Abstract
In order to understand the clinical manifestations and incidence of gastrointestinal symptoms of coronavirus disease (COVID-19) in children and discuss the importance of fecal nucleic acid testing.We retrospectively analyzed studies on gastrointestinal symptoms and fecal nucleic acid detection in pediatric COVID-19 patients from January 1, 2020 to August 10, 2020, including prospective clinical studies and case reports. The results of fecal nucleic acid detection were analyzed systematically. Stata12.0 software was used for meta-analysis.The results showed that the most common gastrointestinal symptoms in children with COVID-19 were vomiting and diarrhea, with a total incidence of 17.7% (95% Cl 13.9–21.5%). However, the prevalence of gastrointestinal symptoms in other countries (21.1%, 95% CI 16.5–25.7%) was higher compared to China (12.9%, 95% CI 8–17.7%). In Wuhan, the pooled prevalence was much higher (41.3%, 95% CI 3.2–79.4%) compared to areas outside Wuhan in China (7.1%, 95% CI 4.0–10.3%). The positive rate of fecal nucleic acid testing in COVID-19 children was relatively high at 85.8% (91/106). Additionally, 71.2% (52/73) were still positive for fecal nucleic acid after respiratory tract specimens turned negative. One and two weeks after the respiratory tract specimens turned nucleic acid-negative, 45.2% (33/73) and 34.2% (25/73) patients, respectively, remained fecal nucleic acid-positive. The longest interval between the respiratory tract specimens turning negative and fecal specimens turning negative exceeded 70 days. Conclusions and relevance: gastrointestinal symptoms in pediatric COVID-19 are relatively common. Attention should be paid to the detection of fecal nucleic acids in children. Fecal nucleic acid-negative status should be considered as one of the desegregation standards.
Subject terms: Gastrointestinal system, Paediatrics
Introduction
Currently, coronavirus disease (COVID-19) has become a global pandemic. There are reports of a large number of cases across the world1,2. It mainly infects the middle-aged and elderly, and the mortality rate is the highest in patients with comorbid diseases3,4. Therefore, it was believed that children were not easily infected during the early stages of the pandemic. However, with the development of the pandemic, pediatric cases of COVID-19, including severe cases, began to emerge5. Consequently, people began to pay attention to the pandemic in children. The main clinical manifestations of COVID-19 are fever, dry cough, and fatigue. Majority of the COVID-19 pediatric patients have nasal obstruction, fever, runny nose, pharyngalgia, muscle pain, and other symptoms6. Therefore, there is greater focus on patients with respiratory tract infection symptoms as the presenting symptoms. Since gastrointestinal symptoms are not typical in children, especially as the initial symptoms, they are often ignored. The detection of viral nucleic acid in respiratory tract specimens is the main priority when treating children with COVID-19 in the clinic, while fecal nucleic acid detection is often neglected. Therefore, children with gastrointestinal symptoms as the predominant manifestation of COVID-19 are often misdiagnosed. Currently, there are no large prospective double-blind controlled studies on the gastrointestinal symptoms of COVID-19 and fecal nucleic acid detection in children. Therefore, this study aimed to summarize the gastrointestinal symptoms and fecal nucleic acid detection in children with COVID-19.
PROSPERO registration number: CRD42020190358.
Materials and methods
Literature retrieval strategy
PubMed, Web of Science, Embase, Johns Hopkins University published data, as well as the Chinese databases CNKI, Wanfang, and Chongqing Weipu data were searched electronically to collect literature reporting the characteristics of gastrointestinal symptoms of COVID-19 in children, and the retrieval period was from January 1, 2020 to August 10, 2020. Both online database retrieval and manual retrieval were used, and the references included in the literature were traced. Subject words as well as free words were used for retrieval, and adjustments were made according to the characteristics of different databases without any limitations regarding the language, race, and region.
The Pubmed search strategy was as follows
#1 (children) OR (child) OR (kid) OR (pediatric).
#2 (clinical feature) OR (epidemiology) OR (vomiting) OR (diarrhea) OR (stomachache).
#3 (2019-nCoV) OR (COVID-19) OR (SARS-CoV-2) OR (Corona Virus Disease).
#1 AND #2 AND #3
Literature screening and data extraction
Two researchers independently searched and screened the literature and collected and cross-checked the relevant data. If there was any dispute, it was discussed or resolved with the help of a third researcher.
Inclusion criteria 1. Research types: cohort study, case–control study, and case analysis; 2. Subjects: children with COVID-19; 3. Observation index: clinical manifestations of COVID-19 in children including gastrointestinal symptoms such as diarrhea and vomiting.
Exclusion criteria 1. Repeated publication of the same research; 2. studies on adults; 3. incomplete or missing data or analysis, and inability to obtain the data literature.
Study of bias risk assessment
We included a case series study, using the National institute for Clinical Optimization.
Clinical excellence (NICE) for quality evaluation7. The evaluation items were as follows: The cases included in the case series should (1) come from different levels of medical institutions that carry out multi-center research; (2) clearly describe the research hypothesis or purpose; (3) have clear exclusion criteria; (4) have a clear definition of the measurement of results; (5) present collected data that achieves the expected purpose; (6) accurately describe that patients are continuously recruited; (7) describe the main findings clearly; (8) analyze and report the results in layers. One point is given for each item, and a total score ≥ 4 out of 8 points is considered as high-quality research. Two researchers independently evaluated the quality and cross-checked the results.
Statistical analysis
Meta-analysis was performed using the Stata 12.0 software. First, the original ratio (R) was transformed by double arcsine to make it conform to a normal distribution, and then the transform ratio (TR) was analyzed by meta-analysis. Subsequently, the final rate (r) with the 95% confidence interval (CI) were obtained by converting the results with the formula R = (sin[tr/2])2. Meta-analysis was carried out by using the random effect model for all studies. The funnel chart was utilized to assess publication bias, and the meta-analysis significance level was designated as α = 0.05.
Ethics
As this is a systematic review, ethical approval was not required.
Results
Gastrointestinal symptoms of COVID-19
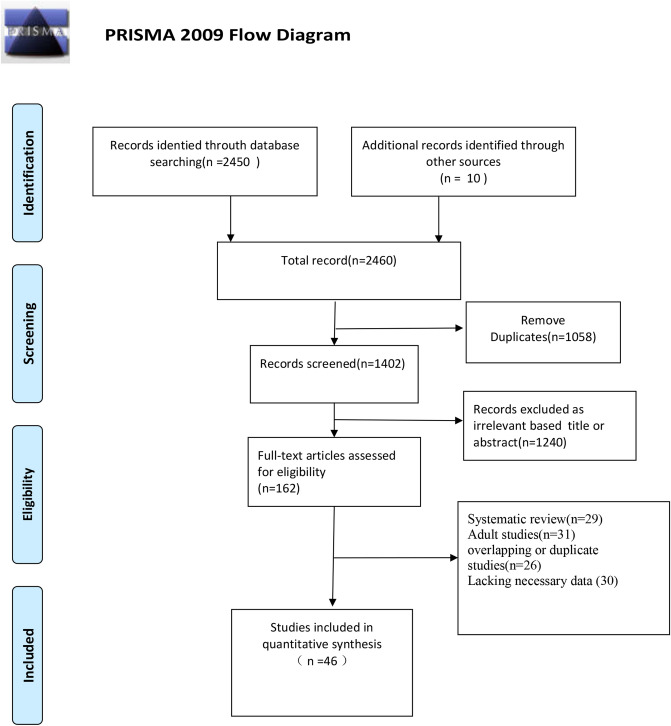
Figure 1 summarizes the article retrieval and abstraction method using the PRISMA guidelines.
Figure 1.
Flow diagram for identification of selected studies in the meta-analysis.
Most research data are concentrated in articles published by China, the United States, and Europe. A total of 46 studies were included in this analysis8–53, 38 of which described the gastrointestinal symptoms of patients (Supplementary Table S1). A total of 3028 patients were evaluated in the study, among whom 536 had digestive tract symptoms, accounting for 17.7% (95% CI 13.9–21.5%) of the patients (Fig. 2). Vomiting and diarrhea were the most common gastrointestinal symptoms. When analyzing by country (studies from China versus studies from other countries), the prevalence of gastrointestinal symptoms in countries outside China was 21.1% (95% CI 16.5–25.7%), which was higher than that in China (12.9%, 95% CI 8–17.7%). Among patients in Wuhan, the pooled prevalence was much higher at 41.3% (95% CI 3.2–79.4%) than in areas outside Wuhan in China (7.1%, 95% CI 4.0%–10.3%) (Table 1).
Figure 2.
Forest plot of the incidence of gastrointestinal symptoms.
Table 1.
Subgroup analysis.
| Region | Number of included studies | Sample size | Heterogeneity | Effect of the model | Meta-analysis results | ||
|---|---|---|---|---|---|---|---|
| P values | I2 | R% (95% CI) | P values | ||||
| China | 21 | 665 | 0 | 78.80% | random | 12.9% (8–17.7%) | 0 |
| Outside China | 17 | 2363 | 0 | 86.70% | random | 21.1% (16.5–25.7%) | 0 |
| Wuhan | 3 | 199 | 0 | 94.50% | random | 41.3% (3.2–79.4%) | 0.034 |
| Outside Wuhan, China | 18 | 466 | 0.023 | 44.40% | random | 7.1% (4–10.3%) | 0 |
| Total | 38 | 3028 | 0 | 87.80% | random | 17.7% (13.9–21.5%) | 0 |
CI confidence interval.
A majority of studies did not describe the stool characteristics or number of bowel movements. Wang Duan et al.10 reported that in the six northern provinces of China, patients had bowel movements 2–6 times per day, while Wu Huaping found that in the Jiangxi province of China, the frequency of diarrhea in affected children was 3–4 times per day21.
Subgroup analysis
The heterogeneity of this study was relatively large. To explore the source of heterogeneity, we analyzed according to the region (country or region) where the research object was located. It was discovered that the analysis results of each subgroup were basically consistent with the overall results, and there were no significant differences between the heterogeneity of each subgroup and the overall heterogeneity. Therefore, was considered that the region of the research object was not the main source of heterogeneity (Table 1).
Fecal testing for viral nucleic acid
Thirteen reports included in the present study described fecal nucleic acid examination (Table 2). The positive rate of fecal nucleic acid testing in COVID-19 patients was 85.8% (91/106). In reports from China19,27,29,34,41,52, the positive rate of all stool samples tested was close to or reached 100%. Additionally, 71.2% (52/73) were still positive for fecal nucleic acid after the respiratory tract specimens turned negative, 45.2% (33/73) were fecal nucleic acid-positive one week after the respiratory tract specimen was nucleic acid-negative, and 34.2% (25/73) were fecal nucleic acid-positive two weeks after a respiratory tract nucleic acid negative test. A study from Anhui Province in China found that the longest interval between the respiratory tract specimen turning negative and fecal specimen turning negative exceeded 70 days34. However, in a study on three neonates27, respiratory tract and fecal nucleic acid tests were positive 2 and 4 days after birth, respectively, and the fecal and respiratory tract specimens were negative on the 6th day after birth.
Table 2.
Stool RT-PCR test of SARS-COV-2 infected patients.
| Authors | Time period of inclusion in the study | Region | Total people | Number of fecal positive results | Age | Clinical picture | Imaging characteristics | The respiratory PCR test was negative, while the stool test was positive | Time difference between fecal NEGATIVE PCR and respiratory tract negative PCR (D) |
|---|---|---|---|---|---|---|---|---|---|
| Park JY22 | February 18, 2020 | Korea | 1 | 1 | 10 years | Low heat, little sputum | The CT findings are mild pneumonia | 0 | > 1 |
| Cui Y23 | January 28, 2020 | Kweichow, China | 1 | 1 | 55 days | Runny nose and dry cough | Flaky shadows and ground-glass opacity | 0 | + 18 |
| Zhang YH24 | January 26, 2020 | Haikou city, China | 1 | 1 | 3 month | Fever | There seems to be a small amount of patchy shadow in the right lower lung field | 0 | + 1 |
| Cai J25 | 19 January to 3 February 2020 | Shanghai, China | 10 | 6 (another 4 cases of fecal nucleic acid were not tested) | Median 74 m(range 3–131 m) | Symptoms of respiratory tract infection | Unilateral patchy infiltration occurred in 4 of the 10 patients | 0 |
> 18 − 12 > 12 > 11 > 12 > 15 All of them were discharged from hospital on February 19 |
| Zeng LK26 | February 5, 2020 | Wuhan Children's Hospital | 1 | 1 | 17 days | Sneezing, vomiting milk | Small bands of fuzzy shadows are seen in both lung fields on CT | 0 | > 1 |
| Xu Y19 | 22 January to 20 February 2020 | Guangzhou Women and Children Medical Center | 10 | 8 | 2 month–15 years | Fever, cough, diarrhea | Isolated or patchy hyaline opacity occurred in 5 patients | 0 |
+ 19 + 1 > 18 + 5 > 18 > 19 + 4 > 3 |
| Zeng L27 | January to February 2020 | Wuhan Children's Hospital | 3 | 3 | 1 days | All had fever and pneumonia | Chest radiographs suggested pneumonia | 0 | 0 |
| Xing Y29 | 17 January to 23 February 2020 | Qingdao Maternal and Child Health Care Hospital, Qingdao, China | 3 | 3 | NA | Mild fever | NA | NA |
+ 8 + 20 + 20 |
| Hua CZ34 | By 29 February 2020 | Zhejiang province, China | 35 | 32 | From 3 months 20 days to 14 years with a mean of 8.16 years (SD: 4.07) | One had diarrhea, others were fever, cough and asymptomatic | Pneumonia or no abnormality | NA |
18 > 1d 14 > 7d 12 > 14d 1 > 70d |
| De Ioris MA38 | 16 March 2020 to 8 April 2020 | Bambino Gesù Children Hospital, Rome, Italy | 22 | 15 | Median age was 84 months (range, 8 days to 210 months) | All were characterized by fever and diarrhea | NA | NA | NA |
| Wu Q41 | January 20 to February 27 of 2020 | Qingdao Women and Children’s Hospital and Wuhan Children’s Hospital. China | 10 | 10 | median (range) 6.00 (0.10–15.08) |
Diarrhea 3 Anorexia 3 |
NA | NA |
8 > 1d 2 > 23d median of 11 days |
| Zhang B52 | Feb1-May7 | First Affiliated Hospital of Jinan University and Dongguan Ninth People's Hospital | 3 | 3 |
14 years 13 years 10 month |
Asymptomatic Asymptomatic Pneumonia |
Pneumonia |
+ 1 + 1 |
NA |
| Du W53 | January 23, 2020 to March 9, 2020 | Jinan Infectious Disease Hospital Affiliated to Shandong University | 10 | 7 | Median age of 5.08 years (range, 9 month-14 years) | NA | NA | NA |
+ 8 + 17 + 13 + 17 Na Na NA |
Publication bias
The funnel plot (Fig. 3) shows the presence of a possible publication bias. Most of the research quality scores were not high. Our confidence in the pooled estimates of prevalence was reduced because of concerns regarding risk of bias (selection bias, detection bias, and attrition bias), heterogeneity of the tested patient populations (inconsistency), as well as issues pertaining to indirectness (the majority of studies included primarily symptomatic hospitalized patients instead of all patients with COVID-19). Additionally, most of the studies were retrospective cohort series and did not specify if consecutive patients were included in the analysis. These factors may have contributed to the heterogeneity of findings across studies.
Figure 3.

Funnel plot assessing publication bias.
Discussion
Incidence of gastrointestinal symptoms
In the early stages of the pandemic, there was a shared misconception that children were not easily infected1. However, with the spread of the pandemic, the number of infected children is increasing and several severe pediatric cases have been reported48. It is sometimes difficult to distinguish the gastrointestinal symptoms of pediatric COVID-19 from those caused by another viral illness, side effects of drugs, and digestive tract symptoms such as nausea and diarrhea caused by the disturbance of gastrointestinal flora by the fever itself. Some studies49 have found that 20.4% children use antibiotics that cause diarrhea, and the diarrhea is more severe in younger patients with lower respiratory tract infections treated with intravenous antibiotics. Moreover, we discovered that the total incidence of gastrointestinal symptoms in children with COVID-19 was 17.7%; unfortunately, not all the studies described a control group when investigating the incidence of gastrointestinal symptoms in an antibiotic treatment group and non-antibiotic treatment group. In a meta-analysis50 of predominantly adult studies, 60 studies (including 4243 patients with COVID-19) were analyzed and the incidence of gastrointestinal symptoms was found to be 17.6%, which is almost equal to 17.7% found in this study. In addition, we discovered that the incidence of gastrointestinal symptoms in other countries (21.1%) was significantly higher than that in China (12.9%). One reason may be that the gastrointestinal symptoms were not paid attention to in the early stage of the epidemic. However, once the literature was published, gastrointestinal symptoms were described in detail.
Pathogenesis of COVID-19
Regarding the mechanism of infection of the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), it is currently believed that the major determinant of SARS-CoV-2 infection is the S protein, which binds to membrane receptors on host cells and mediates the fusion of the virus and cell membrane. Angiotensin converting enzyme 2 (ACE2) is a homolog of ACE and one of the important receptors on the cell membrane of host cells. The interaction between the S protein and ACE2 promotes the invasion of host cells by SARS-CoV-2. The structure of the SARS-CoV-2 S protein is highly similar to that of the SARS coronavirus (SARS-CoV) S protein; however, SARS-CoV-2 S protein binds to ACE2 with a higher affinity than the SARS-CoV S protein, indicating that SARS-CoV-2 possesses a stronger invasion ability51. ACE2 can control intestinal inflammation and diarrhea, and the interaction between SARS-CoV-2 and ACE2 may lead to diarrhea52. ACE2 is highly expressed in the small intestine, especially in the proximal and distal intestinal epithelial cells; therefore, the small intestine is more vulnerable to SARS-CoV-2 infection. Previous investigations may have underestimated the incidence of diarrhea among those infected with SARS-CoV-2. Further research is needed to determine whether diarrhea has diagnostic value for SARS-CoV-2. In case of the Middle East Respiratory Syndrome coronavirus (MERS-CoV), which is highly homologous to SARS-CoV-2, it is believed that the intestinal tract is another route of infection and the incidence rate of diarrhea is 20–25%53.
Pathological examination
Till date, there have been no endoscopic and pathologic studies of the digestive tract in pediatric COVID-19 cases. However, a study in adults54 demonstrated that there was no obvious damage to the mucosal epithelium of the esophagus, stomach, duodenum, and rectum. In the inherent layers of the stomach, duodenum, and rectum, a large number of infiltrating plasma cells and lymphocytes were seen accompanied by interstitial edema. ACE2, the virus host receptor, is mainly found in the cytoplasm of gastrointestinal epithelial cells and virus nucleocapsid proteins were found in the cytoplasm of duodenal and rectal glandular epithelial cells.
Positive rate and significance of fecal nucleic acids
In a recent study54 on 73 hospitalized adult patients in China, the feces of 53.42% of the patients were positive for the viral RNA, the duration for which positive fecal results were obtained ranged from 1 to 12 days, and 23.29% of the patients were still fecal nucleic acid-positive after being confirmed respiratory nucleic acid-negative. Compared with adults, the present study found that the nucleic acid positivity rate of feces in children was higher (85.8%). A study reported that among 59 patients with COVID-19 in Hong Kong, 15 (25.4%) had gastrointestinal symptoms and nine (15.3%) had positive stool viral RNA test results. The detection rates of fecal viral RNA were 38.5% and 8.7% in people with and without diarrhea, respectively50. At present, there is no relevant study on whether there is a difference in the positive rate of fecal nucleic acid testing in COVID-19 children with and without diarrhea.
In a recent study conducted from January 16, 2020 to February 8, 2020, the Chinese CDC reported 2135 pediatric COVID-19 patients (including confirmed and suspected cases), 94 of whom were asymptomatic (4.4%)55. However, a recent study from New York56 claimed that 29 (87.9%) out of 33 pregnant women who tested positive for SARS-CoV-2 on admission did not have symptoms of COVID-19 at the time of treatment. This is very worrying data, because it shows that there are more asymptomatic than symptomatic patients; therefore, controlling asymptomatic patients is the key to controlling the pandemic. In children with asymptomatic COVID-19, there is no relevant study on whether the nucleic acid sensitivity of respiratory specimens is higher than that of feces. Furthermore, it remains unknown whether the children in whom the symptoms have resolved and respiratory tract specimens are negative while the stool samples remain positive for viral nucleic acids, are asymptomatic infectious sources. Consequently, it is important to recommend that after recovery and discharge, pediatric patients be isolated at home for more than 2 weeks.
Prognosis of COVID-19 children with gastrointestinal symptoms
In terms of prognosis, a retrospective comparative study was carried out in patients over 18 years old in the United States57. The experimental group included 278 patients with fever and cough due to COVID-19, and the control group included 238 patients with fever and cough attributable to a common respiratory tract infection. The incidence of gastrointestinal symptoms in the two groups was 34.8% and 26.4%, respectively (P = 0.04). In the 278 patients with COVID-19, the course of gastrointestinal symptoms was longer, but the mortality rate and rate of severe disease were lower in patients with gastrointestinal symptoms than in those without such symptoms. At present, there is no prognostic study on children with COVID-19.
Prevention and treatment
Transmission through respiratory droplets and contact are currently considered to be the main routes of transmission of COVID-19. Nevertheless, there is now increasing evidence of fecal–oral transmission58. In clinical practice, doctors mostly pay attention to the manifestations of respiratory infection in children with COVID-19 such as fever, cough, fatigue, etc. For patients in the gastroenterology department who have no respiratory symptoms, it is recommended to adopt the appointment system and time-division diagnosis and treatment to reduce patient aggregation and avoid cross infections. The clinic should be well-ventilated and disinfection of the clinic should be performed daily at the beginning and end of the clinic. Although gastrointestinal symptoms are often ignored, in children with diarrhea, abdominal pain, nausea, vomiting, and other gastrointestinal symptoms accompanied by a low fever, attention should be paid to their epidemiological history with screening of suspected patients. Nucleic acid examination should be performed using throat swabs and anal tests. In daily life, the risk of transmission can be reduced by good hygiene practices, such as washing hands frequently and closing the toilet lid when flushing.
At present, there is no specific drug for COVID-19. Plasma therapy from convalescent patients is considered for those with severe disease48; however, this treatment is controversial59. Dexamethasone has now been proven to be a good treatment option for the COVID-1960. Diarrhea in COVID-19 patients is mostly self-limiting, and symptomatic treatment such as Montmorillonite powder can be used. For critically ill patients, intestinal microecological regulators may be used to maintain the balance of the intestinal flora and prevent secondary infection by intestinal bacterial translocation.
Study limitations
The number of studies included in the meta-analysis was relatively small, with a relatively large proportion of case reports. Most studies did not report on the duration of the gastrointestinal symptoms preceding the presentation. Additionally, the number of patients was relatively small and the description of the gastrointestinal tract of children in the included studies was not sufficiently detailed. The heterogeneity is large and subgroup analysis can not find the source of heterogeneity, which will affect the accuracy of the results. Therefore, it is necessary to conduct a large-scale double-blind randomized controlled study and include additional research factors such as stool frequency, stool characteristics, number of patients with gastrointestinal symptoms and positive fecal nucleic acid test results, length of hospitalization of fecal nucleic acid-positive patients, severity of illness, and the interrelation between respiratory tract sample nucleic acid and stool nucleic acid findings.
Conclusions
Gastrointestinal symptoms in pediatric COVID-19 are relatively common. Attention should be paid to the detection of fecal nucleic acids in children. Especially in high-risk epidemic areas, all children with digestive tract symptoms as the presenting symptoms should be tested for the viral fecal nucleic acid. Fecal viral nucleic acid-negative status should be considered one of the discharge standards.
Supplementary information
Author contributions
J.W. and H.T. contributed to the study design, while H.C. and X.D. contributed to the data collection. Statistical analyses and interpretation of results were performed by J.W. and H.C., whereas J.W. and H.T. drafted the manuscript and edited the language. All the authors participated in the critical revisions, and approved the final version of the manuscript.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
is available for this paper at 10.1038/s41598-020-74913-0.
References
- 1.Novel CPERE. The epidemiological characteristics of an outbreak of 2019 novel coronavirus diseases (COVID-19) in China. Zhonghua Liu Xing Bing Xue Za Zhi. 2020;41:145–151. doi: 10.3760/cma.j.issn.0254-6450.2020.02.003. [DOI] [PubMed] [Google Scholar]
- 2.Spiteri G, et al. First cases of coronavirus disease 2019 (COVID-19) in the WHO European Region, 24 January to 21 February 2020. Euro Surveill. 2020 doi: 10.2807/1560-7917.es.2020.25.9.2000178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Grasselli G, et al. Baseline characteristics and outcomes of 1591 patients infected with SARS-CoV-2 admitted to ICUs of the Lombardy region, Italy. JAMA. 2020;323:1574–1581. doi: 10.1001/jama.2020.5394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lippi G, Mattiuzzi C, Sanchis-Gomar F, Henry BM. Clinical and demographic characteristics of patients dying from COVID-19 in Italy vs China. J. Med. Virol. 2020 doi: 10.1002/jmv.25860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Feng C, Zhisheng L, Furong Z, et al. China's first critically ill child novel coronavirus. Chin. J. Pediatr. 2020;58:1. [Google Scholar]
- 6.National Health Committee. Diagnosis and Treatment Plan for Pneumonia Infected in novel coronavirus (Trial Version 7). 2020.
- 7.Abraham C, Kelly MP, West R, Michie S. The UK National Institute for Health and Clinical Excellence public health guidance on behaviour change: a brief introduction. Psychol. Health Med. 2009;14:1–8. doi: 10.1080/13548500802537903. [DOI] [PubMed] [Google Scholar]
- 8.Disease C. in Children - United States, February 12-April 2, 2020. MMWR Morb. Mortal. Wkly. Rep. 2019;69(422–426):2020. doi: 10.15585/mmwr.mm6914e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lu X, et al. SARS-CoV-2 infection in children. N. Engl. J. Med. 2020;382:1663–1665. doi: 10.1056/NEJMc2005073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang D, et al. Clinical analysis of 31 cases with novel Coronavirus 2019 infection in 6 provinces (autonomous regions) in North China. Chin. J. Pediatr. 2020;58(04):269–274. doi: 10.3760/cma.j.cn112140-20200225-00138. [DOI] [PubMed] [Google Scholar]
- 11.Steinberger S, et al. CT features of coronavirus disease (COVID-19) in 30 pediatric patients. AJR Am. J. Roentgenol. 2020 doi: 10.2214/ajr.20.23145. [DOI] [PubMed] [Google Scholar]
- 12.Xia W, et al. Clinical and CT features in pediatric patients with COVID-19 infection: different points from adults. Pediatr. Pulmonol. 2020;55:1169–1174. doi: 10.1002/ppul.24718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Qiu H, et al. Clinical and epidemiological features of 36 children with coronavirus disease 2019 (COVID-19) in Zhejiang, China: an observational cohort study. Lancet Infect. Dis. 2020;20:689–696. doi: 10.1016/s1473-3099(20)30198-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shen Q, et al. Novel coronavirus infection in children outside of Wuhan, China. Pediatr. Pulmonol. 2020;55:1424–1429. doi: 10.1002/ppul.24762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Han YN, et al. A comparative-descriptive analysis of clinical characteristics in 2019-coronavirus-infected children and adults. J. Med. Virol. 2020 doi: 10.1002/jmv.25835. [DOI] [PubMed] [Google Scholar]
- 16.Zhang L, Huang S. Clinical features of 33 cases in children infected with SARS-CoV-2 in Anhui Province, China: a multi-center retrospective cohort study. Front. Public Health. 2020;8:255. doi: 10.3389/fpubh.2020.00255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lin L, et al. Gastrointestinal symptoms of 95 cases with SARS-CoV-2 infection. Gut. 2020;69:997–1001. doi: 10.1136/gutjnl-2020-321013. [DOI] [PubMed] [Google Scholar]
- 18.Sun D, et al. Clinical features of severe pediatric patients with coronavirus disease 2019 in Wuhan: a single center's observational study. World J. Pediatr. 2020;16:251–259. doi: 10.1007/s12519-020-00354-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Xu Y, et al. Characteristics of pediatric SARS-CoV-2 infection and potential evidence for persistent fecal viral shedding. Nat. Med. 2020;26:502–505. doi: 10.1038/s41591-020-0817-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tagarro A, et al. Screening and severity of coronavirus disease 2019 (COVID-19) in children in Madrid, Spain. JAMA Pediatr. 2020 doi: 10.1001/jamapediatrics.2020.1346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wu HP, et al. Clinical analysis of 23 cases of COVID-19 in children under 18 years of age in Jiangxi. Chin. J. Contemp. Pediatr. 2020;22(05):419–424. doi: 10.7499/j.issn.1008-8830.2003202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Park JY, Han MS, Park KU, Kim JY, Choi EH. First pediatric case of coronavirus disease 2019 in Korea. J. Korean Med. Sci. 2020;35:e124. doi: 10.3346/jkms.2020.35.e124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cui Y, et al. A 55-day-old female infant infected with 2019 novel coronavirus disease: presenting with pneumonia, liver injury, and heart damage. J. Infect. Dis. 2020;221:1775–1781. doi: 10.1093/infdis/jiaa113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhang YH, et al. A case of novel coronavirus infection in a three-month-old baby. Chin. J. Pediatr. 2020;03:182–184. doi: 10.3760/cma.j.issn.0578-1310.2020.03.004. [DOI] [PubMed] [Google Scholar]
- 25.Cai J, et al. A case series of children with 2019 novel coronavirus infection: clinical and epidemiological features. Clin. Infect. Dis. 2020 doi: 10.1093/cid/ciaa198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zeng LK, et al. The first neonatal COVID-19 in China. Chin. J. Pediatr. 2020;58(04):279–280. doi: 10.3760/cma.j.cn112140-20200212-00081. [DOI] [PubMed] [Google Scholar]
- 27.Zeng L, et al. Neonatal early-onset infection with SARS-CoV-2 in 33 neonates born to mothers with COVID-19 in Wuhan, China. JAMA Pediatr. 2020;174:722–725. doi: 10.1001/jamapediatrics.2020.0878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sun S, et al. Analysis of clinical characteristics of 150 cases of novel coronavirus infection in Nanyang City, Henan Province. Chin. J. Tuberc. Respir. Med. 2020;06:503–508. doi: 10.3760/cma.j.cn112147-20200224-00168. [DOI] [PubMed] [Google Scholar]
- 29.Xing Y, et al. Dynamics of faecal SARS-CoV-2 in infected children during the convalescent phase. J. Infect. 2020;81:318–356. doi: 10.1016/j.jinf.2020.03.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Xiong, J. X., et al. Children COVID: 19 patients with CT performance [J/OL]. Chongqing Medical, 1–4 [2020–08–25].
- 31.Brisca G, et al. The early experiences of a single tertiary Italian emergency department treating COVID-19 in children. Acta Paediatr. 2020 doi: 10.1111/apa.15451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fakiri, K. E., Nassih, H., Sab, I. A., Draiss, G. & Bouskraoui, M. Epidemiology and clinical features of coronavirus disease 2019 in Moroccan children. Indian Pediatr. (2020). [DOI] [PMC free article] [PubMed]
- 33.Gaborieau L, et al. Epidemiology and clinical presentation of children hospitalized with SARS-CoV-2 infection in suburbs of Paris. J. Clin. Med. 2020 doi: 10.3390/jcm9072227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hua CZ, et al. Epidemiological features and viral shedding in children with SARS-CoV-2 infection. J. Med. Virol. 2020 doi: 10.1002/jmv.26180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.de Ceano-Vivas M, et al. SARS-CoV-2 infection in ambulatory and hospitalised Spanish children. Arch. Dis. Child. 2020;105:808–809. doi: 10.1136/archdischild-2020-319366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Parri N, et al. Characteristic of COVID-19 infection in pediatric patients: early findings from two Italian Pediatric Research Networks. Eur. J. Pediatr. 2020;179:1315–1323. doi: 10.1007/s00431-020-03683-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bai K, et al. Clinical analysis of 25 COVID-19 INFECTIONS in children. Pediatr. Infect. Dis. J. 2020;39:e100–e103. doi: 10.1097/inf.0000000000002740. [DOI] [PubMed] [Google Scholar]
- 38.De Ioris MA, et al. Dynamic viral severe acute respiratory syndrome coronavirus 2 RNA shedding in children: preliminary data and clinical consideration from a Italian regional center. J. Pediatr. Infect. Dis. Soc. 2020;9:366–369. doi: 10.1093/jpids/piaa065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhang C, et al. Clinical and epidemiological characteristics of pediatric SARS-CoV-2 infections in China: a multicenter case series. PLoS Med. 2020;17:e1003130. doi: 10.1371/journal.pmed.1003130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Otto WR, et al. The epidemiology of SARS-CoV-2 in a pediatric healthcare network in the United States. J. Pediatr. Infect. Dis. Soc. 2020 doi: 10.1093/jpids/piaa074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wu Q, et al. Coinfection and other clinical characteristics of COVID-19 in children. Pediatrics. 2020 doi: 10.1542/peds.2020-0961. [DOI] [PubMed] [Google Scholar]
- 42.Zheng G, et al. Clinical characteristics of acute respiratory syndrome with SARS-CoV-2 infection in children in South China. Pediatr. Pulmonol. 2020 doi: 10.1002/ppul.24921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Korkmaz MF, Türe E, Dorum BA, Kılıç ZB. The epidemiological and clinical characteristics of 81 children with COVID-19 in a pandemic hospital in Turkey: an observational cohort study. J. Korean Med. Sci. 2020;35:e236. doi: 10.3346/jkms.2020.35.e236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zachariah P, et al. Epidemiology, clinical features, and disease severity in patients with coronavirus disease 2019 (COVID-19) in a children's hospital in New York City, New York. JAMA Pediatr. 2020 doi: 10.1001/jamapediatrics.2020.2430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mannheim J, Gretsch S, Layden JE, Fricchione MJ. Characteristics of hospitalized pediatric COVID-19 Cases: Chicago, Illinois, March–April 2020. J. Pediatr. Infect. Dis. Soc. 2020 doi: 10.1093/jpids/piaa070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Armann JP, et al. Hospital admission in children and adolescents with COVID-19. Dtsch. Arztebl. Int. 2020;117:373–374. doi: 10.3238/arztebl.2020.0373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Soltani J, et al. Pediatric coronavirus disease 2019 (COVID-19): an insight from west of Iran. North Clin. Istanb. 2020;7:284–291. doi: 10.14744/nci.2020.90277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pathak EB, Salemi JL, Sobers N, Menard J, Hambleton IR. COVID-19 in children in the United States: intensive care admissions, estimated total infected, and projected numbers of severe pediatric cases in 2020. J. Public Health Manag. Pract. 2020;26:325–333. doi: 10.1097/phh.0000000000001190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Baù M, et al. Risk and protective factors for gastrointestinal symptoms associated with antibiotic treatment in children: a population study. Pediatr. Gastroenterol. Hepatol. Nutr. 2020;23:35–48. doi: 10.5223/pghn.2020.23.1.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cheung KS, et al. Gastrointestinal manifestations of SARS-CoV-2 infection and virus load in fecal samples from a Hong Kong cohort: systematic review and meta-analysis. Gastroenterology. 2020;159:81–95. doi: 10.1053/j.gastro.2020.03.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zhang X, Li S, Niu S. ACE2 and COVID-19 and the resulting ARDS. Postgrad. Med. J. 2020;96:403–407. doi: 10.1136/postgradmedj-2020-137935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Liang W, et al. Diarrhoea may be underestimated: a missing link in 2019 novel coronavirus. Gut. 2020;69:1141–1143. doi: 10.1136/gutjnl-2020-320832. [DOI] [PubMed] [Google Scholar]
- 53.Zhou J, et al. Human intestinal tract serves as an alternative infection route for Middle East respiratory syndrome coronavirus. Sci. Adv. 2017;3:4966. doi: 10.1126/sciadv.aao4966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Xiao F, et al. Evidence for Gastrointestinal Infection of SARS-CoV-2. Gastroenterology. 2020;158:1831–1833.e1833. doi: 10.1053/j.gastro.2020.02.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Dong Y, et al. Epidemiology of COVID-19 among children in China. Pediatrics. 2020 doi: 10.1542/peds.2020-0702. [DOI] [PubMed] [Google Scholar]
- 56.Sutton D, Fuchs K, D'Alton M, Goffman D. Universal screening for SARS-CoV-2 in women admitted for delivery. N. Engl. J. Med. 2020;382:2163–2164. doi: 10.1056/NEJMc2009316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Nobel YR, et al. Gastrointestinal symptoms and coronavirus disease 2019: a case-control study from the United States. Gastroenterology. 2020;159:373–375.e372. doi: 10.1053/j.gastro.2020.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Gu J, Han B, Wang J. COVID-19: gastrointestinal manifestations and potential fecal-oral transmission. Gastroenterology. 2020;158:1518–1519. doi: 10.1053/j.gastro.2020.02.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Duan K, et al. Effectiveness of convalescent plasma therapy in severe COVID-19 patients. Proc. Natl. Acad. Sci. USA. 2020;117:9490–9496. doi: 10.1073/pnas.2004168117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lester M, Sahin A, Pasyar A. The use of dexamethasone in the treatment of COVID-19. Ann. Med. Surg. 2020;56:218–219. doi: 10.1016/j.amsu.2020.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.