Abstract
OBJECTIVE
To examine whether proinflammatory and hyperinsulinemic diets are associated with increased risk of type 2 diabetes.
RESEARCH DESIGN AND METHODS
We prospectively followed 74,767 women from the Nurses’ Health Study (1984–2016), 90,786 women from the Nurses’ Health Study II (1989–2017), and 39,442 men from the Health Professionals Follow-up Study (1986–2016). Using repeated measures of food-frequency questionnaires, we calculated empirical dietary inflammatory pattern (EDIP) and empirical dietary index for hyperinsulinemia (EDIH) scores, which are food-based indices that characterize dietary inflammatory or insulinemic potential based on circulating biomarkers of inflammation or C-peptide. Diagnoses of type 2 diabetes were confirmed by validated supplementary questionnaires.
RESULTS
We documented 19,666 incident type 2 diabetes cases over 4.9 million person-years of follow-up. In the pooled multivariable-adjusted analyses, individuals in the highest EDIP or EDIH quintile had 3.11 times (95% CI 2.96–3.27) and 3.40 times (95% CI 3.23–3.58) higher type 2 diabetes risk, respectively, compared with those in the lowest quintile. Additional adjustment for BMI attenuated the associations (hazard ratio 1.95 [95% CI 1.85–2.05] for EDIP and hazard ratio 1.87 [95% CI 1.78–1.98] for EDIH), suggesting adiposity partly mediates the observed associations. Moreover, individuals in both highest EDIP and EDIH quintiles had 2.34 times higher type 2 diabetes risk (95% CI 2.17–2.52), compared with those in both lowest quintiles, after adjustment for BMI.
CONCLUSIONS
Higher dietary inflammatory and insulinemic potential were associated with increased type 2 diabetes incidence. Findings suggest that inflammation and hyperinsulinemia are potential mechanisms linking dietary patterns and type 2 diabetes development.
Introduction
Type 2 diabetes is a major cause of morbidity, and the Centers for Disease Control and Prevention ranked diabetes as the seventh leading cause of death in the U.S. in 2017 (1). The estimated prevalence of type 2 diabetes was 12–14% among U.S. adults in 2011–2012 (2), and it is projected to increase to 25–28% by 2050 (3). Type 2 diabetes is characterized by insulin resistance, impaired insulin secretion, and hyperglycemia (4). Moreover, evidence suggests that inflammation plays an important role in the pathogenesis of insulin resistance and type 2 diabetes (5). It is well documented that major risk factors of type 2 diabetes such as obesity and physical inactivity upregulate inflammation and insulin resistance, which consequently cause hyperinsulinemia (6,7).
Diets modulating these biological pathways of inflammation and insulin response may influence type 2 diabetes incidence. Epidemiologic studies have found healthy dietary patterns indicated by the Alternate Healthy Eating Index (AHEI), alternate Mediterranean Diet (aMED), and Dietary Approaches to Stop Hypertension (DASH) to be inversely associated with risk of type 2 diabetes (8). Also, greater adherence to the prudent pattern and lower adherence to the Western pattern were associated with reduced risk of type 2 diabetes (8), and higher glycemic index (GI) and glycemic load (GL) have been found to be associated with higher risk of developing type 2 diabetes (9). Although these dietary patterns are reported to be associated with inflammation and insulin response (10–12), they may not comprehensively capture one’s dietary inflammatory and insulinemic potential, which are important mediators linking diet and type 2 diabetes. Recently, we developed and validated an empirical food-based dietary inflammatory pattern (13) and an empirical food-based dietary index for hyperinsulinemia (14) to assess long-term inflammatory and insulinemic potential of usual diets. These empirical dietary indices showed low-to-moderate correlations with conventional dietary pattern scores (AHEI, aMED, and DASH) (r = −0.09 to −0.45) (15). In this study, we investigated the association of dietary inflammatory and insulinemic potential with incidence of type 2 diabetes in three large prospective cohort studies. We also examined whether the association differs by other major risk factors of type 2 diabetes including BMI.
Research Design and Methods
Study Population
The Nurses’ Health Study (NHS) cohort was established in 1976 with 121,701 female nurses aged 30–55 years (16), the Nurses’ Health Study II (NHSII) cohort was established in 1989 with 116,430 female nurses aged 25–42 years (17), and the Health Professionals Follow-up Study (HPFS) cohort was established in 1986 with 51,529 male health professionals aged 40–75 years (18). In these three cohorts, participants completed questionnaires on medical history and lifestyle at enrollment and every 2 years thereafter. The response rate of each follow-up questionnaire cycle exceeded >90% for all cohorts.
In the current study, we excluded participants who had diabetes, cardiovascular disease, or cancer (except nonmelanoma skin cancer) or who had incomplete dietary information or implausible energy intake (<600 or >3,500 kcal/day for women and <800 or >4,200 kcal/day for men) at baseline. The final sample included 74,767 women from the NHS, 90,786 women from the NHSII, and 39,442 men from the HPFS. The study protocol was approved by the institutional review boards of the Brigham and Women’s Hospital and Harvard T.H. Chan School of Public Health.
Dietary Assessment
Diet was assessed using a validated semiquantitative food-frequency questionnaire (FFQ) (∼130 items) every 4 years, starting from 1984 in NHS, 1991 in NHSII, and 1986 in HPFS (19–22). Participants were asked to report how often, on average, they consumed each food item during the previous year, with a standard portion size, using nine response categories ranging from “never or less than once per month” to “six or more times per day.”
Assessment of Empirical Dietary Inflammatory Pattern and Empirical Dietary Index for Hyperinsulinemia
Empirical dietary inflammatory pattern (EDIP) and empirical dietary index for hyperinsulinemia (EDIH) scores were developed and validated to capture overall inflammatory and insulinemic potential, respectively, of whole diets (13,14). Briefly, EDIP was derived based on 39 predefined food groups from FFQs using reduced-rank regression followed by stepwise linear regression models to identify a dietary pattern most predictive of three plasma inflammatory biomarkers including interleukin-6, C-reactive protein (CRP), and tumor necrosis factor (TNF)α receptor 2. Similarly, EDIH was derived based on 39 predefined food groups using stepwise regression models to identify a dietary pattern most predictive of fasting plasma C-peptide, an indicator of insulin secretion. Of note, EDIH was predictive of both fasting and nonfasting C-peptide in a previous study (23). EDIP and EDIH are weighted sums of 18 food groups (9 overlapping), and higher (more positive) scores indicate higher inflammatory or insulinemic potential of diets and lower (more negative) scores indicate lower inflammatory or insulinemic potential of diets (Supplementary Table 1). Detailed distributions of nutrients and food groups are shown in Supplementary Table 2. In independent data sets, EDIP and EDIH were validated using biomarkers of inflammation and insulin response (13,14,24). For each participant, we calculated EDIP and EDIH scores using updated FFQ data in each 4-year cycle. We also calculated dietary insulin index (II) and insulin load (IL) for a secondary analysis. Details of II and IL are provided in the Supplementary Material.
Assessment of Covariates
Detailed information on age, race, height, body weight, smoking, and physical activity was collected at baseline and updated biennially. Other information such as menopausal status, postmenopausal hormone use, oral contraceptive use, and family history of diabetes was collected from the biennial questionnaires.
Ascertainment of Type 2 Diabetes
Self-report of type 2 diabetes was assessed via questionnaires every 2 years. Diagnoses were confirmed using a supplemental questionnaire, which asked about type 2 diabetes–related symptoms, medication use, and diagnostic tests. In accordance with the National Diabetes Data Group (25), confirmation of diabetes required at least one or more of the following criteria: 1) at least one classic symptom (excessive thirst, polyuria, weight loss, or hunger) plus fasting blood glucose ≥140 mg/dL (7.8 mmol/L), or random blood glucose ≥200 mg/dL (11.1 mmol/L); 2) no symptoms but elevated blood glucose on two occasions (fasting blood glucose ≥140 mg/dL [7.8 mmol/L] or random blood glucose ≥200 mg/dL [11.1 mmol/L]), or blood glucose ≥200 mg/dL after 2‐h blood oral glucose tolerance testing); and 3) treatment with hypoglycemic drugs (insulin or oral hypoglycemic agent). The diagnostic criteria for fasting blood glucose was changed to ≥126 mg/dL (7.0 mmol/L) in 1998 (26), and glycated hemoglobin (HbA1c) ≥6.5% was further added in the criteria in 2010 (27). This supplemental questionnaire was validated previously (28,29).
Statistical Analysis
Person-years were calculated from the baseline when EDIP and EDIH scores were first available (1984 for NHS, 1991 for NHSII, and 1986 for HPFS) until type 2 diabetes diagnosis, death, censoring, or the end of follow-up (June 2016 for NHS, June 2017 for NHSII, and January 2016 for HPFS). We used cumulative average of EDIP and EDIH scores calculated from repeated measure of FFQs to capture habitual long-term dietary intake and reduce within-person variation. For example, we calculated the average of 1986 and 1990 scores and then formed quintiles and applied this to 1990–1994 follow-up. Then, we calculated the average of 1986, 1990, and 1994 scores and then formed quintiles and applied this to 1994–1998 follow-up and so on. The scores were adjusted for total energy intake using the residual method (30).
We used Cox proportional hazards regression models to estimate hazard ratios (HRs) and 95% CI of type 2 diabetes associated with quintiles of EDIP and EDIH scores. We also evaluated the dose-response relationship of EDIP and EDIH with risk of type 2 diabetes using restricted cubic splines. All analyses were stratified by age in months and calendar years. Multivariable models additionally included race, smoking, postmenopausal hormone use, oral contraceptive use, physical activity, and family history of diabetes. We ran an additional multivariable model further adjusting for BMI. To examine the independent association of each empirical dietary index, we conducted a model further mutually adjusting for EDIP and EDIH. As a secondary analysis, we examined the association between other insulin-related indices (II and IL) and type 2 diabetes risk. All analyses were done separately by cohorts (sex), and results were pooled after testing for heterogeneity (P > 0.05). Pooled analyses were additionally stratified by cohorts.
To examine whether the association between empirical hypothesis-oriented dietary indices and type 2 diabetes differs by potential effect modifiers, we conducted subgroup analyses by age, BMI, physical activity, smoking status, alcohol intake, and family history of diabetes. The Wald test was used to test for interaction between the empirical dietary indices (continuous) and stratification variables. Lastly, we examined joint associations of EDIP (quintiles) and EDIH (quintiles) and of each empirical hypothesis-oriented index (quintiles) and BMI (five categories) with type 2 diabetes risk. We conducted a sensitivity analysis restricted to symptomatic diabetes cases to address potential bias due to screening. All statistical analyses were performed using SAS 9.4 with two-sided tests, and P value < 0.05 was considered statistically significant.
Results
Participants with higher EDIP or EDIH scores had higher BMI and lower physical activity levels (Table 1). Moreover, women with higher EDIP or EDIH scores were more likely to have family history of diabetes. The Spearman correlation between EDIP and EDIH was 0.65 (Sup‐plementary Table 3).
Table 1.
Age-adjusted baseline characteristics by quintiles of EDIP and EDIH*
| Characteristic | Quintiles of dietary pattern | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| NHS (74,767 women) | NHSII (90,786 women) | HPFS (39,442 men) | |||||||
| Q1 | Q3 | Q5 | Q1 | Q3 | Q5 | Q1 | Q3 | Q5 | |
| EDIP | |||||||||
| Age, years | 50.5 (6.9) | 50.6 (7.3) | 49.3 (7.3) | 36.6 (4.5) | 36.1 (4.7) | 35.7 (4.7) | 52.0 (8.8) | 53.3 (9.6) | 52.8 (9.7) |
| BMI, kg/m2 | 23.7 (3.7) | 24.7 (4.3) | 26.4 (5.6) | 23.6 (4.5) | 24.3 (4.9) | 26.1 (6.2) | 24.6 (4.8) | 24.7 (4.6) | 25.4 (5.2) |
| Physical activity, MET h/week | 16.3 (24.0) | 14.3 (21.3) | 12.6 (18.8) | 24.4 (31.0) | 19.9 (25.4) | 19.2 (26.7) | 21.1 (26.3) | 18.8 (26.4) | 17.5 (25.6) |
| White race, % | 98.9 | 98.2 | 96.3 | 98.0 | 96.6 | 94.8 | 96.7 | 95.5 | 93.0 |
| Postmenopause, % | 48.5 | 48.7 | 48.8 | 3.0 | 3.6 | 3.9 | — | — | — |
| Postmenopausal hormone use, % | 11.5 | 11.8 | 10.7 | 0.2 | 0.3 | 0.3 | — | — | — |
| Current smoker, % | 29.6 | 22.6 | 21.9 | 10.4 | 11.3 | 15.5 | 10.5 | 8.0 | 8.0 |
| Family history of diabetes, % | 26.0 | 27.8 | 31.5 | 31.5 | 33.5 | 37.7 | 19.9 | 19.2 | 21.7 |
| Total energy, kcal/day | 1,814 (524) | 1,682 (512) | 1,838 (566) | 1,893 (546) | 1,681 (516) | 1,915 (587) | 2,092 (618) | 1,898 (590) | 2,163 (664) |
| EDIH | |||||||||
| Age, years | 51.7 (6.9) | 50.5 (7.1) | 48.5 (7.1) | 36.6 (4.5) | 36.0 (4.7) | 35.6 (4.7) | 54.5 (9.5) | 53.2 (9.4) | 50.7 (9.1) |
| BMI, kg/m2 | 23.6 (3.6) | 24.7 (4.3) | 26.4 (5.6) | 23.0 (4.0) | 24.4 (5.0) | 26.4 (6.3) | 24.3 (4.8) | 24.9 (4.6) | 25.5 (5.4) |
| Physical activity, MET h/week | 17.5 (24.5) | 13.7 (18.5) | 11.5 (16.7) | 28.0 (33.4) | 19.5 (25.3) | 16.9 (23.5) | 24.3 (30.1) | 18.4 (25.0) | 15.9 (23.2) |
| White race, % | 98.2 | 97.8 | 97.8 | 96.7 | 96.4 | 96.3 | 95.6 | 95.0 | 95.3 |
| Postmenopause, % | 48.9 | 48.7 | 48.6 | 2.9 | 3.5 | 3.9 | — | — | — |
| Postmenopausal hormone use, % | 12.0 | 11.1 | 10.8 | 0.2 | 0.3 | 0.4 | — | — | — |
| Current smoker, % | 25.3 | 23.7 | 25.7 | 9.0 | 11.7 | 16.3 | 7.5 | 8.7 | 10.4 |
| Family history of diabetes, % | 25.8 | 28.0 | 30.6 | 30.5 | 34.5 | 37.8 | 20.0 | 19.6 | 21.0 |
| Total energy, kcal/day | 1,837 (534) | 1,641 (502) | 1,885 (552) | 1,927 (541) | 1,672 (517) | 1,921 (580) | 2,153 (631) | 1,877 (589) | 2,140 (646) |
Data are means (SD) or percentages and are standardized to the age distribution of the study population.
Energy-adjusted dietary pattern.
During 4,949,265 person-years of follow-up, we documented 19,666 incident type 2 diabetes cases. Diets with higher inflammatory potential were significantly associated with higher type 2 diabetes incidence in all three cohorts (Table 2). In the pooled multivariable-adjusted analyses, compared with individuals in the lowest EDIP quintile, those in the highest EDIP quintile had 3.11 times higher risk of type 2 diabetes (95% CI 2.96–3.27). Additional adjustment for BMI attenuated the magnitude of the association, but the strong positive association remained statistically significant (HR 1.95 [95% CI 1.85–2.05]). Similarly, diets with higher insulinemic potential were associated with increased risk of type 2 diabetes in all three cohorts (Table 2). In the pooled multivariable-adjusted analyses, compared with individuals in the lowest EDIH quintile, those in the highest EDIH quintile had 3.40 times higher risk of type 2 diabetes (95% CI 3.23–3.58). When we further adjusted for BMI, we observed an attenuated but strong positive association (HR 1.87 [95% CI 1.78–1.98]). In a mutually adjusted model including both EDIP and EDIH, we still observed significant positive associations for both EDIP (HR 1.59 [95% CI 1.49–1.69]) and EDIH (HR 1.42 [95% CI 1.33–1.51]) (Supplementary Table 4). Moreover, additional adjustment for AHEI did not change the results, and the correlations of AHEI were −0.27 for EDIP and −0.47 for EDIH (Supplementary Tables 3 and 4). Dose-response analyses showed that the associations of EDIP and EDIH with type 2 diabetes deviate from linearity with an accelerated increase in risk at higher scores (P for curvature <0.001 for both scores) (Supplementary Fig. 1). Restricting the analyses to symptomatic diabetes cases did not change the results (data not shown).
Table 2.
HR (95% CI) of type 2 diabetes according to quintiles of EDIP and EDIH*
| Quintiles of dietary pattern | Ptrend† | |||||
|---|---|---|---|---|---|---|
| Q1 | Q2 | Q3 | Q4 | Q5 | ||
| EDIP | ||||||
| NHS | ||||||
| Event | 841 | 1,259 | 1,550 | 2,048 | 3,084 | |
| Person-years | 389,038 | 388,762 | 388,337 | 387,678 | 386,909 | |
| Age adjusted | 1 (ref) | 1.50 (1.38–1.64) | 1.85 (1.70–2.02) | 2.47 (2.28–2.67) | 3.74 (3.46–4.04) | <0.001 |
| Multivariable | 1 (ref) | 1.46 (1.33–1.59) | 1.75 (1.61–1.91) | 2.28 (2.10–2.47) | 3.28 (3.04–3.54) | <0.001 |
| Multivariable + BMI | 1 (ref) | 1.28 (1.18–1.40) | 1.41 (1.30–1.53) | 1.69 (1.56–1.83) | 2.05 (1.90–2.22) | <0.001 |
| NHSII | ||||||
| Event | 654 | 965 | 1,245 | 1,716 | 2,577 | |
| Person-years | 423,858 | 424,132 | 424,011 | 423,297 | 422,183 | |
| Age adjusted | 1 (ref) | 1.51 (1.37–1.67) | 1.99 (1.81–2.19) | 2.78 (2.54–3.05) | 4.25 (3.90–4.63) | <0.001 |
| Multivariable | 1 (ref) | 1.42 (1.28–1.57) | 1.79 (1.63–1.97) | 2.38 (2.17–2.60) | 3.36 (3.08–3.67) | <0.001 |
| Multivariable + BMI | 1 (ref) | 1.27 (1.15–1.40) | 1.42 (1.29–1.56) | 1.62 (1.48–1.78) | 1.87 (1.72–2.04) | <0.001 |
| HPFS | ||||||
| Event | 466 | 613 | 685 | 842 | 1,121 | |
| Person-years | 178,762 | 178,512 | 178,307 | 177,778 | 177,702 | |
| Age adjusted | 1 (ref) | 1.31 (1.16–1.48) | 1.46 (1.30–1.64) | 1.80 (1.61–2.02) | 2.43 (2.18–2.71) | <0.001 |
| Multivariable | 1 (ref) | 1.31 (1.16–1.47) | 1.45 (1.28–1.63) | 1.77 (1.58–1.98) | 2.31 (2.07–2.58) | <0.001 |
| Multivariable + BMI | 1 (ref) | 1.27 (1.13–1.44) | 1.39 (1.23–1.56) | 1.62 (1.44–1.81) | 1.87 (1.67–2.09) | <0.001 |
| Pooled results of three cohorts | ||||||
| Age adjusted | 1 (ref) | 1.46 (1.38–1.55) | 1.81 (1.71–1.91) | 2.41 (2.29–2.54) | 3.59 (3.42–3.78) | <0.001 |
| Multivariable | 1 (ref) | 1.41 (1.33–1.49) | 1.70 (1.61–1.80) | 2.20 (2.09–2.33) | 3.11 (2.96–3.27) | <0.001 |
| Multivariable + BMI | 1 (ref) | 1.28 (1.20–1.35) | 1.41 (1.33–1.49) | 1.65 (1.57–1.74) | 1.95 (1.85–2.05) | <0.001 |
| EDIH | ||||||
| NHS | ||||||
| Event | 833 | 1,213 | 1,606 | 2,081 | 3,049 | |
| Person-years | 389,160 | 388,839 | 388,530 | 387,730 | 386,465 | |
| Age adjusted | 1 (ref) | 1.47 (1.35–1.61) | 1.97 (1.81–2.14) | 2.60 (2.39–2.81) | 3.91 (3.62–4.22) | <0.001 |
| Multivariable | 1 (ref) | 1.41 (1.29–1.54) | 1.82 (1.68–1.98) | 2.32 (2.14–2.52) | 3.33 (3.08–3.60) | <0.001 |
| Multivariable + BMI | 1 (ref) | 1.18 (1.08–1.29) | 1.38 (1.26–1.50) | 1.57 (1.44–1.70) | 1.93 (1.79–2.09) | <0.001 |
| NHSII | ||||||
| Event | 579 | 890 | 1,226 | 1,751 | 2,711 | |
| Person-years | 424,463 | 424,425 | 424,021 | 423,057 | 421,514 | |
| Age adjusted | 1 (ref) | 1.58 (1.42–1.76) | 2.22 (2.01–2.45) | 3.24 (2.95–3.56) | 5.16 (4.72–5.65) | <0.001 |
| Multivariable | 1 (ref) | 1.43 (1.29–1.59) | 1.89 (1.71–2.08) | 2.59 (2.36–2.85) | 3.85 (3.51–4.22) | <0.001 |
| Multivariable + BMI | 1 (ref) | 1.15 (1.03–1.27) | 1.29 (1.17–1.43) | 1.49 (1.36–1.64) | 1.74 (1.59–1.91) | <0.001 |
| HPFS | ||||||
| Event | 420 | 593 | 750 | 811 | 1,153 | |
| Person-years | 178,373 | 178,417 | 178,365 | 178,293 | 177,613 | |
| Age adjusted | 1 (ref) | 1.43 (1.26–1.62) | 1.83 (1.62–2.06) | 2.00 (1.78–2.25) | 2.97 (2.65–3.32) | <0.001 |
| Multivariable | 1 (ref) | 1.37 (1.21–1.56) | 1.73 (1.53–1.95) | 1.86 (1.65–2.10) | 2.70 (2.41–3.02) | <0.001 |
| Multivariable + BMI | 1 (ref) | 1.28 (1.13–1.45) | 1.48 (1.32–1.67) | 1.52 (1.35–1.72) | 1.94 (1.73–2.17) | <0.001 |
| Pooled results of three cohorts | ||||||
| Age adjusted | 1 (ref) | 1.50 (1.41–1.59) | 2.02 (1.90–2.13) | 2.66 (2.52–2.81) | 4.09 (3.89–4.31) | <0.001 |
| Multivariable | 1 (ref) | 1.41 (1.33–1.50) | 1.83 (1.73–1.94) | 2.32 (2.20–2.45) | 3.40 (3.23–3.58) | <0.001 |
| Multivariable + BMI | 1 (ref) | 1.19 (1.12–1.26) | 1.37 (1.30–1.45) | 1.54 (1.46–1.63) | 1.87 (1.78–1.98) | <0.001 |
Multivariable models adjusted for age (month), race (White or non-White), smoking (never, past, or current: 1–14, 15–24, or >24 cigarettes/day), postmenopausal hormone use (women only) (premenopausal, postmenopausal current user, or postmenopausal never/past user), oral contraceptive use (women only) (never, past, or current), physical activity (<3, 3–8.9, 9–17.9, 18–26.9, or ≥27 MET h/week), and family history of diabetes (yes or no). Multivariable + BMI models additionally adjusted for BMI (<21, 21–22.9, 23–24.9, 25–26.9, 27–29.9, 30–34.9, 35–39.9, or ≥40 kg/m2). ref, reference.
Energy-adjusted dietary pattern.
Ptrend was calculated using continuous variables of dietary pattern in the model.
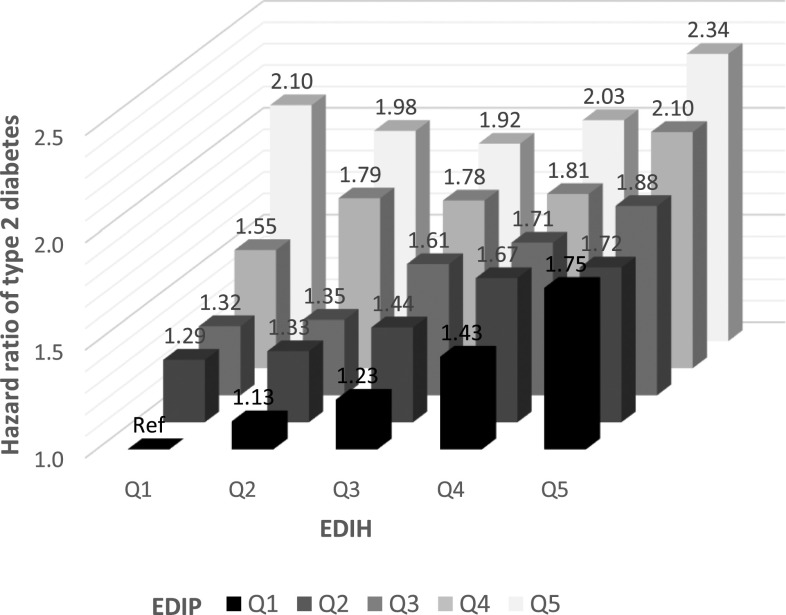
Stratified analyses showed significant positive associations of EDIP and EDIH with type 2 diabetes incidence, regardless of the predefined subgroups (Table 3). However, we observed a stronger positive association between EDIP and type 2 diabetes risk among younger, leaner, or more active adults or those without family history of diabetes (Pinteraction <0.05 for all). Moreover, the positive association between EDIH and type 2 diabetes risk was stronger among younger, leaner, or more active adults, never smokers, or moderate drinkers (Pinteraction <0.05 for all). In the joint analysis of EDIP and EDIH, individuals in both highest EDIP and EDIH quintiles had 4.62 times (95% CI 4.29–4.97) increased risk of type 2 diabetes, compared with those in both lowest quintiles (Supplementary Fig. 2). Additional adjustment for BMI attenuated the association, which remained significant (HR 2.34 [95% CI 2.17–2.52]) (Fig. 1). When we examined the joint association of each empirical hypothesis-oriented dietary index and BMI with type 2 diabetes risk, obese individuals in the highest EDIP or EDIH quintile had 21.8–42.6 times higher risk of type 2 diabetes compared with lean individuals in the lowest quintile (Supplementary Figs. 3 and 4).
Table 3.
HR (95% CI) of type 2 diabetes according to quintiles of EDIP and EDIH by subgroups (pooled results of three cohorts)*
| Quintiles of dietary pattern | Pinteraction† | |||||
|---|---|---|---|---|---|---|
| Q1 | Q2 | Q3 | Q4 | Q5 | ||
| EDIP | ||||||
| Age, years | ||||||
| <60 | 1 (ref) | 1.29 (1.18–1.40) | 1.42 (1.31–1.54) | 1.70 (1.57–1.83) | 2.07 (1.92–2.23) | <0.001 |
| ≥60 | 1 (ref) | 1.27 (1.17–1.37) | 1.41 (1.30–1.52) | 1.62 (1.50–1.74) | 1.83 (1.71–1.97) | |
| BMI, kg/m2 | ||||||
| <25 | 1 (ref) | 1.37 (1.19–1.57) | 1.42 (1.24–1.64) | 1.85 (1.61–2.12) | 2.39 (2.09–2.73) | <0.001 |
| 25–29.9 | 1 (ref) | 1.33 (1.21–1.47) | 1.47 (1.33–1.62) | 1.84 (1.68–2.02) | 2.25 (2.05–2.46) | |
| ≥30 | 1 (ref) | 1.19 (1.09–1.29) | 1.33 (1.23–1.44) | 1.45 (1.35–1.57) | 1.68 (1.56–1.80) | |
| Physical activity, MET h/week | ||||||
| <25 | 1 (ref) | 1.25 (1.17–1.33) | 1.39 (1.31–1.48) | 1.62 (1.53–1.72) | 1.90 (1.79–2.02) | 0.003 |
| ≥25 | 1 (ref) | 1.37 (1.21–1.55) | 1.46 (1.30–1.64) | 1.75 (1.56–1.97) | 2.12 (1.90–2.37) | |
| Smoking | ||||||
| Never | 1 (ref) | 1.25 (1.14–1.36) | 1.42 (1.31–1.55) | 1.62 (1.49–1.76) | 1.94 (1.79–2.10) | 0.21 |
| Past | 1 (ref) | 1.27 (1.17–1.38) | 1.39 (1.28–1.51) | 1.69 (1.56–1.83) | 1.96 (1.82–2.12) | |
| Current | 1 (ref) | 1.46 (1.23–1.74) | 1.41 (1.19–1.68) | 1.76 (1.50–2.08) | 1.99 (1.70–2.32) | |
| Alcohol intake | ||||||
| None | 1 (ref) | 1.32 (1.14–1.52) | 1.34 (1.17–1.54) | 1.64 (1.44–1.86) | 1.90 (1.67–2.15) | 0.05 |
| <1 drink per day | 1 (ref) | 1.23 (1.13–1.33) | 1.35 (1.25–1.46) | 1.54 (1.43–1.66) | 1.83 (1.70–1.98) | |
| ≥1 drink per day | 1 (ref) | 1.16 (1.04–1.30) | 1.39 (1.24–1.56) | 1.60 (1.42–1.80) | 1.62 (1.43–1.82) | |
| Family history of diabetes | ||||||
| No | 1 (ref) | 1.34 (1.24–1.45) | 1.43 (1.32–1.55) | 1.72 (1.60–1.85) | 2.03 (1.89–2.18) | 0.007 |
| Yes | 1 (ref) | 1.20 (1.10–1.30) | 1.38 (1.28–1.50) | 1.58 (1.46–1.70) | 1.85 (1.72–1.99) | |
| EDIH | ||||||
| Age, years | ||||||
| <60 | 1 (ref) | 1.19 (1.08–1.30) | 1.32 (1.21–1.45) | 1.52 (1.40–1.65) | 1.94 (1.79–2.11) | <0.001 |
| ≥60 | 1 (ref) | 1.20 (1.11–1.30) | 1.43 (1.33–1.55) | 1.59 (1.48–1.71) | 1.79 (1.67–1.93) | |
| BMI, kg/m2 | ||||||
| <25 | 1 (ref) | 1.27 (1.11–1.45) | 1.41 (1.23–1.61) | 1.79 (1.57–2.04) | 2.18 (1.92–2.49) | 0.007 |
| 25–29.9 | 1 (ref) | 1.17 (1.06–1.30) | 1.46 (1.33–1.60) | 1.62 (1.47–1.77) | 2.02 (1.84–2.21) | |
| ≥30 | 1 (ref) | 1.14 (1.04–1.25) | 1.26 (1.16–1.37) | 1.39 (1.28–1.51) | 1.67 (1.55–1.81) | |
| Physical activity, MET h/week | ||||||
| <25 | 1 (ref) | 1.14 (1.06–1.22) | 1.33 (1.25–1.42) | 1.47 (1.38–1.56) | 1.79 (1.69–1.90) | 0.007 |
| ≥25 | 1 (ref) | 1.35 (1.20–1.52) | 1.47 (1.30–1.65) | 1.79 (1.59–2.00) | 2.13 (1.91–2.39) | |
| Smoking | ||||||
| Never | 1 (ref) | 1.24 (1.13–1.36) | 1.38 (1.26–1.50) | 1.57 (1.45–1.71) | 1.95 (1.80–2.12) | 0.006 |
| Past | 1 (ref) | 1.16 (1.06–1.27) | 1.38 (1.27–1.50) | 1.57 (1.45–1.70) | 1.87 (1.73–2.03) | |
| Current | 1 (ref) | 1.13 (0.93–1.38) | 1.41 (1.18–1.70) | 1.40 (1.17–1.67) | 1.62 (1.37–1.93) | |
| Alcohol intake | ||||||
| None | 1 (ref) | 1.09 (0.95–1.24) | 1.19 (1.05–1.34) | 1.30 (1.16–1.47) | 1.58 (1.41–1.77) | 0.04 |
| <1 drink per day | 1 (ref) | 1.15 (1.06–1.25) | 1.32 (1.22–1.43) | 1.49 (1.38–1.61) | 1.84 (1.70–1.98) | |
| ≥1 drink per day | 1 (ref) | 1.19 (1.05–1.34) | 1.44 (1.28–1.62) | 1.55 (1.37–1.75) | 1.66 (1.48–1.88) | |
| Family history of diabetes | ||||||
| No | 1 (ref) | 1.15 (1.06–1.25) | 1.36 (1.26–1.47) | 1.53 (1.42–1.65) | 1.86 (1.73–2.00) | 0.70 |
| Yes | 1 (ref) | 1.23 (1.13–1.34) | 1.38 (1.27–1.50) | 1.55 (1.43–1.68) | 1.88 (1.74–2.03) | |
All models were adjusted for age (month), race (White or non-White), smoking (never, past, or current: 1–14, 15–24, or >24 cigarettes/day), postmenopausal hormone use (women only) (premenopausal, postmenopausal current user, or postmenopausal never/past user), oral contraceptive use (women only) (never, past, or current), physical activity (<3, 3–8.9, 9–17.9, 18–26.9, or ≥27 MET h/week), family history of diabetes (yes or no), and BMI (<21, 21–22.9, 23–24.9, 25–26.9, 27–29.9, 30–34.9, 35–39.9, or ≥40 kg/m2). ref, reference.
Energy-adjusted dietary pattern.
Pinteraction was calculated using the Wald test by including the interaction term.
Figure 1.
Joint association of EDIP and EDIH with risk of type 2 diabetes. HRs were calculated in Cox proportional hazards models after adjustment for age (month), race (White or non-White), smoking (never, past, or current: 1–14, 15–24, or >24 cigarettes/day), postmenopausal hormone use (women only) (premenopausal, postmenopausal current user, or postmenopausal never/past user), oral contraceptive use (women only) (never, past, or current), physical activity (<3, 3–8.9, 9–17.9, 18–26.9, or ≥27 MET h/week), and family history of diabetes (yes or no) and BMI (<21.0, 21.0–22.9, 23.0–24.9, 25.0–26.9, 27.0–29.9, 30.0–34.9, 35.0–39.9, or ≥40.0 kg/m2).
In a supplementary analysis using II and IL, we observed weak correlations of II and IL with EDIP (0.14 for II and 0.16 for IL) and EDIH (−0.03 for II and −0.02 for IL) (Supplementary Table 3). Higher II or IL was inversely associated with risk of type 2 diabetes (Supplementary Tables 5 and 6). In the pooled multivariable-adjusted analyses including BMI, individuals in the highest II or IL quintile had ∼10% lower risk of type 2 diabetes compared with those in the lowest quintile.
Conclusions
In three large prospective cohorts, higher dietary inflammatory or insulinemic potential was associated with increased incidence of type 2 diabetes. Notably, the strong positive associations remained significant even after adjustment for BMI and mutual adjustment for the dietary indices including the conventional dietary score (i.e., AHEI). Individuals consuming diets with a high inflammatory and insulinemic potential had a 2.3-fold higher risk of developing type 2 diabetes than those whose dietary patterns have a low inflammatory and insulinemic potential, after adjustment for BMI.
A number of studies have examined the association of various dietary patterns with incidence of type 2 diabetes. A recent meta-analysis of prospective cohort studies reported that a priori–defined “healthy” dietary patterns including AHEI, aMED, and DASH were associated with 21%, 13%, and 19% decreased risk of type 2 diabetes, respectively, in comparisons of extreme quantiles (8). Similar reduction of type 2 diabetes risk (25–35%) was shown with restriction to the same study population (i.e., NHS or HPFS) (8). Evidence indicates that the biological mechanisms by which these dietary patterns influence type 2 diabetes are closely related to inflammation and insulin response (10–12). However, studies have not comprehensively assessed dietary inflammatory or insulinemic potential and its relation with type 2 diabetes. The strong positive associations of EDIP and EDIH scores with type 2 diabetes risk remained robust even after adjustment for the conventional dietary pattern score (AHEI), suggesting unique and strong contributions of the empirical dietary indices in predicting type 2 diabetes risk. Previously, one cross-sectional study using a literature-derived nutrient-based index (Dietary Inflammatory Index [DII]) (31) found a positive association between dietary inflammatory potential and type 2 diabetes in 1,174 Mexican adults (32). Given that DII was calculated using both food items and nutrients (also influenced by supplement use), these results are not directly comparable with our findings using EDIP, which is based exclusively on whole diets. In a previous validation study of 11,053 individuals, EDIP showed greater ability to predict inflammatory markers (i.e., CRP, TNFα receptor 2, and adiponectin) than DII (24). Moreover, the cross-sectional design of that study makes it difficult to assess a temporal relationship between diets and type 2 diabetes.
To our knowledge, studies have not directly examined the association of dietary insulinemic potential with incidence of type 2 diabetes. However, previous studies have used GI and GL as contributors to an immediate insulin response by assessing the influence of carbohydrate-containing foods on postprandial blood glucose (33). In a recent meta-analysis, participants in the highest categories of GI and GL had a 19% and a 16% higher risk of type 2 diabetes, respectively, compared with those in the lowest quintiles (9). Nonetheless, GI and GL have limited capacity to account for noncarbohydrate factors that may affect insulin response, and they do not incorporate effects of diet on insulin resistance, which is a major determinant of insulin response. As an extension of GI and GL, II and IL have been developed to directly quantify the postprandial insulin response to foods independent of insulin resistance (34). However, both GI and II did not predict fasting C-peptide, a marker of β-cell secretory activity (35,36). Moreover, II and IL did not predict risk of colorectal and pancreatic cancers, which are strongly related with hyperinsulinemia and insulin resistance (37,38). Similarly, in our supplementary analyses, II and IL were associated not positively but inversely with risk of type 2 diabetes—a finding that aligns with previous studies showing that II may be inversely associated with long-term insulin exposure (14,23,35), suggesting that the capacity of foods to induce postprandial insulin secretion may have a role in reducing blood glucose and thus risk of type 2 diabetes.
Interestingly, although EDIP and EDIH were correlated, EDIP and EDIH were independently and jointly associated with type 2 diabetes risk, suggesting two distinct biological mechanisms linking diets and type 2 diabetes. Individuals consuming diets with both higher inflammatory and insulinemic potential had the highest risk of type 2 diabetes. A dietary pattern with higher inflammatory and insulinemic potential has higher intakes of red meat, processed meat, nondark (nonfatty) fish, sugar-sweetened (high-energy) beverages, and refined grains and low intakes of wine, leafy green vegetables, and coffee. Overall, our findings show that EDIP and EDIH may be more comprehensive dietary indices that better capture the biologically relevant aspects of diets for type 2 diabetes development.
Diet and adiposity have a complex relationship (39). The effect of diet can be mediated through adiposity. A previous study has shown that both EDIP and EDIH were associated with substantial long-term weight gain (40); thus, the models adjusting for BMI may highlight the adiposity-mediated influence of EDIP and EDIH on type 2 diabetes risk. As expected, we observed largely attenuated associations when we adjusted for BMI (−37% for EDIP and −45% for EDIH), but the strong positive associations remained. Our study shows that although the association of EDIP and EDIH with type 2 diabetes risk may be partly mediated through adiposity, these empirical hypothesis-oriented dietary indices have direct influences on type 2 diabetes, independent of adiposity. In addition, diet may interact with adiposity. Our findings from the joint analysis provide evidence that the combined influence of the dietary index and adiposity can be substantial. Obesity is a major risk factor for type 2 diabetes that is strongly linked to inflammation and insulin resistance. Thus, proinflammatory and/or hyperinsulinemic diets may exacerbate inflammatory and insulin-related pathological pathways, especially among obese individuals who may already be in a condition of increased inflammation and insulin insensitivity, thus leading to higher risk of developing type 2 diabetes.
Additionally, we observed significant interactions between the empirical hypothesis-oriented dietary indices and major risk factors of type 2 diabetes. EDIP showed a stronger positive association with type 2 diabetes risk in younger, leaner, or active adults or those without family history of diabetes. EDIH also showed a stronger positive association in younger or active adults or never smokers. Although the reasons for these significant interactions are not clear, these findings suggest that dietary inflammatory or insulinemic potential may be a stronger predictor of type 2 diabetes in lower risk groups. More research is needed to replicate our findings in diverse racial and ethnic groups. In addition, future studies incorporating genotype for various type 2 diabetes–associated single nucleotide polymorphisms could provide important insights to understand the interaction between individual genetic variants and proinflammatory/hyperinsulinemic diets in relation to type 2 diabetes. Also, integration of these dietary indices with new data including biomarkers and metabolomics can provide opportunity to comprehensively elucidate the underlying biological mechanisms linking dietary patterns and type 2 diabetes development.
Our study has considerable strengths. First, we used validated food-based EDIP and EDIH scores that are strongly correlated with systemic inflammatory biomarkers and C-peptide, respectively, which are related to risk of type 2 diabetes. Second, we had a large sample size with >20 years of follow-up. The large number of type 2 diabetes cases allowed us to obtain precise estimates for stratified and joint analyses. Third, we had detailed prospectively collected information on diets and covariates, which minimizes potential confounding and recall bias. Fourth, repeated measures of diets allowed us to measure long-term effect of diets as well as reduce measurement error due to within-person variation. There are several limitations as well. Self-report diet and other covariates from questionnaires may have measurement errors. However, previous validation studies have shown reasonably good correlation between FFQ and diet records. Moreover, although we thoroughly adjusted for important potential confounding factors in our analyses, we cannot completely rule out the potential for residual confounding by unmeasured variables.
In conclusion, we found that higher dietary inflammatory or insulinemic potential was strongly associated with an increased incidence of type 2 diabetes in U.S. adults. Our study provides strong evidence that inflammation and hyperinsulinemia may mediate the association between diets and type 2 diabetes development. Dietary guidelines and interventions highlighting the importance of reducing or avoiding inflammatory and insulinemic dietary patterns may have great potential for the primary prevention of type 2 diabetes.
Article Information
Funding. This work was supported by the National Institutes of Health (UM1 CA167552, UM1 CA186107, UM1 CA176726, K99 DK122128-01, and R00 CA207736) and the Boston Nutrition Obesity Research Center grant (2P30DK046200-26).
The funders had no role in the design or conduct of the study; collection, management, analysis, or interpretation of the data; preparation, review, or approval of the manuscript; or decision to submit the manuscript for publication.
Duality of Interest. No potential conflicts of interest relevant to this article were reported.
Author Contributions. D.H.L., F.K.T., and E.L.G. designed the research. D.H.L. and J.L. conducted analyses. Y.L., G.L., K.W., S.B., F.B.H., F.K.T., and E.L.G. provided statistical expertise. D.H.L. wrote the first draft of the manuscript. E.B.R., K.M.R., J.E.M., W.C.W., F.B.H., and E.L.G. obtained funding. All authors contributed to the interpretation of the results and critical revision of the manuscript for important intellectual content. All authors approved the final version of the manuscript. D.H.L. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Footnotes
This article contains supplementary material online at https://doi.org/10.2337/figshare.12739601.
D.H.L. and J.L. contributed equally as co–first authors.
F.K.T. and E.L.G. contributed equally as co–last authors.
References
- 1.Heron M. Deaths: leading causes for 2017. Natl Vital Stat Rep 2019;68:1–77 [PubMed] [Google Scholar]
- 2.Menke A, Casagrande S, Geiss L, Cowie CC. Prevalence of and trends in diabetes among adults in the United States, 1988-2012. JAMA 2015;314:1021–1029 [DOI] [PubMed] [Google Scholar]
- 3.Boyle JP, Thompson TJ, Gregg EW, Barker LE, Williamson DF. Projection of the year 2050 burden of diabetes in the US adult population: dynamic modeling of incidence, mortality, and prediabetes prevalence. Popul Health Metr 2010;8:29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stumvoll M, Goldstein BJ, van Haeften TW. Type 2 diabetes: principles of pathogenesis and therapy. Lancet 2005;365:1333–1346 [DOI] [PubMed] [Google Scholar]
- 5.Donath MY, Shoelson SE. Type 2 diabetes as an inflammatory disease. Nat Rev Immunol 2011;11:98–107 [DOI] [PubMed] [Google Scholar]
- 6.Venables MC, Jeukendrup AE. Physical inactivity and obesity: links with insulin resistance and type 2 diabetes mellitus. Diabetes Metab Res Rev 2009;25(Suppl. 1):S18–S23 [DOI] [PubMed] [Google Scholar]
- 7.Esser N, Legrand-Poels S, Piette J, Scheen AJ, Paquot N. Inflammation as a link between obesity, metabolic syndrome and type 2 diabetes. Diabetes Res Clin Pract 2014;105:141–150 [DOI] [PubMed] [Google Scholar]
- 8.Jannasch F, Kröger J, Schulze MB. Dietary patterns and type 2 diabetes: a systematic literature review and meta-analysis of prospective studies. J Nutr 2017;147:1174–1182 [DOI] [PubMed] [Google Scholar]
- 9.Bhupathiraju SN, Tobias DK, Malik VS, et al. Glycemic index, glycemic load, and risk of type 2 diabetes: results from 3 large US cohorts and an updated meta-analysis. Am J Clin Nutr 2014;100:218–232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fung TT, Chiuve SE, McCullough ML, Rexrode KM, Logroscino G, Hu FB. Adherence to a DASH-style diet and risk of coronary heart disease and stroke in women. Arch Intern Med 2008;168:713–720 [DOI] [PubMed] [Google Scholar]
- 11.Fung TT, McCullough ML, Newby PK, et al. Diet-quality scores and plasma concentrations of markers of inflammation and endothelial dysfunction. Am J Clin Nutr 2005;82:163–173 [DOI] [PubMed] [Google Scholar]
- 12.Fung TT, Rimm EB, Spiegelman D, et al. Association between dietary patterns and plasma biomarkers of obesity and cardiovascular disease risk. Am J Clin Nutr 2001;73:61–67 [DOI] [PubMed] [Google Scholar]
- 13.Tabung FK, Smith-Warner SA, Chavarro JE, et al. Development and validation of an empirical dietary inflammatory index. J Nutr 2016;146:1560–1570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tabung FK, Wang W, Fung TT, et al. Development and validation of empirical indices to assess the insulinaemic potential of diet and lifestyle. Br J Nutr 2016;116:1–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lee DH, Fung TT, Tabung FK, et al. Dietary pattern and risk of multiple myeloma in two large prospective US cohort studies. JNCI Cancer Spectr 2019;3:pkz025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Colditz GA, Manson JE, Hankinson SE. The Nurses’ Health Study: 20-year contribution to the understanding of health among women. J Womens Health 1997;6:49–62 [DOI] [PubMed] [Google Scholar]
- 17.Ley SH, Ardisson Korat AV, Sun Q, et al. Contribution of the Nurses’ Health Studies to uncovering risk factors for type 2 diabetes: diet, lifestyle, biomarkers, and genetics. Am J Public Health 2016;106:1624–1630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rimm EB, Giovannucci EL, Willett WC, et al. Prospective study of alcohol consumption and risk of coronary disease in men. Lancet 1991;338:464–468 [DOI] [PubMed] [Google Scholar]
- 19.Yuan C, Spiegelman D, Rimm EB, et al. Relative validity of nutrient intakes assessed by questionnaire, 24-hour recalls, and diet records as compared with urinary recovery and plasma concentration biomarkers: findings for women. Am J Epidemiol 2018;187:1051–1063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yuan C, Spiegelman D, Rimm EB, et al. Validity of a dietary questionnaire assessed by comparison with multiple weighed dietary records or 24-hour recalls. Am J Epidemiol 2017;185:570–584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Feskanich D, Rimm EB, Giovannucci EL, et al. Reproducibility and validity of food intake measurements from a semiquantitative food frequency questionnaire. J Am Diet Assoc 1993;93:790–796 [DOI] [PubMed] [Google Scholar]
- 22.Hu FB, Rimm E, Smith-Warner SA, et al. Reproducibility and validity of dietary patterns assessed with a food-frequency questionnaire. Am J Clin Nutr 1999;69:243–249 [DOI] [PubMed] [Google Scholar]
- 23.Tabung FK, Nimptsch K, Giovannucci EL. Postprandial duration influences the association of insulin-related dietary indexes and plasma C-peptide concentrations in adult men and women. J Nutr 2019;149:286–294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tabung FK, Smith-Warner SA, Chavarro JE, et al. An empirical dietary inflammatory pattern score enhances prediction of circulating inflammatory biomarkers in adults. J Nutr 2017;147:1567–1577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.National Diabetes Data Group Classification and diagnosis of diabetes mellitus and other categories of glucose intolerance. Diabetes 1979;28:1039–1057 [DOI] [PubMed] [Google Scholar]
- 26.Gavin III Jr., Alberti K, Davidson MB, DeFronzo RA. Report of the expert committee on the diagnosis and classification of diabetes mellitus. Diabetes Care 1997;20:1183–1197 [DOI] [PubMed] [Google Scholar]
- 27.American Diabetes Association Standards of medical care in diabetes—2010 [published correction appears in Diabetes Care 2010;33:692]. In Clinical Practice Recommendations, 2010. Diabetes Care 2010;33(Suppl. 1):S11–S61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Manson JE, Rimm EB, Stampfer MJ, et al. Physical activity and incidence of non-insulin-dependent diabetes mellitus in women. Lancet 1991;338:774–778 [DOI] [PubMed] [Google Scholar]
- 29.Hu FB, Leitzmann MF, Stampfer MJ, Colditz GA, Willett WC, Rimm EB. Physical activity and television watching in relation to risk for type 2 diabetes mellitus in men. Arch Intern Med 2001;161:1542–1548 [DOI] [PubMed] [Google Scholar]
- 30.Willett WC, Howe GR, Kushi LH. Adjustment for total energy intake in epidemiologic studies. Am J Clin Nutr 1997;65(Suppl.):1220S–1228S; discussion 1229S–1231S [DOI] [PubMed] [Google Scholar]
- 31.Shivappa N, Steck SE, Hurley TG, Hussey JR, Hébert JR. Designing and developing a literature-derived, population-based dietary inflammatory index. Public Health Nutr 2014;17:1689–1696 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Denova-Gutiérrez E, Muñoz-Aguirre P, Shivappa N, et al. Dietary inflammatory index and type 2 diabetes mellitus in adults: the Diabetes Mellitus Survey of Mexico City. Nutrients 2018;10:385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jenkins DJ, Wolever TM, Taylor RH, et al. Glycemic index of foods: a physiological basis for carbohydrate exchange. Am J Clin Nutr 1981;34:362–366 [DOI] [PubMed] [Google Scholar]
- 34.Holt SH, Miller JC, Petocz P. An insulin index of foods: the insulin demand generated by 1000-kJ portions of common foods. Am J Clin Nutr 1997;66:1264–1276 [DOI] [PubMed] [Google Scholar]
- 35.Nimptsch K, Brand-Miller JC, Franz M, Sampson L, Willett WC, Giovannucci E. Dietary insulin index and insulin load in relation to biomarkers of glycemic control, plasma lipids, and inflammation markers. Am J Clin Nutr 2011;94:182–190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zafar MI, Mills KE, Zheng J, et al. Low-glycemic index diets as an intervention for diabetes: a systematic review and meta-analysis. Am J Clin Nutr 2019;110:891–902 [DOI] [PubMed] [Google Scholar]
- 37.Bao Y, Nimptsch K, Meyerhardt JA, et al. Dietary insulin load, dietary insulin index, and colorectal cancer. Cancer Epidemiol Biomarkers Prev 2010;19:3020–3026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bao Y, Nimptsch K, Wolpin BM, et al. Dietary insulin load, dietary insulin index, and risk of pancreatic cancer. Am J Clin Nutr 2011;94:862–868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Giovannucci E. A framework to understand diet, physical activity, body weight, and cancer risk. Cancer Causes Control 2018;29:1–6 [DOI] [PubMed] [Google Scholar]
- 40.Tabung FK, Satija A, Fung TT, Clinton SK, Giovannucci EL. Long-term change in both dietary insulinemic and inflammatory potential is associated with weight gain in adult women and men. J Nutr 2019;149:804–815 [DOI] [PMC free article] [PubMed] [Google Scholar]