Abstract
Cryptosporidium spp., Giardia duodenalis, and Blastocystis sp. are common intestinal protozoans that infect humans and animals worldwide. A survey that assessed the prevalence, molecular characteristics, and zoonotic potential of these pathogens was conducted on a variety of dogs in Guangzhou, southern China. A total of 651 canine stool samples from household (n = 199), shelter (n = 149), breeding (n = 237), and pet market dogs (n = 66) were collected from eight districts in Guangzhou. Cryptosporidium spp., Giardia duodenalis, and Blastocystis sp. were detected by PCR amplification of the SSU rRNA gene. Giardia duodenalis-positive specimens were further assigned into assemblages using the glutamate dehydrogenase gene. Cryptosporidium spp., G. duodenalis, and Blastocystis sp. were found in 21 (3.2%), 20 (3.1%), and 35 (5.4%) samples, respectively. The overall prevalence of shelter dogs (40.28%, 60/149) was significantly higher than that of household (3.0%, 6/199), breeding (2.1%, 5/237), and pet market dogs (7.5%, 5/66) (χ2 = 154.72, df = 3, P < 0.001). Deworming in the past 12 months had a strong protective effect on the risk of contracting parasite infections (P < 0.001). No significant differences were detected between age or sex groups (P > 0.05). Dog-specific C. canis (n = 19) and zoonotic C. parvum (n = 2) were the only two Cryptosporidium species. Sequence analysis revealed the presence of three G. duodenalis assemblages: dog-specific assemblages D (n = 14) and C (n = 5), and cat-specific F (n = 1). Zoonotic Blastocystis ST3 (n = 28) was the dominant subtype, followed by ST1 (n = 6) and ST10 (n = 1). To our knowledge, this is the first large-scale investigation on the occurrence and molecular characteristics of Blastocystis sp. in dogs in China. Our results indicated that the dogs seemed to play a negligible role as reservoirs for Cryptosporidium spp. and G. duodenalis transmission to humans, but they are potential novel suitable hosts of Blastocystis sp. A strict sentinel surveillance system of dogs should be established to minimise the zoonotic risk of spreading blastocystosis among humans and dogs.
Subject terms: Genotype, Sequencing, Evolution, Genetics, Health care, Risk factors
Introduction
Cryptosporidium spp., Giardia duodenalis, and Blastocystis sp. are cosmopolitan enteric protists with a wide range of hosts, including humans, non-human primates, companion animals, ruminants, birds, and wild mammals1–3. Although the pathogenicity of Blastocystis sp. is under strong debate, it may also be associated with gastrointestinal disease-causing agents like Cryptosporidium spp. and G. duodenalis, which cause issues such as self-limiting diarrhoea, abdominal pain, irritable bowel syndrome, and flatulence1,3–5. In particular, people with compromised immune systems (e.g. AIDS patients and organ transplant recipients) are susceptible to these infections1,6,7. Infections occur mainly by faecal–oral transmission after ingestion of infective forms (oocysts or cysts), usually via water, food, or direct contact1,3,8. Cryptosporidium parvum, C. hominis, C. meleagridis, C. canis, and C. muris are the five most common human pathogenic species of Cryptosporidium, of which C. canis is the most prevalent species in dogs3,9–11. G. duodenalis consists of eight distinct assemblages or genotypes (A–H). Assemblages A and B have a wide host range and are responsible for the majority of known human disease cases, whereas assemblages C–H seem to be host-specific for non-human species1. Dogs are predominantly infected by assemblages C and D1,12–14. Additionally, at least 17 distinct Blastocystis subtypes were identified. ST1–9 and ST12 are the common zoonotic subtypes, and ST10–11 and ST13–17 only infect non-human species15.
Recently, a few molecular epidemiological surveys of Cryptosporidium spp., G. duodenalis, and Blastocystis sp. in dogs have been conducted worldwide, and numerous species/assemblages/subtypes have been detected, such as C. canis, C. parvum, C. muris, C. meleagridis, C. hominis, G. duodenalis assemblages A–F, and Blastocystis ST1–ST6 and ST1010,14 (Tables 1, 2, 3). However, little information on Blastocystis sp. infection and subtype distribution in dogs in China is available16. Additionally, only one pathogen was involved in most of these studies.
Table 1.
Summary of data from studies of Cryptosporidium spp. in dogs worldwide, 2010–2020.
| Location | Period | Methods | No. examined | No. positive (%) | Populations | Species/assemblages/subtypes (n) | References |
|---|---|---|---|---|---|---|---|
| China | 2015 | PCR | 484 | 24 (4.9) | pet | C. canis (20) | 14 |
| China | 2011–2014 | PCR | 485 | 39 (8.0) | household, pet | C. canis (39) | 11 |
| Nigeria | 2017 | PCR | 203 | 5 (2.5) | free-ranging | C. parvum (3), C. muris (2) | 17 |
| China | 2013–2014 | PCR | 267 | 6 (2.2) | pet, stray | C. canis (5), C. ubiquitum (1) | 18 |
| China | 2017–2018 | PCR | 641 | 44 (6.9) | pet | C. canis (42), C. muris (1), C. rat genotype IV (1) | 19 |
| France | 2012–2013 | PCR | 116 | 3 (2.6) | household | C. canis (3) | 20 |
| Japan | 2011–2012 | PCR | 1096 | 111 (10.1) | household, pet | C. canis (111) | 21 |
| Japan | 2014–2017 | PCR | 314 | 66 (21.0) | breeding kennel | C. canis (66) | 22 |
| Spain | 2013–2016 | DFM, PCR | 194 | 8 (4.1) | sheltered | C. canis (5), C. hominis (1) | 23 |
DFM direct fluorescence microscopy.
Table 2.
Summary of data from studies of G. duodenalis in dogs worldwide, 2010–2020.
| Location | Period | Methods | No. examined | No. positive (%) | Populations | Species/assemblages/subtypes (n) | References |
|---|---|---|---|---|---|---|---|
| China | 2015 | PCR | 484 | 62 (12.8) | Pet | D (15), C (5), F (1) | 14 |
| China | 2010–2011 | PCR | 209 | 23 (11) | Pet | D (18), A (5) | 12 |
| China | 2016–2017 | PCR | 527 | 57 (10.8) | Stray | A (26), C (18), D (13) | 13 |
| China | 2011–2014 | PCR | 485 | 127 (26.2) | Household, pet | A (23), B (1), C (26), D (58) | 11 |
| Spain | 2014–2016 | DFM, qPCR | 348 | 127 (36.5) | Sheltered, breeding, hunting, shepherd, pet | A (5), B (8), C (2), D (13) | 24 |
| China | 2013–2014 | PCR | 267 | 12 (4.5) | Pet and stray | C (7), E (5) | 18 |
| China | 2011 | PCR | 205 | 27 (13.2) | Police and farm | A (25), C ( 2) | 25 |
| China | 2017–2018 | PCR | 641 | 60 (9.4) | Pet | C (27), D (26) | 19 |
| Korea | 2017–2018 | PCR | 640 | 99 (15.5) | Sheltered, companion, special purpose | C (16), D (24) | 26 |
DFM direct fluorescence microscopy.
Table 3.
Summary of data from studies of Blastocystis sp. in dogs worldwide, 2010–2020.
| Location | Period | Methods | No. examined | No. positive (%) | Populations | Species/assemblages/subtypes (n) | References |
|---|---|---|---|---|---|---|---|
| China | 2015–2017 | PCR | 136 | 4 (2.9) | Peand farm | ST1 (3), ST4 (1) | 16 |
| USA | 2012 | PCR | 103 | 10 (9.7) | Sheltered, client-owned | ST1 (2), ST10 (2) | 27 |
| Philippines | 2011–2012 | PCR | 145 | 23 (15.8) | Pet | ST1 (1), ST2 (2), ST3 (4), ST4 (3), ST5 (3), unkown (10) | 28 |
| India | 2010–2011 | PCR | 80 | 19 (24) | Stray | ST1 (9), ST4 (2), ST5 (1), ST6 (7) | 29 |
| Australia | 2010–2011 | PCR | 80 | 2 (2.5) | Pet and pound | ST1 (2) | 29 |
| Cambodia | 2010–2011 | PCR | 80 | 1 (1.3) | Semi-domestic | ST2 (1) | 29 |
| Brazil | 2011–2013 | PCR | 49 | 0 (0) | Pet | – | 30 |
| Brazil | 2018 | PCR | 20 | 0 (0) | Pet | – | 31 |
| Brazil | 2013–2014 | CM | 78 | 2 (2.6) | Domestic | – | 32 |
| Colombia | 2013–2014 | PCR | 40 | 15 (37.5) | ST2 (15) | 33 | |
| Spain | 2014 | PCR | 55 | 0 (0) | – | 34 | |
| Turkey | 2010 | PCR | – | – | – | ST1 (1), ST2 (3) | 35 |
| France | 2012–2013 | PCR | 116 | 4 (3.4) | Household | ST2 (2), ST10 (2) | 20 |
CM conventional microscopy.
As intimate companions, dogs have close contact with humans. However, dogs often harbour intestinal protozoa, which can cause mild to severe disease in dogs and lead to zoonotic infections in humans. Among these protozoa, G. duodenalis, Cryptosporidium spp., and Blastocystis sp. are common causes of diarrhoea in dogs worldwide. Guangzhou, southern China, is the third most economically developed city in China and boasts a large population of human residents (i.e. 14.90 million in 2017) and companion animals (i.e. > 10.62 million pets in the city in 2015) (Guangzhou Statistics Bureau; https://tjj.gz.gov.cn/). To date, one study of Cryptosporidium spp. and three molecular epidemiological studies of G. duodenalis have been published on dogs in the region12,13,19,36; however, nothing is known about the occurrence and molecular characterisation of Blastocystis sp.. The purpose of our study was to estimate the overall occurrence of Cryptosporidium spp., G. duodenalis, and Blastocystis sp. in dogs living in areas of Guangzhou and assess the zoonotic potential between humans and dogs.
Methods
Study design
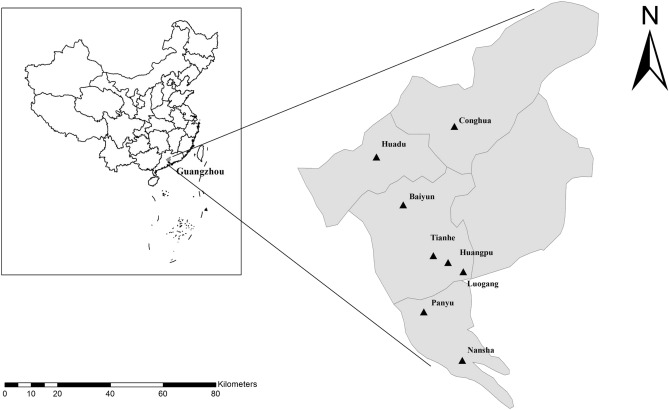
The study was conducted in Guangzhou. Guangzhou is one of the largest metropolitan cities in southern China (coordinates 3° 28′–25° 31′ N and 108° 13′–119° 59′ E); it covers an area of 7434 m2 and has a population of about 140 million. The annual average temperature is 20–22 °C and the average relative humidity is 77% (Guangzhou Statistics Bureau; https://tjj.gz.gov.cn/). A total of 651 fresh faecal samples were randomly collected from 199 household dogs (from four pet hospitals located in four different districts in urban Guangzhou: Tianhe, Baiyun, Huadu, and Panyu Districts), 149 shelter dogs (from two shelters located in suburban Luogang and Huangpu Districts), 237 breeding dogs (from two breeding centres located in suburban Conghua and Nansha Districts), and 66 pet market dogs (from one pet market in urban Tianhe District), with or without a history of illness, on a single occasion between January and December 2018 (Fig. 1). Faecal samples from shelters, breeding centres, and pet markets were collected as soon as practicably possible after defaecation by our research staff, either directly from the floor of the cage or per rectum. Care was taken to avoid sampling faecal material that had contacted the ground at the time of sampling. All samples from household dogs were collected immediately after natural defecation and donated by the dog owners, who provided consent for the use of samples from their animals in the survey. All collected fresh samples had no apparent diarrhoeal symptoms at the time of sampling. The samples were placed into clean plastic bags marked with ID numbers corresponding to the date, origin, age, sex, and whether the dog was dewormed in the past 12 months. The plastic bags were sealed and immediately placed onto ice packs in an insulated container. Samples were transported to the laboratory, stored at 4 °C, and processed no later than 24 h after collection.
Figure 1.
Geographic map of the sampling locations in this study. The figure was originally designed by the authors under the software ArcGIS 10.2. The original vector diagram imported in ArcGIS was adapted from Natural Earth (https://www.naturalearthdata.com).
DNA extraction
A 10-g aliquot of each sample was individually mixed with 30 ml of distilled water and passed through a ~ 250-μm-wide wire mesh sieve. Suspensions were centrifuged at 3000 × g for 5 min and precipitates were used for DNA extraction. Genomic DNA was extracted from 200 mg of each precipitate using an E.Z.N.A. Stool DNA Kit (Omega Bio-Tek Inc., Norcross, GA, USA) according to the manufacturer’s instructions. To improve the quality of recovered DNA, the mixtures of stool samples and SLX-Mlus buffers were vibrated at maximum speed for 15 min until the stool samples were thoroughly homogenised. Extracted DNA was stored at − 20 °C.
PCR detection
Cryptosporidium spp. was detected by nested PCR amplification of an approximately 830-bp fragment of the 18S rRNA gene as previously described37. G. duodenalis was detected by nested PCR amplifications of a 290-bp product of the 18S rRNA gene and a 520-bp polymorphic fragment of the glutamate dehydrogenase (GDH) gene as described38,39. To detect Blastocystis sp., an approximately 600-bp fragment of the 18S rRNA gene was amplified by single primer PCR as previously described (see Supplementary Table S1 online)40. Each 25 μl of PCR mixture contained 0.4 μM of each primer, 2.5 μl 10 × Taq Buffer (Mg2+ free; GC Buffer for the G. duodenalis 18S rRNA gene), 2 mM MgCl2, 0.2 mM dNTP mixture, 0.625 U of TaKaRa Taq (TaKaRa Shuzo Co., Ltd., Otsu, Japan), and 1 μl of genomic DNA. Each specimen was analysed in duplicate using positive (cattle-derived DNA) and negative (sterile water) controls.
Sequence and phylogenetic analysis
The positive secondary PCR products were directly sequenced by GENEWIZ (Suzhou, China). Sequence accuracy was confirmed with two-directional sequencing. All raw sequencing data were viewed and aligned by eye in Chromas Pro 1.33 (Technelysium Pty. Ltd., Helensvale, Queensland, Australia). The identity of species/assemblages/subtypes was established by comparing the obtained sequences with reference sequences from the National Center for Biotechnology Information (https://www.ncbi.nlm.nih.gov/) database using Clustal X 2.1 (https://www.clustal.org). Phylogenetic analysis was performed by a neighbour-joining (NJ) analysis in MEGA 7.01 (https://www.megasoftware.net/) based on the Kimura 2-parameter model using 1000 bootstrap replicates. The p-distance model was selected as the most suitable model.
Statistical analysis
Differences between prevalence and the dog’s origin (households, pet market, breeding centres, and shelters), age (≤ 6 months vs. > 6 months), sex, and deworming conditions (dewormed vs. non-dewormed in the past 12 months) were compared using a χ2 test in SPSS 22.0 for Windows (SPSS Inc., Chicago, IL, USA) with 95% confidence intervals. Differences at P < 0.01 were considered significant.
Statement of informed consent, ethics approval and guidelines
Prior to fecal specimen collection, we showed an informed consent to the dogs owner. This informed consent provides some information, including the purpose of the study, research approval number, the benefits and risks that may bring to their animals participating in the study. The informed consent was obtained from the dog owners. Appropriate permission in written form was obtained from the animal owners. During specimen collection, all animal work strictly followed the guidelines relating to the recommendations from the Guide for the Care and Use of LaboratoryAnimals of the Ministry of Health, China. Our protocol in written form was authorized by the Animal Ethics Procedures and Guidelines of the People's Republic of China and the approval of China Guangdong Province Science and Technology Department (Permit Number: SYXK (Yue) 2011–2018).
Results
Overall prevalence of Cryptosporidium spp., G. duodenalis, and Blastocystis sp.
Faecal samples from 651 dogs were tested by PCR for the presence of Cryptosporidium spp., G. duodenalis, and Blastocystis sp. The prevalence and 95% confidence intervals are summarised in Table 4. Cryptosporidium spp., G. duodenalis, and Blastocystis sp. were detected in 21 (3.2%), 20 (3.1%), and 35 (5.4%) of the examined samples, respectively. Co-infection rates significantly differed based on the dogs’ origins. The overall prevalence of shelter dogs (40.28%, 60/149) was significantly higher than that of household (3.0%, 6/199), breeding (2.1%, 5/237), and pet market dogs (7.5%, 5/66) (χ2 = 154.72, df = 3, P < 0.001). Moreover, deworming in the past 12 months had a strong protective effect on the risk of contracting pathogens, with non-dewormed dogs having 15-times higher risk of overall positive infection rates than dewormed animals (χ2 = 101.92, df = 1, P < 0.001). No significant difference was observed between age categories (≤ 6-month-old puppies and > 6-month-old young animals) in the prevalence of Cryptosporidium spp. (3.8% in puppies vs. 2.6% in young animals; χ2 = 0.69, P = 0.406), G. duodenalis (4.1% in puppies vs. 2.0% in young animals; χ2 = 2.40, P = 0.122), or Blastocystis sp. (3.8% in puppies vs. 7.2% in young animals; χ2 = 3.73, P = 0.053). There were also no significant differences in overall (9.1% in male dogs vs. 14.8% in female dogs; χ2 = 5.04, P = 0.025) and pathogen-specific prevalence rates between the two sex groups of dogs (χ2 = 3.49 for G. duodenalis, χ2 = 0.03 for Cryptosporidium spp., and χ2 = 3.58 for Blastocystis sp.; df = 1, P > 0.05).
Table 4.
Risk factors analysis on the prevalence of Cryptosporidium, G. duodenalis and Blastocystis in dogs.
| Factor | Category | No. tested | No. of positive (positive rate %; CI95) | Co-infection | p | ||
|---|---|---|---|---|---|---|---|
| G. duodenalis | Cryptosporidium | Blastocystis | |||||
| Origin | Shelters | 149 | 13 (8.7; 7.0–10.4) | 12 (8.1; 6.4–9.8) | 35 (23.5; 21.4–25.6) | 60 (40.2; 3.9–42.5) | < 0.001 |
| Households | 199 | 2 (1.0; 0.2–1.8) | 4 (2.0; 1.0–3.0) | 0 | 6 (3.0; 1.9–4.1) | ||
| Pet market | 66 | 5 (7.5; 2.2–7.8) | 0 | 0 | 5 (7.5; 2.2–7.8) | ||
| Breeding centers | 237 | 0 | 5 (2.1; 1.2–3.0) | 0 | 5 (2.1; 1.2–3.0) | ||
| Age | ≤ 6 month | 345 | 14 (4.1; 3.3–4.9) | 13 (3.8; 3.0–4.6) | 13 (3.8; 3.0–4.6) | 40 (11.6; 10.6–12.6) | 0.946 |
| > 6 month | 306 | 6 (2.0; 1.3–2.7) | 8 (2.6; 1.8–3.4) | 22 (7.2; 6.2–8.2) | 36 (11.8; 10.7–12.9) | ||
| Sex | Male | 361 | 7 (1.9; 1.3–2.5) | 12 (3.3; 2.6–4.0) | 14 (3.9; 3.2–4.6) | 33 (9.1; 8.2–10.0) | 0.025 |
| Female | 290 | 13 (4.5; 3.6–5.4) | 9 (3.1; 2.3–3.9) | 21 (7.2; 6.2–8.2) | 43 (14.8; 13.6–16.0) | ||
| Deworming | Yes | 436 | 5 (1.1; 0.6–1.6) | 7 (1.6, 1.1–2.1) | 0 | 12 (2.8; 12.0–13.6) | < 0.001 |
| No | 215 | 15 (7.0, 5.7–8.3 ) | 14 (6.5%; 5.3–7.7) | 35 (16.3; 14.8–17.8) | 64 (30.0; 28.3–31.7) | ||
| Total | 651 | 20 (3.1; 2.6–3.6) | 21 (3.2; 2.7–3.7) | 35 (5.4; 4.9–5.9) | 76 (11.7; 11.1–12.3) | ||
Cryptosporidium species
Sequence analysis of the 21 SSU rRNA Cryptosporidium-positive canine samples revealed the presence of C. canis (n = 19) and C. parvum (n = 2) (Table 5). Nucleotide sequences of C. canis and C. parvum detected in this study had 100% similarity to those deposited sequences in GenBank under accession numbers EU754826 and MF074695, respectively.
Table 5.
Species/assemblages/subtypes of Cryptosporidium, G. duodenalis and Blastocystis in dogs.
| Factor | Category | No. tested | Assemblages/species/subtypes/(no. of specimens) | ||
|---|---|---|---|---|---|
| G. duodenalis | Cryptosporidium | Blastocystis | |||
| Site | Shelters | 149 | D (11), C (2) | C. canis (11), C. parvum (1) | ST3 (28), ST1 (6), ST10 (1) |
| Households | 199 | D (1), F (1) | C. canis (4) | – | |
| Pet market | 66 | D (2), C (3) | – | – | |
| Breeding centers | 237 | 0 | C. canis (4), C. parvum (1) | – | |
| Age | ≤ 6 months | 345 | D (8), C (5), F (1) | C. canis (12), C. parvum (1) | ST3 (11), ST1 (2) |
| > 6 months | 306 | D (6) | C. canis (7), C. parvum (1) | ST3 (17), ST1 (4), ST10 (1) | |
| Sex | Male | 361 | D (5), C (1), F (1) | C. canis (12) | ST3 (10), ST1 (3), ST10 (1) |
| Female | 290 | D (9), C (4) | C. canis (7), C. parvum (2) | ST3 (18), ST1 (3) | |
| Deworming | Yes | 436 | D (4), C (1), | C. canis (7) | – |
| No | 215 | D (10), C (4), F (1) | C. canis (12),C. parvum (2) | ST3 (28), ST1 (6), ST10 (1) | |
| Total | 651 | D (14), C (5), F (1) | C. canis (19),C. parvum (2) | ST3 (28), ST1 (6), ST10 (1) | |
G. duodenalis assemblages
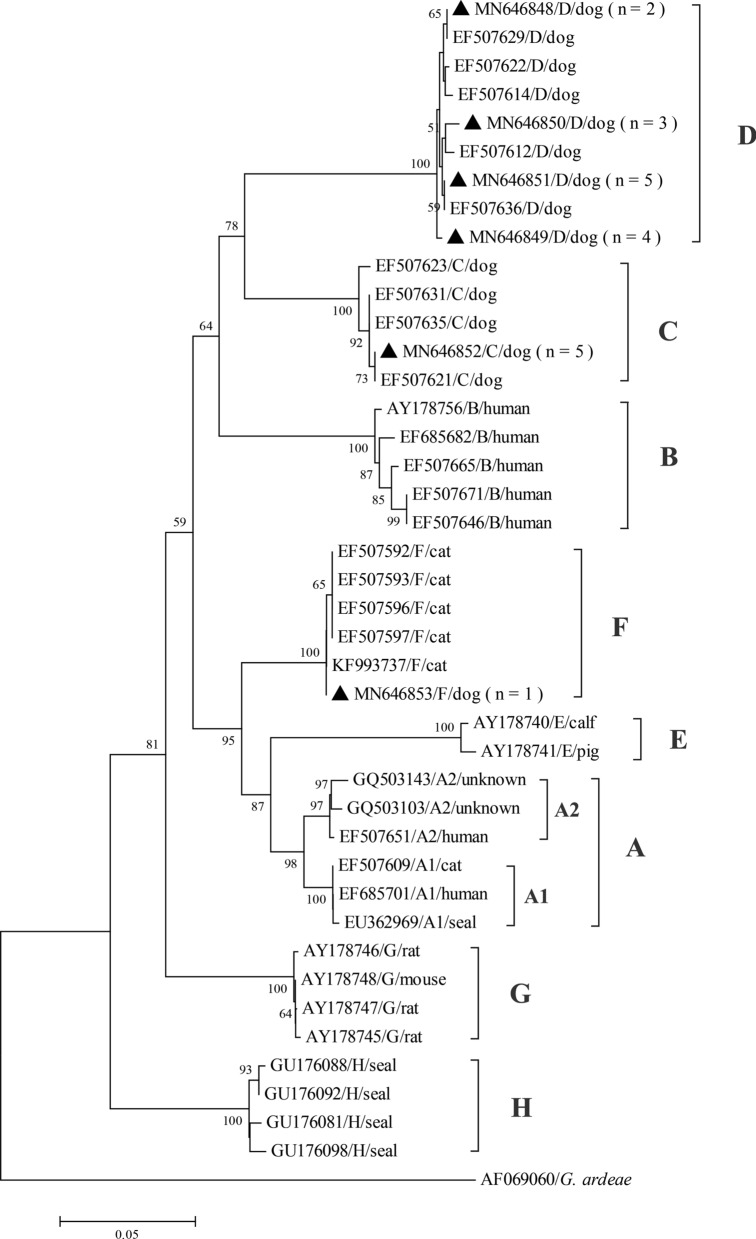
Sequence analysis revealed the presence of three different G. duodenalis assemblages: D (n = 14), C (n = 5), and F (n = 1). Three SSU rRNA nucleotide sequences of G. duodenalis assemblages D, C, and F were 100% identical to the GenBank reference sequences DQ385549, DQ385548, and JX275387, respectively. The genotypes identified by the GDH gene were fully consistent with those identified by the SSU rRNA gene and there were no overlapping nucleotides at any position, as shown by the chromatograms. No mixed assemblage infections were identified (see Supplementary Table S2 online). Moreover, four GDH sub-assemblage D nucleotide sequences were identified in this study. One sub-assemblage D sequence was identical to GenBank EF507636 reference sequences (n = 5), and the remaining three sequences had minor differences from EF507636, including two single nucleotide polymorphism (SNPs) in four specimens (C to T substitution at position 162 and T to G substitution at position 324), three SNPs in two specimens (C to T substitution at position 162, A to G substitution at position 174, and A to T substitution at position 311), and three SNPs in three specimens (T to C substitution at position 109, A to G substitution at position 183, and A to T substitution at position 312). All GDH sub-assemblage C sequences were identical to each other and showed 99% sequence similarity to the EF507621 reference sequence, with one SNP difference at position 12 (T → C). The assemblage F nucleotide sequence was identical to the corresponding KF993737 sequence of from a cat in China (Fig. 2).
Figure 2.
Phylogenetic tree depicting evolutionary relationships among assemblages of G. duodenalis at the gdh locus using the Neighbor-Joining analysis. Bootstrap values lower than 50% were not displayed. Filled circles represent canine sequences from this study. Giardia ardeae was used as outgroup taxa.
Blastocystis subtypes
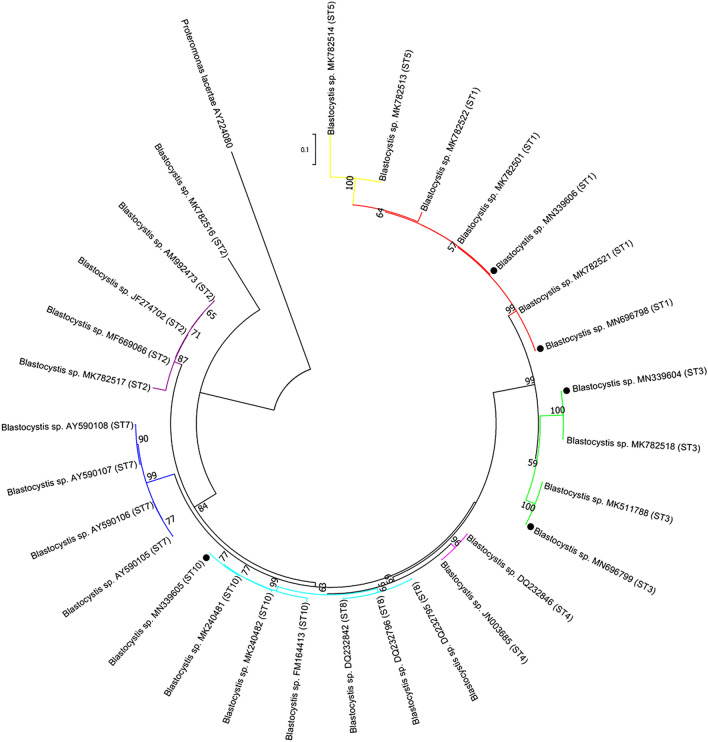
DNA sequencing of the SSU rRNA PCR products from the 35 Blastocystis-positive samples and sequence analysis indicated the existence of subtypes ST3 (n = 27), ST1 (n = 6), ST10 (n = 1), and unknown ST (n = 1). Twenty-seven nucleotide sequences identified as ST3 were identical to each other and had 100% similarity to the MK782518 reference sequence from urticaria patients in Brazil. Six ST1 nucleotide sequences included two different nucleotide sequences with 100% similarity to the GenBank reference sequences MK782501 in four specimens and MK782521 in two specimens. The ST10 nucleotide sequence had 100% similarity to a cattle-derived sequence from Malaysia in GenBank (MK240480). The remaining nucleotide sequence was not assigned a subtype by the sequence typing database, and was 100% homologous with the published sequence MK511788 from Malaysia.
Phylogenetic analysis using NJ analyses clustered Blastocystis subtypes obtained in the present study into three subtypes (ST1, ST3, and ST10), and the unknown subtype was grouped into subtype ST1 (Fig. 3).
Figure 3.
Bootstrap consensus phylogenetic tree for aligned small subunit rDNA sequences from Blastocystis spp. isolated from canines and previously published representative sequences, using Proteromonas lacerate as an outgroup. The tree was derived using the neighbor-joining method based on the Kimura 2-parameter model with 1000 bootstrap replicates. Taxa isolated from canine samples are shown by filled circles.
Discussion
In this study, Cryptosporidium spp., G. duodenalis, and Blastocystis sp. were found at considerably low prevalences (3.2%, 3.1%, and 5.4%, respectively) in the surveyed canine populations. This finding is comparable to the prevalences in Poland (2.0% for G. duodenalis)41, India (3.0% for G. duodenalis)42, the United States (2.0% and 3.8% for Cryptosporidium)43,44, China (3.8% and 4.9% for Cryptosporidium)10,14, Italy (3.3% for Cryptosporidium)9, Australia (2.5% for Blastocystis)29, Brazil (2.6% for Blastocystis)31, and France (3.4% for Blastocystis)20. However, higher prevalences of 21% of Cryptosporidium from Japan, 36.5% of G. duodenalis from Spain, and 37.5% of Blastocystis from Colombia were also previously found in dogs22,24,33. Aside from geographical considerations, many factors can contribute to this difference in the prevalence, including a dog’s age, origin, health status, and examination methods used. Importantly, the true prevalences may be underestimated because of the intermittent shedding of oocysts/cysts, low parasitic burdens, and invalid amplification23. Therefore, it is important to perform a well-designed longitudinal study that includes appropriate sampling methods and a combination of diagnostic tests (e.g. microscopic examination, antigen assay, and PCR assay) to estimate the real prevalence.
In risk factor analysis, the overall and pathogen-specific prevalences of shelter dogs were significantly higher than those of household, breeding, and pet market dogs. Poor care conditions may be a significant factor that contributed to the high prevalence in shelter dogs23. Additionally, these shelter dogs originally roamed free in the nearby streets before they were found by local citizens and sent to the shelters, which increased their exposure to a variety of pathogens. No significant age- and sex-associated differences were detected in the prevalence of these three pathogens, which is consistent with observations of previous studies in China, Japan, Colorado, France11,20,21,44 , and Australia10,12,45. As expected, deworming had a significant negative effect on the risk of overall and single pathogen infections. This could be related to the fact that the anthelminthic ingredients used for dogs in China mainly include ivermectin, pyrantel, praziquantel, pyrantel pamoate, febantel, nitazoxanide, metronidazole, and fenbendazole, most of which are also effective against protozoan infections, such as Giardia spp., Cryptosporidium spp., and Blastocystis sp.46–48. Thus, pet hygiene management is suggested to be a major risk factor for contracting these pathogens in dogs.
The sequencing data revealed that dogs were predominantly infected by the expected host-specific species C. canis. Another interesting outcome was the identification of C. parvum in two of the canine isolates genotyped. Cryptosporidium parvum is the most frequent species known in cryptosporidium infections of humans and has resulted in several zoonotic outbreaks49. However, because of the omnivorous nature of dogs, C. parvum detected from dogs in this study may have been present because of accidental acquisition or mechanical carriage of C. parvum oocysts of anthroponotic origin via environmental contamination. Although the host-specific species C. canis also colonised individuals in hospitals, including children, HIV patients, and even immunocompetent individuals50–52, the infections in humans were likely transient53. Based on our data and that of other studies, we conclude that dogs do not seem to be suitable reservoirs for Cryptosporidium spp. transmission to humans, therefore, posed a limited risk to humans. This is consistent with the findings reported in dog populations in eastern Spain, where most of the genotypes identified seemed to be primarily transmitted within canine cycles24.
Regarding G. duodenalis, dogs were infected by assemblages D and C. Surprisingly, the supposedly cat-specific assemblage F was also found in one household dog, which was consistent with a previous report in Beijing, China14. Considering there was little possibility of the specimen being contaminated with cat faeces, as individual faecal samples were freshly collected by pet dog owners who kept only a single companion animal, it is more likely that the dog was transiently infected by ingesting parasite cysts of cat origin. Assemblages D and C have strong host specificities and have been mainly detected in canines. Both are considered of limited zoonotic relevance, although sporadic cases of human infections have been frequently detected in travellers, children, and diarrhoeal outpatients53–55. The dominant appearances of G. duodenalis assemblages D and C in dogs in our study suggested that zoonotic transmission of giardiasis rarely occurs between humans and dogs.
A high diversity of Blastocystis subtypes has been identified in dogs worldwide and the subtype constitution was observed to differ among geographical regions, such as ST1 and ST4 in China; ST1, ST4, ST5, and ST6 in India; ST1 and ST10 in the USA; ST1, ST3, and ST4 in Australia; and ST1 and ST2 in Thailand27,29,35,56,57. This is the first large-scale survey on the prevalence and genetic characteristics of Blastocystis sp. in dogs of various origins in China, and we detected the presence of subtypes ST3, ST1, and ST10. Among the subtypes in this study, ST3 and ST1 are the two predominant subtypes. The subtype distribution of Blastocystis in dogs in our study consistent with most populations in humans around the world15,57,58. In China, in a cross-sectional survey in humans from four epidemiological settings, ST3 was the predominant type, accounting for 60.4% (n = 116) of 192 positive specimens, followed by subtype ST1, accounting for 24.5% (n = 47)58. A report from Argentina also showed that Blastocystis ST3 was the most prevalent subtype (48 cases) among 76 patients infected with Blastocystis, and other subtypes identified were ST1 (14.9%), ST6 (7.5%), and ST2 (5.9%)57. Moreover, ST3 and ST1 were found in dogs and their owners in Australia, the Philippines, and Turkey15,35,59. All of these studies suggested that ST3 and ST1 might be the important sources of human-to-human or animal-to-human transmission of Blastocystis, although more environmental factors and or other animal sources should be included. Blastocystis ST10 was found in one dog sample; this subtype is frequently identified in common livestock, including cattle, sheep, goats, and deer3,16,60, and occasionally found in wild animals, pigs, dogs, and cats20,27. Taken together, our results revealed that canines are a novel reservoir for Blastocystis.
We conducted partial assessment of the zoonotic potential of our canine isolates using only molecular epidemiological data, because assessing the risk for zoonotic transmission of these pathogens from dogs to/from humans is difficult. The only way to properly determine zoonotic transmission is by conducting case–control studies that assess the genotypes/subtypes of these pathogens by using appropriate molecular typing tools in human and canine populations that maintain permanent close contact in the same spatial and temporal setting23,34,61,62. However, our epidemiological study still generated baseline information and determined the genetic diversity of these pathogens in the investigated region.
Conclusions
The prevalence and molecular characteristics of Cryptosporidium spp., G. duodenalis, and Blastocystis sp. were determined in dogs in Guangzhou. Our risk factor analysis showed that management of pet hygiene may be a major risk factor for contracting these pathogens in dogs. Our results suggest that dogs do not seem to be suitable reservoirs of human giardiasis or cryptosporidiosis in the investigated region, but may act as novel suitable hosts of human blastocystosis. Strict sentinel surveillance of dogs, especially stray dogs, should be established to minimise the risk of spreading blastocystosis among humans and dogs.
Supplementary information
Acknowledgements
This study was supported by Grants from the National Key R&D Program of China (2017YFD0500400), NSFC Grants (31872460, 31602044), NSF Grant of Guangdong Province (2018A030313925), Rural Revitalization Special Strategic Project of Guangdong (201817SY0003), Guangdong Provincial Special Fund for Modern Agriculture Industry Technology Innovation Teams (2019KJ119), the Key Research and Development Program of Guangdong Province (2019B020218004), Special Fund for Scientific Innovation Strategy-construction of High Level Academy of Agriculture Science (No. R2018QD-091 and R2018QD-092), Discipline Team Building Projects of Guangdong Academy of Agricultural Sciences in the 13th Five-Year Period (201623TD), and Science and Technology Program of Guangzhou (201906040005). We also thank Sandra Cheesman, PhD, from Liwen Bianji, Edanz Group China (https://www.liwenbianji.cn/ac), for editing the English text of a draft of this manuscript.
Author contributions
S.L., X.L., Y.S., N.Q., M.L. and G.L. designed the study, M.L., C.W., J.L., J.H., L.Y., H.C. and W.X. performed the isolation, cultivation, and sequencing tasks, S.L., X.L., M.L., C.W., J.L. and L.Y. analyzed the data, S.L. wrote the manuscript, and all authors read and approved the final version of manuscript.
Data availability
The datasets analyzed during the current study are available in the NCBI GenBank repository (https://www.ncbi.nlm.nih.gov/genbank/) under accession numbers: MN646215–MN646217 and MN646848–MN646853 for G. duodenalis, MN696800–MN696801 for Cryptosporidium, and MN339604–MN339606 and MN696798–MN696799 for Blastocystis.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Liao Shenquan and Lin Xuhui.
Contributor Information
Mingfei Sun, Email: smf7810@126.com.
Guoqing Li, Email: gqli@scau.edu.cn.
Supplementary information
is available for this paper at 10.1038/s41598-020-74299-z.
References
- 1.Xiao L. Molecular epidemiology of cryptosporidiosis: An update. Exp. Parasitol. 2010;124:80–89. doi: 10.1016/j.exppara.2009.03.018. [DOI] [PubMed] [Google Scholar]
- 2.Feng Y, Xiao L. Zoonotic potential and molecular epidemiology of Giardia species and giardiasis. Clin. Microbiol. Rev. 2011;24:110–140. doi: 10.1128/CMR.00033-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Stensvold CR, et al. Subtype distribution of Blastocystis isolates from synanthropic and zoo animals and identification of a new subtype. Int. J. Parasitol. 2009;39:473–479. doi: 10.1016/j.ijpara.2008.07.006. [DOI] [PubMed] [Google Scholar]
- 4.Hameed DMA, Hassanin OM, Zuel-Fakkar NM. Association of Blastocystis hominis genetic subtypes withurticaria. Parasitol. Res. 2011;108:553–560. doi: 10.1007/s00436-010-2097-2. [DOI] [PubMed] [Google Scholar]
- 5.Jimenez PA, Jaimes JE, David Ramírez J. A summary of Blastocystis subtypes in North and South America. Parasit. Vectors. 2019;12:376. doi: 10.1186/s13071-019-3641-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Adamu H, et al. Distribution and clinical manifestations of Cryptosporidium species and subtypes in HIV/AIDS patients in Ethiopia. PLoS Negl. Trop. Dis. 2014;8:e2831. doi: 10.1371/journal.pntd.0002831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rao K, Seka U, Iraivan KT, Abraham G, Soundararajan P. Blastocystis hominis—An emerging cause of diarrhoea in renal transplant recipients. J. Assoc. Phys. India. 2003;51:719–721. [PubMed] [Google Scholar]
- 8.Leelayoova S, et al. Drinking water: A possible source of Blastocystis spp. subtype 1 infection in schoolchildren of a rural community in Central Thailand. Am. J. Trop. Med. Hyg. 2008;79:401–406. doi: 10.4269/ajtmh.2008.79.401. [DOI] [PubMed] [Google Scholar]
- 9.Giangaspero A, Iorio R, Paoletti B, Traversa D, Capelli G. Molecular evidence for Cryptosporidium infection in dogs in central Italy. Parasitol. Res. 2006;99:297–299. doi: 10.1007/s00436-006-0169-0. [DOI] [PubMed] [Google Scholar]
- 10.Jian F, et al. Occurrence and molecular characterization of Cryptosporidium in dogs in Henan Province, China. BMC. Vet. Res. 2014;10:26. doi: 10.1186/1746-6148-10-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Xu H, et al. Genotypes of Cryptosporidium spp., Enterocytozoon bieneusi and Giardia duodenalis in dogs and cats in Shanghai, China. Parasit. Vectors. 2016;9:121. doi: 10.1186/s13071-016-1409-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li J, et al. Genotype identification and prevalence of Giardia duodenalis in pet dogs of Guangzhou, Southern China. Vet. Parasitol. 2012;188:368–371. doi: 10.1016/j.vetpar.2012.04.004. [DOI] [PubMed] [Google Scholar]
- 13.Pan W, et al. Prevalence and genotypes of Giardia lamblia from stray dogs and cats in Guangdong, China. Vet. Parasitol. Reg. Stud. Reports. 2018;13:30–34. doi: 10.1016/j.vprsr.2018.03.012. [DOI] [PubMed] [Google Scholar]
- 14.Yu Z, et al. Prevalence of intestinal parasites in companion dogs with diarrhea in Beijing, China, and genetic characteristics of Giardia and Cryptosporidium species. Parasitol. Res. 2018;117:35–43. doi: 10.1007/s00436-017-5631-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Alfellani MA, et al. Variable geographic distribution of Blastocystis subtypes and its potential implications. Acta. Trop. 2013;126:11–18. doi: 10.1016/j.actatropica.2012.12.011. [DOI] [PubMed] [Google Scholar]
- 16.Wang J, et al. Distribution and genetic diversity of Blastocystis subtypes in various mammal and bird species in northeastern China. Parasit. Vectors. 2018;11:522. doi: 10.1186/s13071-018-3106-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ayinmode AB, Obebe OO, Falohun OO. Molecular detection of Cryptosporidium species in street-sampled dog faeces in Ibadan, Nigeria. Vet. Parasitol. Reg. Stud. Reports. 2018;14:54–58. doi: 10.1016/j.vprsr.2018.08.005. [DOI] [PubMed] [Google Scholar]
- 18.Li W, et al. Prevalence and genetic characteristics of Cryptosporidium, Enterocytozoon bieneusi and Giardia duodenalis in cats and dogs in Heilongjiang province, China. Vet. Parasitol. 2015;208:125–134. doi: 10.1016/j.vetpar.2015.01.014. [DOI] [PubMed] [Google Scholar]
- 19.Li J, et al. Genetic characterization of Cryptosporidium spp. and Giardia duodenalis in dogs and cats in Guangdong, China. Parasit. Vectors. 2019;12:571. doi: 10.1186/s13071-019-3822-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Osman M, et al. Prevalence and genetic diversity of the intestinal parasites Blastocystis sp. and Cryptosporidium spp. in household dogs in France and evaluation of zoonotic transmission risk. Vet. Parasitol. 2015;214:167–170. doi: 10.1016/j.vetpar.2015.09.015. [DOI] [PubMed] [Google Scholar]
- 21.Itoh N, et al. Molecular detection and characterization of Cryptosporidium species in household dogs, pet shop puppies, and dogs kept in a school of veterinary nursing in Japan. Vet. Parasitol. 2014;200:284–288. doi: 10.1016/j.vetpar.2013.12.030. [DOI] [PubMed] [Google Scholar]
- 22.Itoh N, et al. Molecular prevalence of Cryptosporidium spp. breeding kennel dogs. Korean. J. Parasitol. 2019;57:197–200. doi: 10.3347/kjp.2019.57.2.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gil H, et al. Detection and molecular diversity of Giardia duodenalis and Cryptosporidium spp. in sheltered dogs and cats in Northern Spain. Infect. Genet. Evol. 2017;50:62–69. doi: 10.1016/j.meegid.2017.02.013. [DOI] [PubMed] [Google Scholar]
- 24.Adell-Aledón M, et al. Occurrence and molecular epidemiology of Giardia duodenalis infection in dog populations in Eastern Spain. BMC. Vet. Res. 2018;14:26. doi: 10.1186/s12917-018-1353-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li W, et al. Molecular characterization of Giardia duodenalis isolates from police and farm dogs in China. Exp. Parasitol. 2013;135:223–226. doi: 10.1016/j.exppara.2013.07.009. [DOI] [PubMed] [Google Scholar]
- 26.Kim HY, et al. Multilocus genotyping and risk factor analysis of Giardia duodenalis in dogs in Korea. Acta. Trop. 2019;99:105113. doi: 10.1016/j.actatropica.2019.105113. [DOI] [PubMed] [Google Scholar]
- 27.Ruaux CG, Stang BV. Prevalence of blastocystis in shelter-resident and client-owned companion animals in the US Pacific Northwest. PLoS ONE. 2014;9:e107496. doi: 10.1371/journal.pone.0107496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Belleza ML, Reyes JC, Tongol-Rivera PN, Rivera WL. Subtype analysis ofBlastocystis sp. isolates from human and canine hosts in an urban community inthe Philippines. Parasitol. Int. 2016;65:291–294. doi: 10.1016/j.parint.2016.02.009. [DOI] [PubMed] [Google Scholar]
- 29.Wang W, et al. Diversity of Blastocystis subtypes in dogs in different geographical settings. Parasit. Vectors. 2013;6:215. doi: 10.1186/1756-3305-6-215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.David E, et al. Molecular characterization of intestinal protozoa in two poor communities in the state of São Paulo, Brazil. Parasit. Vectors. 2015;8:103. doi: 10.1186/s13071-015-0714-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Oliveira-Arbex AP, et al. Blastocystis genetic diversity among children of low-income daycare center in Southeastern Brazil. Infect. Genet. Evol. 2018;57:59–63. doi: 10.1016/j.meegid.2017.11.005. [DOI] [PubMed] [Google Scholar]
- 32.Moura RGF, Oliveira-Silva MB, Pedrosa AL, Nascentes GAN, Cabrine-Santos M. Occurrence of Blastocystis spp. in domestic animals in Triângulo Mineiro area of Brazil. Rev. Soc. Bras. Med. Trop. 2018;51:240–243. doi: 10.1590/0037-8682-0484-2016. [DOI] [PubMed] [Google Scholar]
- 33.Ramírez JD, et al. Blastocystis subtypes detected in humans and animals from Colombia. Infect. Genet. Evol. 2014;22:223–228. doi: 10.1016/j.meegid.2013.07.020. [DOI] [PubMed] [Google Scholar]
- 34.Paulos S, et al. Occurrence and subtype distribution of Blastocystis sp. in humans, dogs and cats sharing household in northern Spain and assessment of zoonotic transmission risk. Zoonoses. Public. Health. 2018;65:993–1002. doi: 10.1111/zph.12522. [DOI] [PubMed] [Google Scholar]
- 35.Eroglu F, Koltas IS. Evaluation of the transmission mode of B. hominis by usingPCR method. Parasitol. Res. 2010;107:841–845. doi: 10.1007/s00436-010-1937-4. [DOI] [PubMed] [Google Scholar]
- 36.Zheng G, et al. Genotyping of Giardia duodenalis isolates from dogs in Guangdong, China based on multi-locus sequence. Korean. J. Parasitol. 2014;52:299–304. doi: 10.3347/kjp.2014.52.3.299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Xiao L, et al. Identification of 5 types of Cryptosporidium parasites in children in Lima. Peru. J. Infec. Dis. 2001;183:492–497. doi: 10.1086/318090. [DOI] [PubMed] [Google Scholar]
- 38.Appelbee AJ, Frederick LM, Heitman TL, Olson ME. Prevalence and genotyping of Giardia duodenalis from beef calves in Alberta, Canada. Vet. Parasitol. 2003;112:289–294. doi: 10.1016/S0304-4017(02)00422-3. [DOI] [PubMed] [Google Scholar]
- 39.Cacciò SM, Beck R, Lalle M, Marinculic A, Pozio E. Multilocus genotyping of Giardia duodenalis reveals striking differences between assemblages A and B. Int. J. Parasitol. 2008;38:1523–1531. doi: 10.1016/j.ijpara.2008.04.008. [DOI] [PubMed] [Google Scholar]
- 40.Scicluna SM, Tawari B, Clark CG. DNA barcoding of Blastocystis. Protist. 2006;157:77–85. doi: 10.1016/j.protis.2005.12.001. [DOI] [PubMed] [Google Scholar]
- 41.Traub RJ, et al. Epidemiological and molecular evidence supports the zoonotic transmission of Giardia among humans and dogs living in the same community. Parasitol. 2004;128:253–262. doi: 10.1017/S0031182003004505. [DOI] [PubMed] [Google Scholar]
- 42.Solarczyk P, Majewska AC. A survey of the prevalence and genotypes of Giardia duodenalis infecting household and sheltered dogs. Parasitol. Res. 2010;106:1015–1019. doi: 10.1007/s00436-010-1766-5. [DOI] [PubMed] [Google Scholar]
- 43.El-Ahraf A, et al. Prevalence of cryptosporidiosis in dogs and human beings in San Bernardino County, California. J. Am. Vet. Med. Assoc. 1991;198:631–634. [PubMed] [Google Scholar]
- 44.Irwin PJ. Companion animal parasitology: A clinical perspective. Int. J. Parasitol. 2002;32:581–593. doi: 10.1016/S0020-7519(01)00361-7. [DOI] [PubMed] [Google Scholar]
- 45.Duda A, Stenzel DJ, Boreham PF. Detection of Blastocystis sp. in domestic dogs and cats. Vet. Parasitol. 1998;76:9–17. doi: 10.1016/S0304-4017(97)00224-0. [DOI] [PubMed] [Google Scholar]
- 46.Foletto VR, et al. Efficacyand security of ivermectin given orally to rats naturally infected withSyphacia spp., Giardia spp. and Hymenolepis nana. Lab. Anim. 2015;49:196–200. doi: 10.1177/0023677214562850. [DOI] [PubMed] [Google Scholar]
- 47.Moron-Soto M, et al. Efficacy of nitazoxanide to treat natural Giardia infections in dogs. Parasit. Vectors. 2017;10:52. doi: 10.1186/s13071-017-1998-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kumarasamy V, et al. Blastocystis sp., parasite associated with gastrointestinal disorders: An overview of its pathogenesis, immune modulation and therapeutic strategies. Curr. Pharm. Des. 2018;24:3172–3175. doi: 10.2174/1381612824666180807101536. [DOI] [PubMed] [Google Scholar]
- 49.Xiao L, Fayer R. Molecular characterisation of species and genotypes of Cryptosporidium and Giardia and assessment of zoonotic transmission. Int. J. Parasitol. 2008;38:1239–1255. doi: 10.1016/j.ijpara.2008.03.006. [DOI] [PubMed] [Google Scholar]
- 50.Feng Y, et al. Extended outbreak of cryptosporidiosis in a pediatric hospital. China. Emerg. Infect. Dis. 2012;18:312–314. doi: 10.3201/eid1802.110666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gatei W, et al. Multilocus sequence typing and genetic structure of Cryptosporidium hominis from children in Kolkata, India. Infect. Genet. Evol. 2007;7:197–205. doi: 10.1016/j.meegid.2006.08.006. [DOI] [PubMed] [Google Scholar]
- 52.Learmonth JJ, et al. Genetic characterization and transmission cycles of Cryptosporidium species isolated from humans in New Zealand. Appl. Environ. Microbiol. 2004;70:3973–3978. doi: 10.1128/AEM.70.7.3973-3978.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Villamizar X, et al. Molecular and descriptive epidemiology of intestinal protozoan parasites of children and their pets in Cauca, Colombia: A cross-sectional study. BMC. Infect. Dis. 2019;19:190. doi: 10.1186/s12879-019-3810-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Broglia A, et al. Molecular typing of Giardia duodenalis isolates from german travellers. Parasitol. Res. 2013;112:3449–3456. doi: 10.1007/s00436-013-3524-y. [DOI] [PubMed] [Google Scholar]
- 55.Liu H, et al. Prevalence and genetic characterization of Cryptosporidium, Enterocytozoon, Giardia and Cyclospora in diarrheal outpatients in China. BMC. Infect. Dis. 2014;14:25. doi: 10.1186/1471-2334-14-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Parkar U, et al. Direct characterization of Blastocystis from faeces by PCR and evidence of zoonotic potential. Parasitol. 2007;134:359–367. doi: 10.1017/S0031182006001582. [DOI] [PubMed] [Google Scholar]
- 57.Casero RD, Mongi F, Sánchez A, Ramírez JD. Blastocystis and urticaria: Examination of subtypes and morphotypes in an unusual clinical manifestation. Acta. Trop. 2015;148:156–161. doi: 10.1016/j.actatropica.2015.05.004. [DOI] [PubMed] [Google Scholar]
- 58.Li LH, et al. Cross-sectional surveys and subtype classification of human Blastocystis isolates from four epidemiological settings in China. Parasitol. Res. 2007;102:83–90. doi: 10.1007/s00436-007-0727-0. [DOI] [PubMed] [Google Scholar]
- 59.Nagel R, et al. Blastocystis subtypes in symptomatic and asymptomatic family members and pets and response to therapy. Intern. Med. J. 2012;42:1187–1195. doi: 10.1111/j.1445-5994.2011.02626.x. [DOI] [PubMed] [Google Scholar]
- 60.Udonsom R, et al. Blastocystis infection and subtype distribution in humans, cattle, goats, and pigs in central and western Thailand. Infect. Genet. Evol. 2018;65:107–111. doi: 10.1016/j.meegid.2018.07.007. [DOI] [PubMed] [Google Scholar]
- 61.de Lucio A, et al. No molecular epidemiological evidence supporting household transmission of zoonotic Giardia duodenalis and Cryptosporidium spp. from pet dogs and cats in the Province of Álava, Northern Spain. Acta. Trop. 2017;170:48–56. doi: 10.1016/j.actatropica.2017.02.024. [DOI] [PubMed] [Google Scholar]
- 62.Rehbein S, et al. Giardia Duodenalis in small animals and their owners in Germany: A pilot study. Zoonoses. Public. Health. 2019;66:117–124. doi: 10.1111/zph.12541. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets analyzed during the current study are available in the NCBI GenBank repository (https://www.ncbi.nlm.nih.gov/genbank/) under accession numbers: MN646215–MN646217 and MN646848–MN646853 for G. duodenalis, MN696800–MN696801 for Cryptosporidium, and MN339604–MN339606 and MN696798–MN696799 for Blastocystis.