Abstract
Myelin Oligodendrocyte Glycoprotein Antibody Disease (MOGAD) represents a demyelinating disorder for which tocilizumab, an anti-IL6 receptor, has been tested to prevent disabling relapses. In a subgroup of patients affected with novel Coronavirus disease (COVID-19), tocilizumab has also increased the survival rate. We present the case of a 31-years-old Caucasian patient who experienced an almost asymptomatic Severe Acute Respiratory Syndrome Coronavirus (SARS-CoV-2) infection during treatment with tocilizumab, which was continued due to the very high risk of relapses of the patient. According to this case, tocilizumab might be not discontinued during COVID-19.
Keywords: COVID-19, SARS-CoV-2, MOGAD, Tocilizumab, Monoclonal antibody, Anti-IL6 receptor
1. Case report
Myelin Oligodendrocyte Glycoprotein (MOG) Antibody Disease (MOGAD) represents a demyelinating disorder characterized by attacks of optic neuritis (ON) and/or episodes of myelitis (Wynford-Thomas et al., 2019). Antibodies to MOG (MOG-Abs) have been consistently identified in several demyelinating syndromes and between them in adults affected with Aquaporin-4 seronegative Neuromyelitis Optica spectrum disorder (Jarius et al., 2016).
Several therapies have been tested in order to prevent the potentially disabling relapses in MOGAD and among them the anti-IL-6 receptor tocilizumab has been newly introduced (Whittam et al., 2020). During the recent pandemy of Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2), this monoclonal antibody has also represented an alternative treatment for a sub-group of patients affected with novel Coronavirus disease (COVID-19) (Toniati et al., 2020).
We describe a case of a 31-year-old Caucasian woman who had a history of paraesthesias in the left upper limb occurred in 2009. In 2014 she experienced an episode of left ON and was diagnosed with multiple sclerosis due to clinical and brain Magnetic Resonance Imaging (MRI) findings. Therefore, subcutaneous Interferon-1β every-other-day was started.
In 2017 she presented acute tetraparesis with left hemianaesthesia. Spinal cord MRI showed multiple gadolinium enhanced lesions suggestive of transverse myelitis and MOGAD diagnosis was formulated according to the presence of MOG-Abs. Thus, the patient received five 1000 mg intravenous rituximab infusions every 24 weeks. In May 2019, a spinal cord MRI revealed new cervical active lesions in association with full CD19+ lymphocytes depletion. Consequently, monthly intravenous tocilizumab was started in June 2019.
On February 24th 2020, the subject presented the sudden onset of anosmia and generalized myalgia lasting 3–4 days, without fever. However, she did not report such symptoms to the treating Neurologist and tocilizumab infusion was routinely administered on the 6th of March. It is important to note that the Italian population was not fully aware of the symptoms of COVID-19 at that moment.
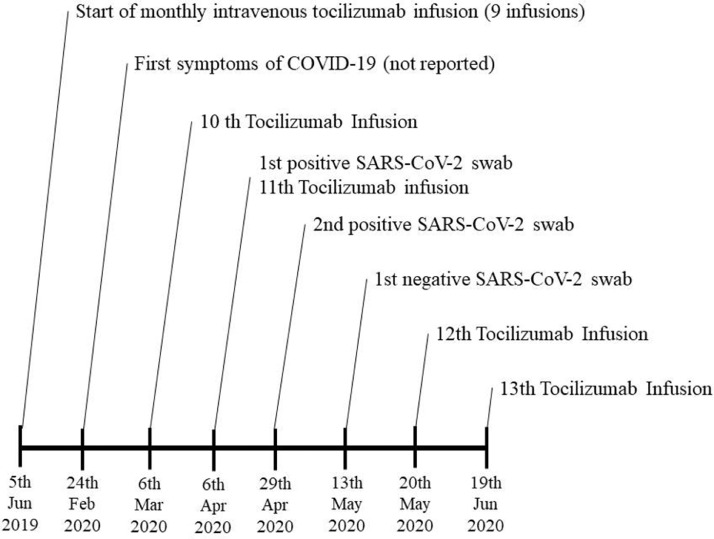
On the 6th of April, the patient referred again to the hospital to undergo a new tocilizumab infusion and, after describing symptoms of COVID-19 in an ad-hoc questionnaire developed to control the access to the hospital, a reverse transcription-polymerase chain reaction (RT-PCR) nasopharyngeal swab was performed, exhibiting a positive result. For the indispensability of the therapy, in a patient at very high risk of relapse, it was decided to perform tocilizumab infusion. The patient, isolated at home, had a new positive finding on the 29th of April and a negative result on May 13th, undergoing a new tocilizumab infusion one week later (see Fig. 1 for further details).
Fig. 1.
Timeline of tocilizumab treatment during Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) infection.
It is important to note that, while the patient was in treatment with tocilizumab, she experienced an almost asymptomatic SARS-CoV-2 infection (confirmed by two positive swabs) and did not need any intervention or hospitalization. Furthermore, her clinical situation, characterized by paraparesis with a Medical Research Council strength 3/5, and an Expanded Disability Status Scale of 6.5, was not modified, and she mantained her own walking ability.
COVID-19 might provoke a cytokine-mediated hyperinflammatory syndrome with rapid onset of respiratory failure and tocilizumab improved survival rate in critically compromised COVID-19 patients (Capra et al., 2020). A purely speculative question arises also on the potential beneficial effect of this anti-IL6 receptor in demodulating a potentially devastating viral infection in a patient with an immuno-mediated condition such as MOGAD.
When considering this single case, it might be deemed that tocilizumab might be not discontinued also during SARS-CoV-2 infection, in particular in a very “active” subject who experienced two previous myelitis and failed Rituximab therapy, in fact being at very high risk of relapses, potentially induced by the cytokine storm deriving from COVID-19.
Another consideration should be done, i.e. if this might have been in any case an almost asymptomatic infection regardless of treatment and primary disease. At the moment, however, the answer to these issues cannot be given and further studies should be performed also in individuals affected by these demyelinating diseases.
Informed consent
Written informed consent was collected from the patient for the inclusion of anonymized clinical data in a scientific publication
Funding statement
No funding was received for this case report.
Submission declaration and verification
This work has not been published previously and it is not under consideration for publication elsewhere. Its publication is approved by all authors. If accepted, it will not be published elsewhere in the same form, in English or in any other language, including electronically without the written consent of the copyright holder.
CRediT authorship contribution statement
Fabio Giuseppe Masuccio: Writing - original draft, Investigation, Resources, Visualization. Marianna Lo Re: Investigation, Resources. Antonio Bertolotto: Supervision, Resources. Marco Capobianco: Investigation, Resources, Supervision, Writing - review & editing. Claudio Solaro: Conceptualization, Investigation, Resources, Supervision, Writing - review & editing.
Declaration of Competing Interest
Fabio Giuseppe Masuccio and Marianna Lo Re declare no conflict of interest. Antonio Bertolotto served on the advisory boards of Alexion, Biogen, Novartis, Sanofi and/or received speaking honoraria from Alexion, Biogen, Novartis, Sanofi and grant support from Almiral, Biogen, Associazione San Luigi Gonzaga ONLUS, Fondazione per la Ricerca Biomedica ONLUS, Novartis and the Italian Multiple Sclerosis Society. Marco Capobianco received personal fees and compensation for speaking at medical meeting and participating in advisory board from Almirall, Biogen, Merck, Sanofi, Roche, Novartis. Claudio Solaro served on the advisory boards of Biogen Idec and Merck Serono. He received speaking honoraria from Bayer Schering, Biogen Idec, Merck Serono, Almirall, Teva and Genzyme. He received research grants and support from the Italian MS Society Research Foundation (Fondazione Italiana Sclerosi Multipla).
References
- Capra R., Rossi N.D., Mattioli F., Romanelli G., Scarpazza C., Pia M., Cossi S. Impact of low dose tocilizumab on mortality rate in patients with COVID-19 related pneumonia. Eur. J. Int. Med. 2020 doi: 10.1016/j.ejim.2020.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarius S., Ruprecht K., Kleiter I., Borisow N., Asgari N., Pitarokoili K., Pache F., Stich O., Beume L.A., Hümmert M.W., Ringelstein M., Trebst C., Winkelmann A., Schwarz A., Buttmann M., Zimmermann H., Kuchling J., Franciotta D., Capobianco M., Siebert E., Lukas C., Korporal-Kuhnke M., Haas J., Fechner K., Brandt A.U., Schanda K., Aktas O., Paul F., Reindl M., Wildemann B. MOG-IgG in NMO and related disorders: a multicenter study of 50 patients. Part 2: epidemiology, clinical presentation, radiological and laboratory features, treatment responses, and long-term outcome. J. Neuroinflamm. 2016;13:1–45. doi: 10.1186/s12974-016-0718-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toniati P., Piva S., Cattalini M., Garrafa E., Regola F., Castelli F., Franceschini F., Airò P., Bazzani C., Beindorf E.A., Berlendis M., Bezzi M., Bossini N., Castellano M., Cattaneo S., Cavazzana I., Contessi G.B., Crippa M., Delbarba A., De Peri E., Faletti A., Filippini M., Frassi M., Gaggiotti M., Gorla R., Lanspa M., Lorenzotti S., Marino R., Maroldi R., Metra M., Matteelli A., Modina D., Moioli G., Montani G., Muiesan M.L., Odolini S., Peli E., Pesenti S., Pezzoli M.C., Pirola I., Pozzi A., Proto A., Rasulo F.A., Renisi G., Ricci C., Rizzoni D., Romanelli G., Rossi M., Salvetti M., Scolari F., Signorini L., Taglietti M., Tomasoni G., Tomasoni L.R., Turla F., Valsecchi A., Zani D., Zuccalà F., Zunica F., Focà E., Andreoli L., Latronico N. Tocilizumab for the treatment of severe COVID-19 pneumonia with hyperinflammatory syndrome and acute respiratory failure: a single center study of 100 patients in Brescia, Italy. Autoimmun. Rev. 2020;19 doi: 10.1016/j.autrev.2020.102568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whittam D.H., Karthikeayan V., Gibbons E., Kneen R., Chandratre S., Ciccarelli O., Hacohen Y., de Seze J., Deiva K., Hintzen R.Q., Wildemann B., Jarius S., Kleiter I., Rostasy K., Huppke P., Hemmer B., Paul F., Aktas O., Pröbstel A.K., Arrambide G., Tintore M., Amato M.P., Nosadini M., Mancardi M.M., Capobianco M., Illes Z., Siva A., Altintas A., Akman-Demir G., Pandit L., Apiwattankul M., Hor J.Y., Viswanathan S., Qiu W., Kim H.J., Nakashima I., Fujihara K., Ramanathan S., Dale R.C., Boggild M., Broadley S., Lana-Peixoto M.A., Sato D.K., Tenembaum S., Cabre P., Wingerchuk D.M., Weinshenker B.G., Greenberg B., Matiello M., Klawiter E.C., Bennett J.L., Wallach A.I., Kister I., Banwell B.L., Traboulsee A., Pohl D., Palace J., Leite M.I., Levy M., Marignier R., Solomon T., Lim M., Huda S., Jacob A. Treatment of MOG antibody associated disorders: results of an international survey. J. Neurol. 2020 doi: 10.1007/s00415-020-10026-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wynford-Thomas R., Jacob A., Tomassini V. Neurological update: MOG antibody disease. J. Neurol. 2019;266:1280–1286. doi: 10.1007/s00415-018-9122-2. [DOI] [PMC free article] [PubMed] [Google Scholar]