Abstract
Epidemiological studies on the intergenerational transmission of hyperglycemia and obesity via in utero exposure have established the scientific foundation for the vicious cycle of diabetes and obesity. The findings compel us to address an urgent public health question: how do we break this vicious cycle and implement upstream prevention strategies that are feasible for patients and health care delivery systems? To address this question, it is necessary to work across a continuum of translational research from basic science, epidemiology, and efficacy trials to pragmatic trials, which, along with evaluations of health programs, may lead to implementation of positive changes in clinical care. Three strategies for translating research on diabetes and obesity in pregnancy into prevention are discussed: 1) identifying diagnostic criteria of gestational diabetes mellitus (GDM) practicable in clinical settings to implement treatment and prevention, 2) examining trends in the prevalence of diabetes in pregnancy and related complications across racial/ethnic groups to plan prevention efforts, and 3) developing and evaluating scalable upstream diabetes and obesity prevention interventions. Upstream preventive interventions aimed at breaking the vicious cycle are discussed. Areas of future research needed to break the vicious cycle are identified. Evaluating the effectiveness of programs for the management of pregnancy hyperglycemia is necessary to reduce complications. Understanding racial/ethnic differences in the pathophysiology of GDM and its complications will be important for risk stratification. Pragmatic trials in real-world clinical settings for upstream prevention are needed to break the vicious cycle at the population level. Finally, leveraging basic science with intergenerational studies will inform targeted interventions.
Introduction
Norbert Freinkel, in his Banting lecture in 1980 (1), was among the first to introduce the concept of the diabetes vicious cycle by describing evidence and hypotheses on how excess pregnancy fuel substrates, resulting from altered glucose and insulin homeostasis, had the potential to lead to adverse effects on the fetus by affecting organogenesis, behaviors, and adiposity. Freinkel hypothesized that among women with gestational diabetes mellitus (GDM) the effect of pregnancy hyperglycemia on the fetal β-cell may have intergenerational effects. This hypothesis was supported by a study of rats with streptozotocin-induced hyperglycemia during pregnancy (F1), which showed that their offspring (F2) exhibited reduced glucose tolerance and insulin secretion during late pregnancy, and the third generation (F3) also showed inadequate β-cell changes during late pregnancy, suggesting that GDM could be an in utero–acquired condition (2).
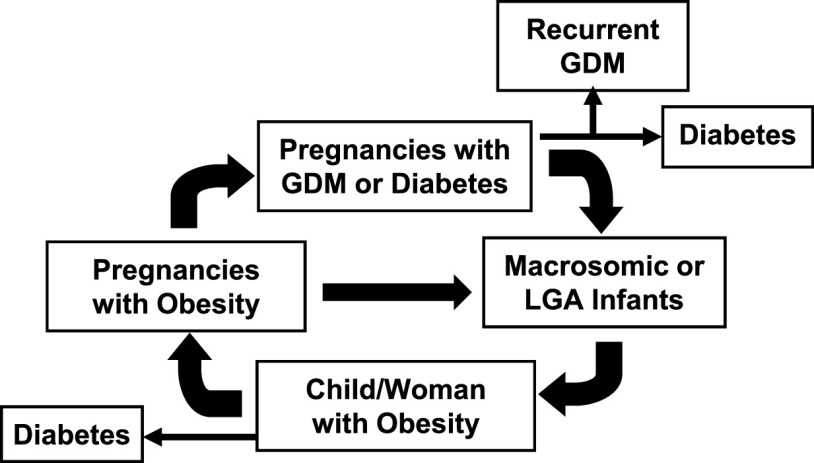
Today, we have several pieces of evidence from large racially and ethnically diverse population-based epidemiological studies on the intergenerational transmission of hyperglycemia and obesity related to in utero exposure to these conditions, thus further substantiating the vicious cycle of diabetes and obesity that was first observed among the Pima Indians (3) (Fig. 1). Women with a pregnancy affected by GDM are at high risk of recurrent GDM in a subsequent pregnancy (4) and type 2 diabetes later in life (5); their offspring are at increased risk of several perinatal complications, including macrosomia and large for gestational age (LGA) (6,7), and adverse health outcomes during childhood and adolescence, such as obesity, early puberty, and type 2 diabetes (8–13). Female offspring are more likely to enter pregnancy with obesity or dysglycemia, factors that predispose women to having GDM (14,15) and a macrosomic or LGA infant (16). The effect of GDM and obesity on these risks is amplified if excess gestational weight gain occurs (17,18). These findings compel us to address an urgent public health question: how do we break the vicious cycle of diabetes and obesity in pregnancy and implement upstream prevention strategies that are feasible for patients and health care delivery systems?
Figure 1.
The vicious cycle of diabetes and obesity.
Here, I will share what I learned about breaking the vicious cycle of diabetes and obesity in pregnancy. I started to work on this area of research when I arrived at the Division of Research at Kaiser Permanente Northern California (KPNC). Doing research there, in a division embedded in an integrated health care delivery system, made it possible to advance and translate my research into a model for prevention. My research was also possible because of the collaboration with valuable colleagues and friends—Drs. Monique Hedderson, Samantha Ehrlich, Susan Brown, Yeyi Zhu, and Charles Quesenberry and the directors of the KPNC Regional Perinatal Service Center (Perinatal Center), Drs. Yvonne Crites and Mara Greenberg. Together we worked across a continuum of translational research. On this continuum, basic science, epidemiology, and efficacy randomized controlled trials (RCTs) all informed pragmatic RCTs that, along with evaluations of health programs, may lead to implementation of positive changes in clinical care and policies. As described below, we had three valuable strategies for translating the research on diabetes and obesity in pregnancy into interventions designed to break the vicious cycle.
1. Identifying Diagnostic Criteria for GDM That Are Practicable in Clinical Settings to Implement Treatment and Prevention
Historically, the first systematic approach to establish diagnostic criteria for GDM was led by O’Sullivan in the late 1950s (19). In 1979 the National Diabetes Data Group (NDDG) proposed modifying upward the glucose thresholds established by O’Sullivan due to a switch from using venous whole-blood samples to plasma or serum samples (20). In 1982 Carpenter and Coustan (CC) proposed glucose thresholds lower than the NDDG thresholds due to the new enzymatic method that reduced measurements of nonglucose substances in the plasma or serum (21). Both the NDDG and CC followed the original O’Sullivan criteria for the diagnosis of GDM by using at least two glucose values during the 100-g, 3-h oral glucose tolerance test (OGTT) above the selected thresholds. Until early 2000, the NDDG thresholds were the most commonly used criteria, given the paucity of evidence on whether lower glucose levels were associated with increased risk of perinatal complications (22).
In 2007 we published the results of three case-control studies with approximately 500 cases for each neonatal complication previously reported to be associated with in utero exposure to hyperglycemia, such as severe macrosomia (birth weight >4,500 g), severe neonatal hypoglycemia (plasma glucose <2.2 mmol/L), and hyperbilirubinemia (serum bilirubin ≥342 μmol/L) (6). Cases and controls were nested within a cohort of more than 45,000 racially and ethnically diverse pregnant women who did not meet the NDDG thresholds and therefore were not treated. We showed that having two or more glucose values meeting the CC thresholds was associated with substantially increased odds of having an infant with macrosomia (odds ratio [OR] 3.40 [95% CI 1.55–7.43]), hypoglycemia (2.61 [0.99–6.92]), or hyperbilirubinemia (2.22 [0.98–5.04]) (Fig. 2A) (6). These results were among the earliest and strongest evidence in support of the associations between the CC criteria and the risk of perinatal complications, and they led to a wider adoption of the CC criteria for the diagnosis of GDM.
Figure 2.
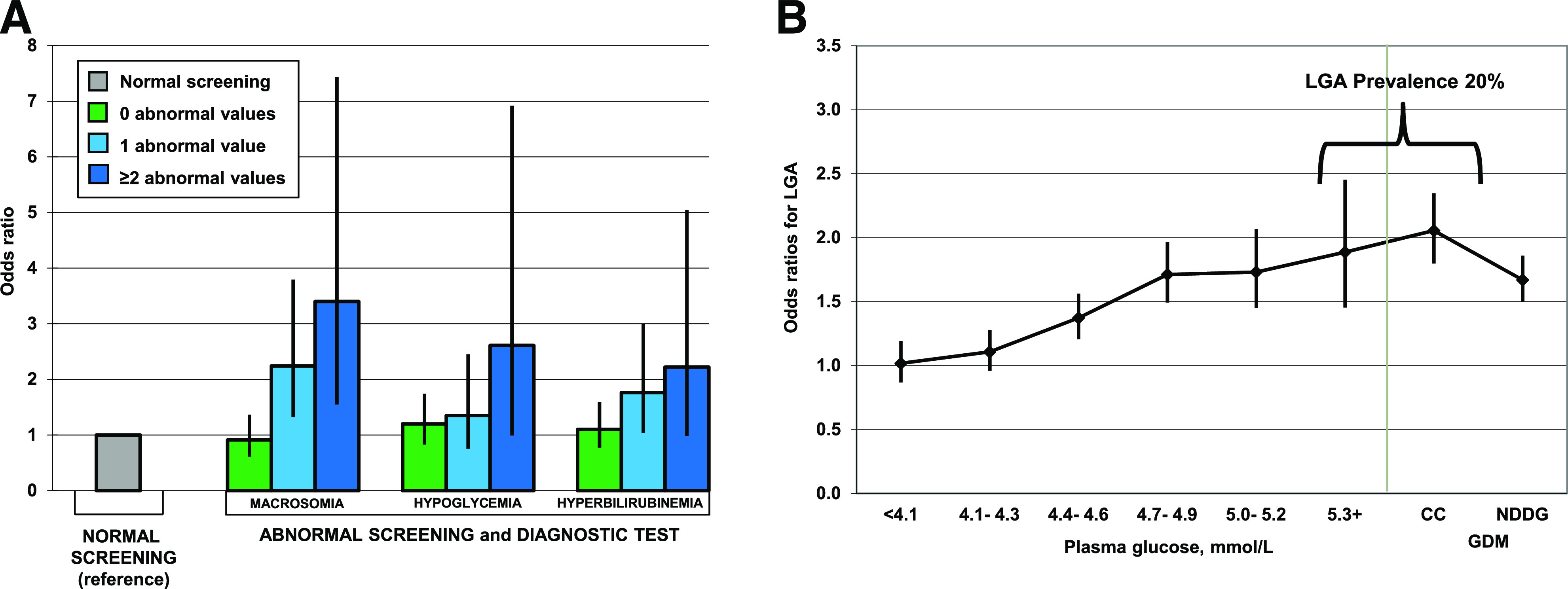
A: ORs and 95% CI for neonatal complications associated with number of glucose values meeting the Carpenter and Couston (CC) thresholds during a diagnostic 100-g, 3-h OGTT among women who did not meet the NDDG criteria. Adapted from Ferrara et al. (6). B: ORs and 95% CI for LGA across categories of fasting plasma glucose. Adapted with permission from Ehrlich et al. (25).
In 2010 the International Association of the Diabetes and Pregnancy Study Groups (IADPSG) proposed new glucose thresholds for the diagnosis of GDM that were based on the results from the Hyperglycemia and Adverse Pregnancy Outcome (HAPO) study (7). The IADPSG-proposed glucose thresholds were lower than the CC thresholds and required only one glucose value obtained during a 75-g, 2-h OGTT at or above those thresholds for the diagnosis of GDM (23). The IADPSG criteria opened a series of debates since their use would substantially increase the prevalence of GDM (24), posing challenges to health care delivery systems. Moreover, in some populations, the IADPSG criteria were not found to be associated with increased risk of perinatal complications (24). Other questions were related to whether the increased odds of perinatal complications that were observed above the IADPSG thresholds were mostly related to values in the upper end of the glucose distribution, which included the CC thresholds.
To provide further information to this ongoing debate, we estimated the odds of LGA across increasing categories of pregnancy glycemia for each of the four time points of a 100-g, 3-h OGTT in a multiethnic cohort of more than 150,000 pregnant women who did not have GDM by the CC criteria and therefore had a glucose value exceeding only one of the CC thresholds (25). We found that the highest prevalence of LGA (i.e., 20%) was observed among women with isolated fasting glycemia at or above the CC threshold (i.e., ≥5.3 mmol/L), and it was similar to that observed in women with GDM by the CC criteria who were not treated. The odds of delivering an LGA infant associated with isolated fasting hyperglycemia ≥5.3 mmol/L was 1.89 (95% CI 1.45–2.45), whereas the odds in women with fasting glycemia in the rage 5.0–5.2 mmol/L, and thus including the IADPSG cut point, was 1.73 (95% CI 1.45–2.07) (Fig. 2B) (25). These results suggested that isolated fasting hyperglycemia may be clinically useful in identifying women with increased odds for delivering an LGA infant, who would benefit from treatment and management of hyperglycemia.
Guided by the continuum of translational research, based on the results we obtained through these epidemiological studies, I collaborated with my colleagues at the KPNC Perinatal Center to implement the CC criteria and isolated fasting hyperglycemia by CC thresholds for the diagnosis and treatment of GDM in our clinical setting. These changes in GDM diagnostic criteria provided opportunities for women with pregnancy hyperglycemia below the NDDG thresholds to receive treatment, with the goal of reducing rates of macrosomia or LGA and breaking the vicious cycle of GDM.
2. Examining Trends in the Prevalence of Diabetes in Pregnancy Across Racial/Ethnic Groups to Plan Treatment and Prevention Efforts
To allow health care delivery systems to allocate resources for treatments and behavioral interventions to prevent perinatal complications among women with GDM or pregestational diabetes, it is important to know trends in the prevalence of these conditions. We were among the first to show that the prevalence of GDM was on the rise when we published a study of 267,051 pregnancies which found that the age- and race/ethnicity-adjusted prevalence of GDM increased by almost 50%, from 5.1% in 1991 to 7.2% in 2000 (Fig. 3A) (26). We also discovered clear racial/ethnic disparities in the burden of GDM, with Asian women having the highest prevalence of GDM, followed by Hispanic women and then African American and White women (26). These racial/ethnic disparities were independent of overall obesity since the prevalence of GDM among Asian women was 10% at a BMI of 22.0–24.9 kg/m2, whereas in Hispanic, White, and Black women, the prevalence was between 8% and 10% at BMIs of 28.0–30.9, 34.0–36.9, and ≥37.0 kg/m2, respectively (27). These data suggest that other factors besides obesity contribute to the high risk of GDM among Asian women. In contrast, we observed that among women with GDM the prevalence of LGA newborns was highest in African Americans (25.1%), lowest in Asians (13.9%), and intermediate among Hispanic (17.3%) and White women (16.4%) (16). These racial/ethnic disparities persisted even after adjustment for sociodemographic factors, glucose values during the 100-g OGTT, and BMI, suggesting that other factors—such as racial/ethnic disparities in achieving optimal glycemic control (28)—may partially explain the observed disparity in having an LGA infant. In addition, we found that among pregnant women with GDM, higher ethnic identity—a measure of attachment to one's ethnic group—was associated with increased physical activity and better diet quality (29), behaviors that we found to be associated with optimal glycemic control in women with GDM (30,31). These racial/ethnic disparities in GDM prevalence, related LGA risk, and protective health behaviors need to be considered when implementing treatment and prevention strategies to ensure their effectiveness across all racial/ethnic groups. They also call for a better understanding of the underlying pathophysiology of GDM across racial/ethnic groups.
Figure 3.
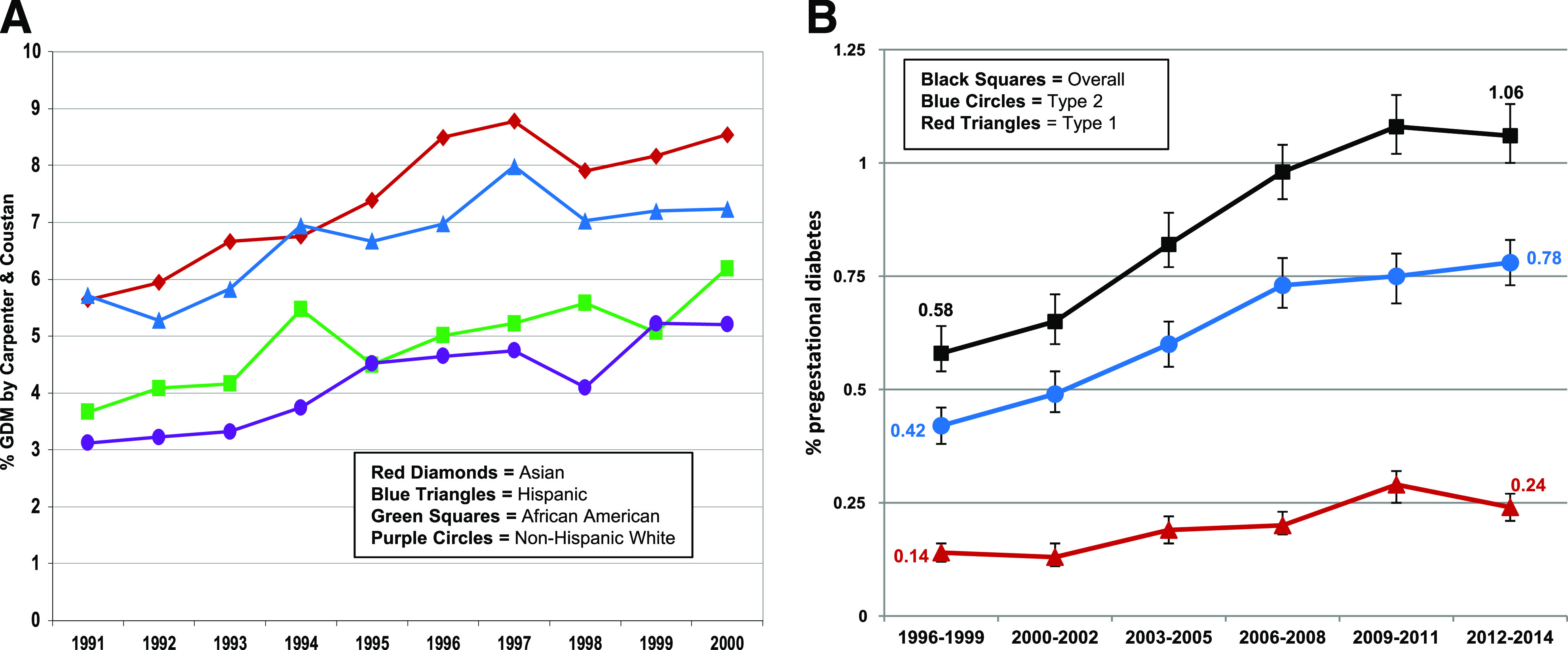
Trends in the age-adjusted prevalence of GDM (A) and pregestational diabetes (B). Panel A adapted from Ferrara et al. (26). Panel B adapted with permission from Peng et al. (32).
We also observed an alarming 82% increase in the prevalence of overall pregestational diabetes between 1996 and 2014, with similar increases for pregestational type 1 and type 2 diabetes (32). The age-adjusted prevalence increased from 0.14% (95% CI 0.12–0.16) to 0.24% (0.21–0.27) for pregestational type 1 diabetes and from 0.42% (0.38–0.46) to 0.78% (0.73–0.83) for pregestational type 2 diabetes (Fig. 3B) (32).
Increases in the prevalence of diabetes in pregnancy are alarming. Given that in utero exposure to diabetes is associated with increased risk of obesity and diabetes in offspring, increases in diabetes in pregnancy suggest a snowballing of the intergenerational transfer of these conditions and thus call for the need for upstream prevention. However, to have an impact at the population level, we need to test upstream diabetes prevention programs that are scalable in clinical settings.
3. Developing and Evaluating Scalable Interventions for Upstream Diabetes and Obesity Prevention
Treatment of Hyperglycemia in Women With GDM
Both the Australian Carbohydrate Intolerance Study in Pregnant Women (ACHOIS) trial (33) and the Maternal-Fetal Medicine Units (MFMU) Network trial (34) showed that treatment of pregnancy hyperglycemia reduced the odds of macrosomia and LGA. These trials provided evidence that the vicious cycle of diabetes in pregnancy can be broken if efficient treatment is implemented. We provided additional evidence on the effectiveness of managing pregnancy hyperglycemia in real-world clinical settings by evaluating the referral of patients with GDM to the KPNC Perinatal Center—a nurse-management program delivering supplemental care to women with GDM by providing telephonic counseling on glucose monitoring and control and encouraging the completion of postpartum screening for diabetes (35). We showed that, as compared with women from KPNC medical facilities that referred <30% of their patients with GDM to the Perinatal Center, women from KPNC medical facilities that referred >70% of their GDM patients had a 25% reduction in the odds of having a macrosomic infant (OR 0.75 [95% CI 0.57–0.98]) and almost three times the odds of completing postpartum screening for diabetes (OR 2.96 [2.56–3.42]) (35). This finding provided further evidence that it is possible to break the vicious cycle of GDM by implementing clinical care to manage pregnancy hyperglycemia. Increasing postpartum diabetes screening followed by patient outreach (36) may promote diabetes prevention or early treatment and possibly reduce the risk of macrosomia in a subsequent pregnancy. Again, guided by the continuum of translational research, results of this clinical care evaluation fully supported the referral of all GDM patients at KPNC to the Perinatal Center. Moreover, it suggested that referral of GDM patients to similar nurse-managed programs has strong potential to reduce rates of macrosomia and interrupt the vicious cycle of GDM.
Lifestyle Interventions in Women With GDM
The Diabetes Prevention Program (DPP) demonstrated that an intensive lifestyle intervention reduced the risk of developing diabetes by approximately 53% in parous women with a history of GDM, whose pregnancies were on average approximately 12 years before enrollment in the trial (37). The DPP lifestyle curriculum was very intensive and required several in-person group sessions, thereby posing challenges for implementation. We adapted the DPP curriculum to be delivered by telephone to meet the needs of pregnant and postpartum women with GDM who may benefit more from early prevention, given their exceptionally high risk of type 2 diabetes, and who juggle the competing demands of having young families. A telephonic intervention also has the potential to be more feasible for implementation in health care delivery settings. Based on evidence showing that excess gestational weight gain is a strong predictor of postpartum weight retention (38) and that postpartum weight retention is linked to type 2 diabetes (39), our DPP-based lifestyle curriculum started soon after the GDM diagnosis and continued through postpartum to help women to manage their weight during these critical periods.
We first tested this lifestyle intervention in our Diet, Exercise, and Breastfeeding Intervention (DEBI) trial, where we randomized 96 women to the intervention and 101 women to usual care soon after the GDM diagnosis (40). During pregnancy, women in the intervention were given the goal to meet the Institute of Medicine (IOM) guidelines for gestational weight gain (41); during postpartum, women were given the goal of returning to their prepregnancy weight, if their prepregnancy BMI was <25.0 kg/m2, or losing an additional 5% of their prepregnancy weight, if their prepregnancy BMI was ≥25.0 kg/m2 (40). We observed that the proportion of women who reached the postpartum weight goal at 12 months postpartum was higher in the intervention condition than in usual care (37.5% vs. 21.4%; 16% absolute condition difference). The intervention was more effective among women who did not exceed the IOM guidelines for gestational weight gain; among these women, the absolute difference in the proportion who reached the postpartum weight goals between conditions was 22.5% (40). In a secondary analysis, we found that, as compared with women who maintained or gained weight, women who lost >2 kg at 12 months postpartum had significantly lower serum glucose levels at fasting and 2 h after a 75-g OGTT (42), suggesting that even modest weight loss in the postpartum period could potentially delay the development of type 2 diabetes in women with GDM. Moving forward on the continuum of translational research, based on the results of the DEBI trial, our nurse-managed program for women with GDM at the KPNC Perinatal Center developed diabetes prevention education materials to be mailed to all women with GDM, thus providing preventive care to these young women at high risk of type 2 diabetes.
This new initiative also offered the opportunity to conduct comparative effectiveness of diabetes prevention strategies in women with GDM. In the Gestational Diabetes’ Effects on Mom (GEM) pragmatic cluster randomized controlled trial (43), we compared mailed prevention materials implemented by our nurse-managed program to the lifestyle intervention adapted from the DPP. The GEM lifestyle intervention had a pregnancy component and a postpartum component. Soon after the diagnosis of GDM, women were mailed a letter including tailored weight goals for the end of pregnancy based on a woman’s prepregnancy BMI, current weight, and gestational weight gain trajectory in relation to the IOM gestational weight gain guidelines, as well as tips on diet and exercise. At 6 weeks postpartum, women were mailed a workbook, derived from the DDP lifestyle curriculum, that was discussed through 13 individual telephone sessions lasting on average 20 min each (43). Women were given the same postpartum weight goals as in the DEBI intervention. We randomized all KPNC medical centers to either the GEM intervention (22 centers, 1,087 women with GDM) or usual care conditions (22 centers, 1,193 women with GDM) (44). As a pragmatic trial, the intervention was presented as a new initiative of our nurse-management program; therefore, the intervention was offered to all women in the medical centers randomized to the intervention. This makes the results of the GEM trial very generalizable, since it was not based on a selected sample of volunteers, as in typical randomized trials. In an intention-to-treat analysis (which included women who did not wish to participate in the intervention), we found that during the 12 months postpartum, women in the medical facilities assigned to the GEM intervention had 28% significantly higher odds of meeting postpartum weight goals than women in the medical facilities assigned to usual care (OR 1.28 [95% CI 1.10–1.47]). The proportion meeting postpartum weight goals was consistently higher in the intervention than in usual care during the follow-up (25.5% vs. 22.4% at 6 weeks, 30.6% vs. 23.9% 6 months, and 33.0% vs. 28.0% at 12 months postpartum) (Fig. 4A) (44). We also found that women in the medical facilities assigned to the GEM intervention were 8% more likely to meet IOM guidelines for weekly rate of gestational weight gain from the GDM diagnosis to delivery (72.6% vs. 67.1%; relative risk 1.08 [95% CI 1.01–1.17]; Fig. 4B) (45). Finally, the proportion of LGA infants was significantly lower in the intervention than usual care condition (9.7% vs. 12.8%) (45). In summary, we found that the GEM intervention improved gestational weight gain, reduced postpartum weight retention, and reduced LGA, providing additional evidence that it is possible to break the vicious cycle of GDM.
Figure 4.
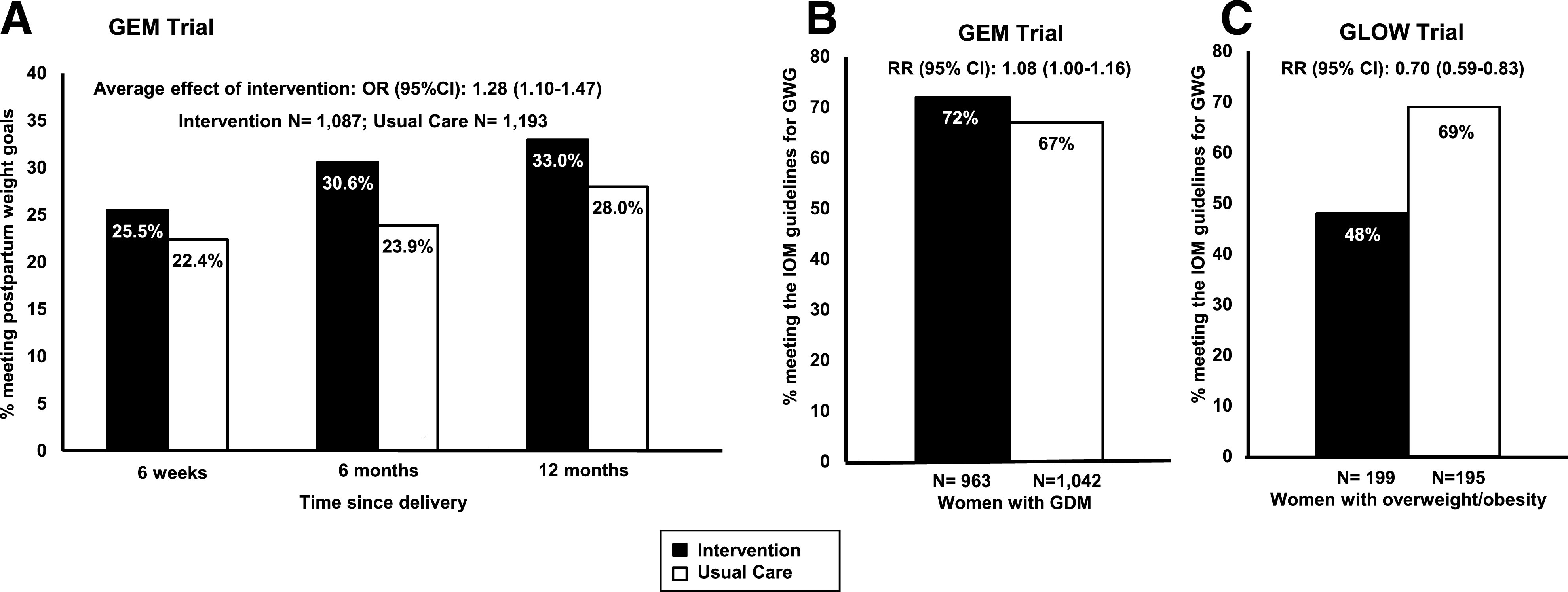
A and B: Effects of lifestyle interventions on the proportion of women with GDM meeting the postpartum weight goals (A) and the IOM guidelines for gestational weight gain (B) in the GEM cluster randomized controlled trial. Panel A adapted with permission from Ferrara et al. (44); panel B adapted with permission from Hedderson et al. (45). C: Effects of lifestyle intervention on the proportion of women with overweight or obesity meeting the IOM guidelines for gestational weight gain in the GLOW randomized controlled trial. Adapted from Ferrara et al. (49). GWG, gestational weight gain.
Lifestyle Interventions for Management of Gestational Weight Gain
The ongoing global epidemics of obesity and excessive gestational weight gain, which synergistically increase the risks of GDM and LGA (18,46), highlight the need for interventions to help women with overweight or obesity to manage gestational weight gain. Weight management interventions in women with overweight or obesity during pregnancy have had limited success. Recently, however, the meta-analysis of the Lifestyle Interventions for Expectant Moms (LIFE-Mom) trials showed that intensive in-person lifestyle interventions in pregnant women with overweight or obesity reduced the proportion of women exceeding the IOM guidelines for weekly rate of gestational weight gain by 17.6% (47). Given that intensive interventions requiring multiple in-person sessions may not be feasible for many pregnant women and may be difficult to implement in health care delivery settings, we developed a behavioral lifestyle intervention delivered primarily by telephone to help pregnant women with overweight or obesity to manage their gestational weight gain (48). We tested this intervention in the GestationaL weight gain and Optimal Wellness (GLOW) trial conducted among pregnant women with overweight or obesity, in which we randomized 199 women to the intervention and 195 to usual care (49). Women in the intervention were provided a workbook that was discussed during two individual in-person sessions and eleven individual telephone sessions starting at approximately 14 weeks of gestation. The GLOW intervention substantially reduced the proportion of women exceeding the IOM guidelines for weekly rate of gestational weight gain by 29.8% (48.2% in the intervention and 68.7% in usual care; relative risk 0.70 [95% CI 0.59–0.83]) (Fig. 4C) (49). The GLOW intervention also significantly reduced the pregnancy-related increases in biomarkers related to diabetes, such as insulin, HOMA of insulin resistance, and leptin, between the first and the third trimester; cord blood levels of leptin and C-peptide were also lower in the intervention group than the usual care group (49).
Both the LIFE-Mom and the GLOW trials did not show intervention effects in reducing LGA, although they were not powered to examine this outcome. Since observational studies have reported an association between first trimester excess gestational weight gain and LGA (50), it may be that interventions should start before conception to help women begin pregnancies with a healthier BMI and a better diet quality to reduce LGA risk. For example, in our Pregnancy Environment and Lifestyle Study (PETALS), with a longitudinal cohort of more than 3,000 pregnant women, we found that a lower healthy eating index score from the immediate preconception period through the first trimester was associated with increased risk of having an LGA infant (51). However, among women with a low healthy eating index score, substituting empty calories with whole grains was associated with a 25% reduction in LGA (OR 0.75 [95% CI 0.66–0.86]), suggesting that diet modifications from the preconception period through the first trimester may have the potential to reduce LGA. We are now following the children of the women from the PETALS cohort in the Environmental influences on Child Health Outcomes (ECHO) program, supported by the National Institutes of Health and including more than 50,000 children across the U.S. Given the large sample size, as well as the assessments of genetics, epigenetics, omics, and other multidimensional factors, the ECHO program may lead to a better understanding of the etiology of childhood obesity associated with in utero exposure to overnutrition (52), and thus it has the potential to inform the development of targeted interventions to break the vicious cycle.
Future Research
We should focus on evaluating the effectiveness of programs delivered in health care systems for management of pregnancy hyperglycemia across levels and diagnostic procedures. These programs need to be rigorously evaluated to provide evidence based on effective treatments to reduce perinatal complications in clinical settings.
We need continuous surveillance of the prevalence of diabetes and obesity in pregnancy and their related complications across diverse racial and ethnic groups to better understand risk factors for future patient risk stratification and tailored interventions. In addition, a better understanding of differences in the pathophysiology of GDM and its related perinatal complications across diverse racial/ethnic groups is needed to better target individual clinical care of pregnancy hyperglycemia. Evaluation of clinical care across racial/ethnic groups is also needed to implement modalities of care that are most effective for each group and to reduce possible health disparities in pregnancy glycemic control and complications.
There remain important gaps in our knowledge on upstream prevention to break the vicious cycle of diabetes and obesity in pregnancy. Trials targeting the treatment of GDM such as the ACHOIS (33) and MFMU (34) trials prevented macrosomia and LGA; however, they did not prevent childhood obesity (53,54). Lifestyle interventions during pregnancy to prevent GDM have reported inconsistent results (55–57). Lifestyle interventions on management of gestational weight gain were successful in reducing excess gestational weight gain; however, they did not reduce the risk for LGA. In contrast, observational studies have showed strong associations between GDM and childhood obesity (8–13), as well as between excess gestational weight gain and LGA and GDM and childhood obesity (14–16).
It is possible that observational studies were unable to control for unmeasured confounding factors and thus overestimated the associations; it is also possible that interventions delivered to women randomized at the individual level included healthier volunteers, as compared with the general population, who were more prone to successfully change health behaviors, thereby improving outcomes. Thus, more pragmatic RCTs—conducted in real-world health care settings using strategies such as telemedicine to potentially increase participation and adherence—are needed to inform upstream prevention strategies to break the vicious cycle of diabetes and obesity. Upstream prevention may require interventions that begin before conception to help women to begin pregnancies with a healthier BMI and a healthier diet to prevent GDM and LGA; this is consistent with our findings that greater weight gain before pregnancy and during the first trimester were associated with increased risk of GDM (4,14,17), and first trimester excess gestational weight gain has been linked to increased risk of LGA (50). Finally, there is the need to merge basic science with intergenerational epidemiological studies. Such work is necessary to better understand the pathophysiology related to complications of in utero exposure to diabetes and obesity in order to inform targeted upstream interventions.
Intergenerational epidemiological studies and the pragmatic randomized controlled intervention trials they may inform are both very time consuming and intensive. To increase our knowledge of how to break the vicious cycle of diabetes and obesity, there is the need to mentor the new generation of scientists who will keep working on the underlying etiologies of the vicious cycle, to develop targeted interventions and evaluate the effectiveness of programs for women with diabetes and obesity in pregnancy at the population level, and finally to advocate for continued scientific investment in the translation and implementation of programs to improve the health of women and their children. In conclusion, the epidemiological evidence tells us that upstream prevention of obesity and diabetes during the preconception period, pregnancy, and postpartum are desperately needed. Prevention strategies need to be feasible and scalable in health care delivery systems in order to break the ongoing vicious cycle of diabetes and obesity.
Article Information
Funding. Funding for this work came from the National Institute of Diabetes and Digestive and Kidney Diseases (R01DK54834, R18DK067334, P30DK092924), Eunice Kennedy Shriver National Institute of Child Health and Human Development (R01HD073572), National Institute of Environmental Health Sciences (R01ES019196), National Institutes of Health Office of the Director (UG3/UH3OD023289), Centers for Disease Control and Prevention (200-95-0953-42, U48/CCU916373, U18DP006289), Agency for Healthcare Research and Quality (HS019367), American Diabetes Association, and Garfield Foundation.
Duality of Interest. No potential conflicts of interest relevant to this article were reported.
Footnotes
The 2019 Norbert Freinkel Award Lecture was presented at the American Diabetes Association’s 79th Scientific Sessions, San Francisco, CA, 9 June 2019.
References
- 1.Freinkel N. Banting lecture 1980. Of pregnancy and progeny. Diabetes 1980;29:1023–1035 [DOI] [PubMed] [Google Scholar]
- 2.Aerts L, Van Assche FA. Is gestational diabetes an acquired condition? J Dev Physiol 1979;1:219–225 [PubMed] [Google Scholar]
- 3.Pettitt DJ, Knowler WC. Diabetes and obesity in the Pima Indians: a cross-generational vicious cycle. J Obes Weight Regulation 1988;7:61–75 [Google Scholar]
- 4.Ehrlich SF, Hedderson MM, Feng J, Davenport ER, Gunderson EP, Ferrara A. Change in body mass index between pregnancies and the risk of gestational diabetes in a second pregnancy. Obstet Gynecol 2011;117:1323–1330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bellamy L, Casas JP, Hingorani AD, Williams D. Type 2 diabetes mellitus after gestational diabetes: a systematic review and meta-analysis. Lancet 2009;373:1773–1779 [DOI] [PubMed] [Google Scholar]
- 6.Ferrara A, Weiss NS, Hedderson MM, et al. . Pregnancy plasma glucose levels exceeding the American Diabetes Association thresholds, but below the National Diabetes Data Group thresholds for gestational diabetes mellitus, are related to the risk of neonatal macrosomia, hypoglycaemia and hyperbilirubinaemia. Diabetologia 2007;50:298–306 [DOI] [PubMed] [Google Scholar]
- 7.Metzger BE, Lowe LP, Dyer AR, et al.; HAPO Study Cooperative Research Group . Hyperglycemia and adverse pregnancy outcomes. N Engl J Med 2008;358:1991–2002 [DOI] [PubMed] [Google Scholar]
- 8.Hillier TA, Pedula KL, Schmidt MM, Mullen JA, Charles MA, Pettitt DJ. Childhood obesity and metabolic imprinting: the ongoing effects of maternal hyperglycemia. Diabetes Care 2007;30:2287–2292 [DOI] [PubMed] [Google Scholar]
- 9.Kubo A, Ferrara A, Laurent CA, et al. . Associations between maternal pregravid obesity and gestational diabetes and the timing of pubarche in daughters. Am J Epidemiol 2016;184:7–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lowe WL Jr, Scholtens DM, Lowe LP, et al.; HAPO Follow-up Study Cooperative Research Group . Association of gestational diabetes with maternal disorders of glucose metabolism and childhood adiposity. JAMA 2018;320:1005–1016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Grunnet LG, Hansen S, Hjort L, et al. . Adiposity, dysmetabolic traits, and earlier onset of female puberty in adolescent offspring of women with gestational diabetes mellitus: a clinical study within the Danish National Birth Cohort. Diabetes Care 2017;40:1746–1755 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ehrlich SF, Hedderson MM, Xu F, Ferrara A. Diagnostic thresholds for pregnancy hyperglycemia, maternal weight status and the risk of childhood obesity in a diverse Northern California cohort using health care delivery system data. PLoS One 2019;14:e0216897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dabelea D, Hanson RL, Lindsay RS, et al. . Intrauterine exposure to diabetes conveys risks for type 2 diabetes and obesity: a study of discordant sibships. Diabetes 2000;49:2208–2211 [DOI] [PubMed] [Google Scholar]
- 14.Hedderson MM, Williams MA, Holt VL, Weiss NS, Ferrara A. Body mass index and weight gain prior to pregnancy and risk of gestational diabetes mellitus. Am J Obstet Gynecol 2008;198:409.e1–409.e7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hedderson MM, Darbinian JA, Quesenberry CP, Ferrara A. Pregravid cardiometabolic risk profile and risk for gestational diabetes mellitus. Am J Obstet Gynecol 2011;205:55.e1–55.e7 [DOI] [PubMed] [Google Scholar]
- 16.Sridhar SB, Ferrara A, Ehrlich SF, Brown SD, Hedderson MM. Risk of large-for-gestational-age newborns in women with gestational diabetes by race and ethnicity and body mass index categories. Obstet Gynecol 2013;121:1255–1262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hedderson MM, Gunderson EP, Ferrara A. Gestational weight gain and risk of gestational diabetes mellitus. Obstet Gynecol 2010;115:597–604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Goldstein RF, Abell SK, Ranasinha S, et al. . Association of gestational weight gain with maternal and infant outcomes: a systematic review and meta-analysis. JAMA 2017;317:2207–2225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.O’Sullivan JB, Mahan CM. Criteria for the oral glucose tolerance test in pregnancy. Diabetes 1964;13:278–285 [PubMed] [Google Scholar]
- 20.National Diabetes Data Group Classification and diagnosis of diabetes mellitus and other categories of glucose intolerance. Diabetes 1979;28:1039–1057 [DOI] [PubMed] [Google Scholar]
- 21.Carpenter MW, Coustan DR. Criteria for screening tests for gestational diabetes. Am J Obstet Gynecol 1982;144:768–773 [DOI] [PubMed] [Google Scholar]
- 22.Magee MS, Walden CE, Benedetti TJ, Knopp RH. Influence of diagnostic criteria on the incidence of gestational diabetes and perinatal morbidity. JAMA 1993;269:609–615 [PubMed] [Google Scholar]
- 23.Metzger BE, Gabbe SG, Persson B, et al.; International Association of Diabetes and Pregnancy Study Groups Consensus Panel . International association of diabetes and pregnancy study groups recommendations on the diagnosis and classification of hyperglycemia in pregnancy. Diabetes Care 2010;33:676–682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McIntyre HD, Jensen DM, Jensen RC, et al. . Gestational diabetes mellitus: does one size fit all? A challenge to uniform worldwide diagnostic thresholds. Diabetes Care 2018;41:1339–1342 [DOI] [PubMed] [Google Scholar]
- 25.Ehrlich SF, Crites YM, Hedderson MM, Darbinian JA, Ferrara A. The risk of large for gestational age across increasing categories of pregnancy glycemia. Am J Obstet Gynecol 2011;204:240.e1–240.e6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ferrara A, Kahn HS, Quesenberry CP, Riley C, Hedderson MM. An increase in the incidence of gestational diabetes mellitus: Northern California, 1991–2000. Obstet Gynecol 2004;103:526–533 [DOI] [PubMed] [Google Scholar]
- 27.Hedderson M, Ehrlich S, Sridhar S, Darbinian J, Moore S, Ferrara A. Racial/ethnic disparities in the prevalence of gestational diabetes mellitus by BMI. Diabetes Care 2012;35:1492–1498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhu Y, Greenberg M, Hedderson M, Feng J, Ngo A, Ferrara A. Racial/ethnic disparities in glycemic control among women with gestational diabetes: a multiracial pregnancy cohort study (abstract). Am J Obstet Gynecol 2020;222:S578–S579 [Google Scholar]
- 29.Brown SD, Ehrlich SF, Kubo A, et al. . Lifestyle behaviors and ethnic identity among diverse women at high risk for type 2 diabetes. Soc Sci Med 2016;160:87–93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ehrlich SF, Hedderson MM, Brown SD, et al. . Moderate intensity sports and exercise is associated with glycaemic control in women with gestational diabetes. Diabetes Metab 2017;43:416–423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gadgil MD, Ehrlich SF, Zhu Y, et al. . Dietary quality and glycemic control among women with gestational diabetes mellitus. J Womens Health (Larchmt) 2019;28:178–184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Peng TY, Ehrlich SF, Crites Y, et al. . Trends and racial and ethnic disparities in the prevalence of pregestational type 1 and type 2 diabetes in Northern California: 1996-2014. Am J Obstet Gynecol 2017;216:177.e1–177.e8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Crowther CA, Hiller JE, Moss JR, McPhee AJ, Jeffries WS, Robinson JS; Australian Carbohydrate Intolerance Study in Pregnant Women (ACHOIS) Trial Group . Effect of treatment of gestational diabetes mellitus on pregnancy outcomes. N Engl J Med 2005;352:2477–2486 [DOI] [PubMed] [Google Scholar]
- 34.Landon MB, Spong CY, Thom E, et al.; Eunice Kennedy Shriver National Institute of Child Health and Human Development Maternal-Fetal Medicine Units Network . A multicenter, randomized trial of treatment for mild gestational diabetes. N Engl J Med 2009;361:1339–1348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ferrara A, Hedderson MM, Ching J, Kim C, Peng T, Crites YM. Referral to telephonic nurse management improves outcomes in women with gestational diabetes. Am J Obstet Gynecol 2012;206:491.e1–491.e5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Brown SD, Fotuhi O, Grijalva CS, et al. . A randomized study of values affirmation to promote interest in diabetes prevention among women with a history of gestational diabetes. Med Care 2019;57:528–535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ratner RE, Christophi CA, Metzger BE, et al.; Diabetes Prevention Program Research Group . Prevention of diabetes in women with a history of gestational diabetes: effects of metformin and lifestyle interventions. J Clin Endocrinol Metab 2008;93:4774–4779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Keppel KG, Taffel SM. Pregnancy-related weight gain and retention: implications of the 1990 Institute of Medicine guidelines. Am J Public Health 1993;83:1100–1103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Peters RK, Kjos SL, Xiang A, Buchanan TA. Long-term diabetogenic effect of single pregnancy in women with previous gestational diabetes mellitus. Lancet 1996;347:227–230 [DOI] [PubMed] [Google Scholar]
- 40.Ferrara A, Hedderson MM, Albright CL, et al. . A pregnancy and postpartum lifestyle intervention in women with gestational diabetes mellitus reduces diabetes risk factors: a feasibility randomized control trial. Diabetes Care 2011;34:1519–1525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Institute of Medicine and National Research Council Weight Gain During Pregnancy: Reexamining the Guidelines. Washington, D.C., The National Academies Press, 2009 [PubMed] [Google Scholar]
- 42.Ehrlich SF, Hedderson MM, Quesenberry CP Jr, et al. . Post-partum weight loss and glucose metabolism in women with gestational diabetes: the DEBI Study. Diabet Med 2014;31:862–867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ferrara A, Hedderson MM, Albright CL, et al. . A pragmatic cluster randomized clinical trial of diabetes prevention strategies for women with gestational diabetes: design and rationale of the Gestational Diabetes’ Effects on Moms (GEM) study. BMC Pregnancy Childbirth 2014;14:21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ferrara A, Hedderson MM, Brown SD, et al. . The comparative effectiveness of diabetes prevention strategies to reduce postpartum weight retention in women with gestational diabetes mellitus: the Gestational Diabetes’ Effects on Moms (GEM) cluster randomized controlled trial. Diabetes Care 2016;39:65–74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hedderson MM, Brown SD, Ehrlich SF, et al. . A tailored letter based on electronic health record data improves gestational weight gain among women with gestational diabetes mellitus: the Gestational Diabetes’ Effects on Moms (GEM) cluster-randomized controlled trial. Diabetes Care 2018;41:1370–1377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.International Weight Management in Pregnancy (i-WIP) Collaborative Group Effect of diet and physical activity based interventions in pregnancy on gestational weight gain and pregnancy outcomes: meta-analysis of individual participant data from randomised trials. BMJ 2017;358:j3119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Peaceman AM, Clifton RG, Phelan S, et al.; LIFE‐Moms Research Group . Lifestyle interventions limit gestational weight gain in women with overweight or obesity: LIFE-Moms prospective meta-analysis. Obesity (Silver Spring) 2018;26:1396–1404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Brown SD, Hedderson MM, Ehrlich SF, et al. . Gestational weight gain and optimal wellness (GLOW): rationale and methods for a randomized controlled trial of a lifestyle intervention among pregnant women with overweight or obesity. BMC Pregnancy Childbirth 2019;19:145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ferrara A, Hedderson MM, Brown SD, et al. . A telehealth lifestyle intervention to reduce excess gestational weight gain in pregnant women with overweight or obesity (GLOW): a randomised, parallel-group, controlled trial. Lancet Diabetes Endocrinol 2020;8:490–500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Broskey NT, Wang P, Li N, et al. . Early pregnancy weight gain exerts the strongest effect on birth weight, posing a critical time to prevent childhood obesity. Obesity (Silver Spring) 2017;25:1569–1576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zhu Y, Hedderson MM, Sridhar S, Xu F, Feng J, Ferrara A. Poor diet quality in pregnancy is associated with increased risk of excess fetal growth: a prospective multi-racial/ethnic cohort study. Int J Epidemiol 2019;48:423–432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tylavsky FA, Ferrara A, Catellier DJ, et al. . Understanding childhood obesity in the US: the NIH environmental influences on child health outcomes (ECHO) program. Int J Obes 2020;44:617–627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gillman MW, Oakey H, Baghurst PA, Volkmer RE, Robinson JS, Crowther CA. Effect of treatment of gestational diabetes mellitus on obesity in the next generation. Diabetes Care 2010;33:964–968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Landon MB, Rice MM, Varner MW, et al.; Eunice Kennedy Shriver National Institute of Child Health and Human Development Maternal-Fetal Medicine Units (MFMU) Network . Mild gestational diabetes mellitus and long-term child health. Diabetes Care 2015;38:445–452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Koivusalo SB, Rönö K, Klemetti MM, et al. . Gestational diabetes mellitus can be prevented by lifestyle intervention: the Finnish Gestational Diabetes Prevention Study (RADIEL): a randomized controlled trial. Diabetes Care 2016;39:24–30 [DOI] [PubMed] [Google Scholar]
- 56.Poston L, Bell R, Croker H, et al.; UPBEAT Trial Consortium . Effect of a behavioural intervention in obese pregnant women (the UPBEAT study): a multicentre, randomised controlled trial. Lancet Diabetes Endocrinol 2015;3:767–777 [DOI] [PubMed] [Google Scholar]
- 57.Simmons D, Devlieger R, van Assche A, et al. . Effect of physical activity and/or healthy eating on GDM risk: the DALI lifestyle study. J Clin Endocrinol Metab 2017;102:903–913 [DOI] [PMC free article] [PubMed] [Google Scholar]