Abstract
Background
There are also differences in survival prognosis among esophageal squamous cell carcinoma (ESCC) patients with a complete regression of the primary tumor (ypT0) after Neoadjuvant chemoradiotherapy (NCRT) followed by surgery. And the purpose of this study was to investigate influencing factors from these different prognostic outcomes and their possible causes.
Methods
The clinical data of 88 cases of ESCC patients with ypT0 after NCRT followed by surgery between 2011 and 2019 were retrospectively analyzed. The clinical and pathological prognostic factors that affect the survival were analyzed.
Results
Sex, number of lymph nodes dissected, and pathologic positivity of lymph nodes may be significant in univariate analysis (P<0.1). Further multivariate analysis suggested that the pathologic positivity of the lymph nodes was an independent factor affecting prognosis (HR: 4.757, 95% CI: 2.195–10.313, P=0.000). Subsequently, the whole group was divided into a positive lymph node group (group LN+) and a negative lymph node group (group LN−) for comparison. The overall survival (OS) of group LN+ was significantly worse (HR: 0.211, 95% CI: 0.0336–0.239; P<0.0001), and recurrence-free survival (RFS) was significantly poorer in the LN+ group (HR: 0.0679, 95% CI: 0.0239–0.1923, P<0.0001). There were 14 cases of recurrence and metastasis in the LN+ group (14/21, 66.7%) and 10 cases in the group LN− (10/67, 14.9%). Among the sites of recurrence and metastasis, there were 10 (10/14, 71.4%) and 4 (4/14, 28.6%) cases of distant metastasis, respectively, and 4 (4/14, 28.6%) cases of local metastasis in the LN+ group; meanwhile, there were 8 (8/10, 80.0%) cases of distant metastasis and 2 (2/10, 20.0%) cases of local metastasis in the LN− group.
Conclusions
The independent risk factor for survival prognosis in ESCC patients with ypT0 after NCRT followed by surgery was positive postoperative pathological lymph nodes. The reason for the shortened survival time associated with this group of patients was their susceptibility to recurrence and metastasis.
Keywords: Esophageal squamous cell carcinoma (ESCC), neoadjuvant chemoradiation, esophagectomy, prognostic factors
Introduction
Esophageal cancer (EC) is one of the most common malignant tumors in the world and one of the six most fatal tumors (1,2). Squamous cell carcinoma is the most common pathological type of EC. In 2012, there were about 398,000 new esophageal squamous cell carcinoma (ESCC) cases, accounting for 87% of EC (3).
Current EC treatment frequently involves multiple treatment modalities, including endoscopic resection (ER), esophagectomy, radiation therapy, chemotherapy (CT), chemoradiotherapy (CRT), immunotherapy (IT), and targeted therapy.
After surgery alone, the prognosis for patients with locally advanced EC remains poor, with a 5-year survival rate of only 25% (4). Recent evidence has suggested neoadjuvant chemoradiotherapy (NCRT) plus surgery may bring long-term survival benefits for locally advanced EC, especially ESCC (5,6).
A number of studies have confirmed that pathological complete response (PCR) remains one of the most important prognostic factors of long-term survival in EC after NCRT followed by surgery (7-10). PCR is defined as there being no evidence of residual tumor cells in the primary site and lymph nodes on operative specimens. However, in actuality, because the lymph nodes of EC often grow across regions, it is impossible to ensure that all the positive lymph nodes are removed during the operation; thus, PCR is only relative, and the condition of patients with a complete regression of primary tumor after NCRT with or without positive lymph nodes (ypT0) in the study seems to be closer to real-world experience.
There are also differences in survival prognosis among ESCC patients with ypT0 after NCRT followed by surgery. Thus, the purpose of this study was to investigate the influencing factors for these different prognostic outcomes and their possible causes.
There was a similar report in the PubMed (11), unlike their study, we used the same chemotherapy regimen and the same surgical procedure for each patient. Therefore, the novel point of view in our article is that even if the bias caused by different treatment schemes is avoided, the conclusion obtained is similar to the above study. We present the following article in accordance with the STROBE reporting checklist (available at http://dx.doi.org/10.21037/atm-20-4864).
Methods
Data source and study population
We reviewed the records of 151 consecutive patients with locally advanced ESCC who received NCRT followed by surgery treatment at the Enze Medical Center (group) of Taizhou city, Zhejiang province, China from January 2011 to June 2019. These data were extracted from our surgical database. According to the following inclusion criteria, a total of 88 cases were eligible for statistical analysis.
The criteria for inclusion were: (I) age between 18 and 70 years, (II) resectable thoracic ESCC clinically staged as T1-4N1M0/T4N0M0 (stage IIB or III) before treatment (12), (III) normal hematologic, renal, and hepatic function, and a Karnofsky performance score (KPS) of ≥90, (IV) a complete regression of the primary tumor (ypT0) after NCRT followed by surgery, (V) no major perioperative complications, and (VI) complete follow-up data.
All procedures performed in this study involving human participants were in accordance with the Declaration of Helsinki (as revised in 2013). This study was approved by the ethics committee of Enze Hospital of the Taizhou Enze Medical Center (group) in Zhejiang province, China. As this was a retrospective study, informed consent was not required.
Treatment plan
Preoperative chemotherapy regimen
Vinorelbine 25 mg/m2 was given intravenously on day 1, 8, 22, and 29, and cisplatin 25 mg/m2 was given intravenously on day 1 to 4 and day 22 to 25.
Preoperative radiotherapy regimen
Gross tumor volume (GTV) included primary esophageal tumor and metastatic lymph nodes, clinical target volume (CTV) included a subclinical lesion (normal esophagus of 3 cm above and below esophageal tumor) and corresponding paraesophageal lymphatic drainage area, and planned target volume (PTV) included CTV plus 8 mm. A total dose of 40.0 Gy was administered in 20 fractions of 2.0 Gy, five fractions per week, starting on the first day of the first cycle of chemotherapy.
Operation plan
About 6 weeks after the end of chemoradiotherapy, a modified McKeown minimally invasive esophagectomy (13), including two-field lymphadenectomy with total mediastinal lymph node dissection, was performed.
Variable collection and definition
General clinical data that could have affected the prognosis were collected, including age, sex, body mass index (BMI), tumor location, clinical stage before treatment, cycles of chemotherapy, the interval between the end of NCRT and surgery, number of lymph nodes dissected, positive lymph nodes dissected, recurrence site, overall survival (OS) time, relapse-free survival (RFS), etc.
The histologic criteria for the response of NCRT was primarily based on postoperative pathology specimens and mainly considers the extent of tumor cell reduction, with a specific reference to the Owaki T. standard which defines the following grades (14): grade I, poor effect on radiation and chemotherapy with no significant change to tumors (necrosis or disappearance of the tumor is present in no more than two thirds of the whole lesion); grade II, tumor obviously changed by radiation and chemotherapy (necrosis or disappearance of the tumor is present in more than two thirds of the whole lesion, but viable tumor cells still remain); and grade III, pathological objective response consisting of complete disappearance of macroscopic and microscopic cancers, with or without the presence of granulation tissue. Esophageal grade primary site grade III was judged as a pathologic complete response of the primary tumor.
OS was defined as the time from date of surgery to death or loss to follow-up. RFS was defined as the time from the date of surgery to objective record of disease recurrence.
Statistical methods
For continuous variables, the number of observations (N), the mean value, the minimum and maximum values, and the 95% confidence interval (CI) of descriptive statistical variables were calculated. T-test or a non-parametric test (Wilcoxon test, etc.) was used for the comparison between the two groups. For discontinuous variables (classification variables), descriptive statistics were used to calculate the number of cases and frequency (percentage) of each type. The two groups were compared using Pearson’s chi-square test or Fisher’s exact test. For the survival follow-up data, Kaplan-Meier analysis was used to estimate the median and 95% CI of OS and RFS, and to plot the survival curve. The differences in RFS and OS between the two groups were compared by using the log-rank method. A Cox proportional hazards risk regression model was used to compare the survival analysis and subgroup analysis of various prognostic factors affecting OS. A P value <0.05 was considered statistically significant.
A multivariate model was constructed by means of a stratified Cox regression analysis, using the variables in the univariate analysis that were found to be significant: variables with a P<0.1 were included in a stepwise conditional forward model. All analyses were performed using IBM SPSS statistics software, version 24 (SPSS Inc., Chicago, IL, USA).
Results
Overall baseline characteristics
As shown in Table 1, 88 patients, including 72 (81.8%) males and 16 (18.2%) females, were enrolled, with an average age of 56 years (31–70 years), including 64 (72.7%) patients aged 60 years or less and 24 (27.3%) patients aged over 60 years. Thirteen (14.8%) patients had a BMI of lower than 18.5 kg/m2, 53 (60.2%) patients had a BMI of 18.5–23.9 kg/m2, and 22 (25.0%) patients had a BMI of more than 24kg/m2. Tumors were located in the proximal third, middle third, and distal third in 10, 65, and 13 cases, respectively. For pretreatment clinical stages, 15, 52, and 21 cases were T2, T3, and T4, respectively; 8 and 80 cases were N0 and N1, respectively; and 15 and 73 cases were stage IIB and III, respectively.
Table 1. Patient characteristics (N=88).
| Variable | Value |
|---|---|
| Age at primary treatment (years) | 56 [31–70] |
| ≤60 y, n (%) | 64 (72.7) |
| >60 y, n (%) | 24 (27.3) |
| Sex, n (%) | |
| Male | 72 (81.8) |
| Female | 16 (18.2) |
| BMI, n (%) | |
| <18.5 kg/m2 | 13 (14.8) |
| 18.5–23.9 kg/m2 | 53 (60.2) |
| ≥24 kg/m2 | 22 (25.0) |
| Tumor location, n (%) | |
| Proximal third | 10 (11.4) |
| Middle third | 65 (73.9) |
| Distal third | 13 (14.8) |
| Clinical T stage, n (%) | |
| cT2 | 15 (17.0) |
| cT3 | 52 (59.1) |
| cT4 | 21 (23.9) |
| Clinical N stage, n (%) | |
| N0 | 8 (9.1) |
| N1 | 80 (90.9) |
| Clinical stage group, n (%) | |
| IIB | 15 (17.0) |
| III | 73 (83.0) |
BMI, body mass index.
Treatment-related prognostic indicators
As shown in Table 2, 7 patients (8.0%) completed 1 cycle of preoperative chemotherapy, and 81 patients (92.0%) completed 2 cycles. The interval between the end of NCRT and surgery was ≤6 weeks in 42 cases (47.7%) and >6 weeks in 46 cases (52.3%). The number of lymph nodes dissected was >19 in 48 cases (54.5%) and ≤19 in 40 cases (45.5%). Postoperative pathological lymph nodes were negative in 67 cases (76.1%) and positive in 21 cases (23.9%).
Table 2. Treatment-related factors (N=88).
| Characteristic | Value |
|---|---|
| Cycles of chemotherapy, n (%) | |
| 1 | 7 (8.0) |
| 2 | 81 (92.0) |
| Interval between the end of NCRT and surgery, n (%) | |
| ≤6 weeks | 42 (47.7) |
| >6 weeks | 46 (52.3) |
| Number of lymph nodes dissected, n (%) | |
| >19 | 48 (54.5) |
| ≤19 | 40 (45.5) |
| Positive lymph nodes dissected, n (%) | |
| No | 67 (76.1) |
| Yes | 21 (23.9) |
NCRT, neoadjuvant chemoradiotherapy.
Univariate analysis and multivariate analysis
Univariate analysis was conducted for age, sex, BMI, tumor location, clinical staging, and cycles of chemotherapy, the interval between the end of NCRT and surgery, number of lymph nodes dissected, and positive lymph nodes dissected.
Multivariate Cox regression analysis included sex (P=0.072), number of dissected lymph nodes (P=0.027), and pathological positive lymph nodes (P=0.000), all elements which exhibited possible influences on prognosis in univariate analysis. Only pathologic lymph node positive was found to be an independent factor affecting prognosis (HR: 4.757,95% CI: 2.195–10.313, P=0.000), as shown in Table 3.
Table 3. Univariate analysis and multivariate analysis of prognostic factors.
| Variable | Univariate analysis | Multivariable analysis | |||
|---|---|---|---|---|---|
| HR (95% CI) | P value | HR (95% CI) | P value | ||
| Age at primary treatment (≤60 vs. >60 y) | 0.266 (0.063–1.125) | 0.357 | |||
| Sex (male vs. female) | 0.266 (0.063–1.125) | 0.072 | 0.301 (0.071–1.277) | 0.103 | |
| BMI (kg/m2) | |||||
| 18.5–23.9 vs. <18.5 | 0.935 (0.345–2.540) | 0.896 | |||
| ≥24 vs. <18.5 | 0.823 (0.260–2.611) | 0.741 | |||
| Tumor location | |||||
| Middle third vs. proximal third | 1.369 (0.411–4.561) | 0.610 | |||
| Distal third vs. proximal third | 0.863 (0.173–4.302) | 0.857 | |||
| Clinical T stage | |||||
| cT3 vs. cT2 | 0.834 (0.303–2.295) | 0.725 | |||
| cT4 vs. cT2 | 1.182 (0.386–3.619) | 0.770 | |||
| Clinical N stage (N0 vs. N1) | 1.375 (0.325–5.809) | 0.665 | |||
| Clinical stage group (IIB vs. III) | 0.929 (0.353–2.444) | 0.881 | |||
| Cycles of chemotherapy (1 vs. 2) | 1.229 (0.291–5.188) | 0.779 | |||
| Interval between the end of NCRT and surgery (≤6 vs. >6 weeks) | 0.596 (0.278–1.274) | 0.181 | |||
| Number of lymph nodes dissected (≤19 vs. >19) | 0.428 (0.202–0.910) | 0.027 | 0.521 (0.243–1.118) | 0.094 | |
| Positive lymph nodes dissected (no vs. yes) | 5.316 (2.453–11.525) | 0.000 | 4.757 (2.195–10.313) | 0.000 | |
BMI, body mass index; NCRT, neoadjuvant chemoradiotherapy.
Group analysis
In the multivariate analysis, positive lymph node pathology was an independent prognostic factor affecting survival; therefore, the whole group was divided into a positive lymph node group (group LN+) and negative lymph node group (group LN−). Compared with the two groups, the OS of Group LN+ was significantly worse (HR: 0.211, 95% CI: 0.0336–0.239; P<0.0001), as shown in Figure 1.
Figure 1.
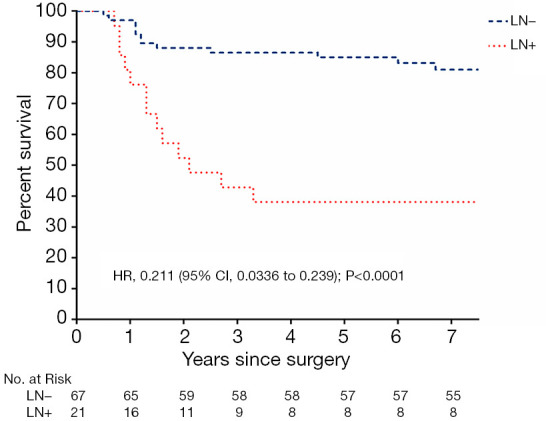
Overall survival (OS) in the LN+ group and the LN− group.
When RFS was compared between the two groups, the prognosis of the LN+ group was poor and there was a significant difference (HR: 0.0679, 95% CI: 0.0239–0.1923, P<0.0001), as shown in Figure 2.
Figure 2.
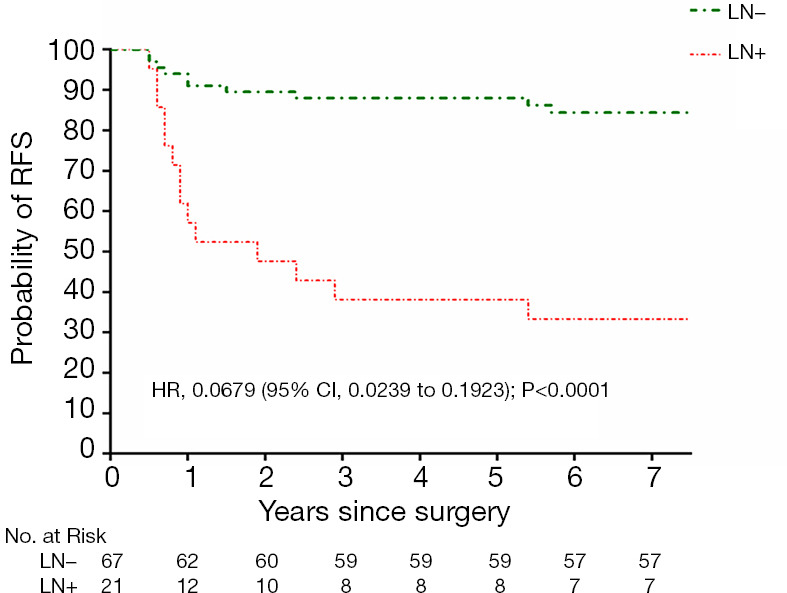
Recurrence-free survival (RFS) in the LN+ group and LN− group.
Recurrence and metastasis analysis
Among the 88 patients in the whole group, 24 (27.3%) had recurrence and metastasis, 21 of whom (21/24,87.5%) had RFS of less than 3 years, and the cumulative recurrence rate was positively correlated with mortality (rs =0.979, P<0.000) (Figure 3). There were 14 cases of recurrence and metastasis in the LN+ group (14/21, 66.7%) and 10 cases in the LN− group (10/67, 14.9%), with a significant difference between the two groups (P<0.000). There were 18 cases of distant metastasis (18/24, 75.0%), 6 cases of local metastasis (6/24, 25.0%), 10 cases of distant metastasis (10/14, 71.4%), and 4 cases of local metastasis (4/14, 28.6%) in the LN+ group, and 8 cases of distant metastasis (8/10, 80.0%) and 2 cases of local metastasis (2/10, 20.0%) in the LN− group, as shown in Figure 4.
Figure 3.
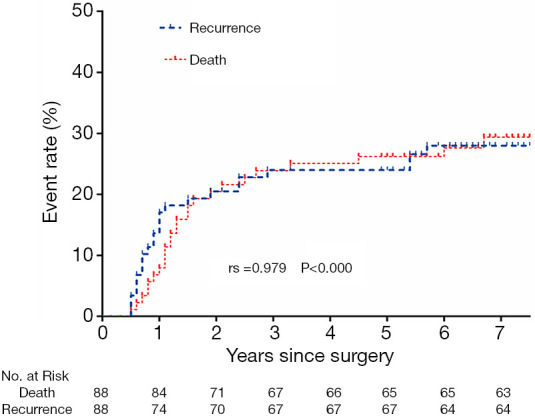
Relationship between cumulative recurrence rate and mortality.
Figure 4.
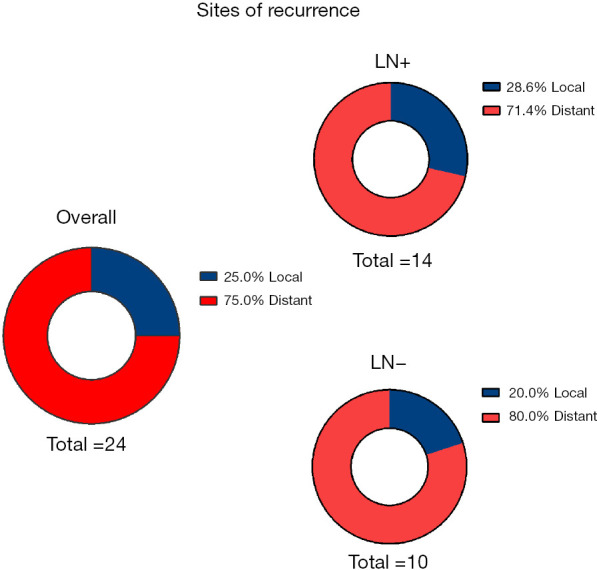
Distribution of recurrence sites.
Discussion
This study found that positive postoperative lymph nodes were found to be an independent risk factor for the survival and prognosis of ESCC patients with ypT0 after NCRT followed by surgery. Positive postoperative lymph nodes were more likely to relapse and metastasize, and the survival time was shortened. However, there appears to be no statistical significance in the relationship between survival and classic prognostic factors including preoperative pathological T stage (6,15). The findings in this study also underline the recommendations for neoadjuvant pathologic staging of cancer of the esophagus and esophagogastric junction for the American Joint Committee on Cancer (AJCC) and the International Union Against Cancer (UICC) (AJCC/UICC) 8th edition staging manuals indicating that ypN+ portends poor survival irrespective of yT− stage (16).
Many studies have proven that NCRT combined with surgery can bring survival benefits to patients with EC, especially SCC (5,6,17), especially for PCR cases with obvious advantages after chemoradiotherapy (7,18). However, due to tumor heterogeneity, the response of primary lesions and lymph nodes to NRCT is not completely consistent, and there are cases of complete and partial pathological response after NCRT. Moreover, EC lymph nodes often grow cross-regionally, and intraoperatively, there is no guarantee that all positive lymph nodes can be removed, rendering PCR rather relative. However, primary focal pathologic mitigation after NCRT can be determined, so for the primary lesion patients with this condition, the situation aligns more with real-world experience. Thus, there remain many unknowns concerning the prognostic factors for survival in patients with ESCC with a PCR of the primary tumor after NCRT followed by surgery (19).
Recently, several studies have explored the prognostic value of pathologic response grades after NCRT in EC. Depypere’s research (18) had similar results to our study, including the suggestion that ypT0N+ in EC patients following NCRT has a poor prognosis and behaves similar to ypT+N+, but their research focused on esophageal adenocarcinoma and the prognosis was related to the number of lymph nodes dissected, while our study focused on ESCC. Although the conclusions were similar in terms of the prognosis of positive lymph nodes, the number of lymph nodes dissected in this study was divided into ≤19 or >19 according to a cutoff value, and seemed to be statistically significant in the univariate analysis, but there was no statistical difference in the multivariate analysis (P=0.094). Two other implications also arise. In relation to lymph node status, if the preoperative positive lymph nodes do not respond to NCRT, there will be a severely negative impact on long-term survival. Second, a thorough lymph node dissection may improve prognosis.
As for the recurrence and metastasis pattern of EC with pathological remission after radiotherapy and chemotherapy, Barbetta et al. (20) showed that although there was a similarity in the cumulative recurrence rate between esophageal adenocarcinoma and ESCC, the recurrence pattern seemed to be different, and ESCC tended to have local recurrence. Conflicting with Barbetta’s study, our findings showed that, ESCC recurrence more commonly manifested as distant metastasis.
This study demonstrated a linear correlation between the cumulative ESCC mortality after NCRT in primary remission and the rate of recurrence and metastasis, which is a pattern that occurs with other solid tumors. A prognosis of postoperative lymph node positive is poor, which makes more prone to recurrence and metastasis. Xi et al. (21) found a similar conclusion with esophageal adenocarcinoma.
This study may provide a basis for certain clinical decisions. To limit the presence of positive postoperative pathological lymph nodes, we believe that (I) It is very important to accurately evaluate lymph node status prior to the initiation of treatment; for example, in addition to enhanced CT, PET-CT, endoscopic ultrasound biopsy and ultrasound bronchoscopy biopsy can be combined according to the situation. (II) For the positive lymph nodes before treatment, local treatment should be strengthened, or combined with immunotherapy, so as to maximize the pathological remission rate of lymph nodes and primary lesions.
This study has some limitations. First, it is a retrospective study with a small sample size. Secondly, a small number of cases that were not included due to incomplete follow-up information might have produced a degree of statistical bias.
Conclusions
This study revealed that an independent risk factor for survival prognosis in ESCC patients with ypT0 after NCRT followed by surgery was the presence of a positive postoperative pathological lymph nodes, which shortened survival time of this group of patients via increased recurrence and metastasis.
Supplementary
The article’s supplementary files as
Acknowledgments
We would like to thank all the members of for the Department of Cardiothoracic Surgery and the Department of Radiotherapy in our hospital who participated in this research.
Funding: None.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study involving human participants were in accordance with the Declaration of Helsinki (as revised in 2013). This study was approved by the ethics committee of Enze Hospital of the Taizhou Enze Medical Center (group) in Zhejiang province, China. As this was a retrospective study, informed consent was not required.
Footnotes
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at http://dx.doi.org/10.21037/atm-20-4864
Data Sharing Statement: Available at http://dx.doi.org/10.21037/atm-20-4864
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/atm-20-4864). The authors have no conflicts of interest to declare.
References
- 1.Torre LA, Bray F, Siegel RL, et al. Global cancer statistics, 2012. CA Cancer J Clin 2015;65:87-108. 10.3322/caac.21262 [DOI] [PubMed] [Google Scholar]
- 2.Ma WJ, Zhang QN, Shi SZ, et al. Preoperative chemoradiation may be more effective for esophageal squamous cell carcinoma compared with adenocarcinoma: results from 15 randomized controlled trials of 2,250 patients. Transl Cancer Res 2018;7:1421-30. 10.21037/tcr.2018.11.04 [DOI] [Google Scholar]
- 3.Arnold M, Soerjomataram I, Ferlay J, et al. Global incidence of oesophageal cancer by histological subtype in 2012. Gut 2015;64:381-7. 10.1136/gutjnl-2014-308124 [DOI] [PubMed] [Google Scholar]
- 4.Shang QX, Yang YS, Hu WP, et al. Prognostic significance and role of thoracic lymph node metastasis based on Chinese expert consensus in esophageal cancer. Ann Transl Med 2019;7:381. 10.21037/atm.2019.07.63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Park IH, Kim JY. Surveillance or resection after chemoradiation in esophageal cancer. Ann Transl Med 2018;6:82. 10.21037/atm.2017.12.16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yang H, Liu H, Chen Y, et al. Neoadjuvant Chemoradiotherapy Followed by Surgery Versus Surgery Alone for Locally Advanced Squamous Cell Carcinoma of the Esophagus (NEOCRTEC5010): A Phase III Multicenter, Randomized, Open-Label Clinical Trial. J Clin Oncol 2018;36:2796-803. 10.1200/JCO.2018.79.1483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wan T, Zhang XF, Liang C, et al. The Prognostic Value of a Pathologic Complete Response After Neoadjuvant Therapy for Digestive Cancer: Systematic Review and Meta-Analysis of 21 Studies. Ann Surg Oncol 2019;26:1412-20. 10.1245/s10434-018-07147-0 [DOI] [PubMed] [Google Scholar]
- 8.van Hagen P, Wijnhoven BP, Nafteux P, et al. Recurrence pattern in patients with a pathologically complete response after neoadjuvant chemoradiotherapy and surgery for oesophageal cancer. Br J Surg 2013;100:267-73. 10.1002/bjs.8968 [DOI] [PubMed] [Google Scholar]
- 9.Soror T, Kho G, Zhao KL, et al. Impact of pathological complete response following neoadjuvant chemoradiotherapy in esophageal cancer. J Thorac Dis 2018;10:4069-76. 10.21037/jtd.2018.06.85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Eyck BM, van der Wilk BJ, Lagarde SM, et al. Neoadjuvant chemoradiotherapy for resectable oesophageal cancer. Best Pract Res Clin Gastroenterol 2018;36-37:37-44. 10.1016/j.bpg.2018.11.007 [DOI] [PubMed] [Google Scholar]
- 11.Hamai Y, Hihara J, Emi M, et al. Evaluation of Prognostic Factors for Esophageal Squamous Cell Carcinoma Treated with Neoadjuvant Chemoradiotherapy Followed by Surgery. World J Surg 2018;42:1496-505. 10.1007/s00268-017-4283-1 [DOI] [PubMed] [Google Scholar]
- 12.Rice TW, Blackstone EH, Rusch VW. 7th edition of the AJCC Cancer Staging Manual: esophagus and esophagogastric junction. Ann Surg Oncol 2010;17:1721-4. [DOI] [PubMed] [Google Scholar]
- 13.Chen B, Zhang B, Zhu C, et al. Modified McKeown minimally invasive esophagectomy for esophageal cancer: a 5-year retrospective study of 142 patients in a single institution. PLoS One 2013;8:e82428. 10.1371/journal.pone.0082428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Owaki T, Matsumoto M, Okumura H, et al. Endoscopic ultrasonography is useful for monitoring the tumor response of neoadjuvant chemoradiation therapy in esophageal squamous cell carcinoma. Am J Surg 2012;203:191-7. 10.1016/j.amjsurg.2011.01.027 [DOI] [PubMed] [Google Scholar]
- 15.Depypere L, Moons J, Lerut T, et al. Neoadjuvant chemoradiation treatment followed by surgery for esophageal cancer: there is much more than the mandard tumor regression score. Acta chirurgica Belgica 2016;116:149-55. 10.1080/00015458.2016.1212500 [DOI] [PubMed] [Google Scholar]
- 16.Rice TW, Ishwaran H, Kelsen DP, et al. Recommendations for neoadjuvant pathologic staging (ypTNM) of cancer of the esophagus and esophagogastric junction for the 8th edition AJCC/UICC staging manuals. Dis Esophagus 2016;29:906-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shapiro J, van Lanschot JJB, Hulshof M, et al. Neoadjuvant chemoradiotherapy plus surgery versus surgery alone for oesophageal or junctional cancer (CROSS): long-term results of a randomised controlled trial. Lancet Oncol 2015;16:1090-8. 10.1016/S1470-2045(15)00040-6 [DOI] [PubMed] [Google Scholar]
- 18.Blum Murphy M, Xiao L, Patel VR, et al. Pathological complete response in patients with esophageal cancer after the trimodality approach: The association with baseline variables and survival-The University of Texas MD Anderson Cancer Center experience. Cancer 2017;123:4106-13. 10.1002/cncr.30953 [DOI] [PubMed] [Google Scholar]
- 19.Depypere LP, Vervloet G, Lerut T, et al. ypT0N+: the unusual patient with pathological complete tumor response but with residual lymph node disease after neoadjuvant chemoradiation for esophageal cancer, what’s up? J Thorac Dis 2018;10:2771-8. 10.21037/jtd.2018.04.136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Barbetta A, Sihag S, Nobel T, et al. Patterns and risk of recurrence in patients with esophageal cancer with a pathologic complete response after chemoradiotherapy followed by surgery. J Thorac Cardiovasc Surg 2018;S0022-5223(18)33122-2. [DOI] [PMC free article] [PubMed]
- 21.Xi M, Hallemeier CL, Merrell KW, et al. Recurrence Risk Stratification After Preoperative Chemoradiation of Esophageal Adenocarcinoma. Ann Surg 2018;268:289-95. 10.1097/SLA.0000000000002352 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The article’s supplementary files as


