Abstract
OBJECTIVE
Glucose response curves (GRCs) during oral glucose tolerance tests (OGTTs) are predictive of type 1 diabetes. We performed a longitudinal analysis in pancreatic autoantibody-positive individuals to assess 1) characteristic GRC changes during progression to type 1 diabetes and 2) GRC changes in relation to β-cell function changes and to combined glucose and C-peptide response curve (GCRC) changes.
RESEARCH DESIGN AND METHODS
Among antibody-positive individuals with serial OGTTs in the TrialNet Pathway to Prevention study, GRC changes from first to last OGTTs were compared between progressors (n = 298) to type 1 diabetes and nonprogressors (n = 2,216). GRC changes from last OGTT before diagnosis to diagnostic OGTTs were studied in progressors.
RESULTS
GRCs changed more frequently from biphasic (two peaks) to monophasic (one peak) GRCs between first and last OGTTs in progressors than in nonprogressors (75.4% vs. 51.0%, respectively; P < 0.001). In contrast, GRCs of progressors changed less frequently from monophasic to biphasic than those of nonprogressors (12.6% vs. 30.6%; P < 0.001). Monotonic (continuous increase) GRCs were present in 47.7% of progressors at diagnosis. The early (30–0 min) C-peptide response decreased in progressors with GRCs changing from biphasic to monophasic between first and last OGTTs (P < 0.001) and from monophasic to monotonic between last and diagnostic OGTTs (P < 0.001). Conversely, the early C-peptide response increased among nonprogressors with GRCs changing from monophasic to biphasic (P < 0.001). Changes in GRCs were related to changes in GCRCs.
CONCLUSIONS
Characteristic GRC changes, biphasic to monophasic to monotonic, occur during the progression to type 1 diabetes. These GRC changes correspond to decreasing β-cell function.
Introduction
Glucose response curves (GRCs) to oral glucose tolerance tests (OGTTs) can assume different forms in both nondiabetes and diabetes states. They are often present in two forms: monophasic (one peak) and biphasic (two peaks). GRCs are of particular interest in the prediabetes state, since they could potentially provide information pertaining to natural history, prediction, and prognosis. Several studies have examined GRCs among those at risk for either type 1 or type 2 diabetes (1–11). However, there is no information regarding longitudinal changes in GRC forms among individuals at risk for type 1 diabetes.
We recently performed a cross-sectional analysis of GRCs in TrialNet Pathway to Prevention (TNPTP) autoantibody-positive participants, of whom many developed type 1 diabetes (1). Our findings showed that the majority had a monophasic GRC, placing them at greater risk for type 1 diabetes than individuals with a biphasic GRC. In addition, those with monophasic GRCs had lower C-peptide levels.
Findings from that cross-sectional study suggested that GRC forms could be indicative of the degree of β-cell pathology. Thus, we have undertaken a more definitive longitudinal study to address three key questions that could not be answered by the prior analyses. 1) Do progressors to type 1 diabetes and nonprogressors have characteristic changes of GRCs over time? 2) Are changes in GRCs related to changes in β-cell function? 3) How do changes in GRCs relate to changes in two-dimensional shapes derived from combined glucose and C-peptide response curves (GCRCs)? To answer these questions, we have used the unique TNPTP database, which includes serial OGTTs in autoantibody-positive relatives of persons with type 1 diabetes.
Research Design and Methods
Subjects
The TNPTP study is the longitudinal, natural history arm of TrialNet, which follows first- and second-degree relatives of people with type 1 diabetes positive for at least one autoantibody. Those individuals are followed longitudinally with OGTTs every 6–12 months for the development of diabetes. As part of the study protocol, the diagnosis of diabetes is defined as a fasting or 2-h glucose level in the diabetes range with a confirmatory OGTT in the diabetes range. However, if a diagnosis can be made on clinical grounds, a confirmatory OGTT is not required. The first OGTT is considered the diagnostic OGTT if findings are confirmed by the subsequent OGTT.
To be included in the analyses, progressors and nonprogressors to type 1 diabetes must have had two OGTTs at least 3 months apart, with complete measurements of glucose and C-peptide at all five time points (0, 30, 60, 90, and 120 min). Additionally, for progressors, data analysis was limited to those who had at least two OGTTs prior to type 1 diabetes development, with the last OGTT within 1 year of diagnosis. Both first and last (prior to diagnosis for progressors) OGTTs must have been in the nondiabetes range as defined by the American Diabetes Association diagnostic criteria for type 1 diabetes (12). Complete OGTT data were available from 2,835 TNPTP antibody-positive participants at study enrollment. Supplementary Fig. 1 is a flowchart for those included in the analysis.
Definitions Used for Classification of the GRCs
We used definitions for “phasic” GRCs as has been previously described by Tschritter et al. (2). Specifically, the glucose curve form was classified as “monophasic” when plasma glucose increased after an oral glucose load to a maximum concentration at 30, 60, or 90 min and then decreased until 120 min with a final decline of ≥0.25 mmol/L (4.5 mg/dL) between 90 and 120 min. Those with glucose curves that decreased by ≥0.25 mmol/L after an initial increase and then increased again by ≥0.25 mmol/L at any time during the second hour of the OGTT were classified as having “biphasic” glucose curves. Based on the glucose assay used in the TNPTP study with mean intra- and interassay coefficients of variation both of <2%, there is 95% confidence that the glucose change of ≥0.25 mmol/L is not due to assay variation. Finally, a monotonic GRC, defined by a continuous rise in glucose until 120 min and considered to be a more pathologic pattern suggestive of imminent diabetes (11), was included in this analysis. There were 321 individuals excluded from the analysis who had unclassified OGTT forms (did not meet definitions and cutoffs for any of the above GRC forms) either at enrollment or during follow-up.
We compared progressors with nonprogressors for changes in GRC forms from first to last OGTTs (before diagnosis for progressors). In addition, among progressors, we compared frequencies of GRC changes from last nondiabetes OGTT result to the diagnostic OGTTs.
Measures of β-Cell Function
C-peptide levels at all OGTT time points (0, 30, 60, 90, and 120 min) and C-peptide area under the curve (AUC) were studied in order to examine the possible contribution of insulin secretion to GRCs. We also assessed the early (30–0 min) C-peptide response, which has been shown to correlate with the first-phase insulin response derived from intravenous glucose tolerance tests (13,14). For further analysis of associations of changes in GRCs with C-peptide changes, the C-peptide index (30–0 min C-peptide/30–0 min glucose) and C-peptide AUC–to–glucose AUC ratio (AUC ratio) were evaluated. The C-peptide index and AUC ratio are OGTT-derived indices of β-cell function that have previously been described and validated against clamp-derived measures of β-cell function (15). Additionally, a metabolic measure (Index60), developed from 2-h OGTTs using the log fasting C-peptide, 60-min C-peptide, and 60-min glucose, was used to assess metabolic risk (16).
Changes in the Combined GCRCs
For further examination of the changes in GRCs over time, mean glucose levels were plotted against mean C-peptide levels at each postglucose load time point (i.e., 30, 60, 90, and 120 min) during the OGTTs on two-dimensional grids (glucose on the y-axis and C-peptide on the x-axis). We then assessed the differences in shapes and locations (on the two-dimensional grid) of these GCRCs over time in progressors and nonprogressors according to changes in GRCs.
Statistical Analysis
Participant demographic and clinical characteristics measured on a nominal or ordinal scale are summarized as counts and percentages, whereas variables measured in the interval scale are summarized as mean ± SD and median (interquartile range). Student t test, Wilcoxon rank sum test, and Pearson χ2 test were used for comparisons. Logistic regression analyses were used with and without adjustments for age, sex, BMI z score, and the interval between OGTTs. For comparisons of GCRCs, centroid coordinates (which define the central point of the GCRC shapes) were calculated for the polygonal shapes formed by the GCRCs after closure with a line connecting the 30-min and 120-min values. Differences between centroids were compared using multivariate ANOVA (MANOVA).
Results
There were 2,216 nonprogressors and 298 progressors for whom complete data were available for analyses. Supplementary Table 1 shows the baseline characteristics of those included in the analyses. At baseline, progressors were significantly younger than nonprogressors (median age 10.5 years [interquartile range 8.1] vs. 12.7 years [17.4], respectively; P < 0.001) with a shorter interval between the first and last OGTT (1.7 years [2.2] vs. 2.1 years [3.4]; P < 0.001) compared with nonprogressors. However, their BMI z score, sex, and racial distribution were similar. The distribution of GRCs at the first OGTT differed significantly between progressors and nonprogressors (P < 0.001). Among nonprogressors, 43.3% had a biphasic pattern, 55.6% monophasic, and 1.1% monotonic. Among progressors, 19.1% had a biphasic pattern, 80.2% monophasic, and 0.7% monotonic (excluding those in the diabetes range).
The analyses described below are separated according to the three key questions pertaining to the evolution of GRC curves during the progression to type 1 diabetes.
1) Do Progressors to Type 1 Diabetes and Nonprogressors Have Characteristic Changes of GRCs Over Time?
Changes in GRCs From First to Last OGTTs in Nonprogressors and Progressors
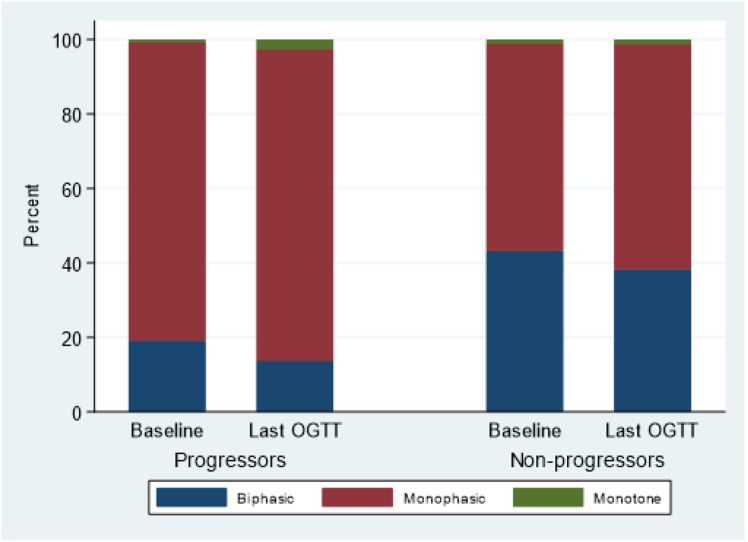
Figure 1 shows the changes in the distribution of the GRCs from the first to the last OGTT. Within progressor and nonprogressor groups, the changes in the distribution of the GRCs from first to last OGTT were significantly different (marginal homogeneity [Stuart-Maxwell] P = 0.043 and P < 0.001, respectively).
Figure 1.
Change in GRC shape over time from first to last OGTT among progressors and nonprogressors. Progressors (change from first to last, n = 298): P = 0.043. Nonprogressors (change from first to last, n = 2,216): P < 0.001.
Compared with GRCs of nonprogressors, GRCs of progressors were significantly more likely to change from biphasic to monophasic before and after adjustments (for age, sex, BMI z score at baseline, and the interval between the first and last OGTT: 75.4% [43 of 57] vs. 51.0% [490 of 960]; P < 0.001 unadjusted and adjusted). In contrast, GRCs of nonprogressors were significantly more likely to change in the reverse direction, from monophasic to biphasic GRCs (12.6% [30 of 239] vs. 30.6% [377 of 1,231]; P < 0.001 unadjusted and adjusted). Supplementary Table 2 shows the odds ratio with 95% CIs for the logistic regression analyses.
The divergent pattern of change between GRCs of nonprogressors and progressors resulted in even greater differences in the GRC distribution at the last OGTT than at the first OGTT. Overall, the distribution of the GRCs was significantly different at the last OGTT between progressors and nonprogressors (P < 0.001). Progressors had a significantly lower proportion of biphasic GRCs at their last OGTT prior to diagnosis compared with nonprogressors (13.8% vs. 38.2%, respectively; P < 0.001).
Changes in GRCs From Last OGTT Prior to Diagnosis to Diagnostic OGTT in Progressors
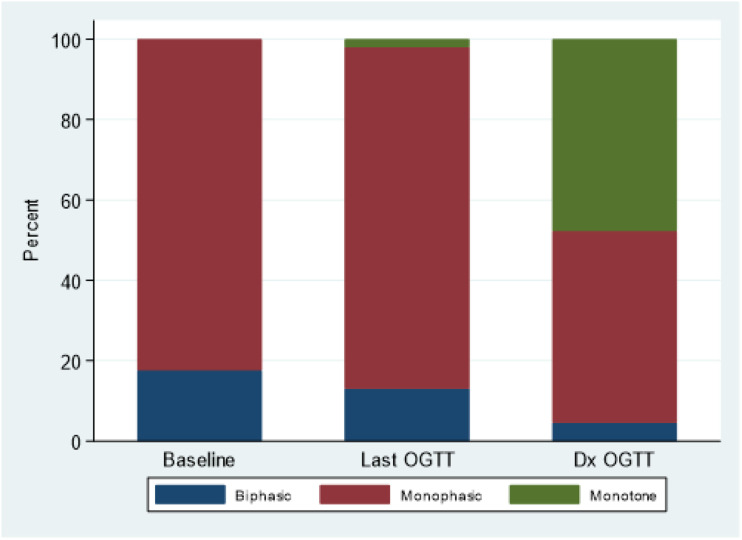
A monotonic GRC was present in a high proportion of progressors who had OGTTs at diagnosis (73 of 153 [47.7%]). Among those with monophasic GRCs at the last OGTT (n = 130), 49.2% (n = 64) had a change to a monotonic shape at diagnosis, while for 47.0% (n = 61) the GRCs remained monophasic at diagnosis and for 3.8% (n = 5) they changed to biphasic. Figure 2 shows changes in distributions of GRC forms from the first OGTT to the OGTT at diagnosis.
Figure 2.
Change in GRC shapes, among progressors who had a diagnostic OGTT for comparison (n = 153), from first to last OGTT and from last OGTT prior to diagnosis to diagnostic OGTT (Dx OGTT). P value <0.001 for the difference in distribution of the GRC shape at each time point.
2) Are Changes in GRCs Related to Changes in β-Cell Function?
Among progressors, those who had GRC changes from biphasic to monophasic (n = 377) had a significant decline in the early C-peptide response (P < 0.001). There were similar declines in the C-peptide index and AUC ratio, while Index60 increased. All of these changes were statistically significant before and after adjustments for changes in age and BMI z score (P < 0.001) (Table 1).
Table 1.
Metabolic changes from first to last OGTT in progressors versus nonprogressors
| N | First OGTT, median (IQR) | Last OGTT, median (IQR) | Signed rank P value | P values (adjusted by change in age and BMI z score) | |
|---|---|---|---|---|---|
| Early C-peptide | |||||
| Progressor | |||||
| BiP to MoP | 43 | 3.37 (2.39) | 2.38 (1.83) | <0.001 | <0.001 |
| MoP to MoT | 5 | 2.20 (2.81) | 1.10 (0.69) | 0.138 | 0.480 |
| MoP to BiP | 30 | 2.22 (1.83) | 2.42 (2.14) | 0.192 | 0.137 |
| MoP to MoP | 204 | 2.33 (1.72) | 1.80 (1.44) | <0.001 | <0.001 |
| Nonprogressor | |||||
| BiP to MoP | 490 | 3.95 (2.77) | 3.88 (2.99) | 0.077 | 0.033 |
| MoP to MoT | 16 | 3.46 (3.93) | 1.83 (2.82) | 0.079 | 0.111 |
| MoP to BiP | 377 | 3.53 (2.85) | 3.97 (2.93) | 0.005 | 0.001 |
| MoP to MoP | 838 | 3.35 (2.67) | 3.27 (2.85) | 0.992 | 0.691 |
| C-peptide index | |||||
| Progressor | |||||
| BiP to MoP | 43 | 0.06 (0.05) | 0.03 (0.03) | <0.001 | <0.001 |
| MoP to MoT | 5 | 0.04 (0.03) | 0.02 (0.05) | 0.893 | 0.413 |
| MoP to BiP | 30 | 0.04 (0.04) | 0.03 (0.03) | 0.530 | 0.468 |
| MoP to MoP | 204 | 0.04 (0.02) | 0.03 (0.02) | <0.001 | <0.001 |
| Nonprogressor | |||||
| BiP to MoP | 490 | 0.078 (0.064) | 0.072 (0.057) | <0.001 | <0.001 |
| MoP to MoT | 15 | 0.076 (0.090) | 0.052 (0.075) | 0.017 | 0.029 |
| MoP to BiP | 377 | 0.065 (0.052) | 0.078 (0.068) | <0.001 | <0.001 |
| MoP to MoP | 837 | 0.058 (0.047) | 0.057 (0.051) | 0.974 | 0.517 |
| Index60 | |||||
| Progressor | |||||
| BiP to MoP | 43 | 0.23 (1.29) | 1.25 (1.33) | <0.001 | <0.001 |
| MoP to MoT | 5 | 1.30 (0.89) | 1.66 (1.18) | 0.893 | 0.625 |
| MoP to BiP | 30 | 1.18 (1.04) | 1.14 (0.73) | 0.614 | 0.886 |
| MoP to MoP | 204 | 1.10 (1.27) | 1.68 (0.88) | <0.001 | <0.001 |
| Nonprogressor | |||||
| BiP to MoP | 490 | −0.223 (1.264) | −0.329 (1.646) | 0.922 | 0.933 |
| MoP to MoT | 16 | −0.241 (2.795) | 0.818 (2.460) | 0.004 | 0.022 |
| MoP to BiP | 377 | −0.070 (1.555) | −0.282 (1.437) | <0.001 | <0.001 |
| MoP to MoP | 838 | 0.099 (1.523) | 0.094 (1.727) | 0.175 | 0.400 |
| AUC ratio | |||||
| Progressor | |||||
| BiP to MoP | 43 | 0.04 (0.02) | 0.03 (0.02) | <0.001 | <0.001 |
| MoP to MoT | 5 | 0.03 (0.02) | 0.02 (0.00) | 0.893 | 0.902 |
| MoP to BiP | 30 | 0.03 (0.02) | 0.03 (0.02) | 0.558 | 0.847 |
| MoP to MoP | 204 | 0.03 (0.02) | 0.02 (0.01) | <0.001 | <0.001 |
| Nonprogressor | |||||
| BiP to MoP | 490 | 0.045 (0.026) | 0.049 (0.029) | <0.001 | <0.001 |
| MoP to MoT | 16 | 0.053 (0.050) | 0.035 (0.042) | 0.003 | 0.012 |
| MoP to BiP | 377 | 0.044 (0.028) | 0.048 (0.027) | 0.001 | <0.001 |
| MoP to MoP | 838 | 0.044 (0.025) | 0.045 (0.028) | 0.002 | <0.001 |
BiP, biphasic; MoP, monophasic; MoT, monotonic.
Those who had a monophasic GRC at their last OGTT prior to diagnosis also showed a significant decline in their early C-peptide response (in ng/mL), whether they changed from monophasic to monotonic (n = 64, median 1.91 [interquartile range 0.97] vs. 0.99 [0.72], P < 0.001) or remained monophasic (n = 61, 2.67 [1.42] vs. 1.92 [1.28], P < 0.001). Similarly, there were significant declines in both groups of the C-peptide index and AUC ratio, while the Index60 increased significantly (P < 0.001 for all) (Supplementary Table 3). These changes remained significant after adjustments for changes in age (P < 0.001). (BMI z score adjustments were precluded by missing values.)
Among nonprogressors with GRCs that changed from monophasic to biphasic at their last OGTT (n = 377), the early C-peptide response, the C-peptide index, and AUC ratio increased significantly, while Index60 decreased significantly (all P < 0.01). This was not evident in progressors, but the number with a change from monophasic to biphasic was much smaller (n = 30) (Table 1).
3) How Do Changes in GRCs Relate to Changes in Two-Dimensional Shapes Derived From GCRCs?
Given the association between the directionality of the change in GRC forms and the associations seen with changes in β-cell function (i.e., improved measures of β-cell function seen in nonprogressors with a GRC that changed from monophasic to biphasic and worsening β-cell function seen in progressors with GRCs that remained monophasic or changed to monotonic over time), we examined associations between GRCs and GCRCs. GCRCs, derived from mean C-peptide and glucose values at OGTT time points (from 30, 60, 90, and 120 min), are displayed on glucose (y-axis)/C-peptide (x-axis) grids in Fig. 3A–C. The two GCRCs in each of the three panels provide insight into how changes in GRCs are related to changes in β-cell function and whether GRCs might be indicative of the more informational GCRCs.
Figure 3.
Two-dimensional grids (glucose on the y-axis and C-peptide on the x-axis) showing the GCRCs from 30 to 120 min and their centroid comparisons (comparison of the central location for each plot) for nonprogressors with GRCs that changed from monophasic to biphasic from first to last OGTT (A), progressors with GRCs that changed from monophasic to monotonic from last to diagnostic OGTTs (B), and progressors with GRCs that remained monophasic from last to diagnostic OGTTs (C). Arrows in each panel point to the change in location of the centroid locations on the grid. *Mean values for glucose and C-peptide at the different time points (30–120 min) during the OGTT.
Figure 3A shows GCRC changes from first to last OGTTs among nonprogressors with GRCs that changed from monophasic to biphasic. The change in the GCRC was indicative of metabolic improvement, as indicated by decreased glucose levels overall. This was mostly suggested by the 30- to 60-min slope changing from a horizontal to a downward direction. These favorable changes are consistent with the improvement in the C-peptide and glucose indices seen in Table 1.
In Fig. 3B, the change from a monophasic to a monotonic GRC (at diagnosis) in progressors was associated with a marked shift and unfolding of the GCRC shape upward and to the left. As the GCRC moved, it also became almost fully straight (i.e., assumes a near monotonic pattern).
Figure 3C shows the changes in the GCRCs of progressors with GRCs that remained monophasic from the last (before diagnosis) OGTT to the diagnostic OGTT. Even though the monophasic GRC persisted, the GCRC moved substantially upward, indicating a marked increase in glucose levels. Evident also was a straightening of the GCRC, suggesting that the GCRC may soon assume the monotonic shape that is present in Fig. 3B. For further confirmation of these visible shifts in the GCRCs, differences in the centroid locations were examined and found to be statistically significant in each panel (all P < 0.001). Lastly, the monophasic GCRCs at the first OGTT in nonprogressors differed markedly in shape compared with monophasic GCRCs of progressors whether at their last OGTT prior to diagnosis or their diagnostic OGTT.
Conclusions
This first longitudinal study of changes in GRCs in individuals at risk for type 1 diabetes provided new findings with implications for future studies. We found that there are characteristic changes in GRCs during the progression to type 1 diabetes. Specifically, compared with nonprogressors, progressors were more likely to have GRCs change from biphasic to monophasic and less likely to have GRCs change from monophasic to biphasic between the first and last visits. In addition, GRCs of a large proportion of progressors changed from monophasic at the last OGTT prior to diagnosis to monotonic at the diagnostic OGTT, resulting in a sizable proportion of monotonic GRCs at diagnosis. Overall, progressors were much more likely to move from biphasic to monophasic to monotonic GRCs than the reverse.
These characteristic GRC changes during the progression to type 1 diabetes were associated with corresponding changes in measures of β-cell function. Progressors and nonprogressors with GRCs that changed from biphasic to monophasic between the first and last visits had significant declines in several measures of β-cell function. Declines were also evident in those with changes from monophasic to monotonic at diagnosis. Interestingly, individuals with GRCs that continued to be monophasic at diagnosis also had marked declines, but β-cell function decline was less severe than in those with GRCs changing from monophasic to monotonic.
This study introduces a novel approach for analyzing GRC changes by assessing how those changes relate to changes in GCRC shapes and their change in position on a two-dimensional glucose/C-peptide grid. Since GRCs per se do not capture changes in β-cell function, relating GRCs to GCRC shapes should aid in better interpreting changes in GRCs.
The underlying basis for the different GRCs and their changes is not well understood, but prior reports in cohorts with type 2 diabetes suggest that changes in GRCs are associated with changes in β-cell function and insulin sensitivity. Studies of individuals at risk for type 2 diabetes consistently show that the monophasic form is associated with lower levels of insulin sensitivity and impaired β-cell function relative to insulin sensitivity (2–11). We assessed whether changes in GRCs are related to HOMA for insulin resistance and BMI z score (as an indicator of insulin resistance). However, our findings were inconsistent (data not shown), which could be attributable to the difficulty in assessing insulin sensitivity in an insulin-deficient state.
The monotonic shape was most prevalent among progressors at diagnosis and was associated with the greatest metabolic decline. However, we cannot say to what extent monotonic GRCs developed prior to diagnosis, since OGTTs were performed at 6-month intervals, which would not capture a transition to monotonic GRCs before diagnosis.
The high prevalence of monotonic GRCs at diagnosis is consistent with findings of previous studies of type 2 diabetes. In a study (11) assessing GRCs at randomization in youth with type 2 diabetes enrolled in the TODAY (Treatment Options for Type 2 Diabetes in Adolescents and Youth) study, compared with those with either monophasic or biphasic GRCs, those with monotonic GRCs at randomization had significantly more impaired β-cell function and higher rates of glycemic failure, as well as accelerated decline in β-cell function independent of diabetes duration and treatment assignment. In adults, a monotonic GRC is characteristic of individuals with impaired glucose tolerance, muscle insulin resistance, and impaired β-cell function (17).
As was evident in Fig. 3C, the progressors with GRCs that remained monophasic from the first to the last OGTT developed a GCRC pattern approaching a monotonic GCRC shape. This straightening of the curve with increasing glucose and C-peptide values from 30 to 90 min is consistent with the finding in our prior cross-sectional study; those who had late-peaking monophasic GRCs (with glucose peaks occurring at 90 min) were at higher risk for type 1 diabetes development compared with those with an earlier peak (1).
A key finding from this study was the sizable percentage of nonprogressors who changed from monophasic to biphasic GRCs. Moreover, that change was accompanied by an improvement in C-peptide responses. This suggests the possibility that the progression to type 1 diabetes is not inevitable, even when there is appreciable metabolic decline. This is a novel finding that suggests β-cell function might be reversed or delayed for some years. If so, OGTTs and the shape of the glucose curve prior to and during intervention therapy could potentially be used to assess the effect of interventions on changes in β-cell function over time. A long-term follow-up study of nonprogressors with monophasic GRCs that changed to biphasic could provide significant insight into our understanding of the natural history of type 1 diabetes, as well as more accurate prognostic information.
Our study has several strengths. The large numbers and the follow-up with serial OGTTs in the TNPTP contributed to this being the first study to describe the pathological evolution of GRCs during the progression to type 1 diabetes. Further, it provided the opportunity to relate GRC changes to changes in β-cell function and to changes in GCRC shapes.
The study had some limitations. Among progressors, not all participants were diagnosed by an OGTT, thus limiting the number of diagnostic OGTTs that could be analyzed. Also, a number of individuals with unclassifiable GRCs were excluded from the analyses. However, in comparisons of this group with individuals with classifiable GRCs, there were no differences in either their demographic or metabolic profile at study enrollment (Supplementary Table 4). Also, we have not assessed the timing of the peak glucoses in the monophasic group. This could help to better define those at risk, as has been shown in our previous work (1). Finally, differences in gastric emptying could have possibly affected the shape of the GRCs. The main strength of the study remains the large amount of longitudinal metabolic information available in TrialNet.
In conclusion, our findings provide answers to the three questions we had posed: 1) Do progressors to type 1 diabetes and nonprogressors have characteristic changes of GRCs over time? Indeed, there appears to be a characteristic evolution of GRC changes during the progression to type 1 diabetes: from biphasic to monophasic and then ultimately to monotonic. However, in contrast to this pattern of progression, an appreciable percentage of nonprogressors had GRCs change from monophasic to biphasic. Additionally, the findings show that there is a small percentage of monotonic GRCs throughout the progression to type 1 diabetes, but the percentage increases markedly at diagnosis. It should be noted that the typical changes of GRCs during progression will not pertain to all individuals. 2) Are changes in GRCs related to changes in β-cell function? C-peptide responses decline as GRCs change from biphasic to monophasic and from monophasic to monotonic in progressors. However, when GRCs of nonprogressors change from monophasic to biphasic, there is improvement in C-peptide responses, suggesting that at least in nonprogressors a decline in β-cell responsiveness can be reversible. 3) How do changes in GRCs relate to changes in two-dimensional shapes derived from GCRCs? The two-dimensional GCRC changes do appear to correspond to GRC changes, and inferences can be made about GCRCs from GRCs.
Our findings suggest that changes in GRCs could potentially serve as biomarkers of the decline in β-cell function during the course of progression to clinical type 1 diabetes. In addition, changes of GRC and GCRCs can provide insights into the natural history of type 1 diabetes and improve the selection of those who might be most appropriate for preventive therapies.
Article Information
Acknowledgments. Members of the Type 1 Diabetes TrialNet Study Group and TrialNet affiliate centers are listed in the supplementary materials. The authors acknowledge the support of the Type 1 Diabetes TrialNet Study Group, which identified study participants and provided samples and follow-up data for this study.
Funding. The Type 1 Diabetes TrialNet Study Group is a clinical trials network funded by the National Institutes of Health (NIH) through the National Institute of Diabetes and Digestive and Kidney Diseases, the National Institute of Allergy and Infectious Diseases, and the Eunice Kennedy Shriver National Institute of Child Health and Human Development, through cooperative agreements U01 DK061010, U01 DK061034, U01 DK061042, U01 DK061058, U01 DK085465, U01 DK085453, U01 DK085461, U01 DK085466, U01 DK085499, U01 DK085504, U01 DK085509, U01 DK103180, U01 DK103153, U01 DK085476, U01 DK103266, U01 DK103282, U01 DK106984, U01 DK106994, U01 DK107013, U01 DK107014, UC4 DK106993, and UC4 DK11700901, and JDRF. This publication was made possible with support from grants KL2TR002530 (A. Carroll, principal investigator [PI]) and UL1TR002529 (A. Shekhar, PI) from the NIH National Center for Advancing Translational Sciences, Clinical and Translational Science Award.
The contents of this article are solely the responsibility of the authors and do not necessarily represent the official views of the NIH or JDRF.
Duality of Interest. No potential conflicts of interest relevant to this article were reported.
Author Contributions. H.M.I., M.A.C., P.X., and J.M.S. conceptualized the analysis, analyzed and interpreted the data, and wrote the manuscript. I.M.L., D.J.B., J.B.M., J.S.S., and J.P.P. contributed to the study design, interpreted the data, and reviewed and edited the manuscript. All authors provided final approval of the manuscript prior to publishing. H.M.I. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Prior Presentation. Parts of this study were presented in abstract form at the 78th Scientific Sessions of the American Diabetes Association, Orlando, FL, 22–26 June 2018.
Footnotes
Members of the Type 1 Diabetes TrialNet Study Group are listed in the supplementary material.
This article contains supplementary material online at https://doi.org/10.2337/figshare.12753662.
References
- 1.Ismail HM, Xu P, Libman IM, et al.; Type 1 Diabetes TrialNet Study Group . The shape of the glucose concentration curve during an oral glucose tolerance test predicts risk for type 1 diabetes. Diabetologia 2018;61:84–92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tschritter O, Fritsche A, Shirkavand F, Machicao F, Häring H, Stumvoll M. Assessing the shape of the glucose curve during an oral glucose tolerance test. Diabetes Care 2003;26:1026–1033 [DOI] [PubMed] [Google Scholar]
- 3.Kanauchi M, Kimura K, Kanauchi K, Saito Y. Beta-cell function and insulin sensitivity contribute to the shape of plasma glucose curve during an oral glucose tolerance test in non-diabetic individuals. Int J Clin Pract 2005;59:427–432 [DOI] [PubMed] [Google Scholar]
- 4.Trujillo-Arriaga HM, Román-Ramos R. Fitting and evaluating the glucose curve during a quasi continuous sampled oral glucose tolerance test. Comput Biol Med 2008;38:185–195 [DOI] [PubMed] [Google Scholar]
- 5.Abdul-Ghani MA, Lyssenko V, Tuomi T, Defronzo RA, Groop L. The shape of plasma glucose concentration curve during OGTT predicts future risk of type 2 diabetes. Diabetes Metab Res Rev 2010;26:280–286 [DOI] [PubMed] [Google Scholar]
- 6.Tura A, Morbiducci U, Sbrignadello S, Winhofer Y, Pacini G, Kautzky-Willer A. Shape of glucose, insulin, C-peptide curves during a 3-h oral glucose tolerance test: any relationship with the degree of glucose tolerance? Am J Physiol Regul Integr Comp Physiol 2011;300:R941–R948 [DOI] [PubMed] [Google Scholar]
- 7.Kim JY, Coletta DK, Mandarino LJ, Shaibi GQ. Glucose response curve and type 2 diabetes risk in Latino adolescents. Diabetes Care 2012;35:1925–1930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bervoets L, Mewis A, Massa G. The shape of the plasma glucose curve during an oral glucose tolerance test as an indicator of beta cell function and insulin sensitivity in end-pubertal obese girls. Horm Metab Res 2015;47:445–451 [DOI] [PubMed] [Google Scholar]
- 9.Nolfe G, Spreghini MR, Sforza RW, Morino G, Manco M. Beyond the morphology of the glucose curve following an oral glucose tolerance test in obese youth. Eur J Endocrinol 2012;166:107–114 [DOI] [PubMed] [Google Scholar]
- 10.Kim JY, Michaliszyn SF, Nasr A, et al. The shape of the glucose response curve during an oral glucose tolerance test heralds biomarkers of type 2 diabetes risk in obese youth. Diabetes Care 2016;39:1431–1439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Arslanian S, El Ghormli L, Young Kim J, et al.; TODAY Study Group . The shape of the glucose response curve during an oral glucose tolerance test: forerunner of heightened glycemic failure rates and accelerated decline in β-cell function in TODAY. Diabetes Care 2019;42:164–172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Expert Committee on the Diagnosis and Classification of Diabetes Mellitus Report of the Expert Committee on the Diagnosis and Classification of Diabetes Mellitus. Diabetes Care 2003;26(Suppl. 1):S5–S20 [DOI] [PubMed] [Google Scholar]
- 13.Sosenko JM, Palmer JP, Rafkin LE, et al.; Diabetes Prevention Trial-Type 1 Study Group . Trends of earlier and later responses of C-peptide to oral glucose challenges with progression to type 1 diabetes in diabetes prevention trial-type 1 participants. Diabetes Care 2010;33:620–625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sosenko JM, Skyler JS, Herold KC, Palmer JP; Type 1 Diabetes TrialNet and Diabetes Prevention Trial–Type 1 Study Groups . The metabolic progression to type 1 diabetes as indicated by serial oral glucose tolerance testing in the Diabetes Prevention Trial–Type 1. Diabetes 2012;61:1331–1337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tura A, Kautzky-Willer A, Pacini G. Insulinogenic indices from insulin and C-peptide: comparison of beta-cell function from OGTT and IVGTT. Diabetes Res Clin Pract 2006;72:298–301 [DOI] [PubMed] [Google Scholar]
- 16.Sosenko JM, Skyler JS, DiMeglio LA, et al.; Type 1 Diabetes TrialNet Study Group; Diabetes Prevention Trial–Type 1 Study Group . A new approach for diagnosing type 1 diabetes in autoantibody-positive individuals based on prediction and natural history. Diabetes Care 2015;38:271–276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Abdul-Ghani MA, Jenkinson CP, Richardson DK, Tripathy D, DeFronzo RA. Insulin secretion and action in subjects with impaired fasting glucose and impaired glucose tolerance: results from the Veterans Administration Genetic Epidemiology Study. Diabetes 2006;55:1430–1435 [DOI] [PubMed] [Google Scholar]