Abstract
OBJECTIVE
To evaluate the incidence and risk factors for diabetic ketoacidosis (DKA) and related adverse events (AEs) in adults with type 1 diabetes treated with sotagliflozin adjunctive to insulin.
RESEARCH DESIGN AND METHODS
Data from two identically designed, 52-week, randomized studies were pooled and analyzed for DKA, changes in β-hydroxybutyrate (BHB), and percentage of patients with BHB >0.6 and >1.5 mmol/L. The patients were administered placebo, sotagliflozin 200 mg, or sotagliflozin 400 mg once daily.
RESULTS
A total of 191 ketosis-related AEs were reported, and 98 underwent adjudication. Of these, 37 events (36 patients) were adjudicated as DKA, with an exposure-adjusted incidence rate of 0.2, 3.1, and 4.2 events per 100 patient-years for placebo, sotagliflozin 200 mg, and sotagliflozin 400 mg, respectively. No patient died of a DKA event. From a baseline BHB of ∼0.13 mmol/L, sotagliflozin treatment led to a small median increase over 52 weeks (≤0.05 mmol/L at all time points). Of sotagliflozin-treated patients, approximately 47% and 7% had ≥1 BHB measurement >0.6 mmol/L and >1.5 mmol/L, respectively (vs. 20% and 2%, respectively, of placebo-treated patients). Subsequent to the implementation of a risk mitigation plan, annualized DKA incidence was lower versus preimplementation in both the sotagliflozin 200 and 400 mg groups.
CONCLUSIONS
In patients with type 1 diabetes, confirmed DKA incidence increased when sotagliflozin was added to insulin compared with insulin alone. A lower incidence of DKA was observed following the implementation of an enhanced risk mitigation plan, suggesting that this risk can be managed with patient education.
Introduction
Diabetic ketoacidosis (DKA) is an acute, serious, and potentially life-threatening metabolic complication of type 1 diabetes (1). Although reported annual incidence of DKA varies widely, it is estimated to affect about 5–8% of adults with type 1 diabetes (2–4). From 2009 to 2014 in the U.S., DKA hospitalization rates increased an average of 6.3% each year (1). DKA incidence is highest in children and tends to decline with age (2,3). Risk factors include acute illnesses such as infection, recent diabetes onset, insulin omission or reduction, insulin pump failure, alcohol intake, and increased strenuous physical activity (4–6).
Sodium–glucose cotransporter 2 (SGLT2) inhibitor use also has been identified as a risk factor for DKA in patients with diabetes (1,7,8). This risk is amplified in those who require insulin therapy, most commonly patients with type 1 diabetes (1,8). In May 2015, the U.S. Food and Drug Administration (FDA) issued a warning that SGLT2 inhibitor use may be associated with an increased risk of DKA, including euglycemic DKA (9,10). After additional review, the FDA updated the prescribing information for SGLT2 inhibitor use in adults with type 2 diabetes to include a warning about increased risk of DKA (11).
Multiple SGLT inhibitors have been investigated in adults with type 1 diabetes (12–20). Sotagliflozin and dapagliflozin were recently approved in the European Union as an adjunct to insulin therapy in adults with type 1 diabetes and a BMI ≥27 kg/m2 (21,22). Sotagliflozin, a dual inhibitor of SGLT1 and SGLT2, improves glycemic control, body weight, and blood pressure with less hypoglycemia. However, DKA incidence rates are higher when added to insulin in patients with type 1 diabetes (12–16). Given the inherent risk of DKA in individuals with type 1 diabetes, a better understanding of DKA incidence and risk factors is necessary, particularly with the adjunct use of SGLT inhibitors. Therefore, we evaluated the incidence of DKA and clinical information related to these events using clinical trial data evaluating sotagliflozin in adults with type 1 diabetes. Because SGLT2 inhibition is associated with increased ketogenesis (23), changes in blood β-hydroxybutyrate (BHB) also were evaluated.
Research Design and Methods
An analysis of the incidence of DKA and related events in patients with type 1 diabetes treated with sotagliflozin as an adjunct to insulin was conducted using data pooled from two 52-week, phase 3 clinical trials. Details of the trial design, methodology, and key efficacy and safety results are published (12,13). The studies were identically designed, with inTandem1 conducted at 75 sites in the U.S. and Canada (March 2015 to February 2017) (12), and inTandem2 conducted at 96 sites across Europe and Israel (May 2015 to June 2017) (13). Both placebo-controlled, double-blind studies enrolled patients (aged ≥18 years) with type 1 diabetes treated with insulin (see detailed inclusion/exclusion criteria in Supplementary Appendix 2). In both trials combined, 1,575 adults with type 1 diabetes were randomized (1:1:1) to receive placebo or sotagliflozin 200 or 400 mg once daily for 52 weeks (12,13).
Investigators were trained at the start of the study and again at the time of study amendment (discussed below) to carefully review and report, at each visit, patient-reported intercurrent illness, infections, generalized weakness, increased weight loss, gastrointestinal symptoms, or other nonspecific symptoms that might be suspicious for metabolic acidosis/ketosis/DKA. At each visit, a random (generally fasting) BHB level was measured, regardless of the presence or absence of symptoms. Observed elevations in BHB were managed with appropriate supportive measures. If blood BHB was >0.6 mmol/L, investigators determined if an assessment for metabolic acidosis was appropriate and, if affirmative, then the possible DKA electronic case report form was completed. All patients were instructed by investigators at each visit to identify the signs and symptoms of DKA and to measure urine ketones and/or blood BHB levels whenever suspicious signs and/or symptoms were present, using sponsor-supplied urine dipsticks, BHB meters, and testing strips, which were provided later as part of an amendment (Supplementary Appendix 3). Patients were considered to be at an increased risk for DKA when home blood BHB levels reached >0.6 mmol/L (24,25). As such, patients were instructed to contact investigators if urine ketones were positive or blood BHB levels were >0.6 mmol/L, at which time patients were instructed to increase hydration, administer additional rapid-acting insulin, and consume oral carbohydrates in larger quantities more frequently (as often as every 2 h), until urine ketones or blood BHB levels were normalized. If the patient’s BHB levels were reduced to ≤0.6 mmol/L following these measures, assessment for metabolic acidosis was not required, per investigator discretion, and no further action was required. However, in cases of persistent BHB elevations >0.6 mmol/L, further assessment for metabolic acidosis was conducted at the discretion of investigators.
In May 2015, the FDA issued a drug safety communication highlighting the increased risk for DKA in patients using adjunct SGLT2 inhibitors (10). This prompted the sponsor to revise and enhance DKA risk mitigation activities, including the following: 1) revision of patient wallet cards with instructions regarding the need for ketone monitoring and correction and not relying on elevated blood glucose to suspect DKA; 2) a protocol amendment emphasizing the importance of patient adherence to urine and blood ketone monitoring (to be used for screening and confirmation, respectively); and 3) the need for early notification of investigators in the event of BHB elevation.
In response to regulatory advice received in December 2015 (11), all reported metabolic acidosis events, in addition to reported DKA events, were submitted for adjudication. Potential cases of metabolic acidosis and DKA were identified using the investigator entry of adverse events (AEs) identified as terms suggestive of possible metabolic acidosis and DKA (Table 1), laboratory values suggestive of persistent ketonemia/DKA (e.g., BHB >0.6 mmol/L), or following review of AE and laboratory data. Ketosis-related events were of special interest and data were collected from the first administration of study medication to 30 days following the last dose of study drug. All possible metabolic acidosis or DKA events were centrally adjudicated by an independent committee and classified as follows: “Yes, with certainty”; “Yes, probably”; “No, unlikely”; “No, with certainty”; “Unclassifiable”; or “Insufficient data.” Events meeting one of the “Yes” criteria were assessed as positively adjudicated. A detailed description of the criteria used by investigators and patients to identify events and the criteria used to positively adjudicate metabolic acidosis and DKA events is provided in Supplementary Appendix 4. The changes in the DKA risk mitigation are outlined in Supplementary Fig. 1.
Table 1.
Preferred terms for identification of DKA/metabolic acidosis
| Terms typically associated with elevated BHB | Terms that may not be associated with elevated BHB |
|---|---|
| Acetonemia | Acidosis |
| Blood ketone body | Acidosis hyperchloremic |
| Blood ketone body increased | Diabetic coma |
| Blood ketone body present | Diabetic hyperglycemia coma |
| DKA | Diabetic metabolic decompensation |
| Diabetic ketoacidotic hyperglycemic coma | Hyperglycemic coma |
| Ketoacidosis | Hyperglycemic seizure |
| Ketosis | Hyperglycemic unconsciousness |
| Urine ketone body | Lactic acidosis |
| Urine ketone body present | Metabolic acidosis |
| Renal tubular acidosis | |
| Uremic acidosis |
Statistical Analyses of Pooled Data
Pooled data were used for the analyses, which were performed using all randomized patients who took at least one dose of the study drug. Full data sets included 52 weeks on treatment plus 30-day follow-up. Descriptive statistics were calculated for baseline characteristics. The incidences of ketosis-related events, adjudicated metabolic acidosis events, and adjudicated DKA events were calculated for each event type as the proportion of patients in each group who experienced at least one event. Exposure-adjusted incidence rates (EAIR) of adjudicated DKA per 100 patient-years of exposure were calculated as the total number of patients who reported at least one positively adjudicated DKA event divided by the total exposure in patient-years for all treated patients multiplied by 100. The 95% CI for the EAIR and the between-group differences in EAIR were based on normal approximation. EAIR were estimated using a similar analysis for selected subgroups, e.g., male versus female, continuous subcutaneous insulin infusion (CSII) versus multiple daily injection, BMI <27 vs. ≥27 kg/m2 to be consistent with the current European Union indication for sotagliflozin (26), baseline insulin use, and change in insulin during study.
Descriptive statistics were performed on BHB levels over time using results from serum chemistries measured during visits at baseline and weeks 4, 12, 24, and 52. At each visit, a two-sample Wilcoxon rank sum test was performed to test the distribution of change in BHB from baseline between sotagliflozin and placebo. The proportion of patients with BHB >0.6 and >1.5 mmol/L were also evaluated for each treatment over 52 weeks (24,25).
While the inTandem trials were open, all sites implemented a revised and enhanced risk mitigation plan by 31 March 2016, as described above and in Supplementary Appendix 3. This plan included approved protocol amendments, distribution of BHB meters and ketone testing strips, and revised informed consent forms. The impact of enhanced monitoring on DKA incidence was evaluated in three patient groups: 1) patients randomized before 31 March 2016 with a DKA event or who completed the study by 31 March 2016; 2) patients randomized before 31 March 2016 with a DKA event or who completed the study after 31 March 2016; and 3) patients randomized after 31 March 2016. The last two groups were considered as impacted by the enhanced risk mitigation plan. The incidence of DKA was calculated for each patient group as the proportion of patients in each group who experienced at least one event.
Results
The pooled data analysis included 526, 524, and 525 patients who received placebo, sotagliflozin 200 mg, and sotagliflozin 400 mg, respectively. Baseline characteristics were similar across groups (Supplementary Table 1). More females used CSII compared with males (48% vs. 38% overall).
Treatment-Emergent, Ketosis-Related AEs
A total of 191 ketosis-related AEs were identified in the pooled data set, with 2.7%, 9.7%, and 13.9% in the placebo and sotagliflozin 200 or 400 mg groups experiencing at least one event (Table 2). There were 98 events that underwent adjudication per protocol. The remaining events did not meet criteria for adjudication based on ketosis reversal with usual measures taken by investigators and/or patients at home or in the clinic. Possible DKA or metabolic acidosis events occurred in 1.3%, 5.7%, and 7.4% of patients treated with placebo and sotagliflozin 200 or 400 mg; of these, 44 events in 43 patients were positively adjudicated as metabolic acidosis (1 patient receiving sotagliflozin 200 mg experienced 2 events). The incidence of positively adjudicated metabolic acidosis was 0.6%, 3.4%, and 4.2% with placebo, sotagliflozin 200 mg, and sotagliflozin 400 mg, respectively (Table 2). Events did not meet the criteria for metabolic acidosis because of normal or marginal laboratory values, or lack of confirmatory laboratory values (e.g., pH or bicarbonate).
Table 2.
Summary of treatment-emergent ketosis-related AEs and positively adjudicated metabolic acidosis and DKA events
| Placebo (n = 526) | Sotagliflozin 200 mg (n = 524) | Sotagliflozin 400 mg (n = 525) | |
|---|---|---|---|
| All ketosis-related AEs | 14 (2.7) | 51 (9.7) | 73 (13.9) |
| Total number of events | 14 | 79 | 98 |
| EOSI of possible DKA/metabolic acidosis events | 7 (1.3) | 30 (5.7) | 39 (7.4) |
| Total number of events | 7 | 32 | 39 |
| Positively adjudicated metabolic acidosis events | 3 (0.6) | 18 (3.4) | 22 (4.2) |
| Total number of events | 3 | 19 | 22 |
| Positively adjudicated DKA events | 1 (0.2) | 15 (2.9) | 20 (3.8) |
| Total number of events | 1 | 16 | 20 |
| EAIR positively adjudicated DKA events per 100 patient-years (95% CI) | 0.2 (0.0, 0.6) | 3.1 (1.5, 4.7) | 4.2 (2.4, 6.0) |
| Difference in EAIR minus placebo (95% CI) | — | 2.9 (1.3, 4.5) | 4.0 (2.1 5.9) |
Data are n (%), unless otherwise noted. EOSI, events of special interest.
A total of 37 events in 36 patients were positively adjudicated as DKA. The incidence of positively adjudicated DKA events was 0.2%, 2.9%, and 3.8% with placebo, sotagliflozin 200 mg, and sotagliflozin 400 mg, respectively. EAIR for positively adjudicated DKA events per 100 patient-years was 0.2 (95% CI 0.0–0.6) in the placebo group, 3.1 (95% CI 1.5–4.7) in the sotagliflozin 200 mg group, and 4.2 (95% CI 2.4–6.0) in the sotagliflozin 400 mg group. The between-group difference in EAIR was statistically significant (Table 2). A numerically higher rate of DKA was observed with sotagliflozin 400 mg group compared with the 200 mg group (absolute difference in EAIR = 1.1 events per 100 patient-years). Events adjudicated as metabolic acidosis but not DKA generally lacked confirmatory ketone values.
All DKA events were classified as serious according to investigators. Nearly all events required hospitalization (34/37 events). Median duration of a DKA event was 3.5 days in the sotagliflozin group and 4 days for the single event in the placebo group. Blood glucose at the time of the DKA event was ≥250 mg/dL for 64% (23/36 events) in the sotagliflozin group and for the single event in the placebo group. No event was associated with a blood glucose value <150 mg/dL. According to the modified American Diabetes Association criteria for DKA severity (regardless of blood glucose level), 43% (16/37 events) were considered severe based on a pH value <7.0 or a bicarbonate level <10 mEq/L (27). Approximately two-thirds of patients restarted treatment with sotagliflozin following resolution of the DKA event. There were no deaths or persistent sequelae due to DKA.
Patients who reported a DKA event with sotagliflozin tended to be younger (mean age 40 years) and were more likely to be female (66%) relative to the entire cohort. In select subgroup analyses, a trend for higher EAIR was noted for sotagliflozin-treated patients using CSII, females, those with BMI <27 kg/m2, and those with baseline total daily insulin dose <0.7 IU/kg relative to the remainder of the study population (Table 3). The single patient in the placebo group with a reported DKA event was a male who had a baseline insulin dose ≥0.7 IU/kg, used CSII, and had a BMI ≥27 kg/m2.
Table 3.
Positively adjudicated DKA by selected subgroups
| EAIR positively adjudicated DKA events per 100 patient-years (95% CI) | |||
|---|---|---|---|
| Placebo (n = 526) | Sotagliflozin 200 mg (n = 524) | Sotagliflozin 400 mg (n = 525) | |
| CSII | 0.5 (0.0, 0.6) | 4.4 (1.5, 7.3) | 6.0 (2.6, 9.3) |
| Total patients | n = 226 | n = 224 | n = 224 |
| MDI | 0 | 2.2 (0.4, 3.9) | 2.9 (0.9, 4.9) |
| Total patients | n = 300 | n = 300 | n = 301 |
| BMI <27 kg/m2 | 0 | 3.5 (0.9, 6.1) | 4.9 (1.7, 8.0) |
| Total patients | n = 228 | n = 219 | n = 212 |
| BMI ≥27 kg/m2 | 0.4 (0.0, 1.9) | 2.9 (0.8, 4.8) | 3.8 (1.5, 6.0) |
| Total patients | n = 298 | n = 305 | n = 313 |
| Male | 0.4 (0.0, 1.2) | 2.9 (0.7, 5.0) | 2.1 (0.3, 4.0) |
| Total patients | n = 271 | n = 265 | n = 253 |
| Female | 0 | 3.4 (1.0, 5.7) | 6.2 (3.1, 9.3) |
| Total patients | n = 255 | n = 259 | n = 272 |
| Baseline insulin dose <0.7 IU/kg | 0 | 3.3 (1.1, 5.5) | 4.4 (1.8, 6.9) |
| Total patients | n = 275 | n = 303 | n = 279 |
| Baseline insulin dose ≥0.7 IU/kg | 0.4 (0.0, 1.3) | 2.9 (0.6, 5.2) | 4.0 (1.4, 6.6) |
| Total patients | n = 251 | n = 221 | n = 244 |
MDI, multiple daily injection.
Changes in insulin dose were evaluated to assess association with DKA events. Because there was a relatively small number of DKA events, investigator-reported events of DKA and serious metabolic acidosis were combined to increase the robustness of this analysis (see Table 2 for events of special interest information). There was a trend toward increased DKA incidence in sotagliflozin-treated patients who had larger reductions in total daily insulin dose (Supplementary Table 2).
A total of 83% (33/36) of the adjudication-confirmed DKA events in the sotagliflozin group were associated with an identified potential contributing factor: 19 were related to reduced/interruption in insulin dosing or pump malfunction, and 14 were related to concomitant illnesses (Supplementary Table 3).
Following implementation of the enhanced DKA risk monitoring and mitigation plan, the incidence of adjudication-confirmed DKA tended to be lower. A lower incidence of DKA was observed following the implementation of an enhanced risk mitigation plan (Supplementary Fig. 2).
Ketones From Central Laboratories
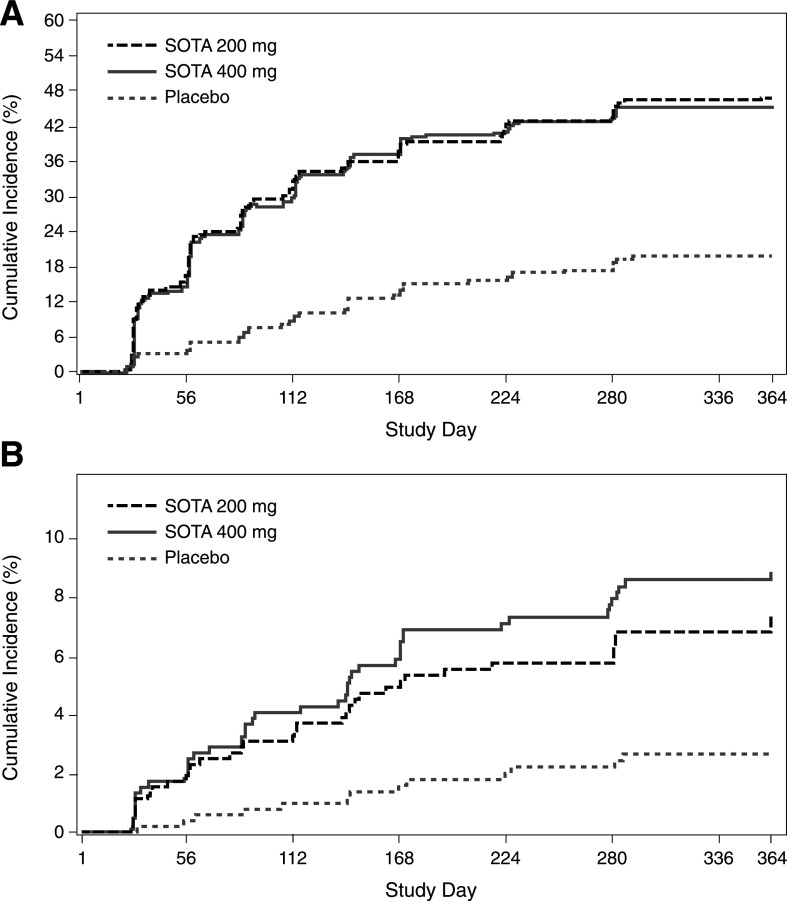
Median baseline BHB values were 0.13 (interquartile range [IQR] 0.10, 0.20), 0.13 (IQR 0.10, 0.22), and 0.14 mmol/L (IQR 0.10, 0.23) for patients in the placebo, sotagliflozin 200 mg, and sotagliflozin 400 mg groups, respectively. Although placebo-treated patients had no change from baseline in BHB values, the median change in BHB from baseline increased by 0.03–0.05 mmol/L with sotagliflozin by 4 weeks and then generally remained stable over 52 weeks (P < 0.001 vs. placebo at each time point) (Supplementary Fig. 3A–C). Per the predefined BHB levels indicative of increased DKA risk, a BHB level >0.6 mmol/L (“ketonemia”) was experienced by 19.8%, 46.9%, and 45.5%, and a BHB level >1.5 mmol/L (“impending DKA”) was experienced by 2.1%, 6.3%, and 8.2% of patients in the placebo, sotagliflozin 200 mg, and sotagliflozin 400 mg groups, respectively. Approximately two-thirds of the BHB >0.6 mmol/L events were observed by week 16, and approximately two-thirds of the BHB >1.5 mmol/L events were observed by week 24 and tended to plateau over the study duration (Fig. 1).
Figure 1.
Cumulative incidence of patients who had a BHB >0.6 mmol/L (A) and >1.5 mmol/L (B) over 52 weeks of treatment. SOTA, sotagliflozin.
Conclusions
In clinical trials, patients with type 1 diabetes who received sotagliflozin 200 or 400 mg added to insulin experienced significant improvements in glycemic control (including A1C, 2-h postprandial glucose, and time in range), and improved body weight, blood pressure, and patient-reported outcomes. Patients receiving sotagliflozin also had a significant reduction in the number of hypoglycemic (blood glucose ≤55 mg/dL) and severe hypoglycemic events (400-mg group only) (12,13,28,29). In these trials, the AE profile was generally consistent with that observed with SGLT2 inhibitors in type 2 diabetes trials, including an increased incidence of genital mycotic infections and DKA.
The current analyses evaluated positively adjudicated DKA and related ketosis events using pooled data from two identically designed 52-week, phase 3 studies. Although these studies enrolled slightly different patient populations, the total number of adjudicated DKA events was comparable between trials. In the pooled analyses, the overall 52-week incidence of DKA with sotagliflozin 200 and 400 mg was <4%, but it was higher than with placebo. This translates to three to four additional events of DKA per 100 patients with type 1 diabetes treated for 1 year. A numerically higher incidence of DKA was noted with sotagliflozin 400 mg compared with 200 mg. No deaths associated with DKA events were reported, and the majority of patients resumed therapy following resolution of the event.
The present analysis focused on these two sotagliflozin trials because of identical design and long duration of follow-up (1 year). The other trials in the inTandem type 1 diabetes clinical trial program had fewer participants, shorter duration of follow-up (12–24 weeks), and/or did not assess all sotagliflozin doses (14–16). Supplementary Table 4 details the incidence of adjudicated DKA in the sotagliflozin type 1 diabetes clinical trial program. Importantly, across trials, no fatal DKA event occurred in any treatment group.
A higher incidence of DKA was observed in patients treated with sotagliflozin on CSII or with lower BMI. Insulin pump–related issues often lead to interruptions in insulin dosing and are a known risk factor for DKA. Lower BMI may be associated with lower insulin requirements, and this coupled with sotagliflozin treatment may lead to increased ketosis or DKA risk (30). Approximately two-thirds of the DKA events occurred in females treated with sotagliflozin. A higher rate of DKA has been reported for females in the T1D Exchange clinic registry (31). The increased incidence of DKA in females and in those on CSII is consistent with results reported with empagliflozin (20), and/or the increased DKA rate in females may be related to their higher use of CSII in this study.
Patients with lower insulin requirements at baseline (<0.7 IU/kg) tended to have a higher DKA rate with sotagliflozin. The incidence of investigator-reported DKA or metabolic acidosis also was higher in sotagliflozin-treated patients who had reductions in total insulin dose ≥10–20% from baseline. Insulin reductions >20% have been associated with larger increases in BHB in dapagliflozin-treated patients with type 1 diabetes (32). This observation emphasizes that reductions >20% in total daily insulin dose should generally be avoided. An increase in carbohydrate intake may be recommended to maintain insulin doses if needed. Patients should adhere to ketone monitoring when insulin doses need to be reduced during SGLT inhibitor treatment.
Potential contributing triggers for DKA events were consistent with established triggers for DKA (9). The most commonly reported were insulin dose reductions, pump-related issues, or concomitant illnesses in those treated with sotagliflozin. Contributing precipitating factors were identified for nearly all events.
Euglycemic DKA is not unique to SGLT2 inhibitors (9). It has been described in other conditions, including chronic liver disease and pregnancy. In an evaluation of U.K.-based patients with type 1 diabetes (N = 334), the background rate of euglycemic DKA (blood glucose <250 mg/dL) in 2014–2015 was 5.4% (33). In the present analyses, one-third of DKA events were associated with blood glucose <250 mg/dL. Regardless, it emphasizes that blood glucose monitoring alone may not be sufficient for early detection of ketosis/ketoacidosis (34). Recent treatment recommendations also emphasize that symptomatic patients treated with SGLT inhibitors should measure their blood or urine ketones and not rely solely on blood glucose (30,35).
By decreasing renal glucose reabsorption, SGLT inhibitors induce rapid increases in urinary glucose excretion that predispose patients to increased ketogenesis (9). The present analyses include BHB measured during scheduled clinic visits over 52 weeks. Although significant compared with placebo, sotagliflozin treatment resulted in only small median BHB increases of ≤0.05 mmol/L over 52 weeks. More sotagliflozin- versus placebo-treated patients experienced clinically important BHB levels (>0.6 mmol/L or >1.5 mmol/L). Importantly, the incidence of DKA (<4% overall on sotagliflozin) relative to the proportion of patients with ≥1 BHB measurement >0.6 mmol/L (∼45%) or >1.5 mmol/L (∼7%), suggests that many elevations in ketones were recognized and managed without progression to DKA.
Following implementation of the enhanced DKA monitoring and mitigation plan in the sotagliflozin type 1 diabetes program, a trend toward lower DKA incidence was observed in patients who received the enhanced mitigation plan compared with those who did not. This decline in DKA may be attributable to the enhanced DKA mitigation program and increased awareness of DKA via publicly announced safety warnings for SGLT2 inhibitors.
In a Truven MarketScan electronic health record analysis of adults with type 1 diabetes, overall DKA incidence was higher among SGLT2 inhibitor users compared with non-SGLT2 inhibitor users. Between 2013 and 2017, in SGLT2 inhibitor users, DKA rate declined from 15.7 to 4.2 events per 100 patient-years. Thus, in 2017, the rate was generally similar to that in non-SGLT2 inhibitor users (5.4 events per 100 patient-years in 2016–2017) (36). This declining incidence is likely related to increased DKA risk awareness and better patient selection (10,11,37). The present results, in addition to those from the Truven analysis, suggest that DKA risk with SGLT inhibitors can be managed with appropriate clinician and patient education (9). Structured education has been shown to reduce risk of DKA in adults with type 1 diabetes (38).
Several diabetes expert groups have published recommendations to manage the risk of DKA in patients with type 1 diabetes receiving SGLT inhibitors (30,35,37,39). These statements generally focus on appropriate patient selection to decrease DKA risk and close monitoring of signs and symptoms and patient self-monitoring of urine or blood ketones for early identification and swift and appropriate management in patients who develop ketosis or DKA. Current recommendations emphasize the STICH protocol: stop the SGLT inhibitor, inject bolus insulin, consume 30 g of carbohydrates, and hydrate (drink water) (39). These publications and associated communications and education can help raise awareness of the risk of DKA with SGLT inhibitors and guide clinicians and patients to their appropriate use.
This analysis has strengths and weaknesses. It provides a comprehensive evaluation of DKA incidence and risk factors in patients with type 1 diabetes using sotagliflozin as an adjunct to insulin. The single DKA event in the placebo group suggests that the insulin optimization strategy and DKA risk mitigation plan were important factors in minimizing DKA risk. However, this single event does not allow a precise estimation of the relative risk of DKA. In addition, because data regarding patients’ dietary carbohydrate intake were not collected prospectively, it was not possible to evaluate whether the combination of a low-carbohydrate diet and SGLT inhibitor use affected DKA risk. Furthermore, data from home BHB measurements performed by patients in response to suspicious symptoms were not systematically collected during the course of the studies, making it difficult to assess frequency of for-cause testing by patients at home.
Finally, the sotagliflozin program did not include several risk mitigation measures that are now covered in European labeling materials (26,40). These include consensus recommendations on avoiding low-carbohydrate diets, management of insulin dosing, and patient involvement in ketone monitoring (before initiation of therapy, in the first few weeks of treatment, and during at-risk situations). Postmarketing studies will provide valuable information on physician education and sotagliflozin safety in clinical practice.
Conclusion
In adults with type 1 diabetes, the incidence of positively adjudicated DKA was increased with sotagliflozin added to insulin compared with insulin alone. Identified risk factors for DKA were consistent with current knowledge. The implementation of an enhanced DKA mitigation plan (education and close monitoring) was associated with a trend in reduced DKA incidence in the sotagliflozin clinical program.
Article Information
Acknowledgments. All investigators are listed in Supplementary Appendix 1. The authors thank the trial investigators, staff, and patients in the studies for their participation. Caitlin Rothermel of Truposha, LLC (Publication Practice Counsel) provided medical writing and editorial support, which was funded by Lexicon Pharmaceuticals, Inc.
Duality of Interest. This study was sponsored and conducted by Lexicon Pharmaceuticals, Inc. A.L.P. is on the advisory board for Eli Lilly, Novo Nordisk, Lexicon Pharmaceuticals, Boehringer Ingelheim, Abbott Diabetes Care, MannKind, and Sanofi; has received research support from Dexcom and vTv Therapeutics; and has stock options from Mellitus Health, Omada Health, Stability Health, Pendulum Therapeutics, and Livongo. D.K.M. has received personal fees from Boehringer Ingelheim, Janssen Research and Development LLC, Sanofi US, Merck & Co., Merck Sharp & Dohme, Eli Lilly USA, Novo Nordisk, GlaxoSmithKline, AstraZeneca, Lexicon Pharmaceuticals, Eisai, Pfizer, Metavant, Applied Therapeutics, Afimmune, and Esperion. T.D. has acted as a consultant, advisory board member, steering committee member, or speaker for Abbott, Medtronic, Roche, Lexicon, Menarini, Boehringer Ingelheim, AstraZeneca, Novo Nordisk, Sanofi, Dexcom, and Eli Lilly and has received research grants from Abbott, AstraZeneca, Novo Nordisk, Medtronic, and Sanofi. J.A.K. serves as medical director of McNair Interests, a private equity group with investments in type 1 diabetes and other chronic illnesses and has served as an advisor for Lexicon and Sanofi in the past. H.W.R. conducts clinical research for AstraZeneca, Gan Lee, Janssen, Lexicon, and Sanofi; is a speaker for AstraZeneca, Lilly, Merck, Novo Nordisk, and Sanofi; and is a consultant for Gan Lee, Lexicon, and Sanofi. K.D. was the independent adjudicator for DKA on the inTandem trials and received honoraria from Sanofi Diabetes. S.S. and M.J.D. were employees of Lexicon Pharmaceuticals, Inc. and may own common stock or may have been granted stock options or other equity incentive awards. P.B., W.J., and P.L. are employees of Lexicon Pharmaceuticals, Inc. and may own common stock or may have been granted stock options or other equity incentive awards.
Author Contributions. A.L.P., D.K.M., T.D., J.A.K., H.W.R., and K.D. contributed to the concept or design of the study; the acquisition, analysis, or interpretation of the data; and the drafting, reviewing, and editing of the manuscript. S.S., P.B., W.J., and P.L. contributed to the concept or design of the study; the acquisition, analysis, or interpretation of the data; the approval of the protocol and amendments; the reviewing of the data quality prior to database lock; the overseeing of the statistical analysis; and the drafting, reviewing, and editing of the manuscript. M.J.D. contributed to the acquisition, analysis, or interpretation of the data and the drafting, reviewing, and editing of the manuscript. All authors had full access to the data in the study, had final responsibility for the decision to publish, provided final approval of the manuscript, and agreed to be accountable for all aspects of the work related to the accuracy or integrity of any part of the work. P.L. is the guarantor of this work, and as such, had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Footnotes
Clinical trial reg. nos. NCT02384941 and NCT02421510, clinicaltrials.gov
The data included in this manuscript have not been previously presented at a scientific meeting or previously published.
This article contains supplementary material online at https://doi.org/10.2337/figshare.12824342.
References
- 1.Dhatariya KK, Glaser NS, Codner E, Umpierrez GE. Diabetic ketoacidosis. Nat Rev Dis Primers 2020;6:40. [DOI] [PubMed] [Google Scholar]
- 2.Beck RW, Tamborlane WV, Bergenstal RM, Miller KM, DuBose SN, Hall CA; T1D Exchange Clinic Network . The T1D Exchange clinic registry. J Clin Endocrinol Metab 2012;97:4383–4389 [DOI] [PubMed] [Google Scholar]
- 3.Fazeli Farsani S, Brodovicz K, Soleymanlou N, Marquard J, Wissinger E, Maiese BA. Incidence and prevalence of diabetic ketoacidosis (DKA) among adults with type 1 diabetes mellitus (T1D): a systematic literature review. BMJ Open 2017;7:e016587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Miller KM, Foster NC, Beck RW, et al.; T1D Exchange Clinic Network . Current state of type 1 diabetes treatment in the U.S.: updated data from the T1D Exchange clinic registry. Diabetes Care 2015;38:971–978 [DOI] [PubMed] [Google Scholar]
- 5.Desai D, Mehta D, Mathias P, Menon G, Schubart UK. Health care utilization and burden of diabetic ketoacidosis in the U.S. over the past decade: a nationwide analysis. Diabetes Care 2018;41:1631–1638 [DOI] [PubMed] [Google Scholar]
- 6.Weinstock RS, Xing D, Maahs DM, et al.; T1D Exchange Clinic Network . Severe hypoglycemia and diabetic ketoacidosis in adults with type 1 diabetes: results from the T1D Exchange clinic registry. J Clin Endocrinol Metab 2013;98:3411–3419 [DOI] [PubMed] [Google Scholar]
- 7.Erondu N, Desai M, Ways K, Meininger G. Diabetic ketoacidosis and related events in the canagliflozin type 2 diabetes clinical program. Diabetes Care 2015;38:1680–1686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Peters AL, Buschur EO, Buse JB, Cohan P, Diner JC, Hirsch IB. Euglycemic diabetic ketoacidosis: a potential complication of treatment with sodium-glucose cotransporter 2 inhibition. Diabetes Care 2015;38:1687–1693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rosenstock J, Ferrannini E. Euglycemic diabetic ketoacidosis: a predictable, detectable, and preventable safety concern with SGLT2 inhibitors. Diabetes Care 2015;38:1638–1642 [DOI] [PubMed] [Google Scholar]
- 10.U.S. Food and Drug Administration FDA warns that SGLT2 inhibitors for diabetes may result in a serious condition of too much acid in the blood, 2015. Accessed 20 January 2020. Available from https://www.fda.gov/media/92185/download
- 11.U.S. Food and Drug Administration FDA Drug Safety Communication: FDA revises labels of SGLT2 inhibitors for diabetes to include warnings about too much acid in the blood and serious urinary tract infections, 2015. Accessed 20 January 2020. Available from https://www.fda.gov/drugs/drug-safety-and-availability/fda-drug-safety-communication-fda-revises-labels-sglt2-inhibitors-diabetes-include-warnings-about
- 12.Buse JB, Garg SK, Rosenstock J, et al. . Sotagliflozin in combination with optimized insulin therapy in adults with type 1 diabetes: the North American inTandem1 study. Diabetes Care 2018;41:1970–1980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Danne T, Cariou B, Banks P, et al. . HbA1c and hypoglycemia reductions at 24 and 52 weeks with sotagliflozin in combination with insulin in adults with type 1 diabetes: the European inTandem2 study. Diabetes Care 2018;41:1981–1990 [DOI] [PubMed] [Google Scholar]
- 14.Garg SK, Henry RR, Banks P, et al. . Effects of sotagliflozin added to insulin in patients with type 1 diabetes. N Engl J Med 2017;377:2337–2348 [DOI] [PubMed] [Google Scholar]
- 15.Bode BW, Banks P, Sawhney S, Strumph P, The Sotagliflozin JDRF Study Writing Group . Efficacy and safety of sotagliflozin, a dual SGLT1 and SGLT2 inhibitor, asadjunct to insulin in young adults with poorly controlled type 1 diabetes (JDRF Study). Diabetologia 2017;60(Suppl. 1):S1–S608 [Google Scholar]
- 16.Baker C, Wason S, Banks P, et al. . Dose-dependent glycometabolic effects of sotagliflozin on type 1 diabetes over 12 weeks: the inTandem4 trial. Diabetes Obes Metab 2019;21:2440–2449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dandona P, Mathieu C, Phillip M, et al.; DEPICT-1 Investigators . Efficacy and safety of dapagliflozin in patients with inadequately controlled type 1 diabetes (DEPICT-1): 24 week results from a multicentre, double-blind, phase 3, randomised controlled trial. Lancet Diabetes Endocrinol 2017;5:864–876 [DOI] [PubMed] [Google Scholar]
- 18.Mathieu C, Dandona P, Gillard P, et al.; DEPICT-2 Investigators . Efficacy and safety of dapagliflozin in patients with inadequately controlled type 1 diabetes (the DEPICT-2 study): 24-week results from a randomized controlled trial. Diabetes Care 2018;41:1938–1946 [DOI] [PubMed] [Google Scholar]
- 19.Rodbard HW, Peters AL, Slee A, Cao A, Traina SB, Alba M. The effect of canagliflozin, a sodium glucose cotransporter 2 inhibitor, on glycemic end points assessed by continuous glucose monitoring and patient-reported outcomes among people with type 1 diabetes. Diabetes Care 2017;40:171–180 [DOI] [PubMed] [Google Scholar]
- 20.Rosenstock J, Marquard J, Laffel LM, et al. . Empagliflozin as adjunctive to insulin therapy in type 1 diabetes: the EASE trials. Diabetes Care 2018;41:2560–2569 [DOI] [PubMed] [Google Scholar]
- 21.Paik J, Blair HA. Dapagliflozin: a review in type 1 diabetes. Drugs 2019;79:1877–1884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Markham A, Keam SJ. Sotagliflozin: first global approval. Drugs 2019;79:1023–1029 [DOI] [PubMed] [Google Scholar]
- 23.Ferrannini E, Mark M, Mayoux E. CV protection in the EMPA-REG OUTCOME trial: a “thrifty substrate” hypothesis. Diabetes Care 2016;39:1108–1114 [DOI] [PubMed] [Google Scholar]
- 24.FreeStyle Precision Neo. Mississauga, ON, Abbott Diabetes Care Ltd., 2014. Available from https://www.freestyle.abbott/ca/en/products/precision_neo.html. Accessed 3 September 2020 [Google Scholar]
- 25.Laffel L. Ketone bodies: a review of physiology, pathophysiology and application of monitoring to diabetes. Diabetes Metab Res Rev 1999;15:412–426 [DOI] [PubMed] [Google Scholar]
- 26.Zynquista (sotagliflozin) [EPAR product information]. Amsterdam, the Netherlands, European Medicines Agency, 2019 [Google Scholar]
- 27.Kitabchi AE, Umpierrez GE, Murphy MB, Kreisberg RA. Hyperglycemic crises in adult patients with diabetes: a consensus statement from the American Diabetes Association. Diabetes Care 2006;29:2739–2748 [DOI] [PubMed] [Google Scholar]
- 28.Danne T, Cariou B, Buse JB, et al. . Improved time in range and glycemic variability with sotagliflozin in combination with insulin in adults with type 1 diabetes: a pooled analysis of 24-week continuous glucose monitoring data from the inTandem program. Diabetes Care 2019;42:919–930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Danne T, Pettus J, Giaccari A, et al. . Sotagliflozin added to optimized insulin therapy leads to lower rates of clinically relevant hypoglycemic events at any HbA1c at 52 weeks in adults with type 1 diabetes. Diabetes Technol Ther 2019;21:471–477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Danne T, Garg S, Peters AL, et al. . International consensus on risk management of diabetic ketoacidosis in patients with type 1 diabetes treated with sodium-glucose cotransporter (SGLT) inhibitors. Diabetes Care 2019;42:1147–1154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shah VN, Wu M, Polsky S, et al.; for T1D Exchange Clinic Registry . Gender differences in diabetes self-care in adults with type 1 diabetes: findings from the T1D Exchange clinic registry. J Diabetes Complications 2018;32:961–965 [DOI] [PubMed] [Google Scholar]
- 32.Henry RR, Dandona P, Pettus J, Mudaliar S, Xu J, Hansen L. Dapagliflozin in patients with type 1 diabetes: a post hoc analysis of the effect of insulin dose adjustments on 24-hour continuously monitored mean glucose and fasting β-hydroxybutyrate levels in a phase IIa pilot study. Diabetes Obes Metab 2017;19:814–821 [DOI] [PubMed] [Google Scholar]
- 33.Macfarlane J, Dhatariya K. Incidence of euglycemic diabetic ketoacidosis in adults with type 1 diabetes in the United Kingdom before the widespread use of sodium glucose cotransporter 2 inhibitors. Mayo Clin Proc 2019;94:1909–1910 [DOI] [PubMed] [Google Scholar]
- 34.Patel NS, Van Name MA, Cengiz E, et al. . Altered patterns of early metabolic decompensation in type 1 diabetes during treatment with a SGLT2 inhibitor: an insulin pump suspension study. Diabetes Technol Ther 2017;19:618–622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Goldenberg RM, Gilbert JD, Hramiak IM, Woo VC, Zinman B. Sodium-glucose co-transporter inhibitors, their role in type 1 diabetes treatment and a risk mitigation strategy for preventing diabetic ketoacidosis: the STOP DKA Protocol. Diabetes Obes Metab 2019;21:2192–2202 [DOI] [PubMed] [Google Scholar]
- 36.Wu C, Li L, Andrews EB, et al. . Diabetic ketoacidosis in adult patients with type 1 diabetes treated off-label with SGLT2 inhibitors in a US claims database: can increased awareness reduce risk over time? Diabetologia 2019;62(Suppl. 1):1–60031384961 [Google Scholar]
- 37.Handelsman Y, Henry RR, Bloomgarden ZT, et al. . American Association of Clinical Endocrinologists and American College of Endocrinology position statement on the association of SGLT-2 inhibitors and diabetic ketoacidosis. Endocr Pract 2016;22:753–762 [DOI] [PubMed] [Google Scholar]
- 38.Elliott J, Jacques RM, Kruger J, et al. . Substantial reductions in the number of diabetic ketoacidosis and severe hypoglycaemia episodes requiring emergency treatment lead to reduced costs after structured education in adults with Type 1 diabetes. Diabet Med 2014;31:847–853 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Garg SK, Peters AL, Buse JB, Danne T. Strategy for mitigating DKA risk in patients with type 1 diabetes on adjunctive treatment with SGLT inhibitors: a STICH protocol. Diabetes Technol Ther 2018;20:571–575 [DOI] [PubMed] [Google Scholar]
- 40.Forxiga (dapagliflozin) [EPAR medicine overview]. Amsterdam, the Netherlands, European Medicines Agency, 2019 [Google Scholar]