Key Points
Question
What is the lifetime prevalence of cervical cancer screening in 55 low- and middle-income countries?
Findings
In this cross-sectional study based on self-reported data collected in 55 countries between 2005 and 2018, the country-level median lifetime prevalence of cervical cancer screening was 43.6% (range, 0.3%-97.4%).
Meaning
Although a wide range of variation in self-reported cervical cancer screening prevalence existed among these countries, the overall findings support the need to increase the rate of screening.
Abstract
Importance
The World Health Organization is developing a global strategy to eliminate cervical cancer, with goals for screening prevalence among women aged 30 through 49 years. However, evidence on prevalence levels of cervical cancer screening in low- and middle-income countries (LMICs) is sparse.
Objective
To determine lifetime cervical cancer screening prevalence in LMICs and its variation across and within world regions and countries.
Design, Setting, and Participants
Analysis of cross-sectional nationally representative household surveys carried out in 55 LMICs from 2005 through 2018. The median response rate across surveys was 93.8% (range, 64.0%-99.3%). The population-based sample consisted of 1 136 289 women aged 15 years or older, of whom 6885 (0.6%) had missing information for the survey question on cervical cancer screening.
Exposures
World region, country; countries’ economic, social, and health system characteristics; and individuals’ sociodemographic characteristics.
Main Outcomes and Measures
Self-report of having ever had a screening test for cervical cancer.
Results
Of the 1 129 404 women included in the analysis, 542 475 were aged 30 through 49 years. A country-level median of 43.6% (interquartile range [IQR], 13.9%-77.3%; range, 0.3%-97.4%) of women aged 30 through 49 years self-reported to have ever been screened, with countries in Latin America and the Caribbean having the highest prevalence (country-level median, 84.6%; IQR, 65.7%-91.1%; range, 11.7%-97.4%) and those in sub-Saharan Africa the lowest prevalence (country-level median, 16.9%; IQR, 3.7%-31.0%; range, 0.9%-50.8%). There was large variation in the self-reported lifetime prevalence of cervical cancer screening among countries within regions and among countries with similar levels of per capita gross domestic product and total health expenditure. Within countries, women who lived in rural areas, had low educational attainment, or had low household wealth were generally least likely to self-report ever having been screened.
Conclusions and Relevance
In this cross-sectional study of data collected in 55 low- and middle-income countries from 2005 through 2018, there was wide variation between countries in the self-reported lifetime prevalence of cervical cancer screening. However, the median prevalence was only 44%, supporting the need to increase the rate of screening.
This cancer epidemiology study characterizes lifetime cervical cancer screening prevalence in low- and middle-income countries overall and by region; per capita gross domestic product; and patient rurality, education, and household wealth.
Introduction
Cervical cancer was estimated to be the fourth most common cause of cancer incidence and mortality among women globally in 2018.1 Deaths due to cervical cancer are largely preventable through regular screening combined with early-stage treatment and, more recently, through vaccination against the human papillomavirus (HPV).2,3 Although scaling up HPV vaccination could prevent many cases of cervical cancer in the future,4,5 HPV vaccination coverage is currently still very low in low- and middle-income countries (LMICs).6,7 Increasing effective screening for cervical cancer in LMICs is, thus, indispensable for a rapid reduction in cervical cancer incidence and mortality.
The World Health Organization (WHO) Director-General’s call for action on cervical cancer in 2018 emphasized the importance of increasing cervical cancer screening in LMICs as being key to eliminating cervical cancer as a public health problem globally.8 Implementing and maintaining effective screening programs requires an in-depth understanding of current screening rates, how they are changing over time, and which population groups within countries are not reached. However, despite its importance for policy makers in LMICs and recommended use as an indicator for measuring progress toward achieving both universal health coverage and global noncommunicable disease goals,9,10,11 the only available international comparison of cervical cancer screening rates with nationally representative data are based on the World Health Surveys.12,13 These surveys were conducted in 2002 and 2003 and are, thus, at least 17 years old.
In an effort to inform the design and monitoring of interventions to improve coverage with cervical cancer screening, this study aimed to determine the proportion of women aged 30 through 49 years in LMICs who self-reported to have ever been screened for cervical cancer and how these estimates vary across regions, countries, and population groups within countries.
Methods
Ethics
This analysis of pseudonymized data (ie, data that could not be linked to individuals without additional information that was not available to the analysts) was considered exempt for nonhuman subjects research by the institutional review board of the Heidelberg University Medical Faculty.
Data Sources
We requested access to the most recent nationally representative WHO STEPwise approach to surveillance (STEPS) survey conducted since 2005 for all countries that the World Bank categorized as low-income, lower middle–income, or upper middle–income at any time since 2005.14 To be included in this study, a country must have been an LMIC (as per the World Bank categorization) at the time of the survey’s data collection (Table and Figure 1). We preferred STEPS surveys because they use the same standardized questionnaire, ask about all commonly applied cervical cancer screening techniques, sample a wide age range of women, and are the official approach developed by the WHO for monitoring noncommunicable disease risk factors at the population level.
Table. Survey Characteristics by Region and Countrya,b.
| Country | ISO code | Survey | Yearc | Women’s response rate, %d | Missing outcome, %e | Sample size (all ages) | Age range, y | Sample size (30-49 y) | Median age (30-49 y) | GDP per capita, int $f | Female population in 2019 (thousands)g |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Latin America and the Caribbean | |||||||||||
| Belize | BLZ | CAMDI | 2005-2006 | 92.7 | 0.8 | 1425 | 19-94 | 562 | 40 | 7924 | 196 |
| Costa Rica | CRI | ENSA | 2006 | 95.0 | 12.1 | 2474 | 18-101 | 772 | 40 | 11 558 | 2525 |
| Bolivia | BOL | DHS | 2008 | 95.9 | 0.6 | 16 699 | 15-49 | 7782 | 38 | 5525 | 5733 |
| El Salvador | SLV | FESAL | 2008 | 90.0 | 0.2 | 11 983 | 15-49 | 6094 | 37 | 6309 | 3430 |
| Jamaica | JAM | RHS | 2008 | 96.7 | 0.5 | 8217 | 15-49 | 4532 | 39 | 8593 | 1485 |
| Paraguay | PRY | ENDSSR | 2008 | 95.1 | 0.1 | 6536 | 15-44 | 2666 | 36 | 9028 | 3464 |
| Chile | CHL | ENS | 2009-2010 | 85.0 | 7.2 | 2916 | 15-100 | 1036 | 40 | 18 924 | 9610 |
| Nicaragua | NIC | ENDESA | 2011 | 93.8 | 0.1 | 15 257 | 15-49 | 7183 | 37 | 4163 | 3320 |
| Honduras | HND | DHS | 2011-2012 | 93.2 | 0.0 | 22 019 | 15-49 | 9677 | 38 | 4028 | 4877 |
| Argentina | ARG | ENFR | 2013 | 70.7 | 0.5 | 17 951 | 18-98 | 6891 | 38 | 19 638 | 22 939 |
| Brazil | BRA | PNS | 2013 | 86.0 | 0.0 | 34 282 | 18-101 | 14 546 | 38 | 15 062 | 107 316 |
| Dominican Republic | DOM | DHS | 2013 | 94.1 | 0.4 | 8990 | 15-49 | 4347 | 39 | 12 183 | 5373 |
| Ecuador | ECU | ENSANUT | 2012 | NA | 0.2 | 17 808 | 15-50 | 10 121 | 38 | 10 286 | 8683 |
| Peru | PER | DHS | 2013 | 97.3 | 7.0 | 20 808 | 15-49 | 11 398 | 39 | 11 734 | 16 362 |
| St Vincent & the Grenadines | VCT | STEPS | 2013 | 67.8 | 0.2 | 1937 | 18-69 | 902 | 39 | 10 259 | 54 |
| Mexico | MEX | SAGE | 2014 | 81.0 | 0.0 | 2799 | 18-98 | 368 | 40 | 17 150 | 65 172 |
| Guatemala | GTM | DHS | 2014-2015 | 96.8 | 0.1 | 25 557 | 15-49 | 11 224 | 38 | 7220 | 8922 |
| Colombia | COL | DHS | 2015 | 86.6 | 0.0 | 26 670 | 21-49 | 17 235 | 38 | 13 115 | 25 626 |
| Guyana | GUY | STEPS | 2016 | 66.7 | 0.1 | 1588 | 18-69 | 690 | 39 | 7285 | 390 |
| Haiti | HTI | DHS | 2016-2017 | 99.3 | 0.0 | 2495 | 35-64 | 1368 | 41 | 1654 | 5705 |
| Europe and Central Asia | |||||||||||
| Russia | RUS | SAGE | 2007-2010 | 87.7 | 1.0 | 2777 | 19-99 | 215 | 41 | 23 063 | 78 269 |
| Kyrgyzstan | KGZ | STEPS | 2013 | NA | 0.7 | 1665 | 25-64 | 840 | 40 | 3117 | 3242 |
| Moldova | MDA | STEPS | 2013 | 83.5 | 11.5 | 2637 | 18-69 | 939 | 39 | 5638 | 2105 |
| Bulgaria | BGR | EHS | 2014 | 72.5 | 13.4 | 2897 | 15-85 | 802 | 40 | 16 324 | 3600 |
| Romania | ROU | EHS | 2014 | NA | 0.0 | 8728 | 15-85 | 2616 | 40 | 19 802 | 9946 |
| Georgia | GEO | STEPS | 2016 | 75.7 | 1.3 | 2903 | 17-70 | 1000 | 40 | 9256 | 2091 |
| Belarus | BLR | STEPS | 2016-2017 | 87.1 | 7.8 | 2692 | 18-69 | 1095 | 41 | 16 978 | 5052 |
| Azerbaijan | AZE | STEPS | 2017 | 97.3 | 5.1 | 1580 | 18-69 | 632 | 40 | 15 929 | 5032 |
| Tajikistan | TJK | STEPS | 2016-2017 | 94.4 | 4.9 | 1539 | 18-70 | 773 | 39 | 2854 | 4623 |
| Mongolia | MNG | SISS | 2018 | 92.0 | 0.3 | 10 765 | 15-49 | 6764 | 39 | 12 209 | 1635 |
| Middle East and Northern Africa | |||||||||||
| Egypt | EGY | DHS | 2015 | 98.9 | 0.0 | 8687 | 15-59 | 3653 | 38 | 10 243 | 49 665 |
| Iraq | IRQ | STEPS | 2015 | 98.8 | 4.0 | 2355 | 18-102 | 1148 | 39 | 14 964 | 19 418 |
| Algeria | DZA | STEPS | 2016-2017 | 93.2 | 2.1 | 3823 | 18-69 | 1928 | 39 | 13 908 | 21 303 |
| Iran | IRN | STEPS | 2016 | 98.4 | 4.5 | 15 260 | 18-100 | 6712 | 38 | 18 664 | 41 024 |
| Lebanon | LBN | STEPS | 2017 | 69.9 | 8.2 | 2167 | 16-70 | 1022 | 39 | 11 647 | 3911h |
| Morocco | MAR | STEPS | 2017 | 89.0 | 4.0 | 3398 | 18-100 | 1535 | 39 | 7509 | 18 379 |
| South Asia, East Asia, and Pacific | |||||||||||
| China | CHN | SAGE | 2008-2010 | 98.9 | 5.2 | 7601 | 18-93 | 785 | 42 | 8683 | 698 159 |
| Philippines | PHL | DHS | 2013 | 98.3 | 0.0 | 24 832 | 15-49 | 12 269 | 39 | 6282 | 53 801 |
| Bhutan | BTN | STEPS | 2014 | 96.9 | 1.9 | 1712 | 18-69 | 887 | 38 | 7954 | 358 |
| Nepal | NPL | SOSAS | 2014 | 97.0 | 2.0 | 1007 | 15-100 | 394 | 38 | 2385 | 15 562 |
| Timor-Leste | TLS | STEPS | 2014 | 96.3 | 7.8 | 1407 | 18-69 | 668 | 39 | 6467 | 640 |
| Indonesia | IDN | IFLS | 2014-2015 | 90.5 | 0.0 | 16 518 | 15-101 | 7151 | 37 | 10 181 | 134 356 |
| India | IND | DHS | 2015-2016 | 96.7 | 0.0 | 677 463 | 15-49 | 331 512 | 38 | 5944 | 656 288 |
| Sri Lanka | LKA | DHS | 2016 | 98.9 | 0.1 | 18 288 | 15-49 | 13 968 | 39 | 11 447 | 11 090 |
| Sub-Saharan Africa | |||||||||||
| Ghana | GHA | SAGE | 2008-2009 | 92.1 | 12.4 | 2407 | 18-114 | 294 | 40 | 2729 | 15 002 |
| Cote d'Ivoire | CIV | DHS | 2011-2012 | 93.0 | 0.3 | 9802 | 15-49 | 4130 | 37 | 5192 | 12 742 |
| Namibia | NAM | DHS | 2013 | 93.8 | 0.9 | 9641 | 15-64 | 3969 | 38 | 9600 | 1286 |
| Botswana | BWA | STEPS | 2014 | 64.0 | 2.3 | 2687 | 15-69 | 1125 | 38 | 16 175 | 1190 |
| Eswatini | SWZ | STEPS | 2014 | 76.0 | 7.3 | 2135 | 15-70 | 821 | 38 | 9309 | 585 |
| Lesotho | LSO | DHS | 2014 | 97.1 | 0.0 | 6211 | 15-49 | 2596 | 37 | 2811 | 1077 |
| Benin | BEN | STEPS | 2015 | 98.6 | 3.5 | 2702 | 18-69 | 1273 | 36 | 1987 | 5910 |
| Kenya | KEN | STEPS | 2015 | 95.0 | 0.3 | 2681 | 18-69 | 1197 | 37 | 2798 | 26 452 |
| Zimbabwe | ZWE | DHS | 2015 | 96.2 | 0.0 | 9481 | 15-49 | 4211 | 37 | 2509 | 7662 |
| South Africa | ZAF | DHS | 2016 | 83.1 | 0.4 | 5939 | 15-95 | 2014 | 38 | 12 246 | 29 699 |
| Sudan | SDN | STEPS | 2016 | 95.0 | 8.2 | 4606 | 18-69 | 2143 | 37 | 4357 | 21 425 |
| Total | NA | NA | NA | 93.8 (86.5-96.8)i | 0.6 (0.1-4.7)i | 1 129 404j | 15-114 | 542 475j | 39 (38-39.5)i | 9256 (5582-12 681)i | 2 259 850j |
Abbreviations: CAMDI, Central America Diabetes Initiative; DHS, Demographic Health and Surveillance Survey; EHS, European Health Survey; ENDESA, Encuesta Nicargaüense de Demografía y Salud; ENDSSR, Encuesta Nacional de Demografía y Salud Sexual y Reproductiva; ENFR, Encuesta Nacional de Factores de Riesgo; ENS, Encuesta Nacional de Salud; ENSA, Encuesta Nacional de Salud; ENSANUT, Encuesta Nacional de Salud y Nutrición; FESAL, Encuesta Nacional de Salud Familiar; GDP, gross domestic product; IFLS-5, Indonesia Family Life Survey Wave 5; ISO, International Organization for Standardization; NA, not available; PNS, Pesquisa Nacional de Saúde; RHS, Reproductive Health Survey; SAGE, Study on Global Aging and Adult Health; SISS, Social Indicator Sample Survey; SOSAS, Surgeons OverSeas Assessment of Surgical need; STEPS, STEPwise approach to Surveillance.
Values are unweighted (ie, do not account for the multistage cluster sampling used by the included surveys).
Sample size, median age, and age ranges are shown for those with a nonmissing outcome variable.
Years in which the data collection for the survey was carried out.
If the women's response rate was unavailable, this shows the combined response rate for both men and women in the survey. This applies to Algeria, Argentina, Azerbaijan, Belarus, Belize, Benin, Bhutan, Botswana, Brazil, Bulgaria, Chile, Eswatini, Georgia, Guyana, Iran, Iraq, Kenya, Lebanon, Mexico, Moldova, Morocco, Nepal, St Vincent & the Grenadines, Sudan, Tajikistan, and Timor-Leste. For Costa Rica and Indonesia, only the household response rate was available.
This is the percent of female participants who had a missing response for the survey question assessing whether she had ever undergone a screening test for cervical cancer.
This is GDP per capita in constant 2011 international dollars (as estimated by the World Bank17) for the year of the survey’s data collection. In case of a multiyear data collection period, we calculated the mean GDP per capita in constant 2011 international dollars across years.
Population in 2019 as estimated by United Nations, Population Division, Department of Economic and Social Affairs.19
Combined number of Lebanese citizens and Syrian refugees living in Lebanon in 2017 as estimated by the United Nations Refugee Agency.20
This is the median value and interquartile range with each country having the same weight.
This is the sum across all countries.
Figure 1. Self-reported Lifetime Prevalence of Cervical Cancer Screening Among Women Aged 30 Through 49 Years.
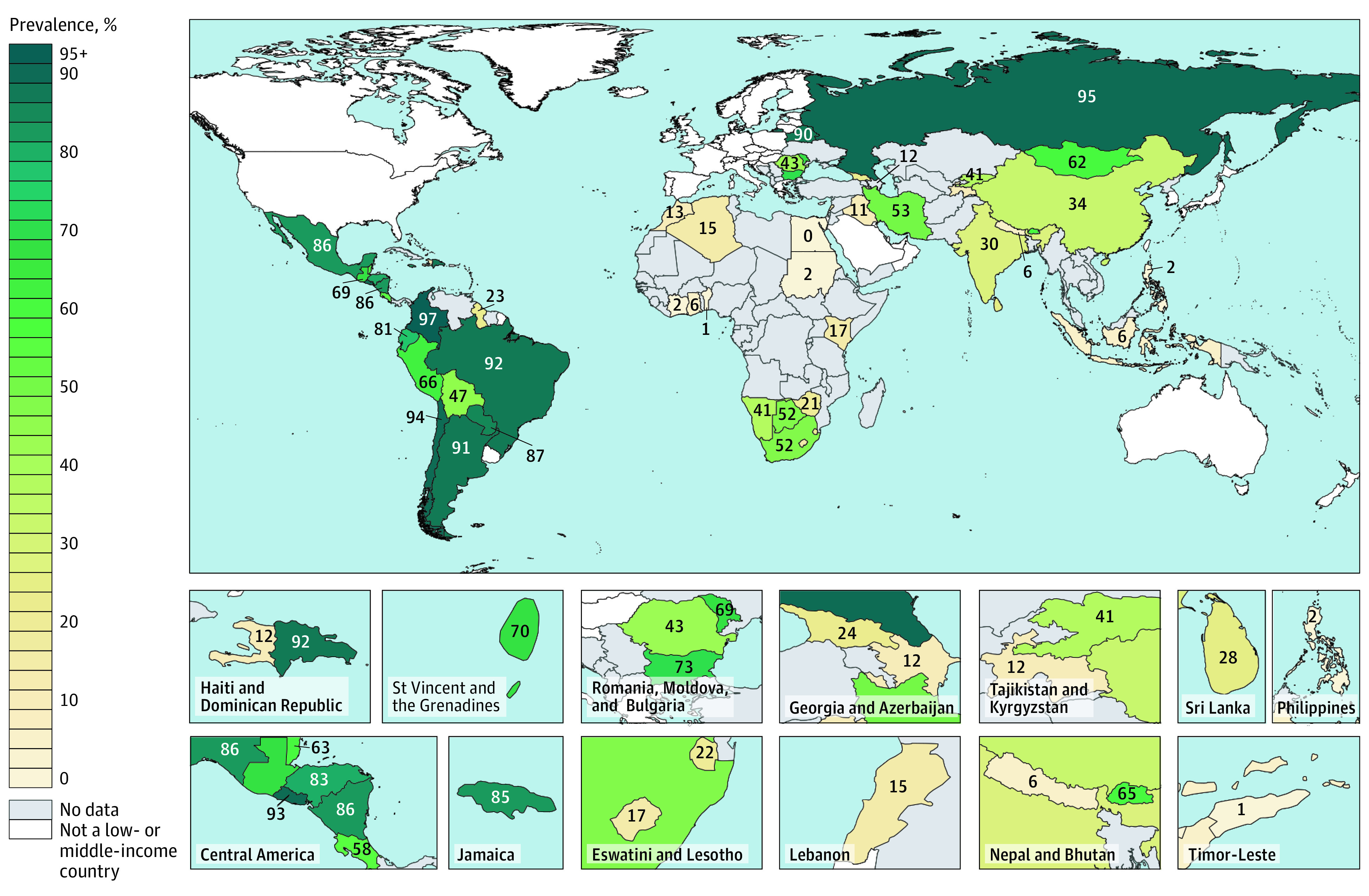
Gray indicates no eligible survey or access to data was unavailable. The numbers indicate prevalence in percent of women aged 30 through 49 years. Prevalence estimates are shown for the survey years listed in the Table. A map with aged-standardized estimates based on the World Health Organization World Standard Population is shown in eFigure 3 in the Supplement.
If an eligible STEPS data set was not available for a country that was an LMIC at any time since 2005 or if we could not gain access to it, we conducted a systematic search in September 2019 using the Google search engine, the International Household Survey Network (IHSN) central data catalog, and the Global Health Data Exchange (GHDx) to identify the most recent nationally representative household survey with data on cervical cancer screening prevalence for that country (eMethods 1 in the Supplement). Surveys were eligible if they were conducted in 2005 or later, collected data on at least three 10-year age groups older than 15 years, and asked female respondents about whether they had ever been screened for cervical cancer. The sampling strategy and response rate calculation for each survey is detailed in eMethods 2 and 3 in the Supplement. Response rate calculations were categorized according to the American Association for Public Opinion Research definitions response rates 1, 2, and 5 and cooperation rate 1.15
Outcome Definition
The outcome for the present analysis was defined as self-reporting to have ever undergone a screening test for cervical cancer or cervical precancerous lesions. The only exception was Peru, for which the survey question asked about screening in the preceding 5 years. The survey questions are detailed in eMethods 4 in the Supplement.
Statistical Analysis
This analysis proceeded in 4 steps. First, we estimated self-reported lifetime prevalence of cervical cancer screening by country and calculated the country-level median prevalence (as well as the range and interquartile range [IQR]) globally and by World Bank region. We restricted the sample for analysis to women aged 30 through 49 years in our primary analysis for this step because the WHO recommends prioritizing cervical cancer screening in this age group.16
Second, to ascertain health system performance for cervical cancer screening relative to a country’s wealth and expenditure on health, we plotted the self-reported lifetime prevalence of cervical cancer screening for women aged 30 through 49 years against the country’s gross domestic product (GDP) per capita and total health expenditure per capita (both in constant 2011 international dollars17) in the year of survey data collection. We show an ordinary least squares regression line through these point estimates, weighting each country equally, for visual orientation only (as opposed to statistical inference).
Third, to explore reasons for differences in screening prevalence between countries, we plotted the self-reported lifetime prevalence of cervical cancer screening for women aged 30 through 49 years separately against each of 8 country-level indicators. We used all country-level indicators as independent variables that we hypothesized may be causally related to a country’s cervical cancer screening prevalence and that were available in the public domain for the majority of the study countries. These indicators were measures of economic development (GDP per capita), human development (the Human Development Index [HDI] and the Gender Development Index [GDI]), investments into the health system (total health expenditure per capita), health worker density (number of nurses and midwives per 1000 people and combined number of physicians, nurses, and midwives per 1000 people), and gender discrimination (the Gender Inequality Index [GII] and the 2014 Social Institutions and Gender Index [SIGI]).
Fourth, to ascertain which population groups were most likely to self-report to have ever been screened, we regressed, separately for each country, self-reporting to have ever had a cervical cancer screening test onto the 10-year age groups, educational attainment, household wealth quintile, rural vs urban residence, and a binary indicator for current self-reported tobacco smoking. The computation of the household wealth quintiles is detailed in eMethods 5 in the Supplement. We fitted covariate-unadjusted and covariate-adjusted Poisson regression models with cluster-robust standard errors (using the sandwich estimator of variance) that were adjusted for clustering at the level of the primary sampling unit. We adhere to the term risk when interpreting the resulting risk ratios (RRs) even though risk in this analysis depicts a desirable (reporting to have undergone screening) rather than an undesirable outcome.
All analyses were complete-case analyses. All primary analyses accounted for the multistage random sampling of the surveys by use of sampling weights and adjusted SEs for clustering at the level of the primary sampling unit. As a robustness check for the fourth step of this analysis and given ongoing debate as to when regression in survey data should account for sampling weights,18 we also fitted Poisson regression models without using sampling weights. We provide further details on the statistical analysis in eMethods 6 in the Supplement. Analyses were conducted in R version 3.6.1 and Stata version 15 (StataCorp).
Results
Sample Characteristics
Out of a total of 142 countries that were classified as an LMIC at any point since 2005, we obtained individual-level STEPS survey data from 20 LMICs and included, from the systematic search, survey data sets from an additional 35 LMICs (eFigures 1 and 2 in the Supplement). Of the 55 included surveys, 20 surveys asked women whether they had ever undergone at least 1 of the 3 commonly used screening modalities (Papanicolaou test, visual inspection of the cervix with acetic acid, or HPV test), 28 surveys asked only about the Papanicolaou test, and 7 surveys asked about cervical cancer screening without specifying a screening modality. One survey (Armenia STEPS 2016) was excluded because the response rate was less than 50%. The survey-level median response rate was 93.8% (IQR, 86.5%-96.8%; range, 64.0%-99.3%; Table). The country-level median percent of women aged 30 through 49 years with missing information on whether they had ever received a cervical cancer screening was 0.5% (IQR, 0.1%-3.4%; range, 0.0%-12.6%). A total of 1 129 404 women with outcome data, of whom 542 475 were aged 30 through 49 years, were included in the analyses (eTable 1 in the Supplement). Detailed sample characteristics are shown in eTables 2, 3, and 4 in the Supplement.
Lifetime Prevalence of Cervical Cancer Screening by Region and Country
A country-level median of 43.6% (IQR, 13.9%-77.3%) of women aged 30 to 49 years self-reported to have ever had a cervical cancer screening test, ranging from 0.3% in Egypt (95% CI, 0.1%-0.6%) to 97.4% in Colombia (95% CI, 97.0%-97.8%). With a country-level median of 84.6% (IQR, 65.7%-91.1%; range, 11.7%-97.4%), countries in Latin America and the Caribbean had the highest self-reported lifetime prevalence of cervical cancer screening, whereas countries in sub-Saharan Africa had the lowest (country-level median, 16.9%; IQR, 3.7%-31.0%; range, 0.9%-50.8%) (Figure 1; eFigures 3, 4, and 5; and eTable 5 in the Supplement). There was substantial variation across countries within regions.
Benchmarking to Countries’ Gross Domestic Product and Total Health Expenditure
Both GDP per capita and total health expenditure per capita appeared to be positively associated with the self-reported lifetime prevalence of cervical cancer screening in a country (Figure 2). Countries that performed well relative to their GDP per capita in the year of the survey included Belarus, Belize, Bhutan, Bolivia, Brazil, Chile, Colombia, the Dominican Republic, Ecuador, El Salvador, Guatemala, Honduras, Jamaica, Moldova, Nicaragua, Peru, and St Vincent and the Grenadines.
Figure 2. Self-reported Lifetime Prevalence of Cervical Cancer Screening by GDP per Capita and Total Health Expenditure per Capita.
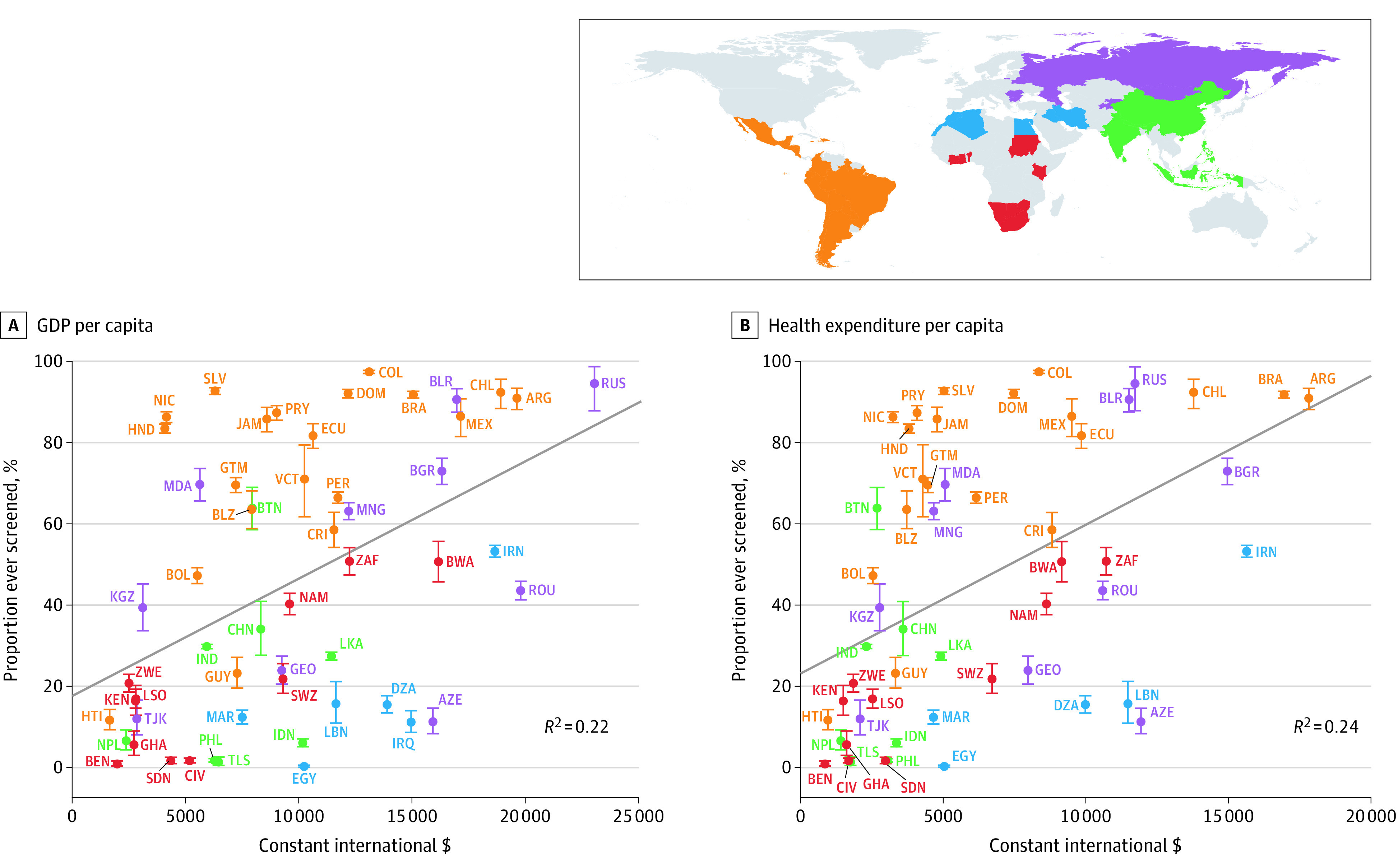
The sample includes only women aged 30 through 49 years; ISO codes are defined in the Table. Gross domestic product (GDP) and total health expenditure per capita is in constant 2011 international dollars for the survey year. Iraq’s health expenditure per capita was unavailable. Error bars indicate the 95% CIs; diagonal lines depict ordinary least-squares regressions (with each country having the same weight) of lifetime cervical cancer screening prevalence in a country onto the GDP or total health expenditure per capita. The standardized regression coefficients were 0.47 (95% CI, 0.23-0.71) for GDP and 0.49 (95% CI, 0.25-0.73) for health expenditure per capita. Estimates among all women and estimates adjusted for differences in individual-level characteristics between countries are shown in eFigures 6, 8, and 10 in the Supplement.
Country-Level Variables Associated With Lifetime Prevalence of Cervical Cancer Screening
In addition to GDP per capita and total health expenditure per capita, a higher HDI and more gender equality as indicated by the GDI, GII, and SIGI appeared to be positively associated with a country’s lifetime prevalence of cervical cancer screening (Figure 3). A higher density of nurses and midwives, as well as of all health care workers, statistically accounted for less of the variability in the self-reported lifetime prevalence of cervical cancer screening between countries (R2 = 0.05 and R2 = 0.09, respectively) than the other country-level variables. The apparent associations shown in Figure 3 were similar when using weighting to adjust for differences in individual-level characteristics between countries (eFigures 8-11 in the Supplement).
Figure 3. Self-reported Lifetime Prevalence of Cervical Cancer Screening by Human Development Index, Gender Equality Indices, and Health Worker Density.
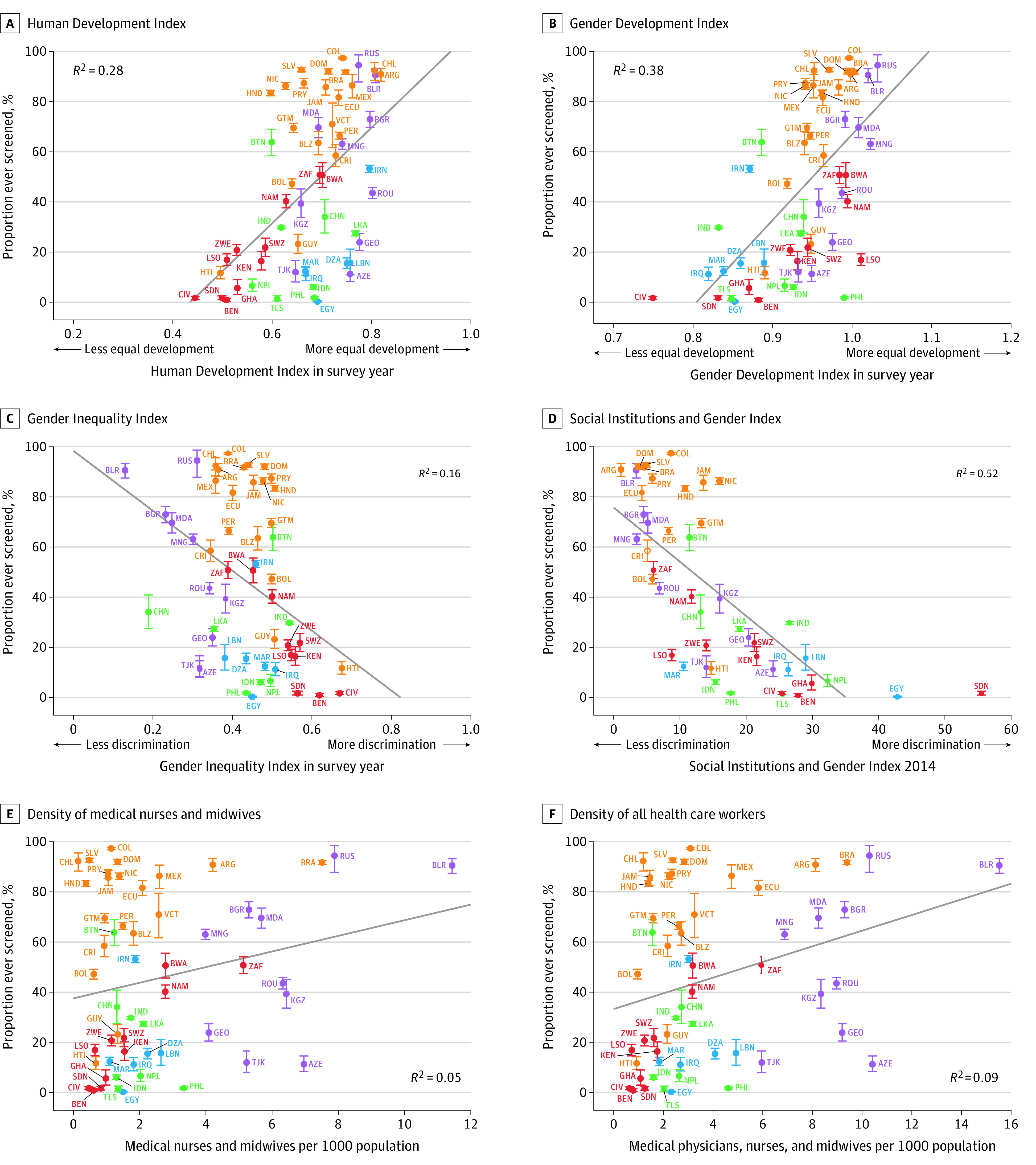
The sample included only women aged 30 through 49 years. ISO codes are defined in the Table. A Gender Development Index value was not available for St Vincent and the Grenadines; a Gender Inequality Index value was not available for Ghana, St Vincent and the Grenadines, and Timor-Leste. Error bars indicate 95% CIs. The diagonal lines depict ordinary least-squares regressions (with each country having the same weight) of lifetime cervical cancer screening prevalence in a country onto the country-level variables of the Human Development Index (0.53; 95% CI, 0.30 to 0.76), Gender Development Index (0.62; 95% CI, 0.41 to 0.83), Gender Inequality Index (−0.40; 95% CI, −0.66 to −0.15), Social Institutions and Gender Index (−0.72; 95% CI, −0.92 to −0.52), density of medical nurses and midwives (0.22; 95% CI, 0.04 to 0.48), and health worker density (0.30; 95% CI, 0.04 to 0.56 ). Estimates among all women and estimates that were adjusted for differences in individual-level characteristics between countries are shown in eFigures 7, 9, and 11 in the Supplement.
Individual-Level Variables Associated With Cervical Cancer Screening
Although some heterogeneity existed across countries, living in an urban area (vs a rural area), having had secondary or tertiary education (vs only having completed primary education or less), being in the 2 highest household wealth quintiles (vs the bottom 2 household wealth quintiles), and being aged 30 through 49 years (vs 20 through 29 years) all appeared to be associated with a higher probability of self-reporting to have ever had a cervical cancer screening test in most countries (Figure 4; eFigures 12 and 13 and eTables 6, 7, 8, 9, 10, and 11 in the Supplement). The relationship between age and self-reported lifetime prevalence of cervical cancer screening had an inverted U shape in all regions, with middle-aged women having the highest self-reported prevalence (eFigure 14 in the Supplement). There was no apparent association between currently smoking (vs having never smoked or smoked in the past) and self-reporting of ever having had a cervical cancer screening test in 32 out of 46 countries that collected smoking data (eFigure 15 and eTable 12 in the Supplement). Currently being married appeared to be associated with a higher probability of self-reporting to have ever had a cervical cancer screening test in 41 out of 55 countries (eFigure 16 and eTable 13 in the Supplement). Risk ratios with 95% CIs from covariate-unadjusted and covariate-adjusted regressions are shown in eTables 14 through 24 in the Supplement. The regression results were similar when not using sampling weights (eFigures 17-24 and eTables 6-13 and 25-35 in the Supplement).
Figure 4. Relative and Absolute Differences in the Probability of Having Ever Been Screened for Cervical Cancer by Sociodemographics .
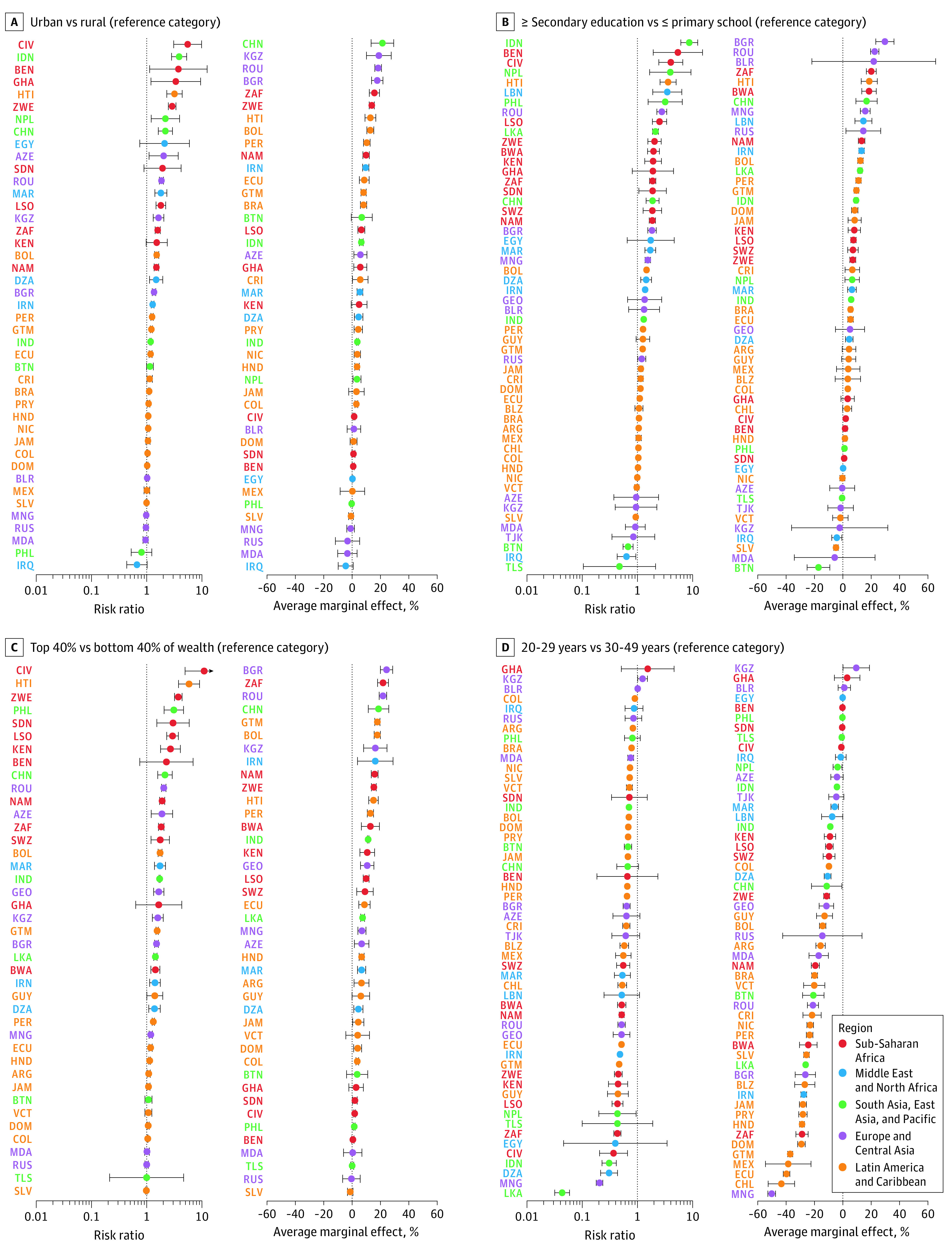
ISO codes are defined in the Table. All regressions were adjusted for age (except D) only, using restricted splines placed at the 5th, 27.5th, 50th, 72.5th, and 95th percentiles. All regressions used sampling weights and adjusted SEs for clustering at the primary sampling unit. Error bars depict 95% CIs. Estimates are in the Supplement.
Countries with a lower GDP per capita at the time of the survey tended to have larger relative differences in lifetime cervical cancer screening prevalence by education, household wealth, and urban vs rural residency than countries with a higher GDP per capita (eFigures 25-30 in the Supplement). This was not the case when examining absolute rather than relative differences (eFigures 25-30 in the Supplement).
Discussion
Overall, the country-level median lifetime prevalence of self-reported cervical cancer screening was 44% in this sample of 55 LMICs, which represent 72% of the world’s population in LMICs.19 Screening prevalence was generally highest among countries in Latin America and the Caribbean and lowest among countries in sub-Saharan Africa. In addition, the highly populous countries of Indonesia (2014-2015 survey), India (2015-2016 survey), and China (2008-2010 survey) had a comparatively low self-reported lifetime screening prevalence among women aged 30 through 49 years. Within countries, women in rural areas and those who were less educated or lived in a less wealthy household tended to be least likely to self-report having ever been screened for cervical cancer.
The low prevalence of self-reported cervical cancer screening identified in this study is especially concerning given that this analysis examined lifetime prevalence of screening as opposed to the prevalence of being screened in the past 3 to 5 years as recommended by the WHO,16 the limited sensitivity of available screening tests,21,22 often poorly functioning referral systems for positive cervical cancer screening tests in LMICs,23,24 and low quality of care for cervical cancer diagnosis and treatment in many of these settings.23,25,26 Nevertheless, even though the majority of countries (37 of 55) included in this study missed the target of 70% cervical cancer screening prevalence proposed by the WHO,27 the analyses identified large differences in self-reported lifetime prevalence among regions and among countries within regions. Relative to their GDP per capita and total health expenditure per capita, many countries in Latin America and the Caribbean, as well as some countries in other regions (eg, Belarus, Bhutan, or Moldova) achieved high self-reported lifetime prevalence levels of cervical cancer screening. Reasons for these countries’ high performance may include having national cervical cancer control programs in place that provide free cervical cancer screening to women in primary health care system structures at the local level,28,29 integration of screening services into comprehensive cervical cancer control activities,29,30 as well as trialing and implementation of programs to reach underserved sociodemographic groups.31,32
Gross domestic product per capita, total health expenditure per capita, HDI, GDI, GII, and SIGI all statistically accounted for a substantial degree of the variation in self-reported lifetime prevalence of cervical cancer screening between countries. The apparent association between indexes of gender equality and self-reported lifetime prevalence of cervical cancer screening suggests that cultural and societal values influence women’s demand for or access to cervical cancer screening.33 The density of nurses and midwives, as well as the density of health care workers in general, statistically accounted for only relatively little (<10%) of the variation between countries, suggesting that other factors may be more important determinants of screening rates, such as the distribution of health care workers within countries, if health care workers have been trained and equipped to conduct cervical cancer screens, and whether women seek out or consent to screenings.34
Limitations
This study has several limitations. First, 28 of the 55 included surveys asked women only whether they had undergone a Papanicolaou test rather than cervical cancer screening more generally. However, available documentation on cervical cancer screening practices in these countries suggests that it is unlikely that a substantial degree of cervical cancer screening was conducted through modalities other than Papanicolaou testing in all but 3 (Guatemala, Mexico, and Nepal) of these 28 countries prior to the data collection period of the included survey (see eMethods 7 and eTable 36 in the Supplement). Nevertheless, this study’s estimates of self-reported lifetime prevalence of cervical cancer screening in these 3 countries may be underestimates of the true prevalence. Second, this study’s estimates relied entirely on self-report. This probably led to an overestimation of the true lifetime cervical cancer screening prevalence because it is likely that most women who had a cervical cancer screening remember the event (given that these screenings are generally perceived as being uncomfortable35,36), while some women who did not have a screening in the past probably reported having had one due to social desirability bias.37 However, because the awareness of the recommendation to have a regular screening and, thus, the expected degree of bias from social desirability bias is fairly low in LMICs,38,39 it is unlikely that social desirability bias led to a substantial overestimation of self-reported cervical cancer screening prevalence in this study. Third, the surveys were conducted in different years ranging from 2005 to 2018. Each country’s performance should thus be interpreted as the performance in the given year rather than as the country’s current performance. Under the assumption that cervical cancer screening prevalence has been increasing in LMICs over time, this study likely underestimates the current prevalence of cervical cancer screening in the study countries. To avoid confounding by time in the analyses with country-level independent variables, this analysis used values for country-level variables for the year of the survey’s data collection. This, however, was not possible for the SIGI, for which values were only available for 2014 and 2019. Fourth, the 55 LMICs in this analysis are unlikely to be representative of all LMICs globally.
Conclusions
In this cross-sectional study of data collected in 55 low- and middle-income countries from 2005 through 2018, there was wide variation between countries in the self-reported lifetime prevalence of cervical cancer screening. However, the median prevalence was only 44%, supporting the need to increase the rate of screening.
eMethods 1. Search method for eligible surveys in low- and middle-income countries for which we were unable to acquire a WHO-STEPS survey
eMethods 2. Country-specific sampling methods
eMethods 3. Response rates
eMethods 4. Survey questions on cervical cancer screening
eMethods 5. Calculation of household wealth quintiles
eMethods 6. Supplementary information on the statistical analysis
eFigure 1. Flowchart for inclusion of STEPS surveys
eFigure 2. Flowchart for inclusion of non-STEPS surveys
eTable 1. Survey characteristics by region and country for women aged 30 to 49 years
eTable 2. Sample characteristics across all countries among (1) all women, and (2) women aged 30 to 49 years
eTable 3. Sample characteristics across all countries among women aged 30 to 49 years who were (1) included in the analysis, and (2) excluded due to a missing outcome variable
eTable 4. Percent missing (among all those with non-missing data on cervical cancer screening) for each individual-level predictor, by country
eFigure 3. Age-standardized lifetime prevalence of cervical cancer screening by country
eFigure 4. Lifetime prevalence of cervical cancer screening among all women
eFigure 5. Lifetime prevalence of cervical cancer screening among women aged 30 to 49 years
eTable 5. Estimates of lifetime cervical cancer screening prevalence by 10-year age group for each country, region, and overall
eFigure 6. Lifetime prevalence of cervical cancer screening among all women by GDP and health expenditure per capita
eFigure 7. Lifetime prevalence of cervical cancer screening among all women by HDI,GDI, GII, SIGI, and health worker density
eFigure 8. Lifetime prevalence of cervical cancer screening among all women by GDP and health expenditure per capita, adjusted for individual-level differences between countries
eFigure 9. Lifetime prevalence of cervical cancer screening among all women by HDI, GDI, GII, SIGI, and health worker density, adjusted for individual-level differences between countries
eFigure 10. Lifetime prevalence of cervical cancer screening among women aged 30 to 49 years by GDP and health expenditure per capita, adjusted for individual-level differences between countries
eFigure 11. Cervical cancer screening prevalence by HDI, GDI, GII, SIGI, and health worker density, adjusted for individual-level differences between countries among women aged 30 to 49 years
eFigure 12. Relative and absolute differences in lifetime prevalence of cervical cancer screening between women aged 50 years and older compared to women aged 30 to 49 years (reference group)
eFigure 13. Relative and absolute differences in lifetime prevalence of cervical cancer screening comparing the top versus the bottom (reference group) household wealth quintile
eTable 6. Relative (risk ratios) and absolute (average marginal effects) differences in lifetime prevalence of cervical cancer screening between urban and rural (reference group)
eTable 7. Relative (risk ratios) and absolute (average marginal effects) differences in lifetime prevalence of cervical cancer screening between women having completed high school or further and those who have completed primary school or less (reference group)
eTable 8. Relative (risk ratios) and absolute (average marginal effects) differences in lifetime prevalence of cervical cancer screening between women in the top two versus the bottom two (reference group) household wealth quintiles
eTable 9. Relative (risk ratios) and absolute (average marginal effects) differences in lifetime prevalence of cervical cancer screening between women in the top versus the bottom (reference group) household wealth quintile
eTable 10. Relative (risk ratios) and absolute (average marginal effects) differences in lifetime prevalence of cervical cancer screening between women aged 20 to 29 years compared to women aged 30 to 49 years (reference group)
eTable 11. Relative (risk ratios) and absolute (average marginal effects) differences in lifetime prevalence of cervical cancer screening between women aged 50 years and older and women aged 30 to 49 years (reference group)
eFigure 14. Country-level median lifetime cervical cancer screening prevalence by five-year age group, grouped by region
eFigure 15. Relative and absolute differences in lifetime prevalence of cervical cancer screening between women who currently smoke and women who do not (reference group)
eTable 12. Relative (risk ratios) and absolute (average marginal effects) differences in lifetime prevalence of cervical cancer screening between women who currently smoke and women who do not (reference group)
eFigure 16. Relative and absolute differences in lifetime prevalence of cervical cancer screening between women who are currently not married and women who are (reference group)
eTable 13. Relative (risk ratios) and absolute (average marginal effects) differences in lifetime prevalence of cervical cancer screening between women who are currently not married and women who are (reference group)
eTable 14. Poisson regressions of reporting to have ever been screened for cervical cancer onto individual-level characteristics (with survey weights) in Europe: Azerbaijan to Kyrgyzstan
eTable 15. Poisson regressions of reporting to have ever been screened for cervical cancer onto individual-level characteristics (with survey weights) in Europe: Moldova to Tajikistan
eTable 16. Poisson regressions of reporting to have ever been screened for cervical cancer onto individual-level characteristics (with survey weights) in Latin America and the Caribbean: Argentina to Chile
eTable 17. Poisson regressions of reporting to have ever been screened for cervical cancer onto individual-level characteristics (with survey weights) in Latin America and the Caribbean: Colombia to El Salvador
eTable 18. Poisson regressions of reporting to have ever been screened for cervical cancer onto individual-level characteristics (with survey weights) in Latin America and the Caribbean: Guatemala to Jamaica
eTable 19. Poisson regressions of reporting to have ever been screened for cervical cancer onto individual-level characteristics (with survey weights) in Latin America and the Caribbean: Mexico to St. Vincent & the Grenadines
eTable 20. Poisson regressions of reporting to have ever been screened for cervical cancer onto individual-level characteristics (with survey weights) in Middle East & Northern Africa
eTable 21. Poisson regressions of reporting to have ever been screened for cervical cancer onto individual-level characteristics (with survey weights) in South Asia, East Asia, and Pacific: Bhutan to Indonesia
eTable 22. Poisson regressions of reporting to have ever been screened for cervical cancer onto individual-level characteristics (with survey weights) in South Asia, East Asia, and Pacific: Nepal to Timor-Leste
eTable 23. Poisson regressions of reporting to have ever been screened for cervical cancer onto individual-level characteristics (with survey weights) in Sub-Saharan Africa: Benin to Kenya
eTable 24. Poisson regressions of reporting to have ever been screened for cervical cancer onto individual-level characteristics (with survey weights) in Sub-Saharan Africa: Lesotho to Zimbabwe
eFigure 17. Relative and absolute differences in lifetime prevalence of cervical cancer screening between urban and rural (reference group) areas (Poisson regression without sampling weights)
eFigure 18. Relative and absolute differences in lifetime prevalence of cervical cancer screening between women having completed high school or further and those who have completed primary school or less (reference group) (Poisson regression without sampling weights)
eFigure 19. Relative and absolute differences in lifetime prevalence of cervical cancer screening between women in the top two versus the bottom two (reference group) household wealth quintiles (Poisson regression without sampling weights)
eFigure 20. Relative and absolute differences in lifetime prevalence of cervical cancer screening between women in the top versus the bottom (reference group) household wealth quintile (Poisson regression without sampling weights)
eFigure 21. Relative and absolute differences in lifetime prevalence of cervical cancer screening between women who are currently not married and women who are (reference group) (Poisson regression without sampling weights)
eFigure 22. Relative and absolute differences in lifetime prevalence of cervical cancer screening between women who currently smoke and women who do not (reference group) (Poisson regression without sampling weights)
eFigure 23. Relative and absolute differences in lifetime prevalence of cervical cancer screening between women aged 20 to 29 years compared to women aged 30 to 49 years (reference group) (Poisson regression without sampling weights)
eFigure 24. Relative and absolute differences in lifetime prevalence of cervical cancer screening between women aged 50 years and older and women aged 30 to 49 years (reference group) (Poisson regression without sampling weights)
eTable 25. Poisson regressions of reporting to have ever been screened for cervical cancer onto individual-level characteristics (without survey weights) in Europe: Azerbaijan to Kyrgyzstan
eTable 26. Poisson regressions of reporting to have ever been screened for cervical cancer onto individual-level characteristics (without survey weights) in Europe: Moldova to Tajikistan
eTable 27. Poisson regressions of reporting to have ever been screened for cervical cancer onto individual-level characteristics (without survey weights) in Latin America and the Caribbean: Argentina to Chile
eTable 28. Poisson regressions of reporting to have ever been screened for cervical cancer onto individual-level characteristics (without survey weights) in Latin America and the Caribbean: Colombia to El Salvador
eTable 29. Poisson regressions of reporting to have ever been screened for cervical cancer onto individual-level characteristics (without survey weights) in Latin America and the Caribbean: Guatemala to Jamaica
eTable 30. Poisson regressions of reporting to have ever been screened for cervical cancer onto individual-level characteristics (without survey weights) in Latin America and the Caribbean: Mexico to St. Vincent & the Grenadines
eTable 31. Poisson regressions of reporting to have ever been screened for cervical cancer onto individual-level characteristics (without survey weights) in Middle East & Northern Africa
eTable 32. Poisson regressions of reporting to have ever been screened for cervical cancer onto individual-level characteristics (without survey weights) in South Asia, East Asia, and Pacific: Bhutan to Indonesia
eTable 33. Poisson regressions of reporting to have ever been screened for cervical cancer onto individual-level characteristics (without survey weights) in South Asia, East Asia and Pacific: Nepal to Timor-Leste
eTable 34. Poisson regressions of reporting to have ever been screened for cervical cancer onto individual-level characteristics (without survey weights) in Sub-Saharan Africa: Benin to Kenya
eTable 35. Poisson regressions of reporting to have ever been screened for cervical cancer onto individual-level characteristics (without survey weights) in Sub-Saharan Africa: Lesotho to Zimbabwe
eFigure 25. GDP per capita plotted against the relative and absolute difference in lifetime prevalence of cervical cancer screening between urban and rural (reference group) areas in each country (Poisson regression with sampling weights)
eFigure 26. GDP per capita plotted against the relative and absolute difference in lifetime prevalence of cervical cancer screening between urban and rural (reference group) areas in each country (Poisson regression without sampling weights)
eFigure 27. GDP per capita plotted against the relative and absolute difference in lifetime prevalence of cervical cancer screening between women having completed high school or further and those who have completed primary school or less (reference group) in each country (Poisson regression with sampling weights)
eFigure 28. GDP per capita plotted against the relative and absolute difference in lifetime prevalence of cervical cancer screening between women having completed high school or further and those who have completed primary school or less (reference group) in each country (Poisson regression without sampling weights)
eFigure 29. GDP per capita plotted against the relative and absolute difference in lifetime prevalence of cervical cancer screening between women in the top two versus the bottom two (reference group) household wealth quintile in each country (Poisson regression with sampling weights)
eFigure 30. GDP per capita plotted against the relative and absolute difference in lifetime prevalence of cervical cancer screening between women in the top two versus the bottom two (reference group) household wealth quintile in each country (Poisson regression without sampling weights)
eMethods 7. Review of prevailing screening methods in countries that asked about Pap smears only
eTable 36. Review summary of prevailing screening modalities in countries that asked about Pap smear testing only
eTable 37. Poisson regressions run separately for each group of cervical cancer screening modalities as ascertained in the included survey questionnaires
References
- 1.Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394-424. doi: 10.3322/caac.21492 [DOI] [PubMed] [Google Scholar]
- 2.Peirson L, Fitzpatrick-Lewis D, Ciliska D, Warren R. Screening for cervical cancer: a systematic review and meta-analysis. Syst Rev. 2013;2(1):35. doi: 10.1186/2046-4053-2-35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Campos NG, Sharma M, Clark A, et al. The health and economic impact of scaling cervical cancer prevention in 50 low- and lower-middle-income countries. Int J Gynaecol Obstet. 2017;138(suppl 1):47-56. doi: 10.1002/ijgo.12184 [DOI] [PubMed] [Google Scholar]
- 4.Huh WK, Joura EA, Giuliano AR, et al. Final efficacy, immunogenicity, and safety analyses of a nine-valent human papillomavirus vaccine in women aged 16-26 years: a randomised, double-blind trial. Lancet. 2017;390(10108):2143-2159. doi: 10.1016/S0140-6736(17)31821-4 [DOI] [PubMed] [Google Scholar]
- 5.Brisson M, Kim JJ, Canfell K, et al. Impact of HPV vaccination and cervical screening on cervical cancer elimination: a comparative modelling analysis in 78 low-income and lower-middle-income countries. Lancet. 2020;395(10224):575-590. doi: 10.1016/S0140-6736(20)30068-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bruni L, Diaz M, Barrionuevo-Rosas L, et al. Global estimates of human papillomavirus vaccination coverage by region and income level: a pooled analysis. Lancet Glob Health. 2016;4(7):e453-e463. doi: 10.1016/S2214-109X(16)30099-7 [DOI] [PubMed] [Google Scholar]
- 7.Oberlin AM, Rahangdale L, Chinula L, Fuseini NM, Chibwesha CJ. Making HPV vaccination available to girls everywhere. Int J Gynaecol Obstet. 2018;143(3):267-276. doi: 10.1002/ijgo.12656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Adhanom Ghebreyesus T. Cervical cancer: an NCD we can overcome. Published 2018. Accessed October 23, 2019. https://www.who.int/dg/speeches/detail/cervical-cancer-an-ncd-we-can-overcome
- 9.Wagstaff A, Neelsen S. A comprehensive assessment of universal health coverage in 111 countries: a retrospective observational study. Lancet Glob Health. 2020;8(1):e39-e49. doi: 10.1016/S2214-109X(19)30463-2 [DOI] [PubMed] [Google Scholar]
- 10.Hogan DR, Stevens GA, Hosseinpoor AR, Boerma T. Monitoring universal health coverage within the Sustainable Development Goals: development and baseline data for an index of essential health services. Lancet Glob Health. 2018;6(2):e152-e168. doi: 10.1016/S2214-109X(17)30472-2 [DOI] [PubMed] [Google Scholar]
- 11.World Health Organization Noncommunicable diseases global monitoring framework: indicator definitions and specifications. Published 2013. Accessed April 14, 2020. https://www.who.int/nmh/ncd-tools/indicators/GMF_Indicator_Definitions_Version_NOV2014.pdf
- 12.Gakidou E, Nordhagen S, Obermeyer Z. Coverage of cervical cancer screening in 57 countries: low average levels and large inequalities. PLoS Med. 2008;5(6):e132. doi: 10.1371/journal.pmed.0050132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Akinyemiju TF. Socio-economic and health access determinants of breast and cervical cancer screening in low-income countries: analysis of the World Health Survey. PLoS One. 2012;7(11):e48834. doi: 10.1371/journal.pone.0048834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.The World Bank Historical classification by income. World Bank country and lending groups. Published 2020. Accessed May 23, 2020. https://databank.worldbank.org/data/download/site-content/OGHIST.xls
- 15.The American Association for Public Opinion Research Standard definitions: final dispositions of case codes and outcome rates for surveys. Published 2016. Accessed April 14, 2020. https://www.aapor.org/AAPOR_Main/media/publications/Standard-Definitions20169theditionfinal.pdf
- 16.World Health Organization WHO guidelines for screening and treatment of precancerous lesions for cervical cancer prevention. Published 2013. Accessed October 23, 2019. https://apps.who.int/iris/bitstream/handle/10665/94830/9789241548694_eng.pdf [PubMed]
- 17.The World Bank GDP per capita, PPP (constant 2011 international $). Published 2019. Accessed October 23, 2019. https://data.worldbank.org/indicator/NY.GDP.PCAP.PP.KD
- 18.Deaton A. Econometric issues for survey data. In: The Analysis of Household Surveys (Reissue Edition With a New Preface): A Microeconometric Approach to Development Policy. The World Bank; 2019:63-132. doi: 10.1596/978-1-4648-1331-3_ch2 [DOI] [Google Scholar]
- 19.UN Department of Economic and Social Affairs Population division world population prospects 2019. Published 2019. Accessed October 23, 2019. https://population.un.org/wpp/Download/Standard/Population/
- 20.United Nations High Commissioner for Refugees (NHCR) Syria regional refugee response. Operational Portal Refugees Situations. Published 2019. Accessed October 23, 2019. https://data2.unhcr.org/en/situations/syria/location/71
- 21.Fokom-Domgue J, Combescure C, Fokom-Defo V, et al. Performance of alternative strategies for primary cervical cancer screening in sub-Saharan Africa: systematic review and meta-analysis of diagnostic test accuracy studies. BMJ. 2015;351(July):h3084. doi: 10.1136/bmj.h3084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chen C, Yang Z, Li Z, Li L. Accuracy of several cervical screening strategies for early detection of cervical cancer: a meta-analysis. Int J Gynecol Cancer. 2012;22(6):908-921. doi: 10.1097/IGC.0b013e318256e5e4 [DOI] [PubMed] [Google Scholar]
- 23.Maza M, Schocken CM, Bergman KL, Randall TC, Cremer ML. Cervical precancer treatment in low- and middle-income countries: a technology overview. J Glob Oncol. 2016;3(4):400-408. doi: 10.1200/JGO.2016.003731 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Maza M, Matesanz S, Alfaro K, et al. Adherence to recommended follow-up care after high-grade cytology in El Salvador. Int J Healthc. 2016;2(2). doi: 10.5430/ijh.v2n2p31 [DOI] [Google Scholar]
- 25.Drummond JL, Were MC, Arrossi S, Wools-Kaloustian K. Cervical cancer data and data systems in limited-resource settings: challenges and opportunities. Int J Gynaecol Obstet. 2017;138(suppl 1):33-40. doi: 10.1002/ijgo.12192 [DOI] [PubMed] [Google Scholar]
- 26.Catarino R, Petignat P, Dongui G, Vassilakos P. Cervical cancer screening in developing countries at a crossroad: emerging technologies and policy choices. World J Clin Oncol. 2015;6(6):281-290. doi: 10.5306/wjco.v6.i6.281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.World Health Organization Draft: global strategy towards the elimination of cervical cancer as a public health problem. Drafted December 16, 2019. Accessed January 15, 2020. https://www.who.int/docs/default-source/cervical-cancer/cerv-cancer-elimn-strategy-16dec-12pm.pdf
- 28.Dhendup T, Tshering P. Cervical cancer knowledge and screening behaviors among female university graduates of year 2012 attending national graduate orientation program, Bhutan. BMC Womens Health. 2014;14(1):44. doi: 10.1186/1472-6874-14-44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.World Health Organization International Agency for Research on Cancer Current status and future directions of breast and cervical cancer prevention and early detection in Belarus. Published 2012. Accessed October 30, 2019. https://publications.iarc.fr/_publications/media/download/4048/987fae663fb0fde0b9f31245163bba6e7f2051cb.pdf
- 30.United Nations Population Fund (UNFPA) Cervical cancer prevention project in the Republic of Moldova. Published 2017. Accessed October 30, 2019. https://moldova.unfpa.org/en/publications/cervical-cancer-prevention-project-republic-moldova
- 31.Maza M, Alfaro K, Garai J, et al. Cervical cancer prevention in El Salvador (CAPE)-an HPV testing-based demonstration project: changing the secondary prevention paradigm in a lower middle-income country. Gynecol Oncol Rep. 2017;20:58-61. doi: 10.1016/j.gore.2017.02.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Baussano I, Tshering S, Choden T, et al. Cervical cancer screening in rural Bhutan with the careHPV test on self-collected samples: an ongoing cross-sectional, population-based study (REACH-Bhutan). BMJ Open. 2017;7(7):e016309. doi: 10.1136/bmjopen-2017-016309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Williams-Brennan L, Gastaldo D, Cole DC, Paszat L. Social determinants of health associated with cervical cancer screening among women living in developing countries: a scoping review. Arch Gynecol Obstet. 2012;286(6):1487-1505. doi: 10.1007/s00404-012-2575-0 [DOI] [PubMed] [Google Scholar]
- 34.Maseko FC, Chirwa ML, Muula AS. Health systems challenges in cervical cancer prevention program in Malawi. Glob Health Action. 2015;8(1):26282. doi: 10.3402/gha.v8.26282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chorley AJ, Marlow LAV, Forster AS, Haddrell JB, Waller J. Experiences of cervical screening and barriers to participation in the context of an organised programme: a systematic review and thematic synthesis. Psychooncology. 2017;26(2):161-172. doi: 10.1002/pon.4126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Armstrong N, James V, Dixon-Woods M. The role of primary care professionals in women’s experiences of cervical cancer screening: a qualitative study. Fam Pract. 2012;29(4):462-466. doi: 10.1093/fampra/cmr105 [DOI] [PubMed] [Google Scholar]
- 37.Callergo M. Social desirability In: Lavrakas P, ed. Encyclopedia of Survey Research Methods. Sage Publications Inc; 2008. [Google Scholar]
- 38.Chidyaonga-Maseko F, Chirwa ML, Muula AS. Underutilization of cervical cancer prevention services in low and middle income countries: a review of contributing factors. Pan Afr Med J. 2015;21:231. doi: 10.11604/pamj.2015.21.231.6350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Devarapalli P, Labani S, Nagarjuna N, Panchal P, Asthana S. Barriers affecting uptake of cervical cancer screening in low and middle income countries: a systematic review. Indian J Cancer. 2018;55(4):318-326. doi: 10.4103/ijc.IJC_253_18 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eMethods 1. Search method for eligible surveys in low- and middle-income countries for which we were unable to acquire a WHO-STEPS survey
eMethods 2. Country-specific sampling methods
eMethods 3. Response rates
eMethods 4. Survey questions on cervical cancer screening
eMethods 5. Calculation of household wealth quintiles
eMethods 6. Supplementary information on the statistical analysis
eFigure 1. Flowchart for inclusion of STEPS surveys
eFigure 2. Flowchart for inclusion of non-STEPS surveys
eTable 1. Survey characteristics by region and country for women aged 30 to 49 years
eTable 2. Sample characteristics across all countries among (1) all women, and (2) women aged 30 to 49 years
eTable 3. Sample characteristics across all countries among women aged 30 to 49 years who were (1) included in the analysis, and (2) excluded due to a missing outcome variable
eTable 4. Percent missing (among all those with non-missing data on cervical cancer screening) for each individual-level predictor, by country
eFigure 3. Age-standardized lifetime prevalence of cervical cancer screening by country
eFigure 4. Lifetime prevalence of cervical cancer screening among all women
eFigure 5. Lifetime prevalence of cervical cancer screening among women aged 30 to 49 years
eTable 5. Estimates of lifetime cervical cancer screening prevalence by 10-year age group for each country, region, and overall
eFigure 6. Lifetime prevalence of cervical cancer screening among all women by GDP and health expenditure per capita
eFigure 7. Lifetime prevalence of cervical cancer screening among all women by HDI,GDI, GII, SIGI, and health worker density
eFigure 8. Lifetime prevalence of cervical cancer screening among all women by GDP and health expenditure per capita, adjusted for individual-level differences between countries
eFigure 9. Lifetime prevalence of cervical cancer screening among all women by HDI, GDI, GII, SIGI, and health worker density, adjusted for individual-level differences between countries
eFigure 10. Lifetime prevalence of cervical cancer screening among women aged 30 to 49 years by GDP and health expenditure per capita, adjusted for individual-level differences between countries
eFigure 11. Cervical cancer screening prevalence by HDI, GDI, GII, SIGI, and health worker density, adjusted for individual-level differences between countries among women aged 30 to 49 years
eFigure 12. Relative and absolute differences in lifetime prevalence of cervical cancer screening between women aged 50 years and older compared to women aged 30 to 49 years (reference group)
eFigure 13. Relative and absolute differences in lifetime prevalence of cervical cancer screening comparing the top versus the bottom (reference group) household wealth quintile
eTable 6. Relative (risk ratios) and absolute (average marginal effects) differences in lifetime prevalence of cervical cancer screening between urban and rural (reference group)
eTable 7. Relative (risk ratios) and absolute (average marginal effects) differences in lifetime prevalence of cervical cancer screening between women having completed high school or further and those who have completed primary school or less (reference group)
eTable 8. Relative (risk ratios) and absolute (average marginal effects) differences in lifetime prevalence of cervical cancer screening between women in the top two versus the bottom two (reference group) household wealth quintiles
eTable 9. Relative (risk ratios) and absolute (average marginal effects) differences in lifetime prevalence of cervical cancer screening between women in the top versus the bottom (reference group) household wealth quintile
eTable 10. Relative (risk ratios) and absolute (average marginal effects) differences in lifetime prevalence of cervical cancer screening between women aged 20 to 29 years compared to women aged 30 to 49 years (reference group)
eTable 11. Relative (risk ratios) and absolute (average marginal effects) differences in lifetime prevalence of cervical cancer screening between women aged 50 years and older and women aged 30 to 49 years (reference group)
eFigure 14. Country-level median lifetime cervical cancer screening prevalence by five-year age group, grouped by region
eFigure 15. Relative and absolute differences in lifetime prevalence of cervical cancer screening between women who currently smoke and women who do not (reference group)
eTable 12. Relative (risk ratios) and absolute (average marginal effects) differences in lifetime prevalence of cervical cancer screening between women who currently smoke and women who do not (reference group)
eFigure 16. Relative and absolute differences in lifetime prevalence of cervical cancer screening between women who are currently not married and women who are (reference group)
eTable 13. Relative (risk ratios) and absolute (average marginal effects) differences in lifetime prevalence of cervical cancer screening between women who are currently not married and women who are (reference group)
eTable 14. Poisson regressions of reporting to have ever been screened for cervical cancer onto individual-level characteristics (with survey weights) in Europe: Azerbaijan to Kyrgyzstan
eTable 15. Poisson regressions of reporting to have ever been screened for cervical cancer onto individual-level characteristics (with survey weights) in Europe: Moldova to Tajikistan
eTable 16. Poisson regressions of reporting to have ever been screened for cervical cancer onto individual-level characteristics (with survey weights) in Latin America and the Caribbean: Argentina to Chile
eTable 17. Poisson regressions of reporting to have ever been screened for cervical cancer onto individual-level characteristics (with survey weights) in Latin America and the Caribbean: Colombia to El Salvador
eTable 18. Poisson regressions of reporting to have ever been screened for cervical cancer onto individual-level characteristics (with survey weights) in Latin America and the Caribbean: Guatemala to Jamaica
eTable 19. Poisson regressions of reporting to have ever been screened for cervical cancer onto individual-level characteristics (with survey weights) in Latin America and the Caribbean: Mexico to St. Vincent & the Grenadines
eTable 20. Poisson regressions of reporting to have ever been screened for cervical cancer onto individual-level characteristics (with survey weights) in Middle East & Northern Africa
eTable 21. Poisson regressions of reporting to have ever been screened for cervical cancer onto individual-level characteristics (with survey weights) in South Asia, East Asia, and Pacific: Bhutan to Indonesia
eTable 22. Poisson regressions of reporting to have ever been screened for cervical cancer onto individual-level characteristics (with survey weights) in South Asia, East Asia, and Pacific: Nepal to Timor-Leste
eTable 23. Poisson regressions of reporting to have ever been screened for cervical cancer onto individual-level characteristics (with survey weights) in Sub-Saharan Africa: Benin to Kenya
eTable 24. Poisson regressions of reporting to have ever been screened for cervical cancer onto individual-level characteristics (with survey weights) in Sub-Saharan Africa: Lesotho to Zimbabwe
eFigure 17. Relative and absolute differences in lifetime prevalence of cervical cancer screening between urban and rural (reference group) areas (Poisson regression without sampling weights)
eFigure 18. Relative and absolute differences in lifetime prevalence of cervical cancer screening between women having completed high school or further and those who have completed primary school or less (reference group) (Poisson regression without sampling weights)
eFigure 19. Relative and absolute differences in lifetime prevalence of cervical cancer screening between women in the top two versus the bottom two (reference group) household wealth quintiles (Poisson regression without sampling weights)
eFigure 20. Relative and absolute differences in lifetime prevalence of cervical cancer screening between women in the top versus the bottom (reference group) household wealth quintile (Poisson regression without sampling weights)
eFigure 21. Relative and absolute differences in lifetime prevalence of cervical cancer screening between women who are currently not married and women who are (reference group) (Poisson regression without sampling weights)
eFigure 22. Relative and absolute differences in lifetime prevalence of cervical cancer screening between women who currently smoke and women who do not (reference group) (Poisson regression without sampling weights)
eFigure 23. Relative and absolute differences in lifetime prevalence of cervical cancer screening between women aged 20 to 29 years compared to women aged 30 to 49 years (reference group) (Poisson regression without sampling weights)
eFigure 24. Relative and absolute differences in lifetime prevalence of cervical cancer screening between women aged 50 years and older and women aged 30 to 49 years (reference group) (Poisson regression without sampling weights)
eTable 25. Poisson regressions of reporting to have ever been screened for cervical cancer onto individual-level characteristics (without survey weights) in Europe: Azerbaijan to Kyrgyzstan
eTable 26. Poisson regressions of reporting to have ever been screened for cervical cancer onto individual-level characteristics (without survey weights) in Europe: Moldova to Tajikistan
eTable 27. Poisson regressions of reporting to have ever been screened for cervical cancer onto individual-level characteristics (without survey weights) in Latin America and the Caribbean: Argentina to Chile
eTable 28. Poisson regressions of reporting to have ever been screened for cervical cancer onto individual-level characteristics (without survey weights) in Latin America and the Caribbean: Colombia to El Salvador
eTable 29. Poisson regressions of reporting to have ever been screened for cervical cancer onto individual-level characteristics (without survey weights) in Latin America and the Caribbean: Guatemala to Jamaica
eTable 30. Poisson regressions of reporting to have ever been screened for cervical cancer onto individual-level characteristics (without survey weights) in Latin America and the Caribbean: Mexico to St. Vincent & the Grenadines
eTable 31. Poisson regressions of reporting to have ever been screened for cervical cancer onto individual-level characteristics (without survey weights) in Middle East & Northern Africa
eTable 32. Poisson regressions of reporting to have ever been screened for cervical cancer onto individual-level characteristics (without survey weights) in South Asia, East Asia, and Pacific: Bhutan to Indonesia
eTable 33. Poisson regressions of reporting to have ever been screened for cervical cancer onto individual-level characteristics (without survey weights) in South Asia, East Asia and Pacific: Nepal to Timor-Leste
eTable 34. Poisson regressions of reporting to have ever been screened for cervical cancer onto individual-level characteristics (without survey weights) in Sub-Saharan Africa: Benin to Kenya
eTable 35. Poisson regressions of reporting to have ever been screened for cervical cancer onto individual-level characteristics (without survey weights) in Sub-Saharan Africa: Lesotho to Zimbabwe
eFigure 25. GDP per capita plotted against the relative and absolute difference in lifetime prevalence of cervical cancer screening between urban and rural (reference group) areas in each country (Poisson regression with sampling weights)
eFigure 26. GDP per capita plotted against the relative and absolute difference in lifetime prevalence of cervical cancer screening between urban and rural (reference group) areas in each country (Poisson regression without sampling weights)
eFigure 27. GDP per capita plotted against the relative and absolute difference in lifetime prevalence of cervical cancer screening between women having completed high school or further and those who have completed primary school or less (reference group) in each country (Poisson regression with sampling weights)
eFigure 28. GDP per capita plotted against the relative and absolute difference in lifetime prevalence of cervical cancer screening between women having completed high school or further and those who have completed primary school or less (reference group) in each country (Poisson regression without sampling weights)
eFigure 29. GDP per capita plotted against the relative and absolute difference in lifetime prevalence of cervical cancer screening between women in the top two versus the bottom two (reference group) household wealth quintile in each country (Poisson regression with sampling weights)
eFigure 30. GDP per capita plotted against the relative and absolute difference in lifetime prevalence of cervical cancer screening between women in the top two versus the bottom two (reference group) household wealth quintile in each country (Poisson regression without sampling weights)
eMethods 7. Review of prevailing screening methods in countries that asked about Pap smears only
eTable 36. Review summary of prevailing screening modalities in countries that asked about Pap smear testing only
eTable 37. Poisson regressions run separately for each group of cervical cancer screening modalities as ascertained in the included survey questionnaires


