Abstract
Climate change has already altered the environmental conditions of the world’s oceans. Here we report declines in gastropod abundances and recruitment of mussels (Mytilus edulis) and barnacles (Semibalanus balanoides) over the last two decades that are correlated with changes in temperature and ocean conditions. Mussel recruitment is declining by 15.7% per year, barnacle recruitment by 5.0% per year, and abundances of three common gastropods are declining by an average of 3.1% per year (Testudinalia testudinalis, Littorina littorea, and Nucella lapillus). The declines in mussels and the common periwinkle (L. littorea) are correlated with warming sea temperatures and the declines in T. testudinalis and N. lapillus are correlated with aragonite saturation state, which affects rates of shell calcification. These species are common on shores throughout the North Atlantic and their loss is likely to lead to simplification of an important food web on rocky shores.
Subject terms: Climate-change ecology, Zoology
Petraitis and Dudgeon report a decline in mussel and gastropod abundance and recruitment on rocky intertidal shores of the Gulf of Maine over a period of 20 years. These declines are correlated with increased water temperatures and aragonite saturation state, which affects rates of shell calcification, and suggests environmental impacts on the food webs of rocky shores.
Introduction
Predictions about how climate change will affect marine ecosystems have been largely based on extrapolations from short-term experiments of physiological changes or retrospective surveys1–5. While the effects of climate change on the oceans are predicted to lead to declines in consumer biomass at mid and higher trophic levels and simplification of food webs6–8, there are few long-term, longitudinal surveys of populations in the wild to support these predictions9,10. Here, we provide evidence for decadal declines of five common benthic species (the tortoiseshell limpet, Testudinalia testudinalis, the common periwinkle, Littorina littorea, the dogwhelk, Nucella lapillus, the blue mussel, Mytilus edulis, and the barnacle, Semibalanus balanoides) on rocky shores of the NW Atlantic Ocean, and link these declines to changes in oceanic conditions in the Gulf of Maine due to climate change. Because of climate change, the Gulf of Maine has recently warmed faster than 99.9% of the global oceans11 and has a relatively low pH and aragonite saturation state (ΩAR), rendering the Gulf of Maine poorly buffered against ocean acidification12. Changes in water temperature, pH, and ΩAR affect the development, growth, calcification, and survivorship of marine organisms1,3, and in particular, changes in ocean pH and ΩAR can slow the calcification of molluscan shells4. Our observations are one of the few continuous long-term studies to document declines in situ and to link those declines to climate change. The declines are surprising in that the five species have been intensively studied for more than 50 years.
These species are key members of intertidal ecosystems throughout the North Atlantic, and their declines will have profound effects on intertidal food webs. Mussels and barnacles are dominant competitors for space on hard surfaces and major prey items for many invertebrate and vertebrate predators. Dogwhelks, periwinkles, and limpets are not only important consumers but also important prey. These species were the basis of one of the first diagrams of a food web13,14, and have been used as model organisms in ground-breaking ecological experiments on competition, predation, and community structure15–18. We hypothesize the declines will cause structural shifts in the food web by enhancing primary producers (i.e., seaweeds and microalgae) and depressing the importance of invertebrate consumers. Continued losses of mid-trophic level consumers will likely lead to simplification of this iconic food web with profound implications for secondary production and stability of rocky shore ecosystems throughout the North Atlantic6,19,20.
Results
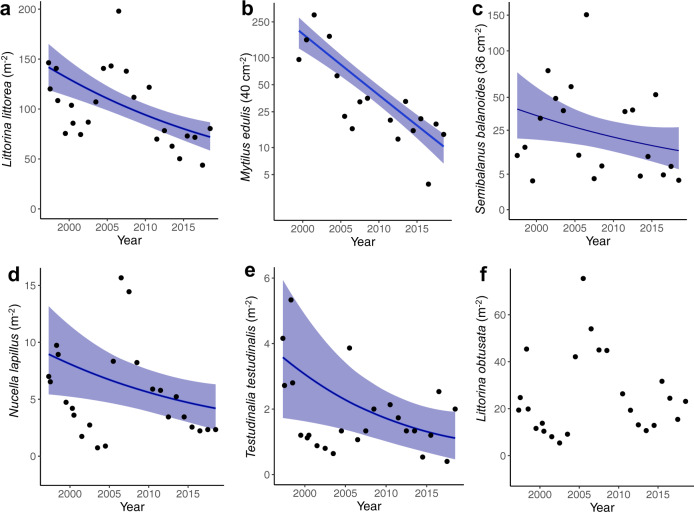
Our observations come from a larger long-term experiment for which we have been sampling the abundances of gastropods and recruitment by barnacles and mussels since 1997. The data are from 12 control plots that are distributed across four bays on Swans Island, Maine, USA. The control plots have not been manipulated, are in their natural state, and any pair are >0.5 km apart. From 1997 to 2018, recruitment of mussels (M. edulis) and barnacles (S. balanoides), and abundances of periwinkles (L. littorea), limpets (T. testudinalis), and dogwhelks (N. lapillus) declined (Fig. 1 and Supplementary Tables 1 and 2). Bayesian estimates of per year rates of decline, ranked from fastest to slowest, are: 15.7% for mussel recruitment (95% credible interval: 13.3–18.2%), 6.0% for limpet abundance (1.9–9.5%), 5.0% for barnacle recruitment (0.1–9.7%), 3.8% for dogwhelk abundance (2.0–6.5%), and 3.2% for periwinkle abundance (2.2–4.3%). Changes in abundance of smooth periwinkle (L. obtusata) are indistinguishable from zero (an average annual increase of 1.0%, CI: −0.9 to +2.7%).
Fig. 1. Changes in snail abundances and recruitment of mussels and barnacles versus year.
Curves are Bayesian estimates of Poisson regressions, and shaded regions are 95% credible intervals (Supplementary Table 1). a Mean abundance of the common perwinkle (L. littorea). b Mean recruitment of mussels (M. edulis); data plotted on a log10 scale. c Mean recruitment of barnacles (S. balanoides); data plotted on a square-root scale. d Mean abundance of the common dogwhelk (N. lapillus). e Mean abundance of the common tortoiseshell limpet (T. testudinalis). f Mean abundance of the smooth periwinkle (L. obtusata). The estimate of slope for L. obtusata is indistinguishable from zero, and so data are plotted without a curve or shaded region.
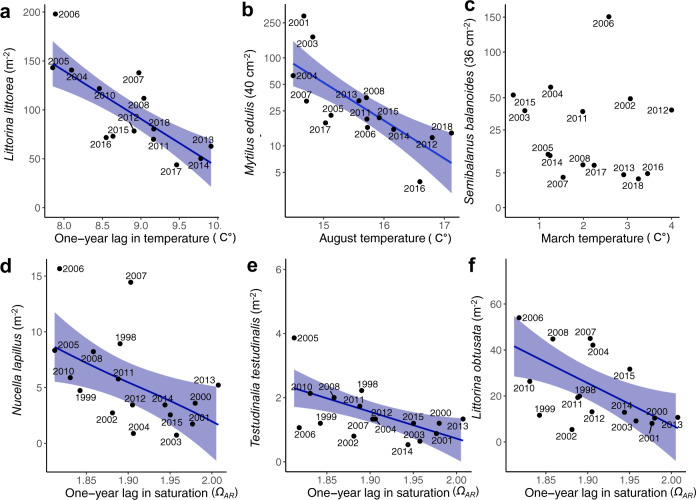
Declines in mussels and the common periwinkle, both of which have planktonic larvae, are correlated with warmer oceanic temperatures in the Gulf of Maine (Supplementary Tables 3–5 and Fig. 2). The decline in mussel recruitment is best explained by water temperature in August, which is when mussel larvae are present in the water column. August water temperature has increased at a rate of 0.085 °C year−1 (95% credible interval: 0.022–0.148; Supplementary Table 6 and Supplementary Fig. 1). For every half-degree increase in temperature, which occurs approximately every 6 years, there is approximately a tenfold decline in mussel recruitment (Fig. 2b; P < 0.0002). The decline in the common periwinkle (L. littorea) is best explained by a 1-year lag in the annual average water temperature (Fig. 2a; P < 0.0001). Note that we usually sampled abundances in July of each year, and for annual average water temperature, we used the average of monthly mean water temperatures from June of the preceding year to July of the current year. We saw a loss of 50 individuals per m2 for each 1 °C increase. The 1-year lag suggests vulnerability in the planktonic stage takes at least 1 year to be evident as a decline in adult populations. The annual mean water temperature increased at a rate of 0.069 °C year−1 (95% credible interval: 0.005–0.128; Supplementary Fig. 1 and Supplementary Table 6), and a one-degree increase would take 14.5 years. In contrast, a decline in the barnacle S. balanoides, which also has a long planktonic phase, could not be linked to changes in water temperature, pH or ΩAR (Fig. 2c and Supplementary Table 5).
Fig. 2. Changes in snail abundances and recruitment of mussels and barnacles versus environmental conditions.
Curves are based on pooled estimates from ten iterations of the imputation of missing values, and shaded regions are the 95% confidence limits (Supplementary Table 3). a Mean abundance of the common perwinkle (L. littorea) versus a 1-year lag in mean annual ocean temperatures. b Mean recruitment of mussels (M. edulis) versus mean August ocean temperatures; data plotted on a log10 scale. c Mean recruitment of barnacles (S. balanoides) versus mean March ocean temperatures; data plotted on a square-root scale. The intercept-only model is best supported (Supplementary Table 5), and so data are plotted without a curve or shaded region. d Mean abundance of the common dogwhelk (N. lapillus) versus a 1-year lag in mean annual saturation ratios. e Mean abundance of the common tortoiseshell limpet (T. testudinalis) versus a 1-year lag in mean annual saturation ratios. f Mean abundance of the smooth periwinkle (L. obtusata) versus a 1-year lag in mean annual saturation ratios.
A 1-year lag in annual ΩAR is the best predictor of the changes in the remaining gastropod species (Fig. 2d–f). Abundances of the limpet T. testudinalis, the dogwhelk N. lapillus and the smooth periwinkle L. obtusata decline with larger values of ΩAR. Gastropods show lower rates of shell calcification at lower ΩAR values2–4, yet we observed larger abundances occurring at smaller values for ΩAR. Notably, ΩAR in the Gulf of Maine covaries with not only acidification, warming, and salinity but also local nearshore conditions21, and so we hypothesize ΩAR may be a proxy for the combined and adverse effects of acidification, warming, and salinity.
Two additional observations suggest local, small-scale characteristics, such as the aspect of the shore and tidal flow within specific bays, also play a role. First is a large amount of spatial variability. Between 20 and 50% of the total variance in abundances and recruitment is due to variation among sites (Supplementary Table 7). Second, the residuals from the predicted declines are correlated (Supplementary Table 8). Four of the six pairwise correlations of abundances among the gastropods are significant and range from +0.154 to +0.418 (P < 0.01 in all cases). There are also clear peaks in 2006 in abundances of N. lapillus, L. littorea, and L. obtusata, and in barnacle recruitment (Fig. 2a, c, d, f, respectively). These are more likely due to mesoscale processes rather than localized conditions, but we have no evidence for a potential driver.
Discussion
The rates of declines we have observed are sobering. Over the last two decades, abundances of limpets, periwinkles, and dogwhelks have declined by at least 50%, and we predict over the next 10–20 years, there will be an additional 50% reduction in (Fig. 1 and Supplementary Table 2). More alarming is the loss of mussel beds, which we think has been driven by nearly a 16% decline in recruitment per year.
Southward’s data on the abundance of S. balanoides and other barnacle species from 1951 to 1990 are the only other comparable data from the north Atlantic Ocean22. While this remarkable set of data has been expanded by other researchers and used in a number of studies, none report a decline in barnacle recruitment or abundances5,23–25. We suspect the one reason why we have seen such dramatic declines not seen by Southward and his co-workers is the environmental shifts in the Gulf of Maine due to climate change have been more rapid and extreme than elsewhere11,12,21.
Our results suggest the effects of climate change on molluscs depend on larval life history. The species with pelagic larvae (mussels and the common periwinkle) are affected by temperature, while species with larvae that are not planktonic (dogwhelks and the smooth periwinkle), or that have a very short planktonic stage (the tortoiseshell limpet), are affected by a combination of factors for which ΩAR may serve as a proxy (e.g., acidification, warming, increased salinity). For molluscs with pelagic larvae, warmer oceanic waters affect metabolic rates, development times, and the composition of the plankton, on which larvae depend on for food3. For dogwhelks, smooth periwinkles, and tortoiseshell limpets, it is quite likely reproductive success and abundance depend on conditions in the local intertidal habitat, as it is well known that changes in acidification, temperature, and salinity can be quite extreme in nearshore environments4.
In contrast, the decline in barnacle recruitment could not be linked to oceanic conditions, and the intercept-only model was the best-supported model (Supplementary Table 5). Lab studies have shown a combination of warming and lower pH can slow the embryonic development of S. balanoides26, but we were unable to detect a relationship with either pH or sea temperature in situ. It is notable that barnacles, which are crustaceans and unlike molluscs, do not begin calcification as pelagic larvae. The decline in barnacle recruitment, therefore, may be less affected by changes in pH and ΩAR. In addition, the lack of a relationship between recruitment and ocean temperature may be due to the timing of when adult barnacles release larvae into the plankton. In the Gulf of Maine, larvae are usually released in early spring and are linked to the timing of the spring phytoplankton bloom27. Moreover, our estimate of the rate of change in ocean temperature in March shows a warming trend, although the credible interval is quite large and encompasses estimates of cooling trends (mean rate of 0.062 °C year−1; CI: −0.048–0.179 °C year−1; see Supplementary Table 6).
The relationships between warming ocean temperatures and declines in species with pelagic larvae should not be surprising given how quickly temperatures are rising in the Gulf of Maine. A modeling effort using sea surface temperature (SST) data throughout the Gulf of Maine shows an increase of 0.03 °C year−1 between 1982 and 2003 and then a dramatic jump to a rate of 0.23 °C year−1 starting in 200411. These rates are among the most rapid increases seen worldwide. Even so, our estimates of the rate of increase in ocean temperature are even larger (Supplementary Table 6). Our estimate, based on data from an offshore buoy close to our sites, is 0.069 °C year−1 (CI: 0.005–0.128 °C year−1) from 2002 to 2018. However, we do not see a jump in the rate when we use only the data from 2004 to 2014. Our estimate for this decade is 0.126 °C year−1, and the credible interval includes not only the published estimate of 0.23 °C year−1 but also cooling of ocean temperatures (CI: −0.009–0.249 °C year−1).
The Gulf of Maine has also seen declines in pH and aragonite saturation state (ΩAR) in the Gulf of Maine; from 1981 to 2014, and the rates of decline for pH and ΩAR have been 0.0018 year−1 and 0.049 year−1, respectively12,21. However, our observations of the links between the changes in water chemistry and declines in species abundances should be viewed with caution for several reasons. The values for pH and ΩAR, which were extracted from the literature, are average yearly estimates across the entire Gulf of Maine. In the Gulf of Maine, values for pH and, in particular, ΩAR, show large annual and decadal variation, and covary with changes in salinity, total alkalinity, and water temperature21. Moreover, freshwater inflows from rivers also affect pH, salinity, and total alkalinity. Even so, our findings raise interesting questions and suggest declines in species abundances, and recruitment may vary from site to site because of very localized differences in water chemistry.
The declines in mussel and barnacle recruitment are likely to have major impacts because both are important prey and foundation species of rocky intertidal ecosystems throughout the western North Atlantic Ocean15,17. Both species can occupy broad swathes of the shore, and their losses as common prey items will affect consumers. Mussels have already disappeared from many intertidal shores throughout the North Atlantic20,28; a loss that has been widely attributed to the recent rise in the predatory green crab, Carcinus maenas29,30. Yet, our results suggest recruitment failure associated with processes in the water column is also playing an important role in the decline of mussels throughout the North Atlantic.
The loss of gastropods, which are important consumers, will affect a number of trophic interactions and likely lead to a simplification of the food web19. The dogwhelk preys upon barnacles and mussels, and so the loss of barnacles and mussels may hasten the decline of dogwhelks. Herbivorous gastropods (periwinkles and limpets) strongly affect the distribution of macroalgae, especially ephemeral species16,17,31. Unlike what has been predicted for the shores of Great Britain and western Europe, it is unlikely that southern species will expand their ranges northward to fill the gaps10,32. Recolonization of rocky intertidal shores in the Gulf of Maine after the last glacial retreat was by European species rather than range expansions of southern species33,34. Moreover, most of the shoreline south of Cape Cod, MA, does not include rocky shores and so there is simply not the species pool to support an expansion from the south. One interesting possibility is that the loss of the common periwinkle L. littorea may return the food web to its state prior to 1860. The common periwinkle, which is now the most common gastropod herbivore from Nova Scotia to Cape Cod, MA, was not found in the Gulf of Maine prior to 186035.
There may very well be other unanticipated impacts. For example, there may be profound effects not only on the flow of carbon through food webs on rocky intertidal shores throughout the North Atlantic but also the export of carbon to offshore ecosystems. The loss of filter feeders (mussels and barnacles) will certainly break the benthic–pelagic coupling between phytoplankton and fecal input into the benthos36,37. Moreover, while increases in dissolved pCO2 and water temperatures due to climate change will fuel increases in primary production38, the loss of consumers may reduce the export of carbon as the links among phytoplankton, filter feeders, and consumer are broken. This is only one of many potential effects, and we anticipate there will be many more surprises in how nearshore ecosystems will respond to the combined effects of climate change and the loss of species.
Methods
Description of study plots, data collection, and data availability
Data were collected from 12 unmanipulated control plots on Swans Island (44°10’ N, 68°25’ W), a small island (approx. 36 km2 in area) in the Gulf of Maine, USA. The control plots are part of a larger, long-term study started in 1996, and each control plot is located at one of the 12 sites on Swans Island. Descriptions of the study sites, the location of each plot, methods of data collection and experimental design, and data for recruitment from 1997 to 2012 and for abundances from 1996 to 2008 are available elsewhere18,39–41. The 12 sites are spread over four different bays on Swans Island, and most are more than 1 km apart. The two closest sites are slightly <1 km apart. All plots are in the mid intertidal zone, which is dominated by stands of the fucoid rockweed, Ascophyllum nodosum interspersed with small patches of the rockweed Fucus vesiculosus. The species composition of invertebrates and algae at these sites is typical of sheltered rocky shores in the north Atlantic42.
Recruitment data for Semibalanus balaniodes and Mytilus edulis have been collected annually since 1997. Abundances of the four most common gastropods (Testudinalia testudinalis, Littorina littorea, Littorina obtusata, and Nucella lapillus) have been sampled at least once a year since 1996. Note that until 2010, Testudinalia testudinalis was previously named Tectura testudinalis43.
Environmental data for pH, aragonite saturation ratio (ΩAR), and temperature were extracted from published papers and online databases. Yearly averages in pH and ΩAR from 1997 to 2014 were taken from published figures21. Monthly average temperatures at 1 m depth and from 2002 to 2018 were downloaded from NERACOOS for the three buoys that are the closest to Swans Island (http://neracoos.org/datatools/climatologies_display). The buoys are E01 (Central Maine Shelf), F01 (Penobscot Bay), and I01 (Eastern Maine Shelf). Averages among the buoys were highly correlated, and all three pairwise correlations were greater than 0.942. Buoy F01 was missing the fewest observations (April 2007, May 2007, and October 2008), and so these data were used for analysis. Monthly and yearly averages for recruitment, abundances, and environmental data are given in Supplementary Data 1; raw data and R script for analyses are available online44.
Regressions of changes in recruitment and abundance over time
Regressions were done assuming Poisson error distribution using the R package MCMCglmm45. The package fits generalized linear mixed models using Markov chain Monte Carlo techniques. The date of sampling is a fixed factor, and the 12 sites are treated as a random effect. We set the thinning rate to 100, the burn-in number to 6000, and the total number of iterations to 206,000. This gives an effective sampling of ~2000 for all parameters in most regressions. We used default priors. Sampling was done annually, except in 1997, 1998, and 2000 when sites were sampled in spring and summer. Spring samples were coded as occurring in April (e.g., 1997.3), and summer samples were coded as occurring mid-year (e.g., 1997.5). Data for all species are converted to standardized units and treated as integers (number per m2 for gastropods, per 36 cm2 for barnacles, and per 40 cm2 for mussels). Units are based on the area or surface used to sample each species40,41; for example, the tile for sampling barnacle recruitment is circular with an area of 39.6 cm2.
In three cases, data were transformed or dropped because models using the full dataset failed to converge. Data for barnacle (S. balanoides) recruitment were square-root transformed. For mussel recruitment (M. edulis), 1997 data were dropped because of extremely large values (average 1161 per 40 cm2, range: 32–5424). For limpet abundance (T. testudinalis), data from two sites were dropped because limpets were absent or very rare from 2000 to 2018, and the datum for one site in 1998 for which abundance was >10 per m2.
Regressions of changes in water temperature over time
Data for the regressions were the monthly average temperatures from Buoy F01 at 1 m depth and from 2002 to 2018. Regressions were done using yearly maximum, minimum, and median water temperatures, which usually occurred in August, February, and May or November, respectively. Regressions were done assuming Gaussian error distribution using the R package MCMCglmm45. Month and year were entered as a continuous number (e.g., February 2002 was coded as 2002.125). The thinning rate was set to 100, the burn-in number to 6000, and the total number of iterations to 206,000. This gives an effective sampling of 2000. We used default priors.
Regressions of changes in recruitment and abundance versus environmental conditions
The relationships between environmental variables and the abundances of snails and the recruitment of mussels and barnacles were assessed in two steps. First, corrected Akaike information criteria (AICc), deltas, and Akaike weights were used to assess the best model containing one or two environmental variables46. Entries in Supplementary Data 1 were used as the data, and years (i.e., rows) with missing values were dropped. The dredge function in MuMIn package was used to analyze all possible models47. The best-supported models are shown in Supplementary Tables 4 and 5.
The second step involved imputing the missing values and using the imputed values to compile pooled estimates of the best-supported model. Pooled estimates were based on ten iterations of imputation. Missing values were imputed using the R package Amelia48, and the pooled estimates were calculated using the R package Zelig49,50. Pooled estimates are given in Supplementary Table 3 and were used to make Fig. 2. Note that estimates in Supplementary Table 3 differ from estimates in Supplementary Tables 4 and 5.
Because sampling of abundances and recruitment were done at different times of the year, different subsets of the environmental data were used for the dredge function. Snail abundances were usually sampled between early July and early August, and thus for water temperature, we used the yearly average based on the previous year (e.g., for densities sampled in July 2001,) we used the yearly average based on data from July 2000 to June 2001. We used the current year averages for pH and saturation ratio. We also expected changes in densities might lag behind changes in the environment, and so included lags of 1 and 2 years. For the recruitment data, we used monthly averages from the current year for temperature and the current year data for pH and saturation ratios. Recruitment surfaces for barnacles were usually put in the water in late March-early April and picked up in late May; the dredge model included monthly temperature averages for February, March, April, and May. Recruitment pads for mussels were placed out in late May and picked up in late August-early September; the dredge model included monthly temperature data for May, June, July, and August.
Statistics and reproducibility
A sampling of abundances and recruitment was done 24 times between 1997 and 2018 at 12 sites for a possible sample size of 288 observations per species. Sample sizes for abundances of all gastropods except limpets are 288 observations per species. There were 49 outliers in the limpet data (N = 239 observations); at two sites were individuals were extremely rare (usually zero counts), and for one site in 1998, the mean count was >10. There are missing data for the recruitment of barnacles (N = 245) and mussels (N = 211). Monthly means for ocean temperature data from August 2001 to December 2018 are from NERACOOS buoy F01; there are three missing means (N = 206 out of a possible total of 209). Yearly means for pH and aragonite saturation ratio (ΩAR) from 1997 to 2014 are from the literature21 (N = 18 for both variables). Poisson regressions of abundances and recruitment versus time were done Markov chain Monte Carlo techniques for generalized linear mixed models with time as a continuous variable and sites as a random factor45. The best environmental predictors (temperature, pH, and ΩAR) of changes in abundances and recruitment using multimodel inference46,47, and missing values were imputed48,49. See “Methods” section for more details about the data and statistical analyses.
Reporting summary
Further information on research design is available in the Nature Research Reporting Summary linked to this article.
Supplementary information
Description of Additional Supplementary Files
Acknowledgements
We thank Ms. Erika Rhile for her assistance with the collection and entry of the data, and Professors Maria Byrne and Roberto Salguero-Gómez for reading the paper and providing comments. This material is based upon work supported by the National Science Foundation under Grant Nos. OCE-9529564, DEB-0314980, DEB-1020480, and DEB-1555641.
Author contributions
P.S.P. and S.R.D. developed the hypothesis and collected the data. P.S.P. conducted the statistical analyses. P.S.P. prepared the initial draft, and both authors edited the paper.
Data availability
The data sets generated and analyzed during the current study are archived and available online via the portal for the Environmental Data Initiative44. Three csv data files are available, which are: abundance and recruitment data; monthly means for ocean temperatures from August 2001 to December 2018, and yearly means for pH and aragonite saturation ratio (ΩAR) from 1997 to 2014.
Code availability
R scripts used to analyze data are archived and available online via the portal for the Environmental Data Initiative44. R scripts were run using RStudio and R version 3.6.1 (2019-07-05). R packages used include MCMCglmm, MuMIn, Amelia, and Zelig.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Peter S. Petraitis, Email: ppetrait@sas.upenn.edu
S. R. Dudgeon, Email: steve.dudgeon@csun.edu
Supplementary information
Supplementary information is available for this paper at 10.1038/s42003-020-01326-0.
References
- 1.Kroeker KJ, et al. Impacts of ocean acidification on marine organisms: quantifying sensitivities and interaction with warming. Glob. Change Biol. 2013;19:1884–1896. doi: 10.1111/gcb.12179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pfister, C. A. et al. Historical baselines and the future of shell calcification for a foundation species in a changing ocean. Proc. Royal Soc. B-Biol. Sci.283, 10.1098/rspb.2016.0392 (2016). [DOI] [PMC free article] [PubMed]
- 3.Przeslawski R, Byrne M, Mellin C. A review and meta-analysis of the effects of multiple abiotic stressors on marine embryos and larvae. Glob. Change Biol. 2015;21:2122–2140. doi: 10.1111/gcb.12833. [DOI] [PubMed] [Google Scholar]
- 4.Waldbusser GG, Salisbury JE. Ocean acidification in the coastal zone from an organism’s perspective: multiple system parameters, frequency domains, and habitats. Annu. Rev. Mar. Sci. 2014;6:221–247. doi: 10.1146/annurev-marine-121211-172238. [DOI] [PubMed] [Google Scholar]
- 5.Helmuth B, Mieszkowska N, Moore P, Hawkins SJ. Living on the edge of two changing worlds: forecasting the responses of rocky intertidal ecosystems to climate change. Annu. Rev. Ecol. Evolution Syst. 2006;37:373–404. doi: 10.1146/annurev.ecolsys.37.091305.110149. [DOI] [Google Scholar]
- 6.Kordas, R. L., Donohue, I. & Harley, C. D. G. Herbivory enables marine communities to resist warming. Sci. Adv.3, 10.1126/sciadv.1701349 (2017). [DOI] [PMC free article] [PubMed]
- 7.Kroeker, K. J., Kordas, R. L. & Harley, C. D. G. Embracing interactions in ocean acidification research: confronting multiple stressor scenarios and context dependence. Biol. Lett.13, 10.1098/rsbl.2016.0802 (2017). [DOI] [PMC free article] [PubMed]
- 8.Jellison BM, Gaylord B. Shifts in seawater chemistry disrupt trophic links within a simple shoreline food web. Oecologia. 2019;190:955–967. doi: 10.1007/s00442-019-04459-0. [DOI] [PubMed] [Google Scholar]
- 9.Wootton JT, Pfister CA, Forester JD. Dynamic patterns and ecological impacts of declining ocean pH in a high-resolution multi-year dataset. Proc. Natl Acad. Sci. USA. 2008;105:18848–18853. doi: 10.1073/pnas.0810079105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Burrows MT, et al. Global-scale species distributions predict temperature-related changes in species composition of rocky shore communities in Britain. Glob. Change Biol. 2020;26:2093–2105. doi: 10.1111/gcb.14968. [DOI] [PubMed] [Google Scholar]
- 11.Pershing AJ, et al. Slow adaptation in the face of rapid warming leads to collapse of the Gulf of Maine cod fishery. Science. 2015;350:809–812. doi: 10.1126/science.aac9819. [DOI] [PubMed] [Google Scholar]
- 12.Wang ZHA, et al. The marine inorganic carbon system along the Gulf of Mexico and Atlantic coasts of the United States: insights from a transregional coastal carbon study. Limnol. Oceanogr. 2013;58:325–342. doi: 10.4319/lo.2013.58.1.0325. [DOI] [Google Scholar]
- 13.Colton HS. On some varieties of Thais lapillus in the Mount Desert region, a study of individual ecology. Proc. Acad. Natl Sci. Phila. 1916;68:440–454. [Google Scholar]
- 14.Fisher, J. A. D. Exploring ecology’s attic: overlooked ideas on intertidal food webs. Bull. Ecol. Soc. Am. 86, 145–151 (2005).
- 15.Connell JH. Effects of competition, predation by Thais lapillus, and other factors on natural populations of barnacle Balanus balanoides. Ecol. Monogr. 1961;31:61–104. doi: 10.2307/1950746. [DOI] [Google Scholar]
- 16.Lubchenco J. Plant species diversity in a marine intertidal community—importance of herbivore food preference and algal competitive abilities. Am. Naturalist. 1978;112:23–39. doi: 10.1086/283250. [DOI] [Google Scholar]
- 17.Lubchenco J, Menge BA. Community development and persistence in a low rocky intertidal zone. Ecol. Monogr. 1978;48:67–94. doi: 10.2307/2937360. [DOI] [Google Scholar]
- 18.Petraitis PS, Dudgeon SR. Variation in recruitment and the establishment of alternative community states. Ecology. 2015;96:3186–3196. doi: 10.1890/14-2107.1. [DOI] [PubMed] [Google Scholar]
- 19.Lord JP, Harper EM, Barry JP. Ocean acidification may alter predator-prey relationships and weaken nonlethal interactions between gastropods and crabs. Mar. Ecol. Prog. Ser. 2019;616:83–94. doi: 10.3354/meps12921. [DOI] [Google Scholar]
- 20.Sorte CJB, et al. Long-term declines in an intertidal foundation species parallel shifts in community composition. Glob. Change Biol. 2017;23:341–352. doi: 10.1111/gcb.13425. [DOI] [PubMed] [Google Scholar]
- 21.Salisbury JE, Jönsson BF. Rapid warming and salinity changes in the Gulf of Maine alter surface ocean carbonate parameters and hide ocean acidification. Biogeochemistry. 2018;141:401–418. doi: 10.1007/s10533-018-0505-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Southward AJ. 40 years of changes in species composition and population density of barnacles on a rocky shore near Plymouth. J. Mar. Biol. Assoc. UK. 1991;71:495–513. doi: 10.1017/S002531540005311X. [DOI] [Google Scholar]
- 23.Hawkins SJ, Southward AJ, Genner MJ. Detection of environmental change in a marine ecosystem—evidence from the western English Channel. Sci. Total Environ. 2003;310:245–256. doi: 10.1016/S0048-9697(02)00645-9. [DOI] [PubMed] [Google Scholar]
- 24.Hawkins SJ, et al. Consequences of climate-driven biodiversity changes for ecosystem functioning of North European rocky shores. Mar. Ecol. Prog. Ser. 2009;396:245–259. doi: 10.3354/meps08378. [DOI] [Google Scholar]
- 25.Mieszkowska N, Burrows MT, Pannacciulli FG, Hawkins SJ. Multidecadal signals within co-occurring intertidal barnacles Semibalanus balanoides and Chthamalus spp. linked to the Atlantic Multidecadal Oscillation. J. Mar. Syst. 2014;133:70–76. doi: 10.1016/j.jmarsys.2012.11.008. [DOI] [Google Scholar]
- 26.Findlay HS, Kendall MA, Spicer JI, Widdicombe S. Future high CO2 in the intertidal may compromise adult barnacle Semibalanus balanoides survival and embryonic development rate. Mar. Ecol. Prog. Ser. 2009;389:193–202. doi: 10.3354/meps08141. [DOI] [Google Scholar]
- 27.Thomas AC, Townsend DW, Weatherbee R. Satellite-measured phytoplankton variability in the Gulf of Maine. Continental Shelf Res. 2003;23:971–989. doi: 10.1016/S0278-4343(03)00086-4. [DOI] [Google Scholar]
- 28.Commito, J. A., Jones, B. R., Jones, M. A., Winders, S. E. & Como, S. After the fall: legacy effects of biogenic structure on wind-generated ecosystem processes following mussel bed collapse. Diversity-Basel11, 10.3390/d11010011 (2019).
- 29.Beal BF, et al. Spatial variability in recruitment of an infaunal bivalve: experimental effects of predator exclusion on the softshell clam (Mya arenaria L.) along three tidal estuaries in southern Maine, USA. J. Shellfish Res. 2018;37:27. doi: 10.2983/035.037.0101. [DOI] [Google Scholar]
- 30.Roman J. Diluting the founder effect: cryptic invasions expand a marine invader’s range. Proc. R. Soc. B-Biol. Sci. 2006;273:2453–2459. doi: 10.1098/rspb.2006.3597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vadas, R. L.,Sr. & Elner, R. W. Plant-animal interactions in the north-west Atlantic. In Plant-Animal Interactions in the Marine Benthos (eds. John, D. M., Hawkins, S. J. & Price, J. H.). Systematics Association Special Vol. 46, 33–60 (Oxford: Clarendon Press, 1992).
- 32.Wethey DS, et al. Response of intertidal populations to climate: effects of extreme events versus long term change. J. Exp. Mar. Biol. Ecol. 2011;400:132–144. doi: 10.1016/j.jembe.2011.02.008. [DOI] [Google Scholar]
- 33.Maggs CA, et al. Evaluating signatures of glacial refugia for North Atlantic benthic marine taxa. Ecology. 2008;89:S108–S122. doi: 10.1890/08-0257.1. [DOI] [PubMed] [Google Scholar]
- 34.Wares JP, Cunningham CW. Phylogeography and historical ecology of the North Atlantic intertidal. Evolution. 2001;55:2455–2469. doi: 10.1111/j.0014-3820.2001.tb00760.x. [DOI] [PubMed] [Google Scholar]
- 35.Chapman JW, Carlton JT, Bellinger MR, Blakeslee AMH. Premature refutation of a human-mediated marine species introduction: the case history of the marine snail Littorina littorea in the Northwestern Atlantic. Biol. Invasions. 2007;9:995–1008. doi: 10.1007/s10530-007-9098-9. [DOI] [Google Scholar]
- 36.Cloern JE, Jassby AD. Drivers of changes in estuarine-coast ecosystems: discoveries from four decades of study in San Francisco Bay. Rev. Geophys. 2012;50:33. doi: 10.1029/2012RG000397. [DOI] [Google Scholar]
- 37.Kemp WM, et al. Eutrophication of Chesapeake Bay: historical trends and ecological interactions. Mar. Ecol. Prog. Ser. 2005;303:1–29. doi: 10.3354/meps303001. [DOI] [Google Scholar]
- 38.Kübler, J. E. & Dudgeon, S. R. Prediciting effects of ocean acidification and warming on algae lacking carbon concentrating mechanisms. PLoS ONE10, 10.1371/journal.pone.0132806 (2015). [DOI] [PMC free article] [PubMed]
- 39.Petraitis PS, Liu H, Rhile EC. Densities and cover data for intertidal organisms in the Gulf of Maine, USA, from 2003 to 2007. Ecology. 2008;89:588. doi: 10.1890/07-1325.1. [DOI] [Google Scholar]
- 40.Petraitis PS, Liu H, Rhile EC. Barnacle, fucoid, and mussel recruitment in the Gulf of Maine, USA, from 1997 to 2007. Ecology. 2009;90:571. doi: 10.1890/08-1462.1. [DOI] [Google Scholar]
- 41.Petraitis PS, Vidargas NA. Marine intertidal organisms found in experimental clearings on sheltered shores in the Gulf of Maine, USA. Ecology. 2006;87:796. doi: 10.1890/05-0232a. [DOI] [Google Scholar]
- 42.Lewis, J. R. The Ecology of Rocky Shores (English University Press, 1964).
- 43.Horton, T. et al. World register of marine species (WoRMS). http://www.marinespecies.org (2010).
- 44.Petraitis, P. S. & Dudgeon, S. R. Data and R scripts for analyses of declines in invertebrate species from the Gulf of Maine, USA, 1997–2018 ver 1. Environmental Data Initiative10.6073/pasta/7ad4e0c85ff585199cb2a007eb291c9d (2020).
- 45.Hadfield JD. MCMC methods for multi-response generalized linear mixed models: the MCMCglmm R package. J. Stat. Softw. 2010;33:1–22. doi: 10.18637/jss.v033.i02. [DOI] [Google Scholar]
- 46.Burnham, K. P. & Anderson, D. R. Model Selection and Multi-Model Inference: A Practical Information-Theoretic Approach, Second edn. (Springer, 2002).
- 47.Bartón, K. MuMIn: multi-model inference R package version 1.40.0. https://CRAN.R-project.org/package=MuMIn (2019).
- 48.Honaker J, King G, Blackwell M. Amelia II: a program for missing data. J. Stat. Softw. 2011;45:1–47. doi: 10.18637/jss.v045.i07. [DOI] [Google Scholar]
- 49.Choirat, C., Honaker, J., Imai, K., King, G. & Lau, O. Zelig: everyone’s statistical software. version 5.1.6.1. http://zeligproject.org (2008).
- 50.Imai K, King G, Lau O. Toward a common framework for statistical analysis and development. J. Computational Graph. Stat. 2008;17:892–913. doi: 10.1198/106186008X384898. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Description of Additional Supplementary Files
Data Availability Statement
The data sets generated and analyzed during the current study are archived and available online via the portal for the Environmental Data Initiative44. Three csv data files are available, which are: abundance and recruitment data; monthly means for ocean temperatures from August 2001 to December 2018, and yearly means for pH and aragonite saturation ratio (ΩAR) from 1997 to 2014.
R scripts used to analyze data are archived and available online via the portal for the Environmental Data Initiative44. R scripts were run using RStudio and R version 3.6.1 (2019-07-05). R packages used include MCMCglmm, MuMIn, Amelia, and Zelig.