Abstract
Nonalcoholic fatty liver disease (NAFLD) is the most common cause of chronically elevated liver enzymes. Diagnosis and risk stratification of NAFLD remains clinically challenge as patients with NAFLD are either asymptomatic or have nonspecific presenting complaints and may have normal liver enzymes. Nonalcoholic steatohepatitis (NASH), the clinically aggressive variant of NAFLD, is also difficult to differentiate noninvasively, and a liver biopsy is required to definitively diagnose NASH. Thus, the definitive diagnosis and risk stratification of NAFLD is embedded in histological assessment of the liver. Several clinical aides been investigated in an attempt to risk stratify and identify patients noninvasively as doing a liver biopsy in all patients with NAFLD are not feasible. Since these biomarkers are unable to differentiate NASH from non‐NASH, they have leveraged biochemical changes within the liver as patients progress to varying degree of hepatic fibrosis to identify patients with moderate fibrosis (fibrosis stage 2 or greater) and advanced fibrosis (fibrosis stage 3 or greater) to help guide the need for additional and more definitive workup. These clinical aides span from by‐products of apoptosis to statistical modelling of clinically available data to identify ‘at‐risk’ patients with NAFLD. The current review will focus the diagnostic performance of these noninvasive serum‐based biomarkers in NAFLD.
Keywords: diagnostic test, liver fibrosis, noninvasive biomarkers
Nonalcoholic fatty liver disease (NAFLD) is common, affecting nearly a third of the US population. The current manuscript summarizes serum‐based biomarkers in patients with NAFLD.
1. INTRODUCTION
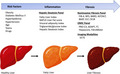
Nonalcoholic fatty liver disease (NAFLD) is characterized by the presence of ectopic fat deposition within the liver not attributable to alcohol or secondary causes of hepatic steatosis. 1 NAFLD, as the aetiology of chronic liver disease, has increased rapidly over the past three decades in the United States and is now the most common cause of chronic liver disease accounting for nearly 75% of all chronic liver diseases and affecting nearly one in three individuals. 2 , 3 NAFLD is commonly associated with obesity, type 2 diabetes mellitus, hyperlipidemia, and hypertension 4 , 5 , 6 and is considered the hepatic manifestation of metabolic syndrome. 7 NAFLD comprises a histological spectrum of nonalcoholic fatty liver (NAFL) characterized by the presence of hepatic steatosis with none or minimal inflammation to nonalcoholic steatohepatitis (NASH), which is characterized by lobular inflammation, ballooning and varying degrees of hepatic fibrosis. 8 Serum liver enzymes, which are often the first clinical clue to presence of liver disease, can be normal or mildly elevated in a majority of patients with NAFLD, therefore making it difficult to detect NAFLD, which has led to underdiagnosis of NAFLD. 9 , 10 This, coupled with the lack of therapeutic options for NAFLD, has resulted in increased prevalence of cirrhosis and end‐stage liver disease associated with NAFLD. 11 , 12 Thus, there is great interest in risk stratification of patients with NAFLD to optimize clinical care. The current review will focus on serum‐based models and biomarkers validated in patients with NAFLD.
1.1. Patients at risk
The distinction between NAFL and NASH is clinically important as patients with NASH are at higher risk for fibrosis progression, cirrhosis and hepatocellular carcinoma. 13 , 14 , 15 Natural history studies of patients with biopsy‐proven NAFLD have demonstrated decreased survival when compared to non‐NASH patients. 16 , 17 In addition to NASH, presence of advanced fibrosis has also been associated with increased risk of fibrosis progression to cirrhosis, death and need for liver transplantation (LT). 18 Recent studies highlight the importance of even moderate fibrosis (ie fibrosis stage 2) as a key risk factor for liver‐related outcomes, reduced survival and need for LT. 19 , 20 The diagnosis of NASH and fibrosis quantification is anchored in a histological assessment of the liver and a liver biopsy. 8 However, liver biopsy is limited by sampling variability, invasiveness, cost and complications, such as pain, bleeding and, in rare instances, death. 21 , 22 , 23 Due to the widespread prevalence of NAFLD, liver biopsy is not practical approach to aid in risk stratification of all patients with NAFLD. This underscores the urgent need for noninvasive, point‐of‐care and cost‐effective biomarkers to identify high‐risk patients. The current review will focus on the diagnostic accuracy of noninvasive blood‐based biomarkers to predict presence of NASH (vs NAFL) and varying fibrosis stages.
1.1.1. Hepatic steatosis
Presence of hepatic steatosis is the prerequisite for diagnosing NAFLD. 24 Several serum‐based biomarkers have been evaluated to predict presence of hepatic steatosis including SteatoTest, Fatty liver index (FLI), NAFLD liver fat score (NAFLD‐LFS), visceral adiposity index (VAI), triglyceride × glucose (TyG) index and Hepatic steatosis index (HSI) (Table 1). 25 , 26 , 27 , 28 , 29 , 30 , 31 These biomarkers have acceptable diagnostic performance for detecting hepatic steatosis. 32 However, their ability to distinguish between steatosis grades is suboptimal. 25 , 26 , 27 , 28 , 29 , 31 Furthermore, these models of hepatic steatosis are unable to differentiate between the presence of NASH vs non‐NASH. 32 In a head‐to‐head comparison using liver biopsy as the reference standard, VAI outperformed other models with an AUROC of 0.92, sensitivity of 79%, specificity of 92%, NPV 16% and PPV of 99%. 32 Given their diagnostic performance, the biomarkers may have a role for identifying hepatic steatosis in population‐based studies but are unable to be used in clinical practice.
TABLE 1.
Noninvasive steatosis biomarkers
| Steatosis model | Formula |
|---|---|
| Fatty liver index 25 | (e0.953 × loge (triglycerides) + 0.139*BMI + 0.718 × loge (ggt) + 0.053 × waist circumference − 15.745)/(1 + e0.953 × loge (triglycerides) + 0.139 × BMI + 0.718 × loge (ggt) + 0.053 × waist circumference − 15.745) × 100 |
| NAFLD liver fat score 26 | −2.89 + 1.18 × metabolic syndrome (yes = 1/no = 0) + 0.45 × type 2 diabetes (yes = 2/no = 0) + 0.15 × insulin (mU/L) + 0.04 × AST(U/L) − 0.94 × AST/ALT |
| Visceral adiposity index 30 |
Male: [WC/39.68 + (1.88 × BMI)] × (triglycerides/1.03) × (1.31/HDL Female: [WC/36.58 + (1.89 × BMI) × (triglycerides/0.81) × (1.52/HDL) |
| Triglyceride/glucose index 28 | Log (fasting triglycerides [mg/dL] × fasting glucose [mg/dL]/2) |
| Hepatic steatosis index 29 | 8 × ALT/AST + BMI (+2, if type 2 diabetes; +2, if female) |
2. NONINVASIVE FIBROSIS MODEL
Noninvasive fibrosis models utilize readily available clinical and laboratory information in an attempt to noninvasively risk stratify patients with NAFLD. While not all of these models were exclusively developed in NALFD, they have been extensively evaluated in NAFLD using histology as the reference standard. 33 , 34 , 35 Most of these models are constructed to take advantage of the published literature demonstrating that older patients and those with diabetes are at higher risk for fibrosis progression, and as patient progress to cirrhosis, they will have a decline in platelet count and rise in the serum AST to ALT ratio. 20 Fibrosis 4 index (FIB‐4), NAFLD fibrosis score (NFS), AST/platelet ratio index (APRI), AST/ALT ratio, and body mass index (BMI), aspartate aminotransferase (AST):alanine aminotransferase (ALT), diabetes (BARD) score are among the most common noninvasive fibrosis models studied (Table 2). 36 , 37 , 38 , 39 , 40 , 41 The diagnostic accuracy for NASH, mild to severe fibrosis and cirrhosis among the most common noninvasive fibrosis models are discussed below.
TABLE 2.
Noninvasive fibrosis model
| Fibrosis model | Formula |
|---|---|
| FIB‐4 36 | (Age × AST)/(Platelets × log [ALT]) |
| NAFLD fibrosis score 37 | −1.675 + (0.037 × age [y]) + (0.094 × BMI [kg/m2]) + (1.13 × IFG/diabetes [yes = 1, no = 0]) + (0.99 × AST/ALT ratio) − (0.013 × platelet count [×109/L]) − (0.66 × albumin [g/dL]) |
| BARD 38 | BMI ≥ 28: No = 0, Yes = 1; AST/ALT ratio: ≥0.8 No = 0, Yes = 2; and Diabetes: No = 0, Yes = 1 |
| AST/ALT ratio 39 | AST in IU/L)/(ALT in IU/L) |
| BAAT 40 | AST/ALT ratio: ≥0.8 = 2 points; a BMI: ≥28 = 1 point; and the presence of diabetes: 1 point |
| APRI 41 | (AST in IU/L)/(AST Upper Limit of Normal in IU/L)/(Platelets in 109/L) |
2.1. FIB‐4
FIB‐4 was developed to model liver fibrosis in HIV/HCV co‐infection using platelet count (PLT), age, AST, and ALT 36 and has been extensively validated in NAFLD. 33 , 34 , 35 , 42 , 43 The diagnostic performance of FIB‐4 to identify varying degrees of hepatic fibrosis is variable and has the greatest accuracy in patients with advanced fibrosis or cirrhosis. 33 , 34 , 42 , 43 In a multicentre cohort of 1904 patients, the diagnostic accuracy of FIB‐4 to identify any fibrosis (fibrosis stage ≥ 1) was moderate with AUROC of 0.72 with PPV of 87%. 43 Its diagnostic accuracy for identifying moderate fibrosis (F ≥ 2‐4) was similar with AUROC of 0.73 and PPV of 70%; however, the diagnostic performance of FIB‐4 improved for identifying advanced with AUROC of 0.80. 43 It is important to note that while the PPV for FIB‐4 is marginal, the NPV is high at 88%, thereby allowing for FIB‐4 to be potentially incorporated in clinical practice to ‘rule‐out’ patients not at risk for significant disease. 43 Since the PPV is modest, those with higher FIB‐4 values require additional diagnostic workup to confirm these findings. 44 The ability of FIB‐4 to differentiate NASH vs non‐NASH has also been evaluated in published literature and is suboptimal to be incorporated into clinical practice. 33 , 45 Finally, FIB‐4 value of 2.67 or greater had a hazard ratio (HR) of 14.6 and 7 for liver‐related events (progression to cirrhosis, hepatic decompensation or need for LT) and death, respectively. 46
Recently, the diagnostic performance of FIB‐4 to predict fibrosis progression was evaluated in the NASH‐CRN cohort of patients with longitudinal biopsy. 43 In patients with nonadvanced fibrosis at baseline, FIB‐4 had an AUROC of 0.81 and NPV of 97% for predicting progression to advanced fibrosis even while accounting for age, BMI, hypertension, dyslipidemia and hypertension. Thus, delta FIB‐4 can be used in clinical practice to identify potential patients at risk for fibrosis progress, in whom additional confirmatory testing might be necessary to optimize clinical care.
2.2. NAFLD fibrosis score
The NFS incorporates patient's age, history of diabetes, weight, platelet count, albumin and AST/ALT ratio to identify NAFLD patients with advanced fibrosis. 37 Similar to FIB‐4, the diagnostic performance of NFS in patients with minimal fibrosis and moderate fibrosis is suboptimal. The AUROC, sensitivity, specificity, NPV and PPV for NFS are 0.69, 64%, 66%, 85% and 37% for predicting any fibrosis (fibrosis stage ≥ 1) and 0.73, 57%, 77%, 70% and 67% in moderate fibrosis (fibrosis stage ≥ 2), respectively. 43 The performance of NFS to identify patients with advanced fibrosis improves with an AUROC of 0.78, sensitivity of 70%, specificity of 74%, PPV of 51% and NPV of 86%, thus yielding an accurate test for excluding advanced fibrosis and dichotomizing patients for further investigation. 43 Similar to FIB‐4, NFS can also detect change in hepatic fibrosis with an AUROC of 0.80 and PPV and NPV of 39% and 95%, respectively. 43 Finally, NFS is unable to differentiate between patients with NASH vs non‐NASH.
The NFS ability to predict clinically relevant outcomes including death, need for LT and hepatic decompensation has been investigated previously. 46 , 47 , 48 In a cohort of 320 patients, an elevated NFS had an AUROC of 0.86 and 0.70 to predict liver‐related events and death/LT, respectively. 46 Importantly, the HR for liver events in intermediate (score between − 1.455 and 0.676) and high (score > 0.676) NFS risk categories was 7.7 and 34.2, respectively. The HR for mortality in intermediate and high risk was 4.2 and 9.8, respectively. 46 These findings regarding NFS ability to predict all‐cause mortality were confirmed in a cohort of Chinese population. 47 Similarly, a meta‐analysis by Salomone et al 48 correlated NAFLD patients with NFS > 0.676 a four‐fold higher risk of death.
2.3. BARD score
The BARD score models hepatic fibrosis using BMI, AST/ALT, and presence of diabetes. 38 In the initial study, the reported AUROC was 0.81 for predicting presence of advanced fibrosis with PPV and NPV of 43% and 96%, respectively. 38 However, BARD is limited by an ordinal scoring system which presents expected challenges when determining optimal cut‐off values to identify the at‐risk population. 49 BARD diagnostic performance of identifying presence of any and moderate fibrosis is low with AUROC of 0.67 and 0.69, respectively. 42 Furthermore, its NPV and PPV for patients with any fibrosis is also low (PPV 80% and NPV 43%) making it less useful clinically. The diagnostic performance for predicting the presence of advanced fibrosis is better than lower fibrosis stages prediction; however, it is less accurate when compared to other noninvasive fibrosis scores (ie NFS and FIB‐4) with a sensitivity and NPV of 39% and 73%, respectively. 42 The performance of BARD in predicting fibrosis progression is unknown. Similar to other noninvasive fibrosis models, BARD is unable to distinguish between patients with NASH vs non‐NASH. 42 The ability of BARD score to predict future clinically relevant outcomes is less than that of NFS and APRI, with an AUROC of just 0.73 for liver‐related events and 0.66 for mortality or LT. 46 The overall performance of BARD is substandard to both FIB‐4 and NFS, thus favouring limited use in clinical practice.
2.4. AST/ALT ratio
The AST/ALT ratio was developed to predict liver fibrosis in patients with chronic HCV infection by exploring the variation in liver enzymes from liver injury and was subsequently validated in NAFLD. 39 , 50 In a multicentre cohort of patients with histologically confirmed NAFLD, the diagnostic performance of AST/ALT ratio to predict presence of any fibrosis was marginal with AUROC 0.59, sensitivity of 38%, specificity of 76%, PPV of 83% and NPV of 28%. 43 The diagnostic performance improved slightly when identifying advanced fibrosis with an AUROC of 0.68, sensitivity of 54%, specificity 73%, PPV of 44% and NPV of 80%. 43 Since the performance of AST/ALT ratio is dependent on the premise of elevated aminotransferase and reversal of serum ALT to AST concentrations, it may not be as accurate in patients with less advanced disease. As a result, its isolated use is less prevalent and the ratio itself has been incorporated as part of other noninvasive fibrosis panel, such as FIB‐4 and NFS, and the omics approach. 36 , 37 , 51 The AST to ALT ratio can detect progression to advanced fibrosis, although inferior to APRI, FIB‐4 and NFS model score, with an a AUROC of 0.68, sensitivity of 71%, specificity of 76%, PPV of 36% and NPV of 93%. 43 The AST/ALT ratio's ability to differentiate between NASH vs non‐NASH is suboptimal as it is with other noninvasive models. 26 Furthermore, the ability of AST/ALT ratio to predict future risk of hepatic decompensation, death or need for LT remains unknown.
2.5. BAAT score
The BMI, age, ALT, triglyceride (BAAT) score is a noninvasive model that was developed in overweight patients to identify at‐risk patients with NAFLD. 40 Similar to BARD score, the clinical utility of BAAT score is limited by its ordinal scoring system. The diagnostic performance of BAAT in patients with any fibrosis is limited with an AUROC, sensitivity, specificity, PPV and NPV of 0.67, 90%, 35%, 78% and 60%, respectively. 40 Furthermore, in advanced fibrosis or cirrhosis the AUROC was 0.62; however, the sensitivity and NPV were 95% and 90%, respectively, making it an appropriate fibrosis model for advanced fibrosis and cirrhosis. In a head‐to‐head comparison with other noninvasive fibrosis model, BAAT score was inferior to other noninvasive models underscoring the limited increase in serum aminotransferases and lack of reciprocation of AST changes in patients with NAFLD. 42 BAAT is also not able to differentiate between patients with and without NASH. 42 The performance of BAAT in predicting fibrosis progression and clinically significant outcomes is unknown.
2.6. APRI
The APRI was developed to predict liver cirrhosis in chronic HCV patients leveraging that progression to cirrhosis and development of cirrhosis is associated with a decline in platelet count and an increase in serum AST levels. 41 Since its inception, APRI has been validated extensively in patients with NAFLD. 33 , 42 , 52 , 53 Similar to other noninvasive fibrosis models, APRI is unable to differentiate between NASH and non‐NASH. 42 The diagnostic accuracy of APRI for detecting any fibrosis is limited with an AUROC 0.75, sensitivity 63%, specificity 76%, PPV 89% and NPV of 40%. 43 Similarly, its diagnostic accuracy limitation is also present for detecting moderate fibrosis with an AUROC of 0.73, sensitivity of 65%, specificity of 71%, PPV of 67% and NPV of 69%. The diagnostic accuracy of APRI improves in patients with advanced fibrosis and cirrhosis with AUROC of 0.76 and sensitivity, specificity, PPV and NPV of 75%, 65%, 46% and 87%, respectively. 43 Although the diagnostic accuracy of APRI is superior to BAAT, BARD and AST/ALT ratio for detecting advanced fibrosis, it is inferior to that of FIB‐4 and NFS in a head‐to‐head comparison. APRI ability to detect fibrosis progression to advance fibrosis was recently established and predicts it with an AUROC of 0.82 with PPV of 34% and NPV of 97%. 43 Similar to FIB‐4, delta APRI can be used in clinical practice to identify risk for fibrosis progression. Finally, the HR of APRI to predict liver‐related events and death in high‐risk patient was 20.9 and 3.1, respectively. 46
3. BLOOD‐BASED BIOMARKERS
3.1. Enhanced liver fibrosis test
The enhanced liver fibrosis (ELF) test is a panel that measures by‐products of the fibrotic process that include tissue inhibitor of matrix metalloproteinase 1 (TIMP1), hyaluronic acid and aminoterminal peptide of pro‐collagen III (PIIINP). 54 In a multicentre cohort of 196 patients, ELF predicted presence of any fibrosis with an AUROC of 0.76 and limited sensitivity, specificity, PPV and NPV of 61%, 80%, 81% and 79%, respectively. The diagnostic accuracy in moderate fibrosis and severe fibrosis or cirrhosis improved with an AUROC of 0.82 and 0.90, respectively. 55 ELF test performance in moderate fibrosis had a sensitivity of 70%, specificity of 80%, PPV of 70% and NPV of 80%. 55 Furthermore, its performance in advanced fibrosis or cirrhosis improved with a sensitivity, specificity, PPV and NPV of 80%, 90%, 71% and 94%, respectively. 55 The diagnostic accuracy and overall performance of ELF in moderate and advanced fibrosis can be improved by supplementing it with clinical and laboratory information. 55 , 56 The combined panel's ability to detect moderate fibrosis improved with AUROC of 0.93, sensitivity of 89%, specificity of 86%, and NPV and PPV of 94% and 75%, respectively. 55 Its performance in severe fibrosis or cirrhosis was also better, yielding an AUROC of 0.98, sensitivity of 91%, specificity of 96%, NPV of 77% and PPV of 99%; however, further validation is needed. 55 Overall, the ELF test can predict clinical outcomes in patients with chronic liver disease but the data specifically to patients with NAFLD are unknown. 57 Moreover, its performance in identifying fibrosis progression remains unknown. ELF, similarly to most noninvasive fibrosis models test, has adequate performance in advanced fibrosis or cirrhosis and can be utilized for excluding advanced NAFLD.
3.2. Cytokeratin‐18
Cytokeratins are keratin‐containing proteins that comprise the structure of cytoskeletons of hepatocytes. 58 During apoptosis, circulating keratin 18 (CK18) is cleaved and generate detectable fragments with immunoassays, echoing liver injury in NAFLD. 59 , 60 , 61 CK‐18 fragments can be measured using either the M30 or M65 kit. The M30 kit measures caspase‐cleaved CK18 produced during apoptosis, while the M65 kit measures the levels of both caspase‐cleaved and intact CK18. 62 , 63 In a multicentre study, CK‐18 fragments measured by M30 ELISA kit had moderate performance in differentiating NASH from non‐NASH with AUROC of 0.83, specificity of 92% and sensitivity of 65%. 64 These findings were further validated in a meta‐analysis where CK18 fragments, measured by M30 ELISA kit, predicted NASH with a 60%‐88% sensitivity, 66%‐97% specificity and AUROC 0.70‐0.87. 65 Similar diagnostic accuracy has been reported with M65 testing of CK‐18 for detection of NASH. 66 Yet, only changes measured with M65 have been shown to detect mild fibrosis and predicting both NASH and fibrosis progression. 67 In a recent meta‐analysis, the diagnostic accuracy of CK‐18 to detect NASH was suboptimal with a pooled AUROC of 0.65. 68 To improve the diagnostic accuracy of CK‐18 in NASH, it has been combined with other biomarkers including ALT, platelets and triglycerides; adiponectin; and soluble Fas, with the latter providing the best diagnostic accuracy and overall performance with an AUROC of 0.93, sensitivity of 88%, specificity of 89%, PPV of 86% and NPV of 91%. 69 , 70 , 71 Most recently, CK‐18 fragment level was combined with FIB‐4 fibrosis model to yield a new scoring system with the accuracy and flexibility of predicting both NASH and presence of liver fibrosis. 72 Further validation of these combined panels is required.
3.3. Omics approach
The omics approach in NAFLD utilizes blood biomarkers that stems from genomics, transcriptomics, epigenomics, proteomics, lipidomics, and metabolomics and identify liver fibrosis by analysing patient's genetic expression, RNA transcripts production, genetic modification, change in protein production, fatty acid derivatives and body metabolism, respectively. 73 , 74 Type III procollagen (PRO‐C3), FibroTest® (FT), Hepascore and Fibrometer are among the most common panels derived from the omics approach. The diagnostic accuracy of the most common omics approach panels in NAFLD is discussed below.
3.4. Type III procollagen
The PRO‐C3 is a derived fragment of type III collagen liver deposition cleavage. 75 Its utility in NAFLD has mostly been shown in patients with advanced fibrosis. In a recent multicentre cohort of 449 patients with biopsy‐proven NAFLD, PRO‐C3 had an AUROC of 0.73 and sensitivity and specificity of 60% and 74%, respectively, for predicting presence of advanced fibrosis. 76 PRO‐C3 has been incorporated with clinical or serological markers to improve its performance. 76 , 77 FIBC3 is a combined panel, based on PRO‐C3, age, BMI, diabetes type 2 and platelet count, that has been tested with improved overall performance with an AUROC of 0.89, sensitivity of 83%, specificity of 80%, PPV of 74% and NPV of 88% in advanced liver fibrosis. 76 PRO‐C3 performance for predicting fibrosis progression has been validated on other advanced liver disease but its role in NAFLD is unknown. 78 , 79
3.5. Age, diabetes, PRO‐C3 and platelet count
The age, diabetes, PRO‐C3 and platelet count (ADAPT) score is PRO‐C3 based algorithm that additionally incorporates clinical and metabolic parameters associated with liver disease. 80 (Daniels, ADAPT) Similarly to PRO‐C3, its applicability in NAFLD is identifying patients with advanced fibrosis. In a multicentre cohort of 431 patients with biopsy‐proven NAFLD, the diagnostic accuracy of ADAPT was found to be superior to APRI, FIB‐4 and NFS noninvasive fibrosis models with an AUROC of 0.86 and a PPV and NPV of 48% and 97%, respectively. 80 ADAPT performance in identifying NAFLD fibrosis progression is unknown.
3.6. FibroTest®
The FT is a test based on α2‐macroglobulin, apolipoprotein A1, haptoglobin, total bilirubin and γ‐glutamyl transpeptidase (GGT) to predict fibrosis. 81 Multiple cohort studies have validated FT performance in NAFLD population. 82 , 83 , 84 In initial study, its performance in F ≥ 2 fibrosis yielded an AUROC of 0.86 and sensitivity and NPV of 77% and 90%, respectively. 40 Furthermore, its diagnostic accuracy in advanced fibrosis improved with AUROC, sensitivity and NPV of 0.92, 92% and 98%, respectively. 40 FT fibrosis model may have a role in assessing liver fibrosis progression; however, further validation is required. 85
3.7. Hepascore
The Hepascore is a serum‐based model that was developed to predict fibrosis severity in chronic liver disease. 86 This panel incorporates clinical variables, such as age and gender, and serum biomarkers including bilirubin, GGT, hyaluronic acid and α2‐macroglobulin. 86 In a cohort of 242 subjects with NAFLD, Hepascore diagnostic accuracy for moderate fibrosis was marginal with an AUROC of 0.73 in comparison to advanced fibrosis (AUROC of 0.80) or cirrhosis (AUROC of 0.86). 35 Furthermore, the sensitivity, specificity, PPV and NPV of Hepascore in advanced fibrosis was 76%, 84%, 57% and 92%, respectively. 35 Hepascore performance in predicting liver‐related mortality and morbidity has been validated in other chronic liver disease but its role in NAFLD is unknown. 87 Finally, as in other omics derived fibrosis tests, Hepascore performance in fibrosis progression is unknown.
3.8. FibroMeter
FibroMeter is a serum‐based panel that integrates glucose, AST, ALT, ferritin, platelet, body weight and age to predict fibrosis in NAFLD patients. 51 In a cohort of 235 patients, FibroMeter diagnostic accuracy was high in identifying significant (AUROC of 0.94). Furthermore, its sensitivity, specificity, PPV and NPV was 79%, 96%, 88% and 92%, respectively. The overall diagnostic performance of FibroMeter is high and may have a role in both the screening and diagnosing of fibrosis. However, FibroMeter appears to be limited by its inability to differentiate between the degrees of fibrosis. Thus, further study validating FibroMeter is required. 88 Lastly, its role for predicting long‐term outcome or fibrosis progression is unknown.
3.8.1. Vibration‐controlled transient elastography
Vibration‐controlled transient elastography (VCTE) is a noninvasive biomarker that utilizes shear wave speed across the liver to derive its stiffness and thus hepatic fibrosis. 89 Similar to noninvasive models and blood‐based biomarkers, VCTE performance has been validated in accurately distinguishing advanced from early fibrosis in several multicenter cohort's studies. 90 , 91 , 92 , 93 , 94 In a recent prospective cohort study of 393 patients with biopsy‐proven NAFLD, VCTE performed with a sensitivity of 90% and 90% and NPV of 91% and 99% at excluding advance fibrosis and cirrhosis, respectively. Furthermore, VCTE diagnostic accuracy was high with an AUROC of 0.83. 90 In a head‐to‐head comparison to the most validated noninvasive models, VCTE outperformed APRI, FIB4, BARD and NFS at excluding advanced fibrosis with similar performance as mentioned above. 92 , 95 Nevertheless, noninvasive models are a simple and more accessible tools to primary health provider that can easily be extracted from readily available clinical and laboratory information and accurately exclude advanced fibrosis and cirrhosis.
4. CONCLUSION
In summary, noninvasive fibrosis models, blood‐based biomarkers and combined panels derived from omics approach can potentially be used in clinical practice as point‐of‐care test to risk stratify patients with NAFLD. Noninvasive diagnostic tools, in particular FIB‐4 and NFS, are great screening tools that can accurately exclude advanced fibrosis and cirrhosis, predict fibrosis, and dichotomize patient for further workup with readily available clinical and laboratory information without additional cost. Further testing and validation is required before considering these fibrosis models as the standard of care in primary care to impact referral and additional diagnostic workup.
CONFLICT OF INTEREST
Nothing to declare.
AUTHOR CONTRIBUTIONS
Jose Hernandez Roman drafted the manuscript. Mohammad S. Siddiqui was involved in the critical revision of the manuscript.
ETHICAL APPROVAL
No funding/financial support was received for the conduct of this research or for the preparation of this article.
Hernandez Roman J, Siddiqui MS. The role of noninvasive biomarkers in diagnosis and risk stratification in nonalcoholic fatty liver disease. Endocrinol Diab Metab. 2020;3:e00127 10.1002/edm2.127
REFERENCES
- 1. Siddiqui MS, Harrison SA, Abdelmalek MF, et al. Case definitions for inclusion and analysis of endpoints in clinical trials for nonalcoholic steatohepatitis through the lens of regulatory science. Hepatology. 2018;67(5):2001‐2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Younossi ZM, Koenig AB, Abdelatif D, Fazel Y, Henry L, Wymer M. Global epidemiology of nonalcoholic fatty liver disease—Meta‐analytic assessment of prevalence, incidence, and outcomes. Hepatology. 2016;64(1):73‐84. [DOI] [PubMed] [Google Scholar]
- 3. Younossi ZM, Stepanova M, Afendy M, et al. Changes in the prevalence of the most common causes of chronic liver diseases in the United States from 1988 to 2008. Clin Gastroenterol Hepatol. 2011;9(6):524‐530.e1; quiz e60. [DOI] [PubMed] [Google Scholar]
- 4. Portillo‐Sanchez P, Bril F, Maximos M, et al. High prevalence of nonalcoholic fatty liver disease in patients with type 2 diabetes mellitus and normal plasma aminotransferase levels. J Clin Endocrinol Metab. 2015;100(6):2231‐2238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. DeFilippis AP, Blaha MJ, Martin SS, et al. Nonalcoholic fatty liver disease and serum lipoproteins: the Multi‐Ethnic Study of Atherosclerosis. Atherosclerosis. 2013;227(2):429‐436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ma J, Hwang S‐J, Pedley A, et al. Bidirectional relationship between fatty liver and cardiovascular disease risk factors. J Hepatol. 2017;66(2):390‐397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kim CH, Younossi ZM. Nonalcoholic fatty liver disease: a manifestation of the metabolic syndrome. Cleve Clin J Med. 2008;75(10):721‐728. [DOI] [PubMed] [Google Scholar]
- 8. Kleiner DE, Brunt EM, Van Natta M, et al. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology. 2005;41(6):1313‐1321. [DOI] [PubMed] [Google Scholar]
- 9. Siddiqui MS, Sterling RK, Luketic VA, et al. Association between high‐normal levels of alanine aminotransferase and risk factors for atherogenesis. Gastroenterology. 2013;145(6):1271‐1279.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Blais P, Husain N, Kramer JR, Kowalkowski M, El‐Serag H, Kanwal F. Nonalcoholic fatty liver disease is underrecognized in the primary care setting. Am J Gastroenterol. 2015;110(1):10‐14. [DOI] [PubMed] [Google Scholar]
- 11. Wong RJ, Cheung R, Ahmed A. Nonalcoholic steatohepatitis is the most rapidly growing indication for liver transplantation in patients with hepatocellular carcinoma in the U.S. Hepatology. 2014;59(6):2188‐2195. [DOI] [PubMed] [Google Scholar]
- 12. Wong RJ, Aguilar M, Cheung R, et al. Nonalcoholic steatohepatitis is the second leading etiology of liver disease among adults awaiting liver transplantation in the United States. Gastroenterology. 2015;148(3):547‐555. [DOI] [PubMed] [Google Scholar]
- 13. Starley BQ, Calcagno CJ, Harrison SA. Nonalcoholic fatty liver disease and hepatocellular carcinoma: a weighty connection. Hepatology. 2010;51(5):1820‐1832. [DOI] [PubMed] [Google Scholar]
- 14. Ong JP, Younossi ZM. Epidemiology and natural history of NAFLD and NASH. Clin Liver Dis. 2007;11(1):1‐16. [DOI] [PubMed] [Google Scholar]
- 15. Argo CK, Northup PG, Al‐Osaimi AMS, Caldwell SH. Systematic review of risk factors for fibrosis progression in non‐alcoholic steatohepatitis. J Hepatol. 2009;51(2):371‐379. [DOI] [PubMed] [Google Scholar]
- 16. Adams LA, Lymp JF, Sauver JS, et al. The natural history of nonalcoholic fatty liver disease: a population‐based cohort study. Gastroenterology. 2005;129(1):113‐121. [DOI] [PubMed] [Google Scholar]
- 17. Ekstedt M, Hagström H, Nasr P, et al. Fibrosis stage is the strongest predictor for disease‐specific mortality in NAFLD after up to 33 years of follow‐up. Hepatology. 2015;61(5):1547‐1554. [DOI] [PubMed] [Google Scholar]
- 18. Younossi ZM, Stepanova M, Rafiq N, et al. Pathologic criteria for nonalcoholic steatohepatitis: interprotocol agreement and ability to predict liver‐related mortality. Hepatology. 2011;53(6):1874‐1882. [DOI] [PubMed] [Google Scholar]
- 19. Hagström H, Nasr P, Ekstedt M, et al. Fibrosis stage but not NASH predicts mortality and time to development of severe liver disease in biopsy‐proven NAFLD. J Hepatol. 2017;67(6):1265‐1273. [DOI] [PubMed] [Google Scholar]
- 20. Angulo P, Kleiner DE, Dam‐Larsen S, et al. Liver fibrosis, but no other histologic features, is associated with long‐term outcomes of patients with nonalcoholic fatty liver disease. Gastroenterology. 2015;149(2):389‐397.e10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Rockey DC, Caldwell SH, Goodman ZD, Nelson RC, Smith AD. Liver biopsy. Hepatology. 2009;49(3):1017‐1044. [DOI] [PubMed] [Google Scholar]
- 22. Castéra L, Nègre I, Samii K, Buffet C. Pain experienced during percutaneous liver biopsy. Hepatology. 1999;30(6):1529‐1530. [DOI] [PubMed] [Google Scholar]
- 23. Piccinino F, Sagnelli E, Pasquale G, Giusti G. Complications following percutaneous liver biopsy. A multicentre retrospective study on 68,276 biopsies. J Hepatol. 1986;2(2):165‐173. [DOI] [PubMed] [Google Scholar]
- 24. Adams LA, Angulo P, Lindor KD. Nonalcoholic fatty liver disease. CMAJ. 2005;172(7):899‐905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Bedogni G, Bellentani S, Miglioli L, et al. The fatty liver index: a simple and accurate predictor of hepatic steatosis in the general population. BMC Gastroenterol. 2006;6:33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kotronen A, Peltonen M, Hakkarainen A, et al. Prediction of non‐alcoholic fatty liver disease and liver fat using metabolic and genetic factors. Gastroenterology. 2009;137(3):865‐872. [DOI] [PubMed] [Google Scholar]
- 27. Amato MC, Giordano C, Galia M, et al. Visceral adiposity index: a reliable indicator of visceral fat function associated with cardiometabolic risk. Diabetes Care. 2010;33(4):920‐922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Guerrero‐Romero F, Simental‐Mendía LE, González‐Ortiz M, et al. The product of triglycerides and glucose, a simple measure of insulin sensitivity. Comparison with the euglycemic‐hyperinsulinemic clamp. J Clin Endocrinol Metab. 2010;95(7):3347‐3351. [DOI] [PubMed] [Google Scholar]
- 29. Lee J‐H, Kim D, Kim HJ, et al. Hepatic steatosis index: a simple screening tool reflecting nonalcoholic fatty liver disease. Dig Liver Dis. 2010;42(7):503‐508. [DOI] [PubMed] [Google Scholar]
- 30. Petta S, Amato M, Cabibi D, et al. Visceral adiposity index is associated with histological findings and high viral load in patients with chronic hepatitis C due to genotype 1. Hepatology. 2010;52(5):1543‐1552. [DOI] [PubMed] [Google Scholar]
- 31. Poynard T, Ratziu V, Naveau S, et al. The diagnostic value of biomarkers (SteatoTest) for the prediction of liver steatosis. Comp Hepatol. 2005;4:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Fedchuk L, Nascimbeni F, Pais R, Charlotte F, Housset C, Ratziu V. Performance and limitations of steatosis biomarkers in patients with nonalcoholic fatty liver disease. Aliment Pharmacol Ther. 2014;40(10):1209‐1222. [DOI] [PubMed] [Google Scholar]
- 33. Musso G, Gambino R, Cassader M, Pagano G. Meta‐analysis: natural history of non‐alcoholic fatty liver disease (NAFLD) and diagnostic accuracy of non‐invasive tests for liver disease severity. Ann Med. 2011;43(8):617‐649. [DOI] [PubMed] [Google Scholar]
- 34. McPherson S, Stewart SF, Henderson E, Burt AD, Day CP. Simple non‐invasive fibrosis scoring systems can reliably exclude advanced fibrosis in patients with non‐alcoholic fatty liver disease. Gut. 2010;59(9):1265‐1269. [DOI] [PubMed] [Google Scholar]
- 35. Adams LA, George J, Bugianesi E, et al. Complex non‐invasive fibrosis models are more accurate than simple models in non‐alcoholic fatty liver disease. J Gastroenterol Hepatol. 2011;26(10):1536‐1543. [DOI] [PubMed] [Google Scholar]
- 36. Sterling RK, Lissen E, Clumeck N, et al. Development of a simple noninvasive index to predict significant fibrosis in patients with HIV/HCV coinfection. Hepatology. 2006;43(6):1317‐1325. [DOI] [PubMed] [Google Scholar]
- 37. Kim D, Kim WR, Kim HJ, Therneau TM. Association between non‐invasive fibrosis markers and mortality among adults with non‐alcoholic fatty liver disease in the United States. Hepatology. 2013;57(4):1357‐1365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Harrison SA, Oliver D, Arnold HL, Gogia S, Neuschwander‐Tetri BA. Development and validation of a simple NAFLD clinical scoring system for identifying patients without advanced disease. Gut. 2008;57(10):1441‐1447. [DOI] [PubMed] [Google Scholar]
- 39. Sheth SG, Flamm SL, Gordon FD, Chopra S. AST/ALT ratio predicts cirrhosis in patients with chronic hepatitis C virus infection. Am J Gastroenterol. 1998;93(1):44‐48. [DOI] [PubMed] [Google Scholar]
- 40. Ratziu V, Massard J, Charlotte F, et al. Diagnostic value of biochemical markers (FibroTest‐FibroSURE) for the prediction of liver fibrosis in patients with non‐alcoholic fatty liver disease. BMC Gastroenterol. 2006;6(1):6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Wai C‐T, Greenson JK, Fontana RJ, et al. A simple noninvasive index can predict both significant fibrosis and cirrhosis in patients with chronic hepatitis C. Hepatology. 2003;38(2):518‐526. [DOI] [PubMed] [Google Scholar]
- 42. Siddiqui MS, Patidar KR, Boyett S, Luketic VA, Puri P, Sanyal AJ. Performance of non‐invasive models of fibrosis in predicting mild to moderate fibrosis in patients with non‐alcoholic fatty liver disease. Liver Int. 2016;36(4):572‐579. [DOI] [PubMed] [Google Scholar]
- 43. Siddiqui MS, Yamada G, Vuppalanchi R, et al. Diagnostic accuracy of noninvasive fibrosis models to detect change in fibrosis stage. Clin Gastroenterol Hepatol. 2019;17(9):1877‐1885.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Puri P, Sanyal AJ. Nonalcoholic fatty liver disease: definitions, risk factors, and workup. Clin Liver Dis (Hoboken). 2012;1(4):99‐103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. McPherson S, Hardy T, Henderson E, Burt AD, Day CP, Anstee QM. Evidence of NAFLD progression from steatosis to fibrosing‐steatohepatitis using paired biopsies: Implications for prognosis and clinical management. J Hepatol. 2015;62(5):1148‐1155. [DOI] [PubMed] [Google Scholar]
- 46. Angulo P, Bugianesi E, Bjornsson ES, et al. Simple noninvasive systems predict long‐term outcomes of patients with nonalcoholic fatty liver disease. Gastroenterology. 2013;145(4):782‐9.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Xun Y‐H, Guo J‐C, Lou G‐Q, et al. Non‐alcoholic fatty liver disease (NAFLD) fibrosis score predicts 6.6‐year overall mortality of Chinese patients with NAFLD. Clin Exp Pharmacol Physiol. 2014;41(9):643‐649. [DOI] [PubMed] [Google Scholar]
- 48. Salomone F, Micek A, Godos J. Simple scores of fibrosis and mortality in patients with NAFLD: a systematic review with meta‐analysis. J Clin Med. 2018;7(8):219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Shah AG, Lydecker A, Murray K, Tetri BN, Contos MJ, Sanyal AJ. Comparison of noninvasive markers of fibrosis in patients with nonalcoholic fatty liver disease. Clin Gastroenterol Hepatol. 2009;7(10):1104‐1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Angulo P, Keach JC, Batts KP, Lindor KD. Independent predictors of liver fibrosis in patients with nonalcoholic steatohepatitis. Hepatology. 1999;30(6):1356‐1362. [DOI] [PubMed] [Google Scholar]
- 51. Calès P, Oberti F, Michalak S, et al. A novel panel of blood markers to assess the degree of liver fibrosis. Hepatology. 2005;42(6):1373‐1381. [DOI] [PubMed] [Google Scholar]
- 52. Kruger FC, Daniels CR, Kidd M, et al. A simple bedside marker for advanced fibrosis that can avoid liver biopsy in patients with NAFLD/NASH. S Afr Med J. 2011;101(7):477‐480. [PubMed] [Google Scholar]
- 53. Loaeza‐del‐Castillo A, Paz‐Pineda F, Oviedo‐Cárdenas E, Sánchez‐Avila F, Vargas‐Vorácková F. AST to platelet ratio index (APRI) for the noninvasive evaluation of liver fibrosis. Ann Hepatol. 2008;7(4):350‐357. [PubMed] [Google Scholar]
- 54. Rosenberg WMC, Voelker M, Thiel R, et al. Serum markers detect the presence of liver fibrosis: a cohort study. Gastroenterology. 2004;127(6):1704‐1713. [DOI] [PubMed] [Google Scholar]
- 55. Guha IN, Parkes J, Roderick P, et al. Noninvasive markers of fibrosis in nonalcoholic fatty liver disease: Validating the European Liver Fibrosis Panel and exploring simple markers. Hepatology. 2008;47(2):455‐460. [DOI] [PubMed] [Google Scholar]
- 56. Crespo G, Fernández‐Varo G, Mariño Z, et al. ARFI, FibroScan®, ELF, and their combinations in the assessment of liver fibrosis: a prospective study. J Hepatol. 2012;57(2):281‐287. [DOI] [PubMed] [Google Scholar]
- 57. Parkes J, Roderick P, Harris S, et al. Enhanced liver fibrosis test can predict clinical outcomes in patients with chronic liver disease. Gut. 2010;59(9):1245‐1251. [DOI] [PubMed] [Google Scholar]
- 58. Moll R, Franke WW, Schiller DL, Geiger B, Krepler R. The catalog of human cytokeratins: patterns of expression in normal epithelia, tumors and cultured cells. Cell. 1982;31(1):11‐24. [DOI] [PubMed] [Google Scholar]
- 59. Bantel H, Ruck P, Gregor M, Schulze‐Osthoff K. Detection of elevated caspase activation and early apoptosis in liver diseases. Eur J Cell Biol. 2001;80(3):230‐239. [DOI] [PubMed] [Google Scholar]
- 60. Wieckowska A, Zein NN, Yerian LM, Lopez AR, McCullough AJ, Feldstein AE. In vivo assessment of liver cell apoptosis as a novel biomarker of disease severity in nonalcoholic fatty liver disease. Hepatology. 2006;44(1):27‐33. [DOI] [PubMed] [Google Scholar]
- 61. Feldstein AE, Canbay A, Angulo P, et al. Hepatocyte apoptosis and fas expression are prominent features of human nonalcoholic steatohepatitis. Gastroenterology. 2003;125(2):437‐443. [DOI] [PubMed] [Google Scholar]
- 62. Leers MP, Kölgen W, Björklund V, et al. Immunocytochemical detection and mapping of a cytokeratin 18 neo‐epitope exposed during early apoptosis. J Pathol. 1999;187(5):567‐572. [DOI] [PubMed] [Google Scholar]
- 63. Kramer G, Erdal H, Mertens HJMM, et al. Differentiation between cell death modes using measurements of different soluble forms of extracellular cytokeratin 18. Cancer Res. 2004;64(5):1751‐1756. [DOI] [PubMed] [Google Scholar]
- 64. Feldstein AE, Wieckowska A, Lopez AR, Liu Y‐C, Zein NN, McCullough AJ. Cytokeratin‐18 fragment levels as noninvasive biomarkers for nonalcoholic steatohepatitis: a multicenter validation study. Hepatology. 2009;50(4):1072‐1078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Kwok R, Tse Y‐K, Wong GL‐H, et al. Systematic review with meta‐analysis: non‐invasive assessment of non‐alcoholic fatty liver disease – the role of transient elastography and plasma cytokeratin‐18 fragments. Aliment Pharmacol Ther. 2014;39(3):254‐269. [DOI] [PubMed] [Google Scholar]
- 66. He L, Deng L, Zhang Q, et al. Diagnostic value of CK‐18, FGF‐21, and related biomarker panel in nonalcoholic fatty liver disease: a systematic review and meta‐analysis. Biomed Res Int. 2017;2017:1‐12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Shen J, Chan HL‐Y, Wong GL‐H, et al. Assessment of non‐alcoholic fatty liver disease using serum total cell death and apoptosis markers. Aliment Pharmacol Ther. 2012;36(11–12):1057‐1066. [DOI] [PubMed] [Google Scholar]
- 68. Cusi K, Chang Z, Harrison S, et al. Limited value of plasma cytokeratin‐18 as a biomarker for NASH and fibrosis in patients with non‐alcoholic fatty liver disease. J Hepatol. 2014;60(1):167‐174. [DOI] [PubMed] [Google Scholar]
- 69. Tamimi TIA‐R, Elgouhari HM, Alkhouri N, et al. An apoptosis panel for nonalcoholic steatohepatitis diagnosis. J Hepatol. 2011;54(6):1224‐1229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Younossi ZM, Jarrar M, Nugent C, et al. A novel diagnostic biomarker panel for obesity‐related nonalcoholic steatohepatitis (NASH). Obes Surg. 2008;18(11):1430‐1437. [DOI] [PubMed] [Google Scholar]
- 71. Cao W, Zhao C, Shen C, Wang Y. Cytokeratin 18, alanine aminotransferase, platelets and triglycerides predict the presence of nonalcoholic steatohepatitis. PLoS ONE. 2013;8(12):e82092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Tada T, Kumada T, Toyoda H, Saibara T, Ono M, Kage M. New scoring system combining the FIB‐4 index and cytokeratin‐18 fragments for predicting steatohepatitis and liver fibrosis in patients with nonalcoholic fatty liver disease. Biomarkers. 2018;23(4):328‐334. [DOI] [PubMed] [Google Scholar]
- 73. Hasin Y, Seldin M, Lusis A. Multi‐omics approaches to disease. Genome Biol. 2017;18(1):83 10.1186/s13059-017-1215-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Martel C, Esposti DD, Bouchet A, Brenner C, Lemoine A. Non‐alcoholic steatohepatitis: new insights from OMICS studies. Curr Pharm Biotechnol. 2012;13(5):726‐735. [DOI] [PubMed] [Google Scholar]
- 75. Nielsen MJ, Nedergaard AF, Sun S, et al. The neo‐epitope specific PRO‐C3 ELISA measures true formation of type III collagen associated with liver and muscle parameters. Am J Transl Res. 2013;5(3):303‐315. [PMC free article] [PubMed] [Google Scholar]
- 76. Boyle M, Tiniakos D, Schattenberg JM, et al. Performance of the PRO‐C3 collagen neo‐epitope biomarker in non‐alcoholic fatty liver disease. JHEP Rep. 2019;1(3):188‐198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Daniels SJ, Nielsen MJ, Krag A, et al. Serum Pro‐C3 combined with clinical parameters is superior to established serological fibrosis tests at identifying patients with advanced fibrosis among patients with non‐alcoholic fatty liver disease. J Hepatol. 2017;66(1):S671. [Google Scholar]
- 78. Karsdal MA, Henriksen K, Nielsen MJ, et al. Fibrogenesis assessed by serological type III collagen formation identifies patients with progressive liver fibrosis and responders to a potential antifibrotic therapy. Am J Physiol Gastrointest Liver Physiol. 2016;311(6):G1009‐G1017. [DOI] [PubMed] [Google Scholar]
- 79. Nielsen MJ, Veidal SS, Karsdal MA, et al. Plasma Pro‐C3 (N‐terminal type III collagen propeptide) predicts fibrosis progression in patients with chronic hepatitis C. Liver Int. 2015;35(2):429‐437. [DOI] [PubMed] [Google Scholar]
- 80. Daniels SJ, Leeming DJ, Eslam M, et al. ADAPT: an algorithm incorporating PRO‐C3 accurately identifies patients with NAFLD and advanced fibrosis. Hepatology. 2019;69(3):1075‐1086. [DOI] [PubMed] [Google Scholar]
- 81. Poynard T, Imbert‐Bismut F, Munteanu M, et al. Overview of the diagnostic value of biochemical markers of liver fibrosis (FibroTest, HCV FibroSure) and necrosis (ActiTest) in patients with chronic hepatitis C. Comp Hepatol. 2004;3(1):8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Lassailly G, Caiazzo R, Hollebecque A, et al. Validation of noninvasive biomarkers (FibroTest, SteatoTest, and NashTest) for prediction of liver injury in patients with morbid obesity. Eur J Gastro Hepatol. 2011;23(6):499‐506. [DOI] [PubMed] [Google Scholar]
- 83. Poynard T, Lassailly G, Diaz E, et al. Performance of biomarkers FibroTest, ActiTest, SteatoTest, and NashTest in patients with severe obesity: meta analysis of individual patient data. PLoS ONE. 2012;7(3):e30325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Diagnostic performance of FibroTest, SteatoTest and ActiTest in patients with NAFLD using the SAF score as histological reference – Munteanu – 2016 – Alimentary Pharmacology & Therapeutics – Wiley Online Library; 2016. https://onlinelibrary.wiley.com/doi/full/10.1111/apt.13770. Accessed August 18, 2019. [DOI] [PMC free article] [PubMed]
- 85. Poynard T, Munteanu M, Deckmyn O, et al. Validation of liver fibrosis biomarker (FibroTest) for assessing liver fibrosis progression: Proof of concept and first application in a large population. J Hepatol. 2012;57(3):541‐548. [DOI] [PubMed] [Google Scholar]
- 86. Huang Y, Adams LA, Joseph J, Bulsara MK, Jeffrey GP. The ability of Hepascore to predict liver fibrosis in chronic liver disease: a meta‐analysis. Liver International. 2017;37(1):121‐131. [DOI] [PubMed] [Google Scholar]
- 87. Jeffrey AW, Huang Y, de Boer WB, et al. Improved Hepascore in hepatitis C predicts reversal in risk of adverse outcome. World J Hepatol. 2017;9(19):850‐856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Calès P, Lainé F, Boursier J, et al. Comparison of blood tests for liver fibrosis specific or not to NAFLD. J Hepatol. 2009;50(1):165‐173. [DOI] [PubMed] [Google Scholar]
- 89. Wong VW‐S, Vergniol J, Wong GL‐H, et al. Diagnosis of fibrosis and cirrhosis using liver stiffness measurement in nonalcoholic fatty liver disease. Hepatology. 2010;51(2):454‐462. [DOI] [PubMed] [Google Scholar]
- 90. Siddiqui MS, Vuppalanchi R, Van Natta ML, et al. Vibration‐controlled transient elastography to assess fibrosis and steatosis in patients with nonalcoholic fatty liver disease. Clin Gastroenterol Hepatol. 2019;17(1):156‐163.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Eddowes PJ, Sasso M, Allison M, et al. Accuracy of FibroScan controlled attenuation parameter and liver stiffness measurement in assessing steatosis and fibrosis in patients with nonalcoholic fatty liver disease. Gastroenterology. 2019;156(6):1717‐1730. [DOI] [PubMed] [Google Scholar]
- 92. Tovo CV, Villela‐Nogueira CA, Leite NC, et al. Transient hepatic elastography has the best performance to evaluate liver fibrosis in non‐alcoholic fatty liver disease (NAFLD). Ann Hepatol. 2019;18(3):445‐449. [DOI] [PubMed] [Google Scholar]
- 93. Tapper EB, Challies T, Nasser I, Afdhal NH, Lai M. The performance of vibration controlled transient elastography in a US Cohort of patients with nonalcoholic fatty liver disease. Am J Gastroenterol. 2016;111(5):677‐684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Park CC, Nguyen P, Hernandez C, et al. Magnetic resonance elastography vs transient elastography in detection of fibrosis and noninvasive measurement of steatosis in patients with biopsy‐proven nonalcoholic fatty liver disease. Gastroenterology. 2017;152(3):598‐607.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Petta S, Wong VW‐S, Cammà C, et al. Serial combination of non‐invasive tools improves the diagnostic accuracy of severe liver fibrosis in patients with NAFLD. Aliment Pharmacol Ther. 2017;46(6):617‐627. [DOI] [PubMed] [Google Scholar]


