Abstract
OBJECTIVE
The role of fibrosis in early progressive renal decline in type 2 diabetes is unknown. Circulating WFDC2 (WAP four-disulfide core domain protein 2) and matrix metalloproteinase 7 (MMP-7; Matrilysin) are postulated to be biomarkers of renal fibrosis. This study examined an association of circulating levels of these proteins with early progressive renal decline.
RESEARCH DESIGN AND METHODS
Individuals with type 2 diabetes enrolled in the Joslin Kidney Study with an estimated glomerular filtration rate (eGFR) ≥60 mL/min/1.73 m2 were monitored for 6–12 years to ascertain fast early progressive renal decline, defined as eGFR loss ≥5 mL/min/1.73 m2/year.
RESULTS
A total of 1,181 individuals were studied: 681 without and 500 with albuminuria. Median eGFR and albumin-to-creatinine ratio (ACR) at baseline were 97 mL/min/1.73 m2 and 24 mg/g, respectively. During follow-up, 152 individuals experienced fast early progressive renal decline: 6.9% in those with normoalbuminuria and 21% with albuminuria. In both subgroups, the risk of renal decline increased with increasing baseline levels of WFDC2 (P < 0.0001) and MMP-7 (P < 0.0001). After adjustment for relevant clinical characteristics and known biomarkers, an increase by one quartile in the fibrosis index (combination of levels of WFDC2 and MMP-7) was associated with higher risk of renal decline (odds ratio 1.63; 95% CI 1.30–2.04). The association was similar and statistically significant among patients with and without albuminuria.
CONCLUSIONS
Elevation of circulating profibrotic proteins is associated with the development of early progressive renal decline in type 2 diabetes. This association is independent from albuminuria status and points to the importance of the fibrotic process in the development of early renal decline.
Introduction
Early progressive renal decline has been demonstrated to be the most important clinical manifestation of diabetic kidney disease (DKD) in type 1 and type 2 diabetes (1,2). It develops when patients have normal renal function and progresses at a constant rate until the onset of end-stage kidney disease (ESKD). It was recently recognized that progressive renal decline develops in many patients with type 2 diabetes independently from albuminuria (3). An ongoing significant research effort aims to understand the mechanisms of progressive renal decline, particularly in patients with diabetes with impaired renal function or late progressive renal decline (4–6). The development of renal fibrosis is postulated to be a major determinant of progression to ESKD in these patients (7,8). Very little is known, however, about the contribution of renal fibrosis to the development of early progressive renal decline in patients with diabetes with normal renal function, with and without albuminuria.
Fibrosis is a pathological process induced by persistent injurious stimuli and is responsible for organ dysfunction and organ failure (9). Renal fibrosis is characterized by increased fibroblast proliferation and extracellular matrix (ECM) accumulation, and the pathological hallmark of progressive renal disease is the accumulation of ECM in the glomerulus and tubulointerstitium (10). As renal fibrosis develops, injured tubular epithelia lose their regenerative capacity and undergo apoptosis. Glomerular scarring progresses to glomerular sclerosis. These processes lead to tubular atrophy, nonfunctional glomeruli, and, eventually, the progressive loss of kidney function (11). Fibrosis gradually replaces functional nephron units with a scar composed of a variety of ECM proteins (12). In this process, the myofibroblasts are commonly regarded as the predominant effector cells, which act as the main producers of ECM, crosslinking enzymes and inhibitors of matrix-degrading metalloproteinases (13).
Recently, WAP four-disulfide core domain 2 (WFDC2) (14) and matrix metalloproteinase 7 (MMP-7/Matrilysin) (15) were reported to be involved in the pathogenesis of renal fibrosis. WFDC2 belongs to the whey acidic protein (WAP) family and possesses two whey four-disulfide core (WFDC) domains, which are known to suppress the activity of multiple proteases (e.g., serine protease and MMPs) and inhibit their capacity to degrade type I collagen (14). Gene expression of WFDC2 in renal biopsy specimens from chronic kidney disease (CKD) patients shows significant relationships with both tubular cell damage and extent of tubulointerstitial fibrosis (16). Elevated levels of serum WFDC2 were associated with renal decline (17–19) and advanced stage of renal fibrosis (17,18). MMP-7 belongs to a member of a family of zinc-containing enzymes with proteolytic activity against a wide range of extracellular proteins (15,20). In systemic response, the elevated level of serum MMP-7 is independently associated with renal fibrosis and progression of renal decline in patients with IgA nephropathy (21). It is not detected in healthy kidneys but is detected in epithelial cells in the distal tubule region of kidneys from autosomal dominant polycystic kidney disease (20). Elevated circulating levels of MMP-7 also associate with other fibrotic diseases, including lung fibrosis and disease progression in patients with idiopathic pulmonary fibrosis (22).
We postulate that examining these two profibrotic proteins in circulation may enable us to capture the ongoing fibrogenic process in the diabetic kidney, without renal biopsy specimens. Therefore, our study aims to evaluate the association between circulating levels of WFDC2 and MMP-7 at baseline with the development of fast early renal decline in a large cohort of patients with type 2 diabetes monitored for 6–12 years. We also aim to determine whether these proteins are involved in the development of early progressive renal decline not only in individuals with albuminuria but also in those with normoalbuminuria. In the Joslin Kidney Study (JKS) for type 2 diabetes, we previously established the association of early progressive renal decline with multiple determinants, including biomarkers of tubular damage (23). Therefore, this study will incorporate the previous findings and assess the effect of profibrotic proteins on early progressive renal decline in individuals with and without albuminuria.
Research Design and Methods
Patients and Eligibility Criteria
The Joslin Diabetes Center Committee on Human Studies approved the informed consent, recruitment, and examination procedures for the JKS. The JKS is a longitudinal observation that aims to investigate the determinants and to describe the natural history of renal function decline in type 1 and type 2 diabetes. Participants in the JKS who had type 2 diabetes were recruited from patients attending the Joslin Clinic between 2003 and 2009. Residents of New England aged 35–64 years old at study enrollment with type 2 diabetes diagnosed after 30 years of age were eligible. We excluded individuals who were on chronic dialysis, had a renal transplant, or had a history of HIV or hepatitis C infection. This JKS aimed to enroll all eligible participants with albuminuria and a similar number of eligible individuals taken randomly from a much larger pool of participants with normoalbuminuria. We enrolled 1,476 individuals between 2003 and 2009: 743 individuals with albuminuria and 733 individuals with normoalbuminuria. They were examined during routine visits in the clinic as a baseline examination and biannually afterward with blood and urine specimens taken for laboratory determinations and storage in −85°C. Individuals with less frequent clinic visits and those who stopped coming to the clinic were examined at their homes.
To identify ESKD and deaths in the JKS, we queried the U.S. Renal Data System and the National Death Index covering all events up to the end of 2015. The U.S. Renal Data System maintains a roster of U.S. patients receiving renal replacement therapy that includes dates of dialysis and transplantation. The National Death Index is a comprehensive roster of deaths in the U.S. and includes the date and cause of death.
For the current study, only individuals with a normal baseline estimated glomerular filtration rate (eGFR) (≥60 mL/min/1.73 m2) and at least 6 years of follow-up were included. This yielded 1,181 individuals: 681 with normoalbuminuria and 500 with albuminuria. This study includes 149 more individuals than the previous study (23). The increase in individual count resulted from improved follow-up and completion of missing data in the JKS.
Assessment of Abnormalities in Urinary Albumin Excretion
According to the median values of the urinary albumin-to-creatinine ratio (ACR) from two or more consecutive urine samples obtained during the 2-year period preceding enrollment (baseline), two groups were assembled: albuminuria (ACR ≥30 mg/g) and normoalbuminuria (ACR <30 mg/g). We attempted to enroll all individuals with albuminuria and similar individuals with normoalbuminuria.
Assessment of Renal Function
Measurements of serum creatinine performed at routine clinic visits or during special examinations were used to determine renal function at baseline and its changes during follow-up. Protocols to calibrate serum creatinine measurements over time were described previously (24). From 2013 through 2015, serum specimens obtained at baseline and during follow-up in individuals participating in the study were retrieved and used to measure creatinine concentrations. Creatinine measurements were performed at the University of Minnesota Advanced Research and Diagnostic Laboratory using the Roche Enzymatic Assay (Product No. 11775685) on a Roche/Hitachi Mod P analyzer (Roche Diagnostics, Indianapolis, IN). The Chronic Kidney Disease Epidemiology Collaboration formula was used to estimate eGFR (25). For the current study, only individuals with normal baseline eGFR and at least 6 years of follow-up and six serum creatinine measurements were included.
Definition of Fast Early Progressive Renal Decline
The primary outcome was fast early progressive renal decline during 6–12 years of follow-up, defined as annual eGFR loss of ≥5 mL/min/1.73 m2/year. This threshold was previously used by others (2,26). Individuals with eGFR loss of ≥5 mL/min/1.73 m2/year were at risk for developing ESKD within 3–20 years of follow-up (2). We refer to this outcome as “fast early renal decline.” To estimate the slope of eGFR decline, we extracted the linear component of every individual’s eGFR trajectory using individual-specific linear regression models.
Laboratory Procedures to Measure Candidate Proteins in Circulation and in Urine
Protocols to measure levels of plasma tumor necrosis factor receptor 1 (TNF-R1), plasma kidney injury molecule 1 (KIM-1), urinary monocyte chemotactic peptide 1 (MCP-1), and urinary epidermal growth factor (EGF) were described in a previous publication (23). Serum collected at baseline from 1,181 study individuals was used for measurements of WFDC2 (catalog no. DHE400; R&D Systems, Minneapolis, MN) and MMP-7 (catalog no. DMP700; R&D Systems) with ELISAs. Frozen samples were aliquoted for each assay on wet ice and run in duplicate, and averages are reported.
It needs to be recognized that the ELISA used in our study is not recommended to measure MMP-7 concentration in plasma due to plasma-chelating properties (https://resources.rndsystems.com/pdfs/datasheets/dmp700.pdf). It is suggested that EDTA-plasma extraction of zinc from the metalloprotease MMP-7 could cause conformational changes in the protein that hamper accuracy of its measurements. On the other hand, multiple reports suggest that determination of MMP-7 in serum is reliable (27,28).
Statistical Analysis
Baseline characteristics are presented as median with first (Q1) and third (Q3) quartiles or number and percent, where applicable. Differences between the two groups were tested using the Wilcoxon rank sum test for continuous variables and the χ2 test for categorical variables. Spearman rank correlation was performed to examine intercorrelations between the eGFR slope, clinical characteristics, and biomarkers. To construct the fibrosis index as a summary variable associated with multifaceted fibrosis pathway, we assessed the strength of correlations between serum WFDC2 and MMP-7. After verifying the moderate correlations between each other, we included them both as components of the fibrosis index.
The fibrosis index was created using a logistic regression model of fast renal decline as an outcome variable. First, serum WFDC2 and MMP-7 were individually modeled in relation to fast renal decline as continuous variables. Each biomarker was natural log transformed, and linearity assumption was tested using the log-likelihood model goodness-of-fit test comparing the model with and without quadratic terms of each biomarker. Second, we included serum WFDC2 and MMP-7 in the appropriate forms into the model of fast renal decline. The index was calculated as a sum of log-transformed levels of the two contributing biomarkers weighted by their β-coefficients, according to the formula:
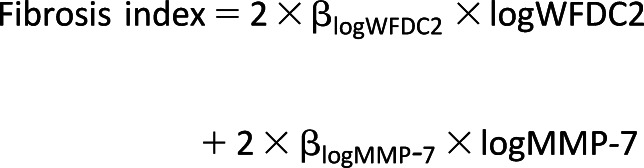 |
The β-coefficients were multiplied by 2 in order to scale the index, such that its one unit was approximately equal to one quartile of its distribution for easier interpretability of effect estimates. We derived the fibrosis index from the entire cohort and estimated its effect on fast renal decline in the subgroups classified by albuminuria status.
Multivariable logistic regression models were used to estimate the effects of the covariates and biomarkers on fast renal decline. In the models, the effects of ACR, plasma TNF-R1, plasma KIM-1, and the urinary EGF–to–MCP-1 ratio were estimated per one quartile increase. To evaluate the fibrosis-associated markers and the fibrosis index, we first constructed a model with the clinical covariates already included in our previous report (23): ACR, systolic blood pressure (BP), and risk biomarkers plasma TNF-R1, plasma KIM-1, and the urinary EGF–to–MCP-1 ratio. In addition to these determinants, we included eGFR and HbA1c at baseline. Subsequently, we examined the effects of profibrotic proteins and the fibrosis index on the risk of fast renal decline. To examine the effect of renal fibrosis on fast renal decline according to albuminuria status, we performed the same analyses in subgroups of patients with or without albuminuria and tested the homogeneity of effect estimates using interaction terms with the albuminuria stratum indicator. The discriminatory and predictive model performance was assessed with C statistics and the Nagelkerke R2 for logistic regression models, which approximates the proportion of variance explained by a model in a given data set. Statistical analyses were performed using SAS for Windows 9.4 software (SAS Institute, Cary, NC). Two-sided P values <0.05 were considered statistically significant.
Results
Characteristics of Study Groups
A total of 1,181 patients with type 2 diabetes and eGFR ≥60 mL/min/1.73 m2 were examined. Their clinical characteristics and baseline values of biomarkers are summarized in Table 1 and stratified by albuminuria status. During 6–12 years of follow-up, 152 patients (12.9%) developed fast early renal decline, defined as eGFR loss ≥5 mL/min/1.73 m2/year (referred to as decliners), 33 (2.7%) developed ESKD, and only 13 (1%) died of ESKD-unrelated causes. In patients with albuminuria, there were more men; patients with albuminuria had higher BMI and systolic BP, were more frequently treated with antihypertensive drugs, had higher HbA1c, and by design had a higher level of ACR. Importantly, patients with normoalbuminuria and albuminuria at baseline had similar age, duration of diabetes, proportion of treatment with insulin, and similar eGFR. With regard to baseline levels of biomarkers, patients with albuminuria had significantly higher serum levels of profibrotic proteins, plasma levels of TNF-R1 and KIM-1, and lower urinary level of EGF–to–MCP-1 ratio. During 6–12 years of follow-up, 105 patients (21.0%) with albuminuria and 47 (6.9%) with normoalbuminuria developed fast early renal decline (P < 0.0001). A similar significant difference between the two subgroups was seen for the development of ESKD.
Table 1.
Clinical characteristics and biomarker levels in patients with type 2 diabetes in the entire study cohort and in normoalbuminuria and albuminuria subgroups
| All patients | Normoalbuminuria | Albuminuria | P value¶ | |
|---|---|---|---|---|
| (N = 1,181) | (n = 681) | (n = 500) | ||
| Characteristics at baseline | ||||
| Age, years | 57 (50; 62) | 57 (51; 62) | 57 (50; 61) | 0.110 |
| Men, % | 58 | 48 | 71 | <0.0001 |
| Duration of diabetes, years | 10 (6; 15) | 10 (7; 15) | 10 (5; 15) | 0.066 |
| BMI, kg/m2 | 31 (27; 36) | 30 (26; 35) | 32 (29; 37) | <0.0001 |
| Systolic BP, mmHg | 131 (120; 142) | 130 (120; 140) | 134 (122; 145) | <0.0001 |
| ACE inhibitor or ARB Rx, % | 65 | 55 | 79 | <0.0001 |
| Insulin Rx, % | 57 | 60 | 53 | 0.021 |
| HbA1c, % | 7.6 (6.9; 8.6) | 7.5 (6.8; 8.3) | 7.8 (6.9; 9.0) | <0.0001 |
| eGFR, mL/min/1.73 m2 | 96 (83; 105) | 95 (84; 105) | 97 (82; 106) | 0.392 |
| Baseline ACR, mg/g | 13 (6; 42) | 7 (5; 11) | 53 (26; 159) | <0.0001 |
| Biomarkers at baseline | ||||
| Profibrotic proteins | ||||
| Serum WFDC2, ng/mL | 6.7 (5.6; 8.4) | 6.5 (5.4; 7.9) | 6.7 (5.9; 9.4) | <0.0001 |
| Serum MMP-7, ng/mL | 3.9 (3.0; 5.1) | 3.5 (2.7; 4.5) | 4.2 (3.4; 6.0) | <0.0001 |
| Other biomarkers† | ||||
| Plasma TNFR-1, pg/mL | 1,250 (1,048; 1,603) | 1,183 (993; 1,450) | 1,404 (1,140; 1,880) | <0.0001 |
| Plasma KIM-1, pg/mL | 10 (5; 17) | 9 (5; 13) | 13 (6; 22) | <0.0001 |
| Urinary EGF–to–MCP-1 ratio | 39 (24; 76) | 46 (27; 102) | 33 (20; 54) | <0.0001 |
| Outcomes during 6–12 years of follow-up | ||||
| eGFR slope, mL/min/1.73 m2/year | −1.6 (−3.3; −0.5) | −1.2 (−2.4; −0.3) | −2.2 (−4.2; −0.9) | <0.0001 |
| Fast renal decliner§, n (%) | 152 (12.9) | 47 (6.9) | 105 (21.0) | <0.0001 |
| Incidence of ESKD, n (%) | 33 (2.7) | 3 (0.4) | 30 (6.0) | <0.0001 |
| Deaths unrelated to ESKD, n (%) | 13 (1.0) | 6 (0.9) | 7 (1.4) | 0.398 |
Data are presented as the median (Q1; Q3) or as indicated. Subgroups of normoalbuminuria (ACR <30 mg/g) and albuminuria (ACR ≥30 mg/g) were determined according to the median values of ACR from two or more consecutive urine samples obtained during the 2-year period preceding enrollment (baseline). Rx, treatment.
P value for comparison of patients with normoalbuminuria versus albuminuria. †Our previous publication included biomarkers of inflammation and tubular damage, which were associated with early progressive renal decline: plasma TNF-R1, plasma KIM-1, and urinary EGF–to–MCP-1 ratio (23). §Fast renal decline is defined as eGFR loss ≥5 mL/min/1.73 m2/year calculated over a median 7-year follow-up.
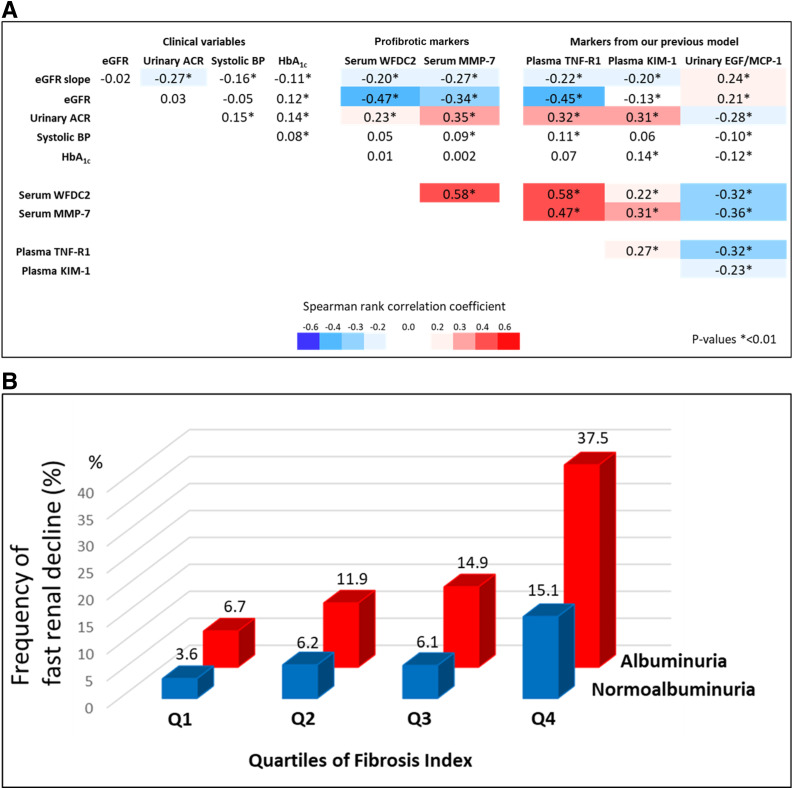
Figure 1A shows matrix correlation of clinical characteristics and levels of examined biomarkers in the entire cohort. The eGFR slope was weakly correlated with clinical variables (except for no correlation with baseline eGFR) and all examined biomarkers. Baseline eGFR was moderately or strongly correlated with profibrotic proteins and TNF-R1, but not with other biomarkers. The baseline ACR was weakly or moderately correlated with all biomarkers. Baseline levels of systolic BP and HbA1c were not correlated with any of the examined biomarkers. Serum WFDC2, serum MMP-7, and plasma TNF-R1 were strongly intercorrelated. These three biomarkers had weak or moderate correlation with the other biomarkers, and, similarly, the other biomarkers had only weak or moderate correlation among themselves.
Figure 1.
Associations of two profibrotic proteins with other variables. A: Spearman rank correlation coefficient matrix of clinical variables and biomarkers. B: Frequency of fast renal decline (%) according to quartiles of the fibrosis index.
Profibrotic Proteins and Risk of Fast Early Renal Decline
Risk of fast early renal decline during 6–12 years of follow-up was examined according to quartiles of the two profibrotic proteins in the entire cohort. The results are shown in Supplementary Fig. 1. Individuals with low baseline values of WFDC2 (Q1) and low values of MMP-7 (Q1) had a very low risk of fast renal decline during follow-up. In contrast, individuals with high baseline levels of both profibrotic proteins had risk for fast renal decline increasing in a multiplicative way. Overall, these two proteins showed largely independent effects. To analyze the joint effect of both WFDC2 and MMP-7 on the risk of fast early renal decline, we developed the fibrosis index as a summary variable, according to the formula described in research design and methods:
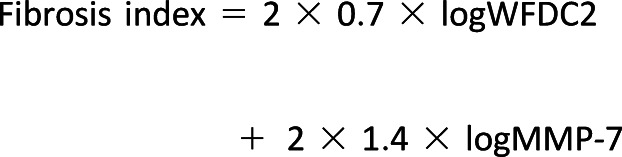 |
The risk of fast early renal decline during follow-up in subgroups according to albuminuria status and according to quartiles of the fibrosis index is shown in Fig. 1B. The risk of renal decline was increasing with quartiles of the fibrosis index in both subgroups, although it was more than twice as high in individuals with albuminuria compared with those with normoalbuminuria. Multivariable logistic analysis was used to further examine the association between the fibrosis index and fast renal decline in individuals in the two subgroups controlling for clinical characteristics and the biomarkers we previously reported (23). The odds ratios (ORs) for variables examined in the logistic analysis are provided in Table 2. In the entire cohort, six variables reported in the previous publication had significant independent effects on risk of fast early renal decline. The fibrosis index added to the same logistic model showed a very strong association with risk of fast early renal decline (OR 1.64, 95% CI 1.31–2.05). The ORs changed for two variables: the OR increased for eGFR and declined for TNF-R1, becoming statistically nonsignificant. The effect of the fibrosis index on risk of fast early renal decline was statistically significant in individuals with normoalbuminuria (OR 1.40; 95% CI 1.02–1.94) and albuminuria (OR 1.92; 95% CI 1.39–2.66). Supplementary Table 1 reports results of similar analyses as in Table 2 using both profibrotic proteins instead of the fibrosis index. While almost all results were the same, the effects of two profibrotic proteins were significant only in individuals with albuminuria. They were not significant in individuals with normoalbuminuria.
Table 2.
Effect estimates of examined variables at baseline on risk of fast early renal decline in patients with type 2 diabetes during 6–12 years of follow-up
| Entire cohort (N = 1,181) | ||||||||
|---|---|---|---|---|---|---|---|---|
| Without fibrosis index | With fibrosis index | Normoalbuminuria (n = 681) | Albuminuria (n = 500) | |||||
| Variable | OR | (95% CI) | OR | (95% CI) | OR | (95% CI) | OR | (95% CI) |
| eGFR | 1.14 | (0.99–1.30) | 1.21 | (1.05–1.39) | 1.28 | (1.00–1.62) | 1.17 | (0.99–1.40) |
| Systolic BP | 1.36 | (1.15–1.62) | 1.38 | (1.16–1.64) | 1.37 | (1.03–1.82) | 1.39 | (1.12–1.74) |
| Albuminuria† | 1.89 | (1.26–2.85) | 1.68 | (1.11–2.55) | ||||
| HbA1c | 1.11 | (1.00–1.24) | 1.14 | (1.02–1.27) | 1.18 | (0.96–1.45) | 1.14 | (1.00–1.30) |
| TNF-R1 | 1.36 | (1.10–1.68) | 1.16 | (0.93–1.46) | 1.37 | (0.96–1.96) | 1.04 | (0.77–1.40) |
| KIM-1 | 1.45 | (1.21–1.75) | 1.36 | (1.13–1.65) | 1.22 | (0.91–1.65) | 1.47 | (1.15–1.88) |
| EGF–to–MCP-1 ratio | 0.62 | (0.51–0.75) | 0.67 | (0.55–0.82) | 0.57 | (0.42–0.77) | 0.75 | (0.58–0.98) |
| Fibrosis index | 1.64 | (1.31–2.05) | 1.40 | (1.02–1.94) | 1.92 | (1.39–2.66) | ||
Multivariable logistic regression was used to estimate ORs of change in predictors (including the fibrosis index) on the risk of renal decline in the entire cohort and separately in normoalbuminuria and albuminuria subgroups (see also Supplementary Table 1). ORs in bold are statistically significant. There was no evidence for a statistically significant interaction between albuminuria status and the effect of any of the examined baseline variables. The effects of eGFR and systolic BP on fast renal decline were estimated per 10 mL/min/1.73 m2 and per 10 mmHg increase, respectively. The effect of HbA1c on fast renal decline was estimated per 1% increase. The effects of plasma TNF-R1, plasma KIM-1, the urinary EGF–to–MCP-1 ratio, and the fibrosis index on fast renal decline were estimated per one quartile increase.
Albuminuria is modeled as a categorical variable (normoalbuminuira = 1, albuminuria = 2).
Profibrotic Proteins as Predictors of Fast Progressive Renal Decline
Although the aim of the study was to examine the effect of profibrotic circulating proteins on the risk of early progressive renal decline (etiological model) in individuals with normoalbuminuria and albuminuria, we also evaluated how much measurements of these proteins could improve the prediction of fast early renal decline in patients with type 2 diabetes (Table 3). We did not have statistical power to examine this issue in normoalbuminuria and albuminuria separately; therefore, the analysis was conducted in the entire cohort without stratification. As a reference, model 1 included clinical variables (eGFR, systolic BP, ACR, and HbA1c), model 2 additionally included the fibrosis index, and model 3 included model 2 predictors together with TNF-R1, a biomarker of inflammation, KIM-1, and the EGF–to–MCP-1 ratio as biomarkers of tubular damage, as reported in our previous study (23). Table 3 reports the effect estimates for variables included in each model. Clinical variables had a strong effect on the risk of fast renal decline in all models, except for eGFR. The fibrosis index had a strong effect on the risk of fast renal decline in both model 2 and model 3. Among the other biomarkers, plasma KIM-1 and the urinary EGF–to–MCP-1 ratio had a strong independent effect on risk of fast renal decline, but the effect of plasma TNF-R1 was nonsignificant in the presence of the fibrosis index.
Table 3.
Comparison of multivariable logistic regression models with regard to the prediction of fast early renal decline in patients with type 2 diabetes during 6–12 years of follow-up
| Model 1 (reference) | Model 2 | Model 3 | ||||
|---|---|---|---|---|---|---|
| Baseline variables | OR | (95% CI) | OR | (95% CI) | OR | (95% CI) |
| eGFR | 0.91 | (0.82–1.02) | 1.08 | (0.96–1.22) | 1.19 | (1.03–1.37) |
| Systolic BP | 1.34 | (1.13–1.57) | 1.37 | (1.15–1.62) | 1.38 | (1.16–1.64) |
| ACR | 1.83 | (1.54–2.19) | 1.50 | (1.25–1.81) | 1.27 | (1.04–1.56) |
| HbA1c | 1.19 | (1.08–1.32) | 1.20 | (1.08–1.34) | 1.14 | (1.02–1.27) |
| Fibrosis index | 1.98 | (1.60–2.43) | 1.63 | (1.30–2.04) | ||
| TNF-R1 | 1.15 | (0.91–1.44) | ||||
| KIM-1 | 1.36 | (1.12–1.64) | ||||
| EGF–to–MCP-1 ratio | 0.68 | (0.56–0.83) | ||||
| C statistic | 0.725 | 0.766 | 0.791 | |||
| ∆C statistic compared with model 1 | 0.041 (0.011–0.071) | 0.066 (0.031–0.100) | ||||
| Nagelkerke R2 | 0.144 | 0.208 | 0.250 | |||
Model 1: reference; model 2: model 1 + fibrosis index; model 3: model 2 + other biomarkers (plasma TNF-R1, plasma KIM-1, and urinary EGF–to–MCP-1 ratio).
The effects of eGFR and systolic BP on fast renal decline were estimated per 10 mL/min/1.73 m2 and per 10 mmHg increase, respectively. The effect of HbA1c was estimated per 1% increase. The effects of ACR, the fibrosis index, plasma TNF-R1, plasma KIM-1, and the urinary EGF–to–MCP-1 ratio were estimated per one quartile increase.
Compared with model 1, the C statistics of models 2 and 3 were significantly higher, and the C statistic of model 3 was significantly higher by 0.025 (95% CI 0.005–0.045) than that of model 2. The proportion of variation in risk of fast early renal decline explained by these models was evaluated with the Nagelkerke R2. The R2 for model 1 that included only clinical covariates was 0.144. Inclusion of the fibrosis index increased the Nagelkerke R2 to 0.208 (model 2) and further to 0.250 (model 3) by including the previously reported biomarkers (Table 3).
Conclusions
The findings in this study provide novel evidence that circulating profibrotic proteins, biomarkers of renal fibrosis, contribute strongly to early progressive renal decline in type 2 diabetes in patients with and without albuminuria. Previously, renal fibrosis was assumed to be an important factor contributing to late progressive renal decline in patients with impaired renal function (10,29,30). The current findings expand our previous results obtained in the same cohort, indicating that profibrotic proteins have a strong and independent effect on the risk of early progressive renal decline in addition to the biomarkers of glomerular damage measured as ACR and tubular damage measured as circulating KIM-1 and urinary ratio of EGF to MCP-1 (23). Furthermore, since our findings hold strong not only in individuals with albuminuria but also in those with normoalbuminuria, we postulate that abnormalities in profibrotic circulating proteins contribute to the onset/initiation of early progressive renal decline independent of glomerular and tubular damage. It is interesting that the strong effect of circulating TNF-R1 reported in our previous publication (23) was attenuated when the fibrosis index was included in the multivariable model. This may be interpreted as evidence that TNF-R1 is a biomarker of the inflammatory process that precedes initiation of renal fibrosis, which in turn leads to early progressive renal decline. We hypothesize that in a multivariable logistic analysis, the factors proximal to the outcome in the causal chain show a stronger association than more distal factors. From a clinical perspective, elevation in the circulation of the fibrotic markers, TNF receptors, and decrease in the EGF–to–MCP-1 ratio in urine are already present in the absence of the hallmark of DKD, abnormal urinary albumin level.
Elevated levels of circulating WFDC2 and MMP-7 were previously reported in cross-sectional studies (16–18) and in one prospective study (21) as associated with renal fibrosis, respectively. However, our study is the first in which both of these proteins were studied in a prospective manner. We showed that elevated levels of these proteins had independent and additive effects on the risk of early progressive renal decline. It may be interpreted as these two proteins representing distinct but complementary disease processes involved in renal fibrosis, which lead to early progressive renal decline. These proteins could be the signatures of profibrotic exposures, reflecting the excess activation of profibrotic pathways or dysregulation of ECM turnover in kidneys. The fibrosis index used in our study as a summary of the two profibrotic proteins added extra value over two independent proteins in terms of statistical efficiency for an analysis performed in a very low-risk group of patients. The importance of the fibrotic pathway in patients without albuminuria would be overlooked if the proteins were analyzed separately. Additionally, the fibrosis index jointly captures all of the predictive information and probably the pathophysiological mechanisms relayed through these two proteins.
Presently, the source of the circulating profibrotic proteins and the mechanisms of their upregulation are unknown. Also unknown is whether these proteins are causal factors or just markers of fibrogenesis that underlies early progressive renal decline. From limited research, we know that expression of WFDC2 is elevated in human fibrotic kidneys (16–19). LeBleu et al. (14) identified WFDC2 as the most upregulated gene in cultured fibrosis-associated myofibroblasts that are considered the primary site of matrix protein synthesis during the active phase of renal fibrogenesis (12). It is postulated that WFDC2 acts as a panprotease inhibitor and that this activity leads to reduction of type I collagen turnover (14). Accumulation of type I collagen is responsible for chronic structural and functional alterations of the kidney parenchyma and eventual organ failure (29).
Increased MMP-7 causes an imbalance between synthesis and degradation of ECM components, leading to renal fibrosis. In general, MMP-7 is responsible for the turnover of connective tissue, degrading a broad range of ECM substrates and additional noncollagenous glycoproteins (15). MMP-7 is not detected in healthy human renal tubular epithelium (15,31). However, MMP-7 is upregulated in renal tubular cells in response to kidney injury (31,32). The magnitude of MMP-7 induction is closely correlated with the extent of renal fibrotic lesions in mouse models (31). On the other hand, blockade of MMP-7 activity inhibits renal expression of collagen I and fibronectin and reduces myofibroblast activation in mouse models (33). It is postulated that upregulation of MMP-7 promotes renal fibrosis through several pathways; however, the specific mechanisms responsible for elevated levels of the profibrotic proteins in circulation are unknown.
Our study demonstrated the effects of several other factors on the risk of fast renal decline in type 2 diabetes, indicating elevated systolic BP, hyperglycemia, and glomerular and tubular damage (23). It is important to note that the addition of the fibrosis index into the multivariable logistic analysis did not change the effect of these factors on risk of progressive renal decline except for attenuation of the effect of circulating TNF-R1. These findings support the idea that early progressive renal decline in type 2 diabetes is a multifactorial disease process. Considering that many patients with type 2 diabetes follow a nonalbuminuric pathway to renal function loss (34), the findings also implicate fibrosis as an important factor contributing not only to progressive renal decline but also to its onset.
Finally, the strengths and limitations of our study deserve consideration. The major strength was the prospective study design with sufficient 6–12 years of follow-up of a large cohort of patients with type 2 diabetes and normal renal function at baseline. This study design allowed us to reliably ascertain patients with early progressive renal decline. On the other hand, the major limitation was the lack of measurements of the extent of fibrosis in kidney biopsy specimens and the observational nature of our study. The latter limits the interpretation of our findings regarding the causal relationship between upregulation of circulating profibrotic proteins and the onset of early renal decline.
Article Information
Funding and Duality of Interest. This study was supported by the Uehara Memorial Foundation (Postdoctoral Fellowship) and the Japan Society for the Promotion of Science (Overseas Research Fellowship) to H.K.; by the Sunstar Foundation, Japan (Hiroo Kaneda Scholarship), and the Foundation for Growth Science from Japan to E.S.; by JDRF grant “Biomarkers of Diabetic Nephropathy Collaborative Research Initiative (DN-BIO)” no. 3-SRA-2015-106-Q-R to A.S.K.; by National Institutes of Health National Institute of Diabetes and Digestive and Kidney Diseases grant DK-041526 to A.S.K.; by a grant from Eli Lilly and Co. to A.S.K. to study biomarkers of early renal decline in type 2 diabetes; and by National Institutes of Health National Institute of Diabetes and Digestive and Kidney Diseases Diabetes Research Center grant P30 DK-036836. No other potential conflicts of interest relevant to this article were reported.
Author Contributions. K.I. contributed to the study design, performed data analysis, interpreted the results, and wrote the manuscript. K.I. and J.S. worked together on data analysis. J.S. contributed to study design, interpreted the results, and edited the manuscript. H.K., Z.I.M.D., K.O., and E.S. contributed to data collection and reviewed the manuscript. J.M.W. supervised the ELISA determination and read and edited the manuscript. H.S.B. and L.M.B. performed ELISA determinations. M.D.B. reviewed the manuscript. K.L.D. contributed to the study design, interpretation of the findings, and writing and editing of the manuscript. A.S.K. was responsible for the design of the study, supervised data collection and data analysis, and contributed to writing and editing the manuscript. A.S.K. is the guarantor of this work and, as such, had full access to the data for the cohorts and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Footnotes
M.D.B. is currently affiliated with Janssen Cardiovascular and Metabolism, Boston, MA.
This article contains supplementary material online at https://doi.org/10.2337/figshare.12808433.
References
- 1.Krolewski AS. Progressive renal decline: the new paradigm of diabetic nephropathy in type 1 diabetes. Diabetes Care 2015;38:954–962 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Krolewski AS, Skupien J, Rossing P, Warram JH. Fast renal decline to end-stage renal disease: an unrecognized feature of nephropathy in diabetes. Kidney Int 2017;91:1300–1311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vistisen D, Andersen GS, Hulman A, Persson F, Rossing P, Jørgensen ME. Progressive decline in estimated glomerular filtration rate in patients with diabetes after moderate loss in kidney function-even without albuminuria. Diabetes Care 2019;42:1886–1894 [DOI] [PubMed] [Google Scholar]
- 4.Osterby R, Gundersen HJ, Nyberg G, Aurell M. Advanced diabetic glomerulopathy. Quantitative structural characterization of nonoccluded glomeruli. Diabetes 1987;36:612–619 [DOI] [PubMed] [Google Scholar]
- 5.Lane PH, Steffes MW, Fioretto P, Mauer SM. Renal interstitial expansion in insulin-dependent diabetes mellitus. Kidney Int 1993;43:661–667 [DOI] [PubMed] [Google Scholar]
- 6.Niewczas MA, Pavkov ME, Skupien J, et al. . A signature of circulating inflammatory proteins and development of end-stage renal disease in diabetes. Nat Med 2019;25:805–813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Katz A, Caramori MLA, Sisson-Ross S, Groppoli T, Basgen JM, Mauer M. An increase in the cell component of the cortical interstitium antedates interstitial fibrosis in type 1 diabetic patients. Kidney Int 2002;61:2058–2066 [DOI] [PubMed] [Google Scholar]
- 8.Eddy AA. Progression in chronic kidney disease. Adv Chronic Kidney Dis 2005;12:353–365 [DOI] [PubMed] [Google Scholar]
- 9.Rockey DC, Bell PD, Hill JA. Fibrosis--a common pathway to organ injury and failure. N Engl J Med 2015;372:1138–1149 [DOI] [PubMed] [Google Scholar]
- 10.Liu Y. Cellular and molecular mechanisms of renal fibrosis. Nat Rev Nephrol 2011;7:684–696 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Boor P, Ostendorf T, Floege J. Renal fibrosis: novel insights into mechanisms and therapeutic targets. Nat Rev Nephrol 2010;6:643–656 [DOI] [PubMed] [Google Scholar]
- 12.Eddy AA. Serine proteases, inhibitors and receptors in renal fibrosis. Thromb Haemost 2009;101:656–664 [PMC free article] [PubMed] [Google Scholar]
- 13.Falke LL, Gholizadeh S, Goldschmeding R, Kok RJ, Nguyen TQ. Diverse origins of the myofibroblast—implications for kidney fibrosis. Nat Rev Nephrol 2015;11:233–244 [DOI] [PubMed] [Google Scholar]
- 14.LeBleu VS, Teng Y, O’Connell JT, et al. . Identification of human epididymis protein-4 as a fibroblast-derived mediator of fibrosis. Nat Med 2013;19:227–231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ke B, Fan C, Yang L, Fang X. Matrix metalloproteinases-7 and kidney fibrosis [published correction appears in Front Physiol 2017;8:192] Front Physiol 2017;8:21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nakagawa S, Nishihara K, Miyata H, et al. . Molecular markers of tubulointerstitial fibrosis and tubular cell damage in patients with chronic kidney disease. PLoS One 2015;10:e0136994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wan J, Wang Y, Cai G, et al. . Elevated serum concentrations of HE4 as a novel biomarker of disease severity and renal fibrosis in kidney disease. Oncotarget 2016;7:67748–67759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liu L, Ning X, Bai M, et al. . Prognostic and diagnostic value of HE4 expression in patients with chronic kidney diseases. Int J Clin Exp Pathol 2016;9:7930–7940 [Google Scholar]
- 19.Nagy B Jr., Krasznai ZT, Balla H, et al. . Elevated human epididymis protein 4 concentrations in chronic kidney disease. Ann Clin Biochem 2012;49:377–380 [DOI] [PubMed] [Google Scholar]
- 20.Surendran K, Simon TC, Liapis H, McGuire JK. Matrilysin (MMP-7) expression in renal tubular damage: association with Wnt4. Kidney Int 2004;65:2212–2222 [DOI] [PubMed] [Google Scholar]
- 21.Zhang J, Ren P, Wang Y, et al. . Serum matrix metalloproteinase-7 level is associated with fibrosis and renal survival in patients with IgA nephropathy. Kidney Blood Press Res 2017;42:541–552 [DOI] [PubMed] [Google Scholar]
- 22.Bauer Y, White ES, de Bernard S, et al. . MMP-7 is a predictive biomarker of disease progression in patients with idiopathic pulmonary fibrosis. ERJ Open Res 2017;3:00074-2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nowak N, Skupien J, Smiles AM, et al. . Markers of early progressive renal decline in type 2 diabetes suggest different implications for etiological studies and prognostic tests development. Kidney Int 2018;93:1198–1206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Skupien J, Warram JH, Smiles AM, et al. . The early decline in renal function in patients with type 1 diabetes and proteinuria predicts the risk of end-stage renal disease. Kidney Int 2012;82:589–597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Levey AS, Stevens LA, Schmid CH, et al.; CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration) . A new equation to estimate glomerular filtration rate [published correction appears in Ann Intern Med 2011;155:408] Ann Intern Med 2009;150:604–612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kidney Disease: Improving Global Outcomes (KDIGO) CKD Work Group KDIGO 2012 clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Int Suppl 2013;3:1–150 [Google Scholar]
- 27.Ayache S, Panelli MC, Byrne KM, et al. . Comparison of proteomic profiles of serum, plasma, and modified media supplements used for cell culture and expansion. J Transl Med 2006;4:40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jonsson A, Hjalmarsson C, Falk P, Ivarsson ML. Levels of matrix metalloproteinases differ in plasma and serum – aspects regarding analysis of biological markers in cancer. Br J Cancer 2016;115:703–706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Buchtler S, Grill A, Hofmarksrichter S, et al. . Cellular origin and functional relevance of collagen I production in the kidney. J Am Soc Nephrol 2018;29:1859–1873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zeisberg M, Neilson EG. Mechanisms of tubulointerstitial fibrosis. J Am Soc Nephrol 2010;21:1819–1834 [DOI] [PubMed] [Google Scholar]
- 31.He W, Tan RJ, Li Y, et al. . Matrix metalloproteinase-7 as a surrogate marker predicts renal Wnt/β-catenin activity in CKD. J Am Soc Nephrol 2012;23:294–304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tan RJ, Zhou D, Zhou L, Liu Y. Wnt/β-catenin signaling and kidney fibrosis. Kidney Int Suppl (2011) 2014;4:84–90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhou D, Tian Y, Sun L, et al. . Matrix metalloproteinase-7 is a urinary biomarker and pathogenic mediator of kidney fibrosis. J Am Soc Nephrol 2017;28:598–611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.MacIsaac RJ, Ekinci EI. Progression of diabetic kidney disease in the absence of albuminuria. Diabetes Care 2019;42:1842–1844 [DOI] [PubMed] [Google Scholar]