient outcomes. Importantly, post/pre-RT Kyn levels and post/pre-RT Kyn:Trp ratios were significantly correlated with ORR and OS, respectively.
Consequently, IDO may serve as an important target for anticancer agents. At present, multiple clinical trials using various IDO inhibitors are being conducted (16). A trial study (NCT01219348) that used an IDO peptide vaccine in patients with stage III-IV NSCLC after standard chemotherapy produced promising results with no severe toxicity (17). Multiple phases 1/2 trials that involve IDO inhibitors have revealed encouraging outcomes with improved patient response to anti-PD1 immune checkpoint therapies. However, the results from ECHO-301, the first substantial phase 3 trial that evaluated epacadostat, an IDO1-selective enzyme inhibitor, in combination with pembrolizumab, an anti-PD1 antibody, in advanced melanoma, did not indicate the addition of epacadostat resulted in an increased benefit. Several caveats were associated with this failed trial (18). First, it is still uncertain whether the IDO was inhibited. Second, the selective IDO1 blockade was insufficient to relieve tumor immunosuppression durably. Hence, other potential compensatory mechanisms need to be considered. At present, the inhibition of the Trp-Kyn-AhR pathway is still as an attractive therapeutic target. Preclinical models have revealed the heightened activity of the Trp-Kyn-AhR pathway was associated with the impairment of antitumor immunity and tumor growth (19,20). Despite the uncertainty on selective-IDO1 inhibition, there is sufficient preclinical evidence for Trp-Kyn-AhR pathway inhibitors in enhancing antitumor immunity and controlling tumor growth (21).
Despite the advances in delivery techniques, NSCLC has displayed suboptimal outcomes following RT. A solution to improve patient prognosis is multidisciplinary therapy. Animal studies suggested that a novel triple therapy consisting of local RT, systemic IDO blockade, and intratumoral CpG was well tolerated, and reduced intratumoral immunosuppression, producing robust systemic antitumor effects (22). IDO inhibitor in combination with RT could delay tumor growth by reversing T cell exhaustion (23). However, the key problems with the combination of RT and IDO inhibitors, or other immunotherapeutic drugs, are the optimization of RT regimens and the identification of the best time to give the IDO inhibitors. Given the non-invasiveness of blood biomarkers, these could provide ongoing snapshots of the disease during treatment (24). Blood biomarkers can be used to predict the effects of cancer treatment, providing an early warning of possible recurrence and the status of organs at risk during RT (25). The present study, which involved the blood biomarkers of IDO activity, might be clinically useful as a guide for comprehensive multidisciplinary treatment.
There are still some limitations to the present study. First, the number of samples was moderate, especially for the BED <70 Gy group. The results should to be proved by a large sample prospective study. Second, this study only detected IDO activity before RT, and at one week after RT. Future studies should measure the IDO activity at various time points during RT. Third, earlier studies have shown that chemotherapy and IDO inhibitors have synergistic effects in promoting clinical responses, which might be a confounding effect. Lastly, the serum Kyn:Trp ratio cannot fully stand for the activity of IDO. Other catabolizing enzymes that convert Trp to Kyn exist, including Trp 2,3-dioxygen-ase (IDO2). These enzymes might also contribute to the production of Kyn.
Conclusions
It was found that RT alters IDO-mediated antitumor immune activity. Baseline Kyn:Trp ratios strongly correlate with PFS. Furthermore, post/pre-RT Kyn levels and Kyn:Trp ratios correlate with ORR and OS, respectively. However, despite the moderate sample size, the present study shows that assaying for IDO activity could prevent excessive RT and represent an easily assessable biomarker in determining the optimal individualized RT dose for each patient.
Supplementary
The article’s supplementary files as
Acknowledgments
We would like to thank the patients and family members for supplying their consent for presenting the data in this study.
Funding: This work was supported by the National Natural Science Foundation of China (No. 81372438/H1610) and Science Research Foundation of China Ministry of Health -Zhejiang Medicine & Health Key Research Fund (No. 201339868).
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study involving human participants were in accordance with the Declaration of Helsinki (as revised in 2013). The Ethics Review Committee approved this retrospective study of Zhejiang Cancer Hospital (No. 201339868). All patients provided written informed consent before therapy.
Reporting Checklist: The authors have completed the REMARK reporting checklist. Available at http://dx.doi.org/10.21037/atm-20-5634
Data Sharing Statement: Available at http://dx.doi.org/10.21037/atm-20-5634
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/atm-20-5634). The authors have no conflicts of interest to declare.
(English Language Editor: J. Chapnick)
References
- 1.Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018;68:394-424. 10.3322/caac.21492 [DOI] [PubMed] [Google Scholar]
- 2.Smyth MJ, Ngiow SF, Ribas A, et al. Combination cancer immunotherapies tailored to the tumour microenvironment. Nat Rev Clin Oncol 2016;13:143-58. 10.1038/nrclinonc.2015.209 [DOI] [PubMed] [Google Scholar]
- 3.Antonia SJ, Villegas A, Daniel D, et al. Overall Survival with Durvalumab after Chemoradiotherapy in Stage III NSCLC. N Engl J Med 2018;379:2342-50. 10.1056/NEJMoa1809697 [DOI] [PubMed] [Google Scholar]
- 4.Pandey JP. Prognostic immune markers in non-small cell lung cancer--letter. Clin Cancer Res 2011;17:7835; author reply 7836. 10.1158/1078-0432.CCR-11-2103 [DOI] [PubMed] [Google Scholar]
- 5.Mbongue JC, Nicholas D, Torrez TW, et al. The Role of Indoleamine 2, 3-Dioxygenase in Immune Suppression and Autoimmunity. Vaccines (Basel) 2015;3:703-29. 10.3390/vaccines3030703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Karanikas V, Zamanakou M, Kerenidi T, et al. Indoleamine 2,3-dioxygenase (IDO) expression in lung cancer. Cancer Biol Ther 2007;6:1258-62. 10.4161/cbt.6.8.4446 [DOI] [PubMed] [Google Scholar]
- 7.Suzuki Y, Suda T, Furuhashi K, et al. Increased serum kynurenine/tryptophan ratio correlates with disease progression in lung cancer. Lung Cancer 2010;67:361-5. 10.1016/j.lungcan.2009.05.001 [DOI] [PubMed] [Google Scholar]
- 8.Creelan BC, Antonia S, Bepler G, et al. Indoleamine 2,3-dioxygenase activity and clinical outcome following induction chemotherapy and concurrent chemoradiation in Stage III non-small cell lung cancer. Oncoimmunology 2013;2:e23428. 10.4161/onci.23428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang Y, Hu GF, Wang ZH. The status of immunosuppression in patients with stage IIIB or IV non-small-cell lung cancer correlates with the clinical characteristics and response to chemotherapy. Onco Targets Ther 2017;10:3557-66. 10.2147/OTT.S136259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pallotta MT, Orabona C, Volpi C, et al. Indoleamine 2,3-dioxygenase is a signaling protein in long-term tolerance by dendritic cells. Nat Immunol 2011;12:870-8. 10.1038/ni.2077 [DOI] [PubMed] [Google Scholar]
- 11.Nguyen NT, Kimura A, Nakahama T, et al. Aryl hydrocarbon receptor negatively regulates dendritic cell immunogenicity via a kynurenine-dependent mechanism. Proc Natl Acad Sci U S A 2010;107:19961-6. 10.1073/pnas.1014465107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Prendergast GC, Malachowski WJ, Mondal A, et al. Indoleamine 2,3-Dioxygenase and Its Therapeutic Inhibition in Cancer. Int Rev Cell Mol Biol 2018;336:175-203. 10.1016/bs.ircmb.2017.07.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang W, Huang L, Jin JY, et al. IDO Immune Status after Chemoradiation May Predict Survival in Lung Cancer Patients. Cancer Res 2018;78:809-16. 10.1158/0008-5472.CAN-17-2995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bradley JD, Paulus R, Komaki R, et al. Standard-dose versus high-dose conformal radiotherapy with concurrent and consolidation carboplatin plus paclitaxel with or without cetuximab for patients with stage IIIA or IIIB non-small-cell lung cancer (RTOG 0617): a randomised, two-by-two factorial phase 3 study. Lancet Oncol 2015;16:187-99. 10.1016/S1470-2045(14)71207-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Botticelli A, Cerbelli B, Lionetto L, et al. Can IDO activity predict primary resistance to anti-PD-1 treatment in NSCLC? J Transl Med 2018;16:219. 10.1186/s12967-018-1595-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vacchelli E, Aranda F, Eggermont A, et al. Trial watch: IDO inhibitors in cancer therapy. Oncoimmunology 2014;3:e957994. 10.4161/21624011.2014.957994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kjeldsen JW, Iversen TZ, Engell-Noerregaard L, et al. Durable Clinical Responses and Long-Term Follow-Up of Stage III-IV Non-Small-Cell Lung Cancer (NSCLC) Patients Treated With IDO Peptide Vaccine in a Phase I Study-A Brief Research Report. Front Immunol 2018;9:2145. 10.3389/fimmu.2018.02145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Muller AJ, Manfredi MG, Zakharia Y, et al. Inhibiting IDO pathways to treat cancer: lessons from the ECHO-301 trial and beyond. Semin Immunopathol 2019;41:41-8. 10.1007/s00281-018-0702-0 [DOI] [PubMed] [Google Scholar]
- 19.Munn DH, Sharma MD, Hou D, et al. Expression of indoleamine 2,3-dioxygenase by plasmacytoid dendritic cells in tumor-draining lymph nodes. J Clin Invest 2004;114:280-90. 10.1172/JCI21583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Brandacher G, Perathoner A, Ladurner R, et al. Prognostic value of indoleamine 2,3-dioxygenase expression in colorectal cancer: effect on tumor-infiltrating T cells. Clin Cancer Res 2006;12:1144-51. 10.1158/1078-0432.CCR-05-1966 [DOI] [PubMed] [Google Scholar]
- 21.Labadie BW, Bao R, Luke JJ. Reimagining IDO Pathway Inhibition in Cancer Immunotherapy via Downstream Focus on the Tryptophan-Kynurenine-Aryl Hydrocarbon Axis. Clin Cancer Res 2019;25:1462-71. 10.1158/1078-0432.CCR-18-2882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Monjazeb AM, Kent MS, Grossenbacher SK, et al. Blocking Indolamine-2,3-Dioxygenase Rebound Immune Suppression Boosts Antitumor Effects of Radio-Immunotherapy in Murine Models and Spontaneous Canine Malignancies. Clinical cancer research: an official journal of the American Association for Cancer Research. 2016;22:4328-40. [DOI] [PMC free article] [PubMed]
- 23.Liu M, Li Z, Yao W, et al. IDO inhibitor synergized with radiotherapy to delay tumor growth by reversing T cell exhaustion. Molecular medicine reports. 2020;21:445-53. [DOI] [PubMed] [Google Scholar]
- 24.Chen KZ, Lou F, Yang F, et al. Circulating Tumor DNA Detection in Early-Stage Non-Small Cell Lung Cancer Patients by Targeted Sequencing. Sci Rep 2016;6:31985. 10.1038/srep31985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang S, Campbell J, Stenmark MH, et al. Plasma Levels of IL-8 and TGF-beta1 Predict Radiation-Induced Lung Toxicity in Non-Small Cell Lung Cancer: A Validation Study. Int J Radiat Oncol Biol Phys 2017;98:615-21. 10.1016/j.ijrobp.2017.03.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The article’s supplementary files as